Effect of Corrosion on the Bond Behavior of Steel-Reinforced, Alkali-Activated Slag Concrete
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Mix Proportions
2.2. Electrically Accelerated Corrosion Test of Steel Bars
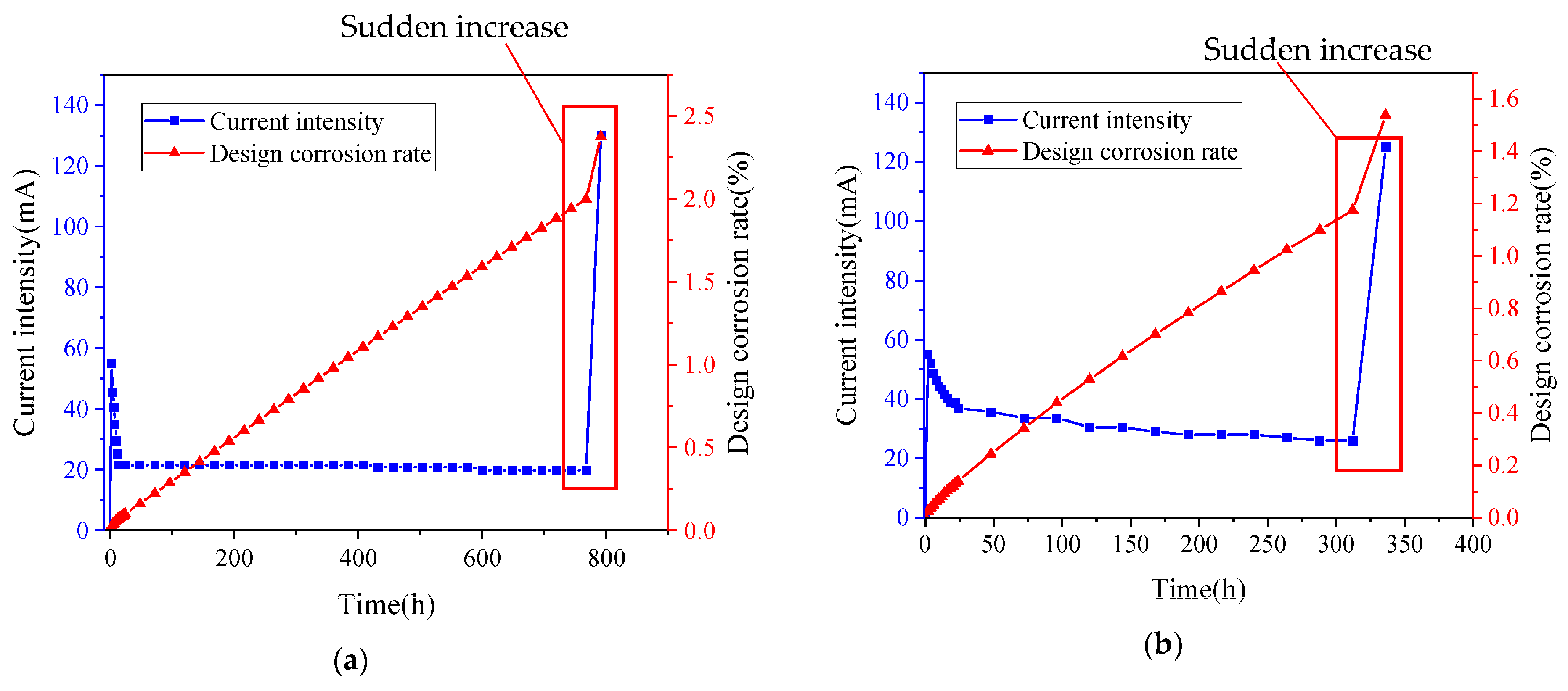
2.2.1. Selection of Corrosion Current Density
