Spherical Attapulgite/Silica Aerogels Fabricated via Different Drying Methods with Excellent Adsorption Performance
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
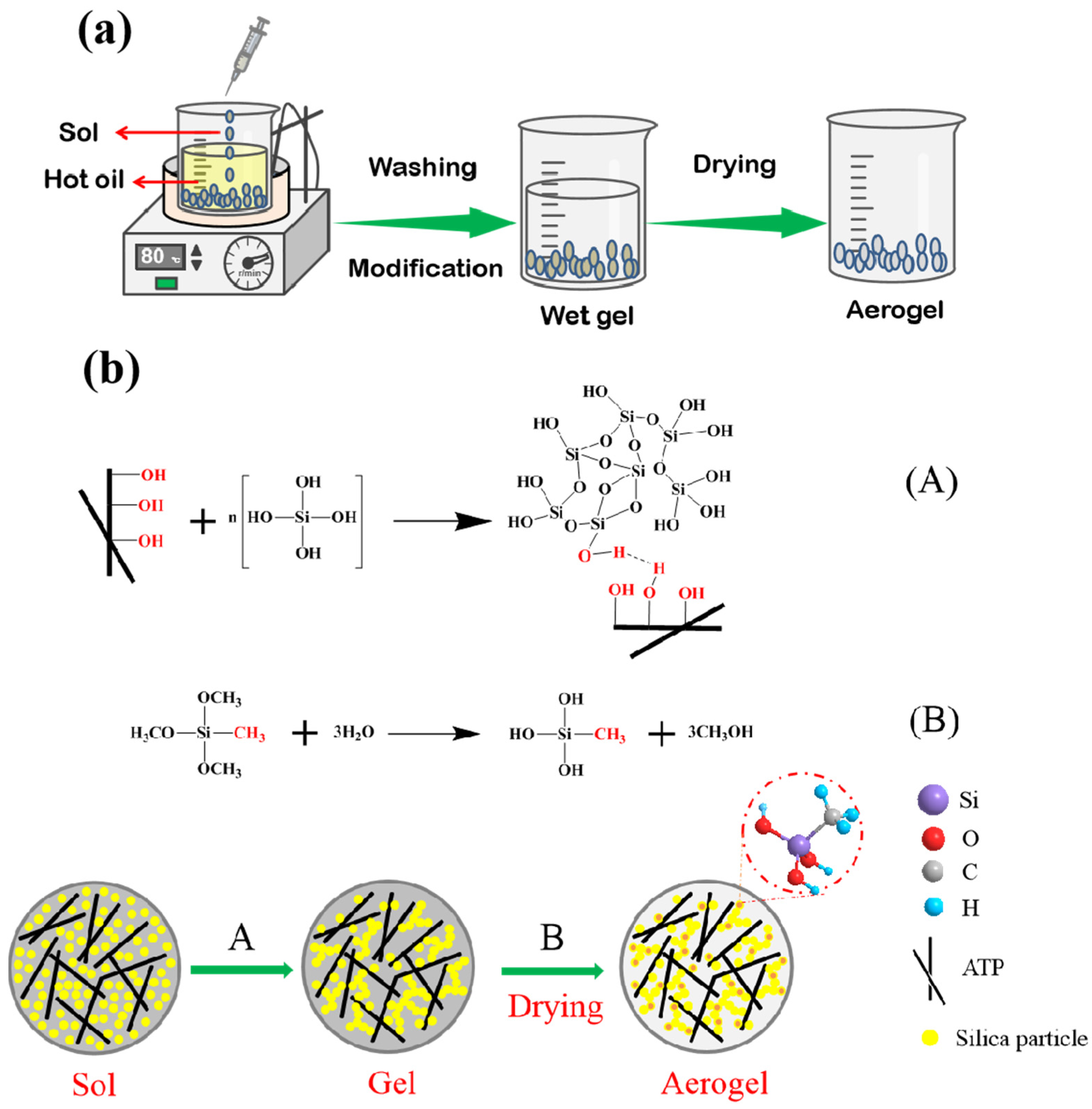