2.2.2. Test Procedure for Electrically Accelerated Corrosion of Steel Bars
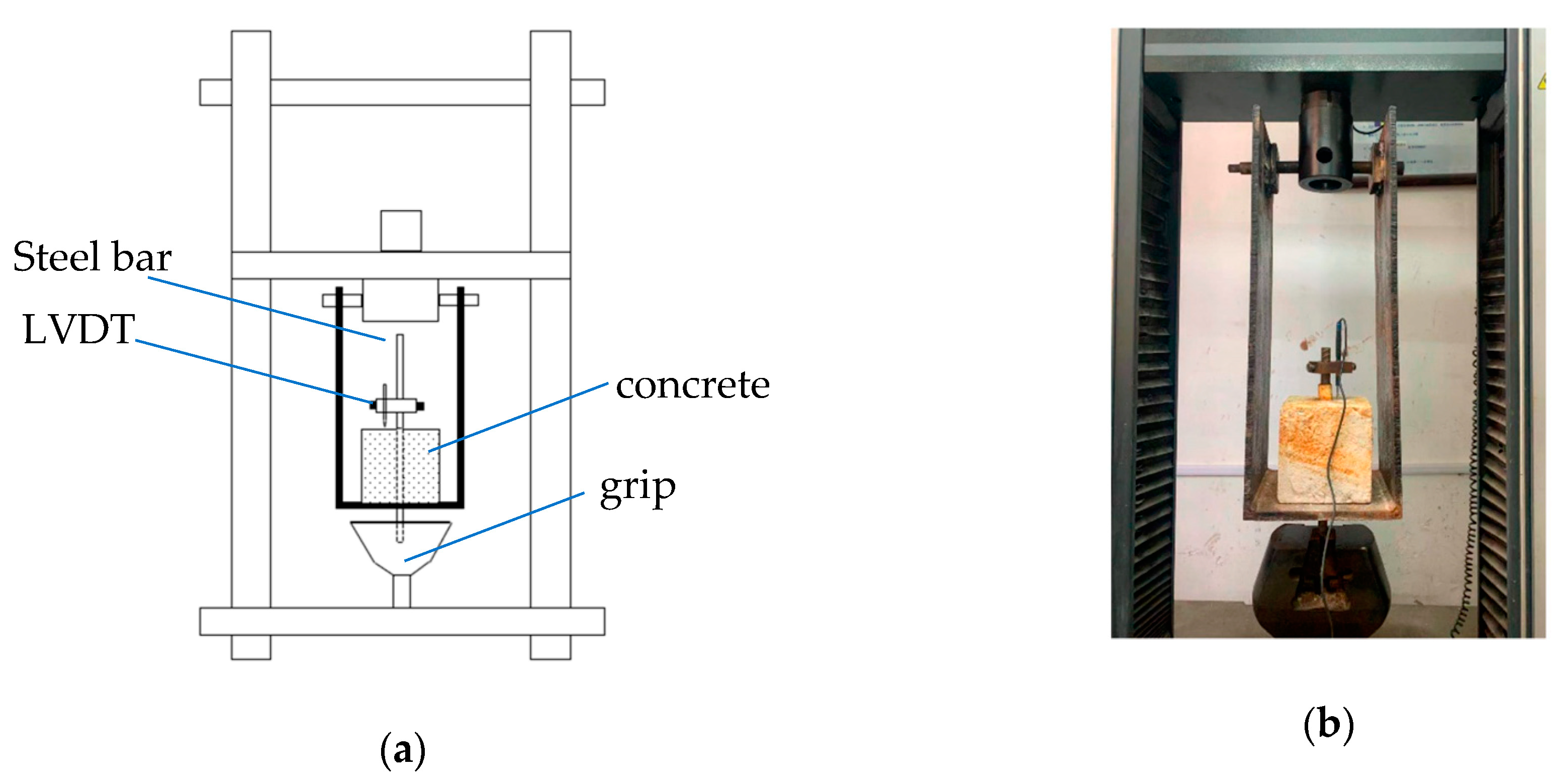
2.3. Pull-Out Test and Measurement of Actual Corrosion
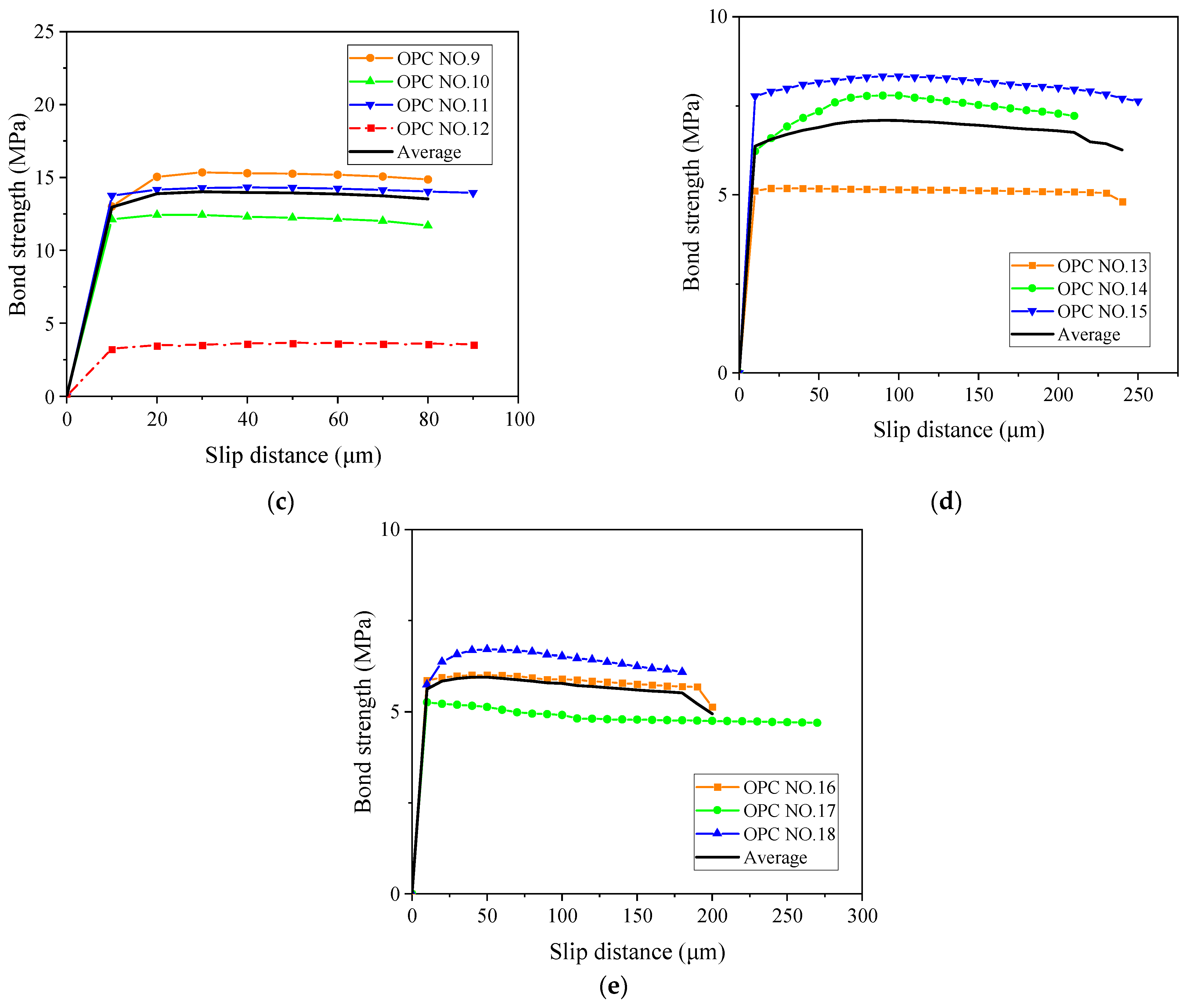
3. Results and Discussion
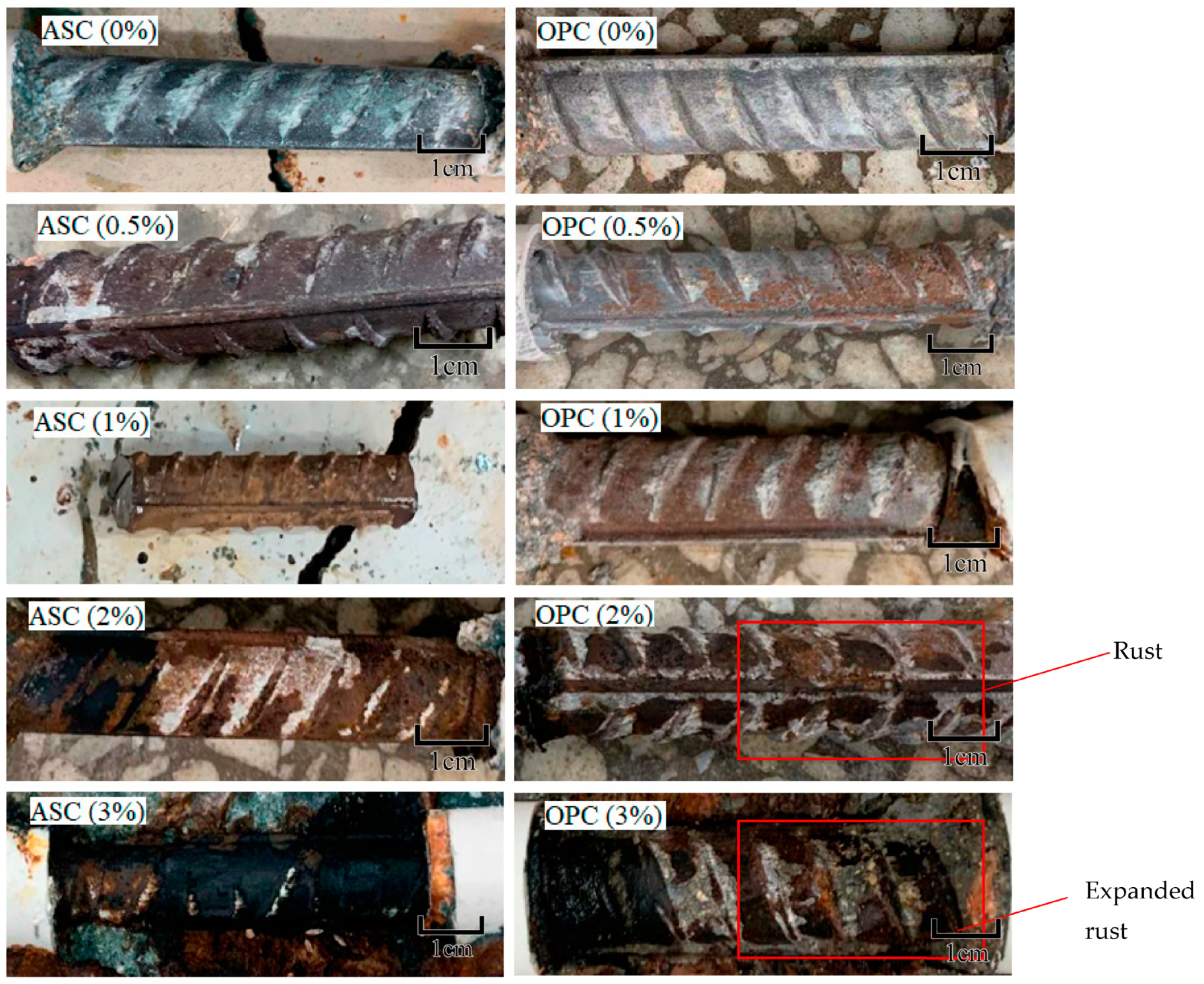
3.1. Corrosion Degree of Steel Bars
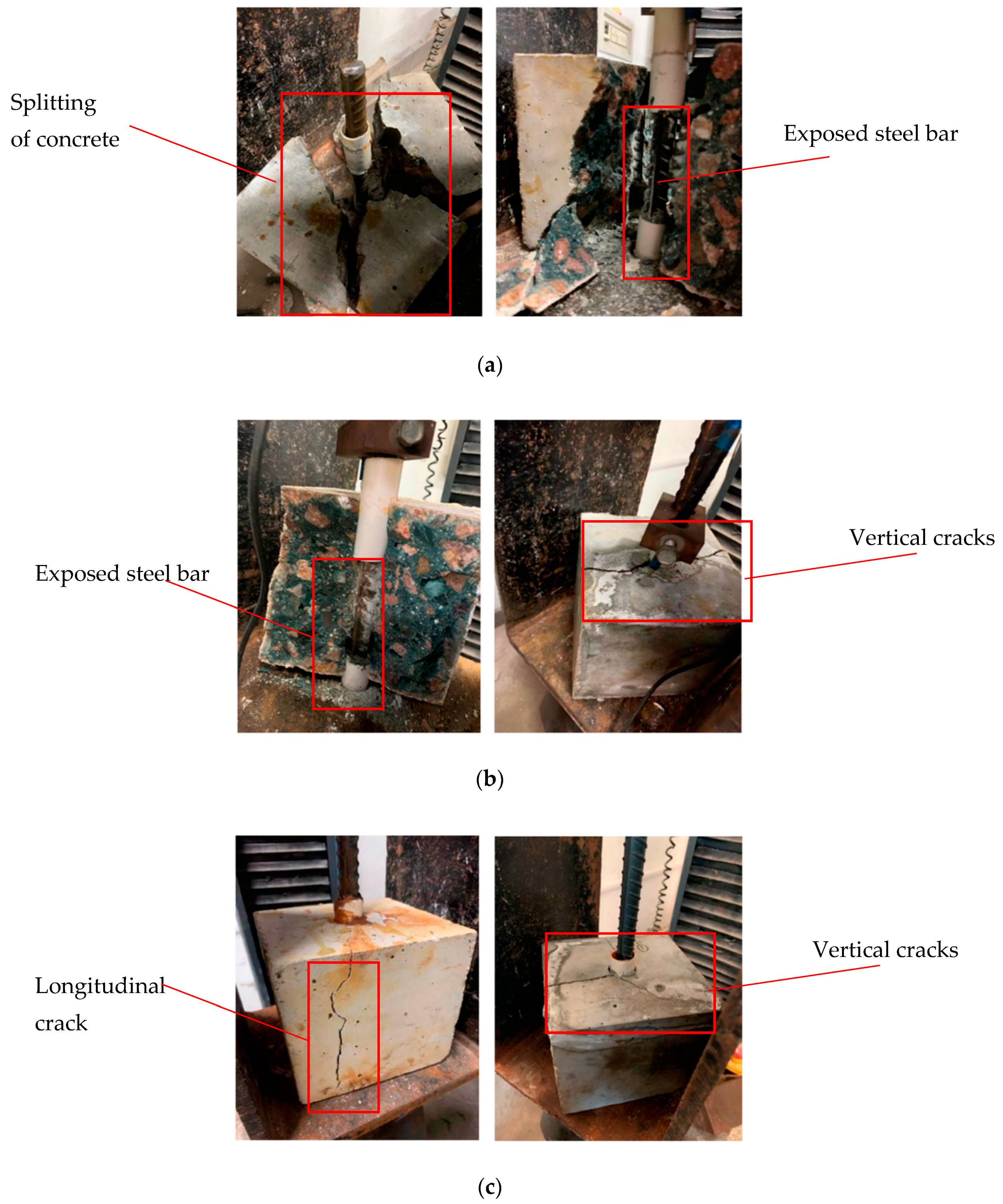
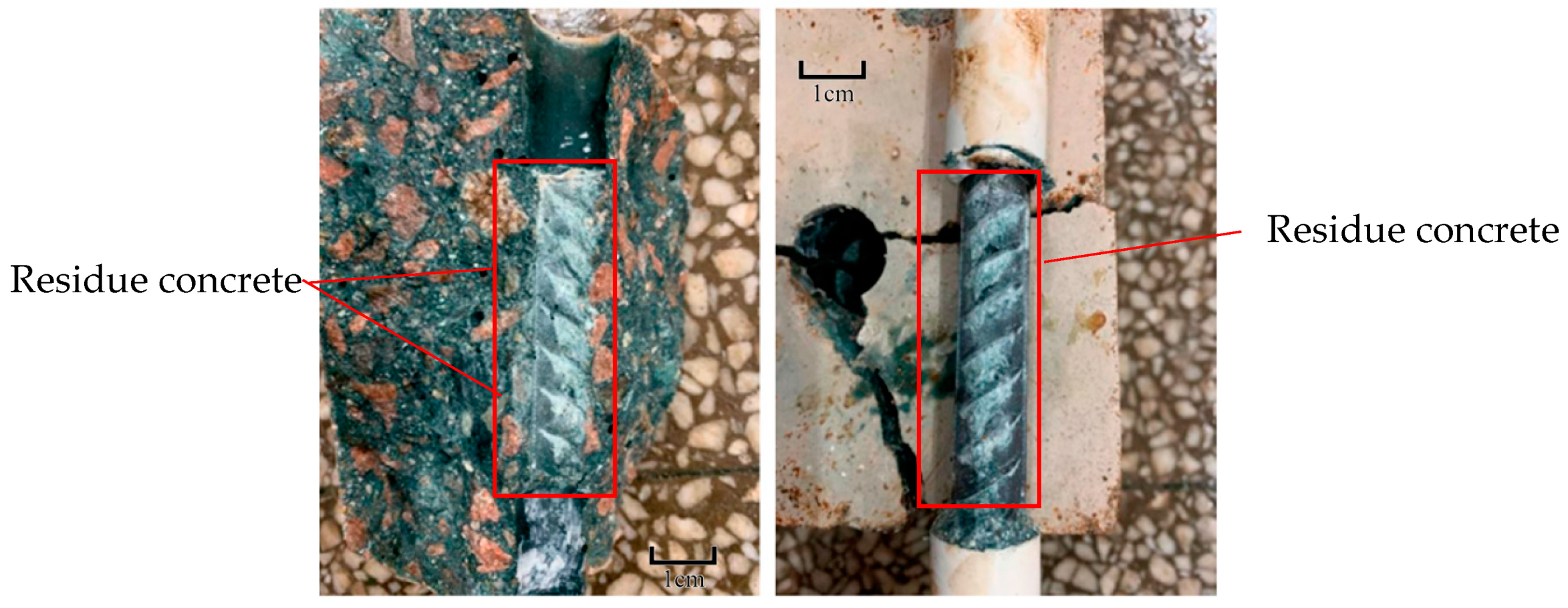
3.2. Failure Modes of the Bond Test
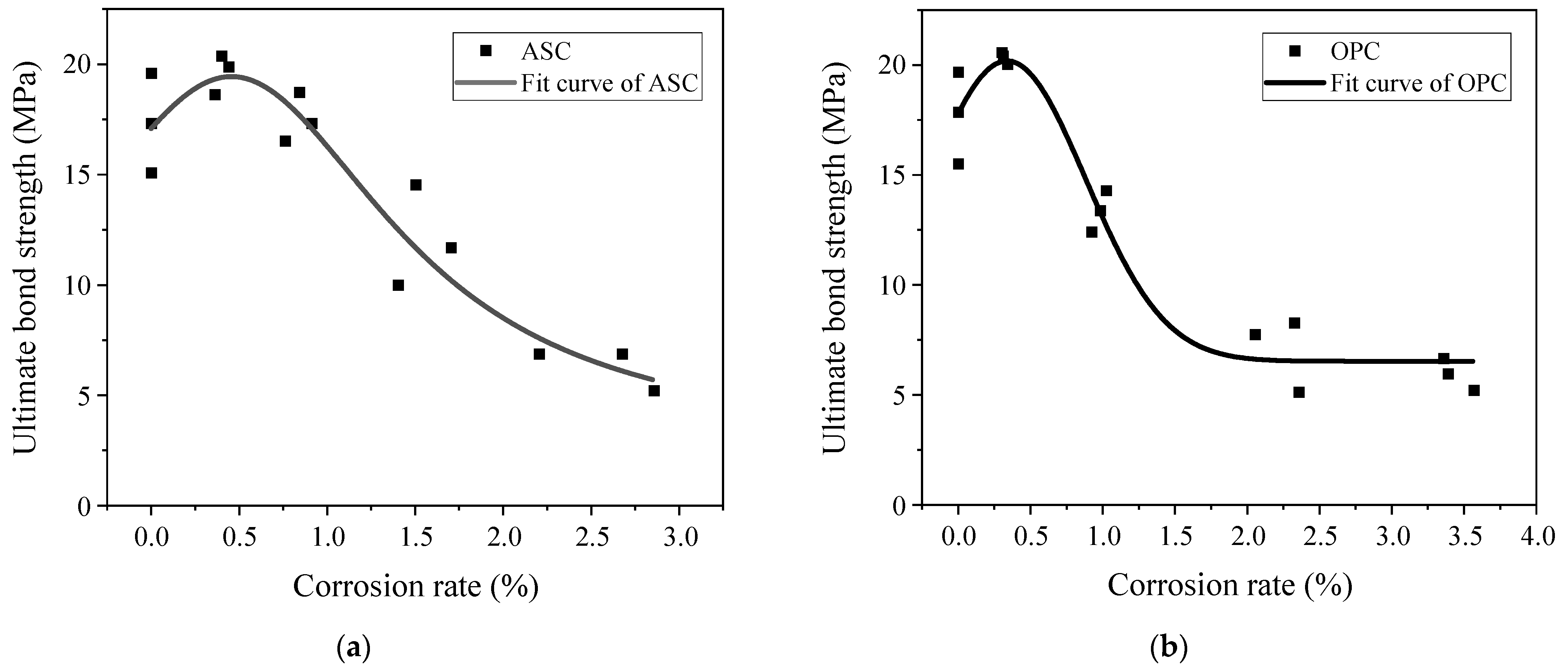
3.3. Effect of Corrosion Degree on Ultimate Bond Strength
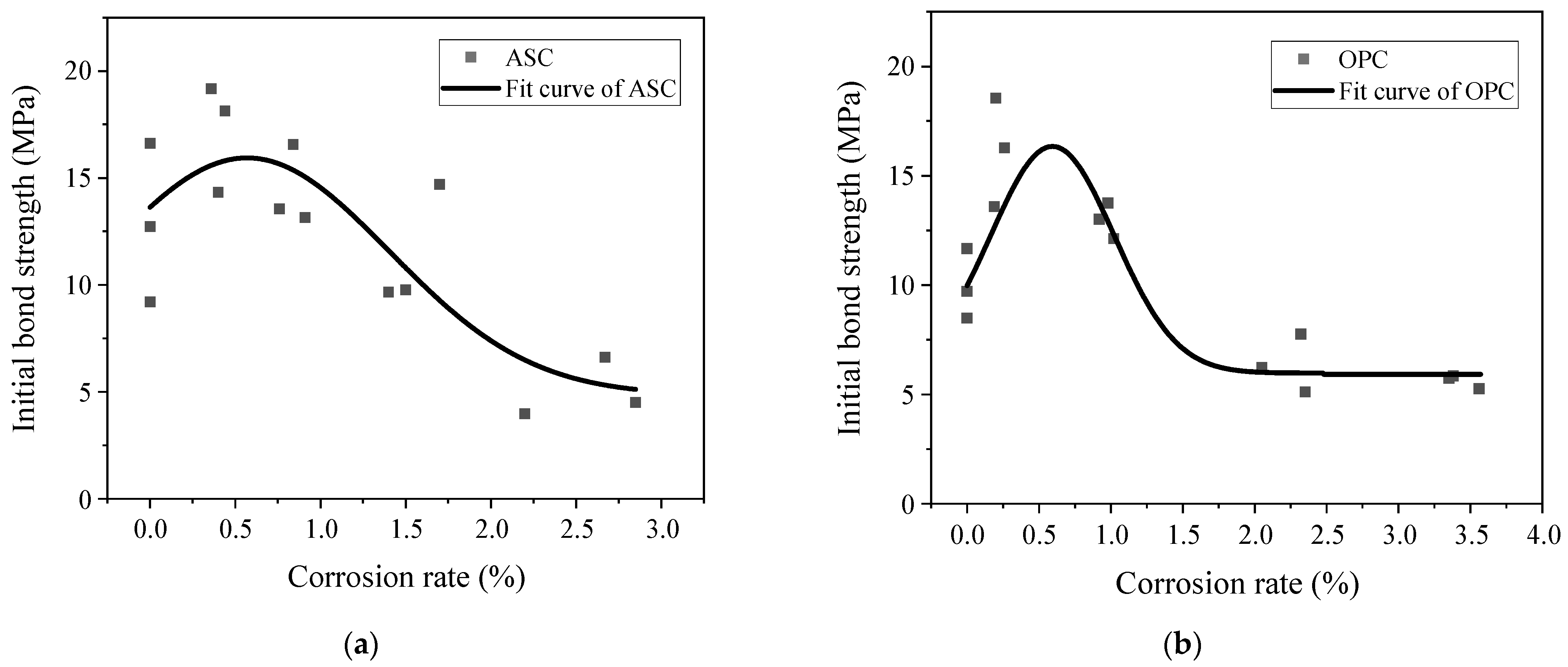
3.4. Effect of Corrosion Degree on Initial Bond Strength
3.5. Effect of Corrosion Degree on Average Bond Strength