2.2.1. Synthesis of Spherical ATP/SiO2 Gels
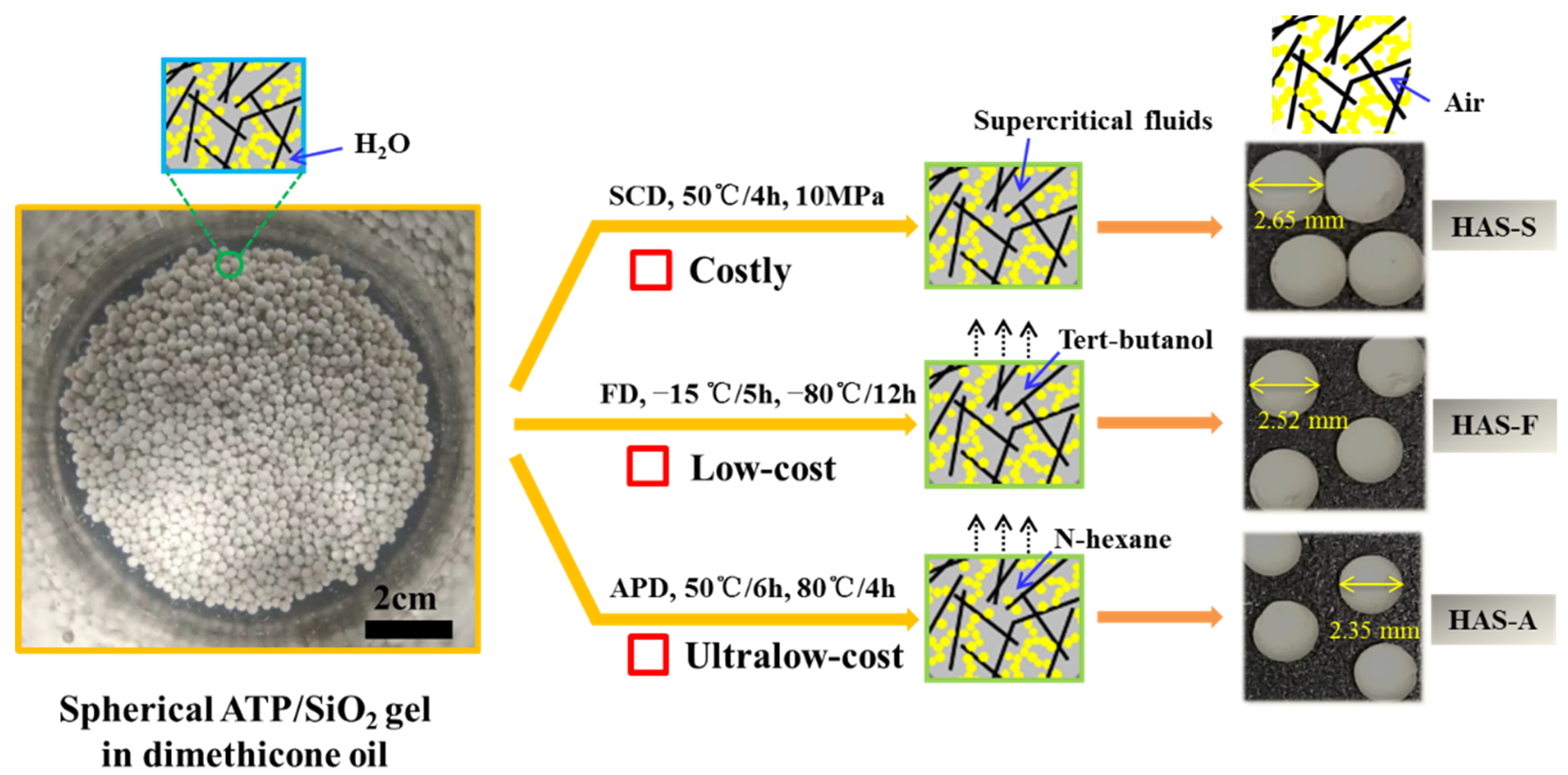
2.2.2. Preparation of Spherical ATP/SiO2 Aerogels Using Different Treatment Techniques
2.3. Characterization
2.4. Adsorption Experiment