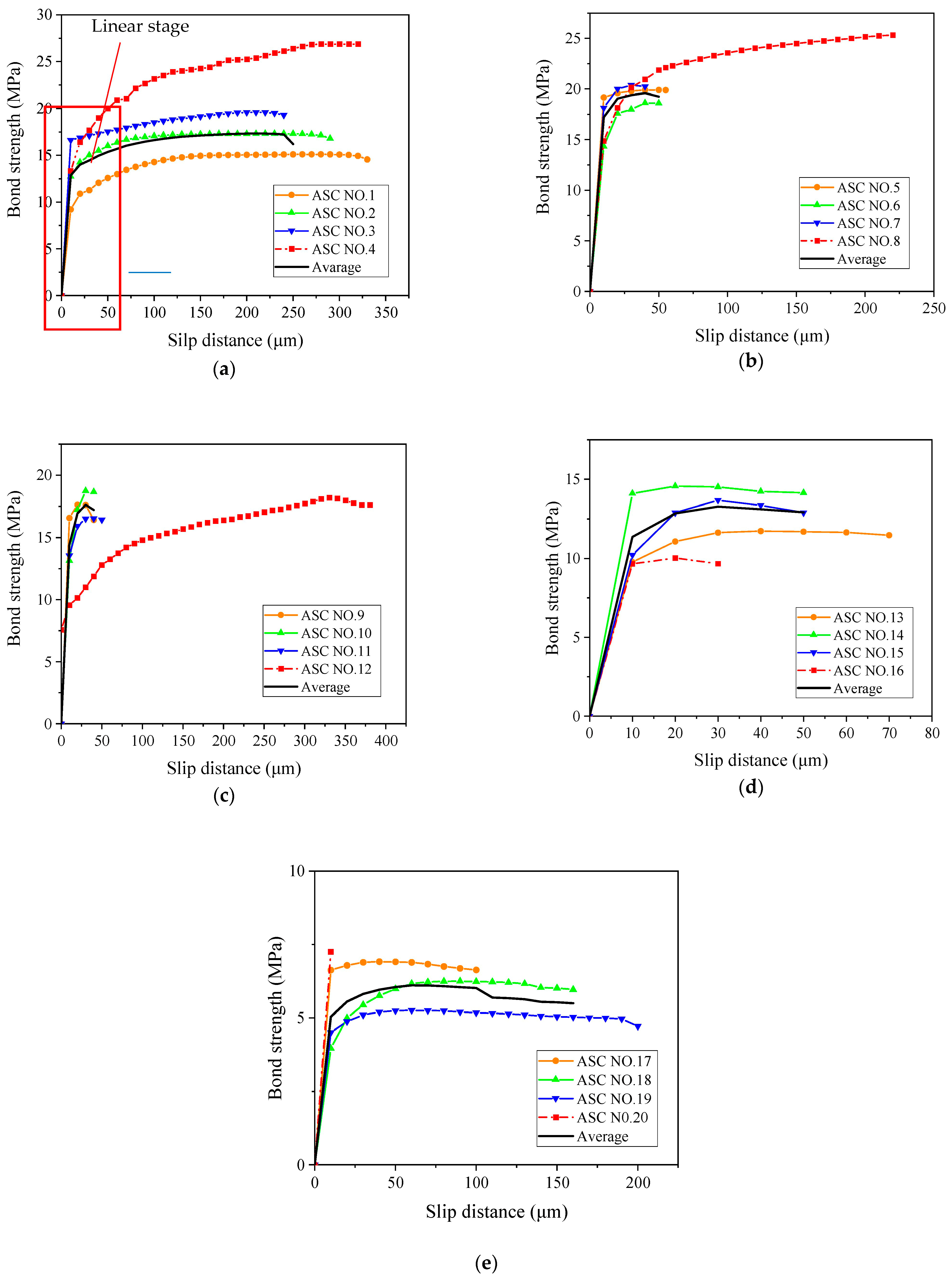
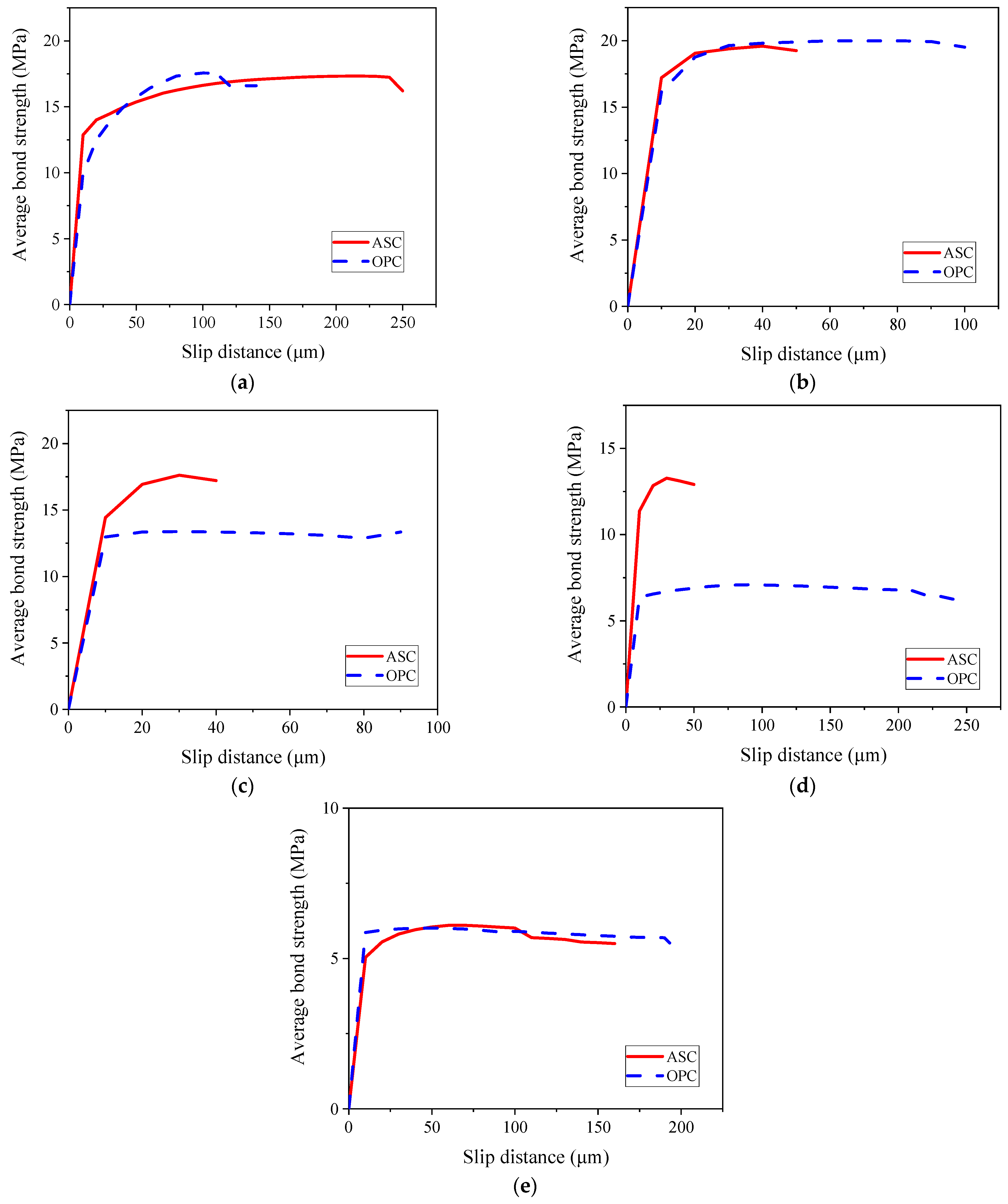
3.6. Comparison of Bond–Slip Curves between ASC and OPC
4. Conclusions
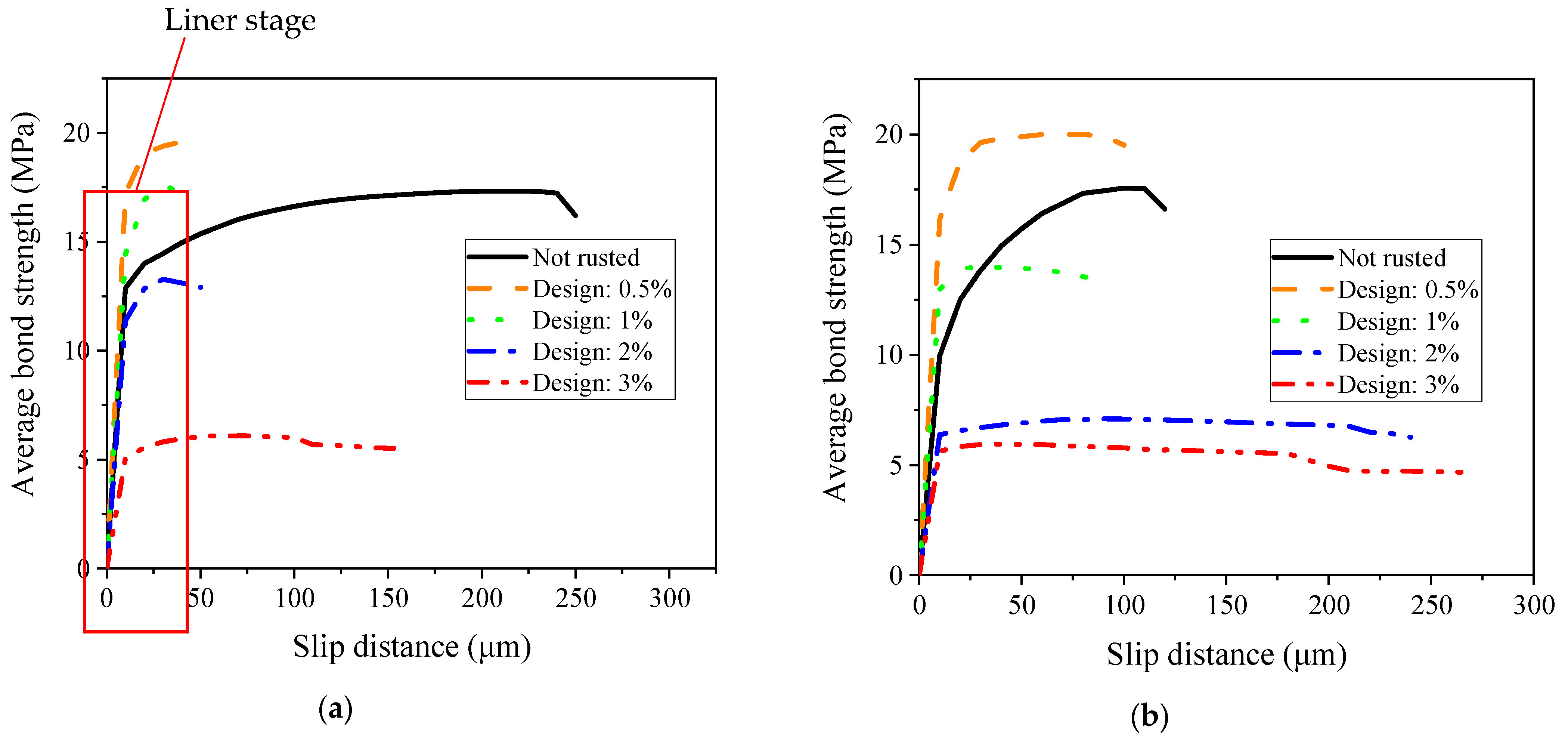
- (a)
- The steel bars were corroded earlier when embedded in OPC. When the corrosion degree of the OPC specimens was approximately 1%, corrosion cracking occurred. The ASC specimens showed corrosion cracking later when the corrosion degree was 2%; this is because of the superior chloride resistance and the compactness of the pore structure of ASC.
- (b)
- The ultimate and initial bond stresses increased with the corrosion degree, up to 0.5%, and then decreased afterward.
- (c)
- The bond strength between ASC and OPC and steel bar is similar for the non-corroded steel bars and at low corrosion levels. As the corrosion degree increased above 0.5%, the OPC specimens showed a sudden drop in bond, while the ASC specimens showed a gradual decrease.
- (d)
- At a 1% corrosion degree, the OPC specimen showed a lower bond strength than the control, while the ASC specimen showed a slightly higher value than the control. This shows the better tolerance of ASC for corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rocha, J.H.A.; Toledo Filho, R.D.; Cayo-Chileno, N.G. Sustainable Alternatives to CO2 Reduction in the Cement Industry: A Short Review. Mater. Today Proc. 2022, 57, 436–439. [Google Scholar] [CrossRef]
- Poudyal, L.; Adhikari, K. Environmental Sustainability in Cement Industry: An Integrated Approach for Green and Economical Cement Production. Resour. Environ. Sustain. 2021, 4, 100024. [Google Scholar] [CrossRef]
- Ameri, F.; Shoaei, P.; Zareei, S.A.; Behforouz, B. Geopolymers vs. Alkali-Activated Materials (AAMs): A Comparative Study on Durability, Microstructure, and Resistance to Elevated Temperatures of Lightweight Mortars. Constr. Build. Mater. 2019, 222, 49–63. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and Carbon Emissions for Geopolymer Pastes in Comparison to Ordinary Portland Cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of Alkali-Activated Binders: A Clear Advantage over Portland Cement or an Unproven Issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Z.; Li, X.; Yu, Q. Effect of Curing Conditions on Freeze-Thaw Resistance of Geopolymer Mortars Containing Various Calcium Resources. Constr. Build. Mater. 2021, 313, 125507. [Google Scholar] [CrossRef]
- Cui, Y.; Qu, S.; Bao, J.; Zhang, P. Bond Performance of Steel Bar and Fly Ash-Based Geopolymer Concrete in Beam End Tests. Polymers 2022, 14, 2012. [Google Scholar] [CrossRef] [PubMed]
- Amer, I.; Kohail, M.; El-Feky, M.S.; Rashad, A.; Khalaf, M.A. A Review on Alkali-Activated Slag Concrete. Ain Shams Eng. J. 2021, 12, 1475–1499. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Hamada, H.M.; Almeshal, I.; Bakar, B.H.A. Durability and Mechanical Properties of Cement Concrete Comprising Pozzolanic Materials with Alkali-Activated Binder: A Comprehensive Review. Case Stud. Constr. Mater. 2022, 17, e01429. [Google Scholar] [CrossRef]
- Fan, L.; Bao, Y. Review of Fiber Optic Sensors for Corrosion Monitoring in Reinforced Concrete. Cem. Concr. Compos. 2021, 120, 104029. [Google Scholar] [CrossRef]
- Saji, V.S. Organic Nanotubes for Smart Anticorrosion and Antibiofouling Coatings. Npj Mater. Degrad. 2022, 6, 30. [Google Scholar] [CrossRef]
- DNV GL. Assessment of Global Cost of Corrosion; Report No. OAPUS310GKOCH (PP110272)-1, Rev. 3, Appendix; DNV: Bærum, Norway, 2015. [Google Scholar]
- Chaparro, W.A.; Ruiz, J.H.B.; Gómez, R.D.J.T. Corrosion of Reinforcing Bars Embedded in Alkali-Activated Slag Concrete Subjected to Chloride Attack. Mater. Res. 2011, 15, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Criado, M.; Provis, J.L. Alkali Activated Slag Mortars Provide High Resistance to Chloride-Induced Corrosion of Steel. Front. Mater. 2018, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wu, M.; Ming, J. Long-Term Corrosion Resistance of Reinforcing Steel in Alkali-Activated Slag Mortar after Exposure to Marine Environments. Corros. Sci. 2021, 179, 109175. [Google Scholar] [CrossRef]
- Farhan, N.A.; Sheikh, M.N.; Hadi, M.N.S. Experimental Investigation on the Effect of Corrosion on the Bond Between Reinforcing Steel Bars and Fibre Reinforced Geopolymer Concrete. Structures 2018, 14, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Huyen Vu, T.; Dang, L.C.; Kang, G.; Sirivivatnanon, V. Chloride Induced Corrosion of Steel Reinforcement in Alkali Activated Slag Concretes: A Critical Review. Case Stud. Constr. Mater. 2022, 16, e01112. [Google Scholar] [CrossRef]
- Lin, F.; Dong, Y.; Kuang, X.; Lu, L. Strain Rate Behavior in Tension of Reinforcing Steels HPB235, HRB335, HRB400, and HRB500. Materials 2016, 9, 1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GB/T 50081; Standard for Test Methods of Concrete Physical and Mechanical Properties. Ministry of Housing and Urban-Rural Development of the PRC: Beijing, China, 2015.
- ZENG Yanhong; GU Xianglin; ZHANG Weiping; HUANG Qinghu Accelerated Corrosion Technique for Reinforcement Steel Bars in Concrete. Struct. Eng. 2009, 1, 101–105.
- Tekle, B.H.; Khennane, A.; Kayali, O. Bond Properties of Sand-Coated GFRP Bars with Fly Ash–Based Geopolymer Concrete. J. Compos. Constr. 2016, 20, 685. [Google Scholar] [CrossRef] [Green Version]
- Van Nguyen, C.; Lambert, P. Effect of Current Density on Accelerated Corrosion of Reinforcing Steel Bars in Concrete. Struct. Infrastruct. Eng. 2018, 14, 1535–1546. [Google Scholar] [CrossRef]
- You, N.; Shi, J.; Zhang, Y. Corrosion Behaviour of Low-Carbon Steel Reinforcement in Alkali-Activated Slag-Steel Slag and Portland Cement-Based Mortars under Simulated Marine Environment. Corros. Sci. 2020, 175, 108874. [Google Scholar] [CrossRef]
- Puertas, F.; de Gutierrez, R.; Fernández-Jiménez, A.; Delvasto, S.; Maldonado, J. Alkaline Cement Mortars. Chemical Resistance to Sulfate and Seawater Attack. Mater. De Constr. 2002, 2002, 326. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.J.; Ariyachandra, E.; Lezama, D.; Peethamparan, S. Comparison of Chloride Permeability Methods for Alkali-Activated Concrete. Constr. Build. Mater. 2018, 165, 16. [Google Scholar] [CrossRef]
- Zhu, Y.; Wan, X.; Han, X.; Ren, J.; Luo, J.; Yu, Q. Solidification of Chloride Ions in Alkali-Activated Slag. Constr. Build. Mater. 2022, 320, 126219. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Yu, T.; Hadi, M.N.S. Factors Affecting the Bond Strength Between the Fly Ash-Based Geopolymer Concrete and Steel Reinforcement. Structures 2018, 14, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Shen, D.; Shi, X.; Zhang, H.; Duan, X.; Jiang, G. Experimental Study of Early-Age Bond Behavior between High Strength Concrete and Steel Bars Using a Pull-out Test. Constr. Build. Mater. 2016, 113, 653–663. [Google Scholar] [CrossRef]
- Gao, K.; Cui, Y.; Zhang, P.; Zhao, T. Research Progress on Reinforcement Corrosion in Alkali Activated Concrete under Chloride Attack. Bull. Chin. Ceram. Soc. 2020, 10, 3070–3077. (In Chinese) [Google Scholar]



| Composition | CaO | SiO2 | Al2O3 | MgO | SO3 | Fe2O3 | K2O | Others |
|---|---|---|---|---|---|---|---|---|
| GGBS | 38.95 | 33.35 | 17.96 | 6.02 | 1.39 | 0.382 | 0.318 | 1.63 |
| Cement | 57.1 | 21.6 | 7.12 | 4.15 | 3.06 | 4.43 | 0.721 | 1.779 |
| Water Glass | Na2O (%) | SiO2 (%) | Baume Degrees | Modulus | Transparency |
|---|---|---|---|---|---|
| 26.96 | 9.02 | 39.5–40.2 | 3.07 | ≥82 |
| NaOH | Purity | Carbonate | Chloride | Sulfate | Al | Ca |
|---|---|---|---|---|---|---|
| Content (%) | ≥99.5 | ≤1.5 | ≤0.005 | ≤0.005 | ≤0.002 | ≤0.01 |
| Material | Cement | Water | Coarse Aggregate | Sand | GGBS | Activator | Compressive Strength |
|---|---|---|---|---|---|---|---|
| OPC | 450 | 157.5 | 1125 | 675 | - | - | 61.0 ± 2.4 |
| ASC | - | - | 1074 | 716 | 400 | 184 | 64.1 ± 1.3 |
| ASC | Design Corrosion Degree (%) | Measured Corrosion Degree (%) | Average Corrosion Degree (%) | OPC | Design Corrosion Degree (%) | Measured Corrosion Degree (%) | Average Corrosion Degree (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 2 | 0 | 0 | ||
| 3 | 0 | 0 | 3 | 0 | 0 | ||
| 4 | 0.5 | 0.4 | 0.4 | 4 | 0.5 | 0.26 | 0.22 |
| 5 | 0.5 | 0.44 | 5 | 0.5 | 0.2 | ||
| 6 | 0.5 | 0.36 | 6 | 0.5 | 0.19 | ||
| 7 | 1 | 0.84 | 0.84 | 7 | 1 | 0.92 | 0.97 |
| 8 | 1 | 0.91 | 8 | 1 | 1.02 | ||
| 9 | 1 | 0.76 | 9 | 1 | 0.98 | ||
| 10 | 2 | 1.5 | 1.53 | 10 | 2 | 2.05 * | 2.24 |
| 11 | 2 | 1.7 | 11 | 2 | 2.32 * | ||
| 12 | 2 | 1.4 | 12 | 2 | 2.35 * | ||
| 13 | 3 | 2.2 * | 2.57 | 13 | 3 | 3.56 * | 3.43 |
| 14 | 3 | 2.85 * | 14 | 3 | 3.35 * | ||
| 15 | 3 | 2.67 * | 15 | 3 | 3.38 * |
| ASC | Corrosion Degree (%) | Ultimate Bond Strength (Mpa) | OPC | Corrosion Degree (%) | Ultimate Bond Strength (Mpa) |
|---|---|---|---|---|---|
| 1 | 0 | 17.11 | 1 | 0 | 15.52 |
| 2 | 0 | 18.60 | 2 | 0 | 17.85 |
| 3 | 0 | 15.11 | 3 | 0 | 19.68 |
| 4 | 0.4 | 18.63 | 4 | 0.26 | 20.04 |
| 5 | 0.44 | 20.24 | 5 | 0.2 | 20.03 |
| 6 | 0.36 | 19.88 | 6 | 0.19 | 20.57 |
| 7 | 0.84 | 17.62 | 7 | 0.92 | 13.40 |
| 8 | 0.91 | 18.74 | 8 | 1.02 | 12.44 |
| 9 | 0.76 | 16.54 | 9 | 0.98 | 14.31 |
| 10 | 1.5 | 11.733 | 10 | 2.05 * | 7.79 |
| 11 | 1.7 | 14.56 | 11 | 2.32 * | 8.32 |
| 12 | 1.4 | 10.02 | 12 | 2.35 * | 5.18 |
| 13 | 2.2 * | 6.24 | 13 | 3.56 * | 5.26 |
| 14 | 2.85 * | 5.25 | 14 | 3.35 * | 6.71 |
| 15 | 2.67 * | 6.91 | 15 | 3.38 * | 6.01 |
| Designed Corrosion Rate (%) | 0 | 0.5 | 1 | 2 | 3 | |
|---|---|---|---|---|---|---|
| Ultimate Bond Strength (MPa) | ||||||
| ASC | 17.34 | 19.62 | 17.54 | 12.1 | 6.36 | |
| OPC | 17.68 | 20.34 | 13.38 | 7.09 | 5.99 | |
| n | 1.01 | 1.03 | 0.76 | 0.58 | 0.94 | |
| ASC | Corrosion Degree (%) | Initial Bond Strength (MPa) | OPC | Corrosion Degree (%) | Initial Bond Strength (MPa) |
|---|---|---|---|---|---|
| 1 | 0 | 12.73 | 1 | 0 | 9.71 |
| 2 | 0 | 16.62 | 2 | 0 | 8.49 |
| 3 | 0 | 9.21 | 3 | 0 | 11.67 |
| 4 | 0.4 | 14.33 | 4 | 0.26 | 16.28 |
| 5 | 0.44 | 18.13 | 5 | 0.2 | 18.55 |
| 6 | 0.36 | 19.17 | 6 | 0.19 | 13.59 |
| 7 | 0.84 | 16.57 | 7 | 0.92 | 13.01 |
| 8 | 0.91 | 13.15 | 8 | 1.02 | 12.12 |
| 9 | 0.76 | 13.55 | 9 | 0.98 | 13.75 |
| 10 | 1.5 | 9.77 | 10 | 2.05 * | 6.22 |
| 11 | 1.7 | 14.69 | 11 | 2.32 * | 7.76 |
| 12 | 1.4 | 9.66 | 12 | 2.35 * | 5.11 |
| 13 | 2.2 * | 3.97 | 13 | 3.56 * | 5.26 |
| 14 | 2.85 * | 4.5 | 14 | 3.35 * | 5.74 |
| 15 | 2.67 * | 6.62 | 15 | 3.38 * | 5.84 |
| Design Corrosion Rate (%) | 0 | 0.5 | 1 | 2 | 3 | |
|---|---|---|---|---|---|---|
| Ultimate Bond Strength (MPa) | ||||||
| ASC | 12.85 | 17.21 | 14.42 | 11.17 | 5.03 | |
| OPC | 9.95 | 16.14 | 12.96 | 6.36 | 5.62 | |
| m | 0.77 | 0.93 | 0.89 | 0.56 | 1.11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Qu, S.; Gao, K.; Tekle, B.H.; Bao, J.; Zhang, P. Effect of Corrosion on the Bond Behavior of Steel-Reinforced, Alkali-Activated Slag Concrete. Materials 2023, 16, 2262. https://doi.org/10.3390/ma16062262
Cui Y, Qu S, Gao K, Tekle BH, Bao J, Zhang P. Effect of Corrosion on the Bond Behavior of Steel-Reinforced, Alkali-Activated Slag Concrete. Materials. 2023; 16(6):2262. https://doi.org/10.3390/ma16062262
Chicago/Turabian StyleCui, Yifei, Shihao Qu, Kaikai Gao, Biruk Hailu Tekle, Jiuwen Bao, and Peng Zhang. 2023. "Effect of Corrosion on the Bond Behavior of Steel-Reinforced, Alkali-Activated Slag Concrete" Materials 16, no. 6: 2262. https://doi.org/10.3390/ma16062262