3. Results and Discussion
3.1. Formation Mechanism of the Spherical ATP/SiO2 Aerogels
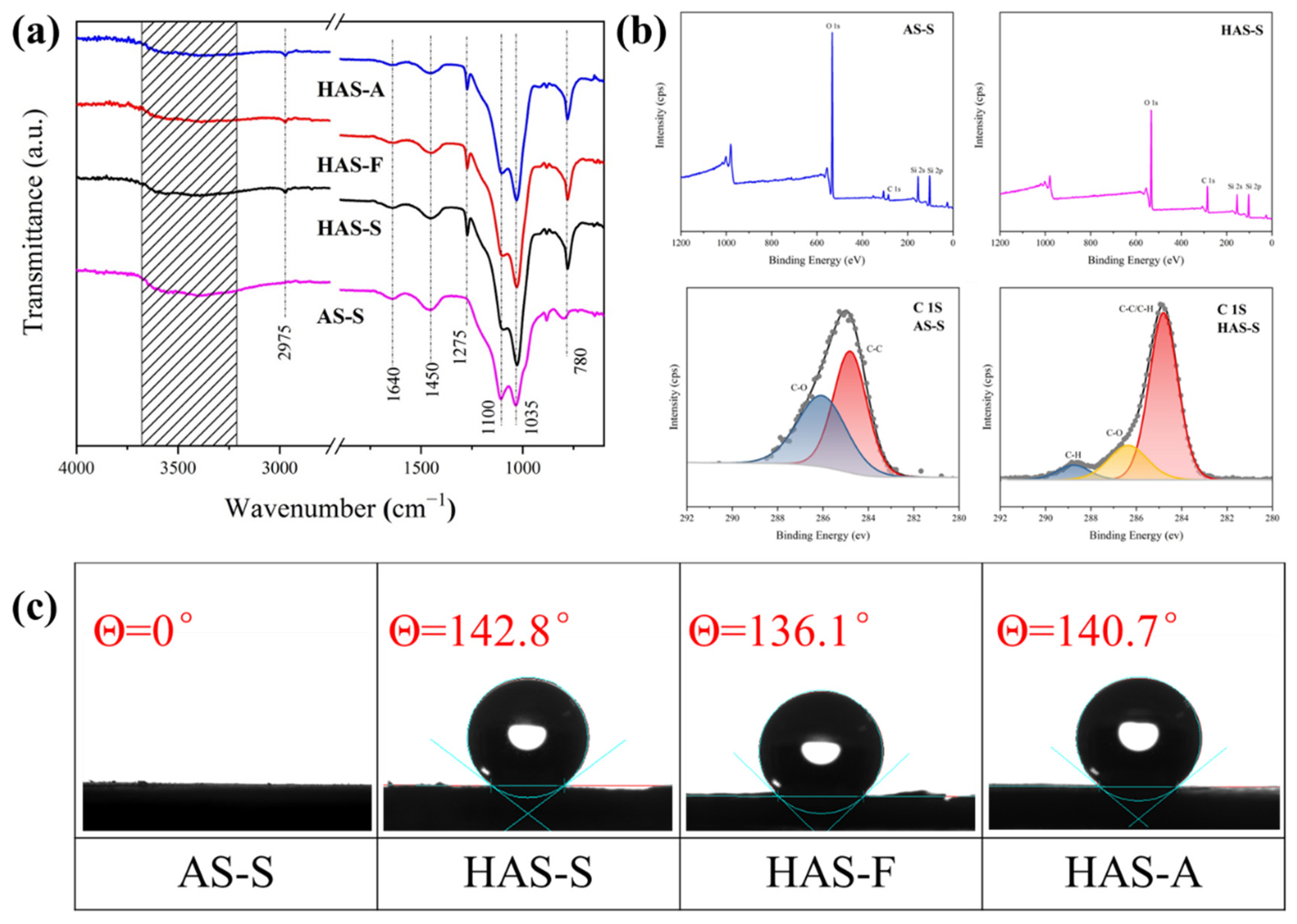
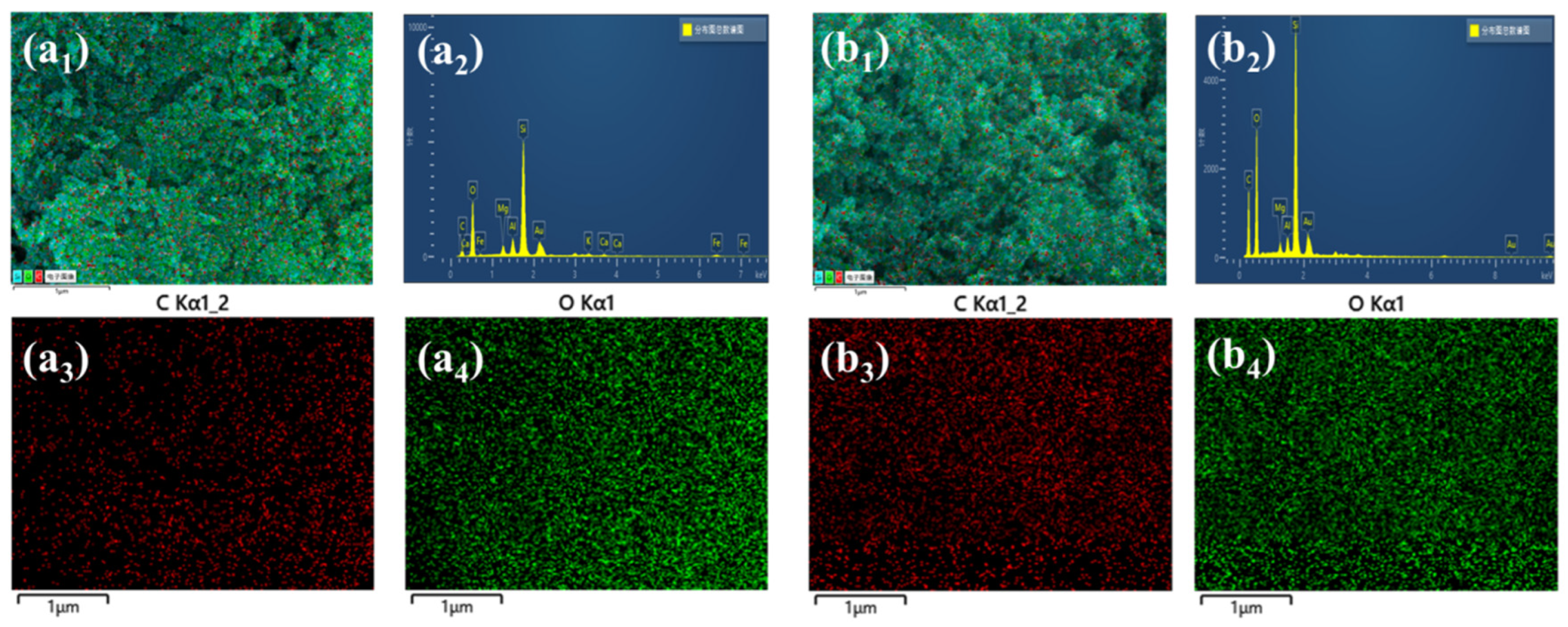
3.2. Effect of Drying Techniques and MTMS Modification on Physical Characteristic and Morphological Properties of Spherical ATP/SiO2 Aerogels
3.3. Effect of Heat Treatment on Physical Characteristic and Morphological Properties of HAS-A
3.4. Adsorption Studies
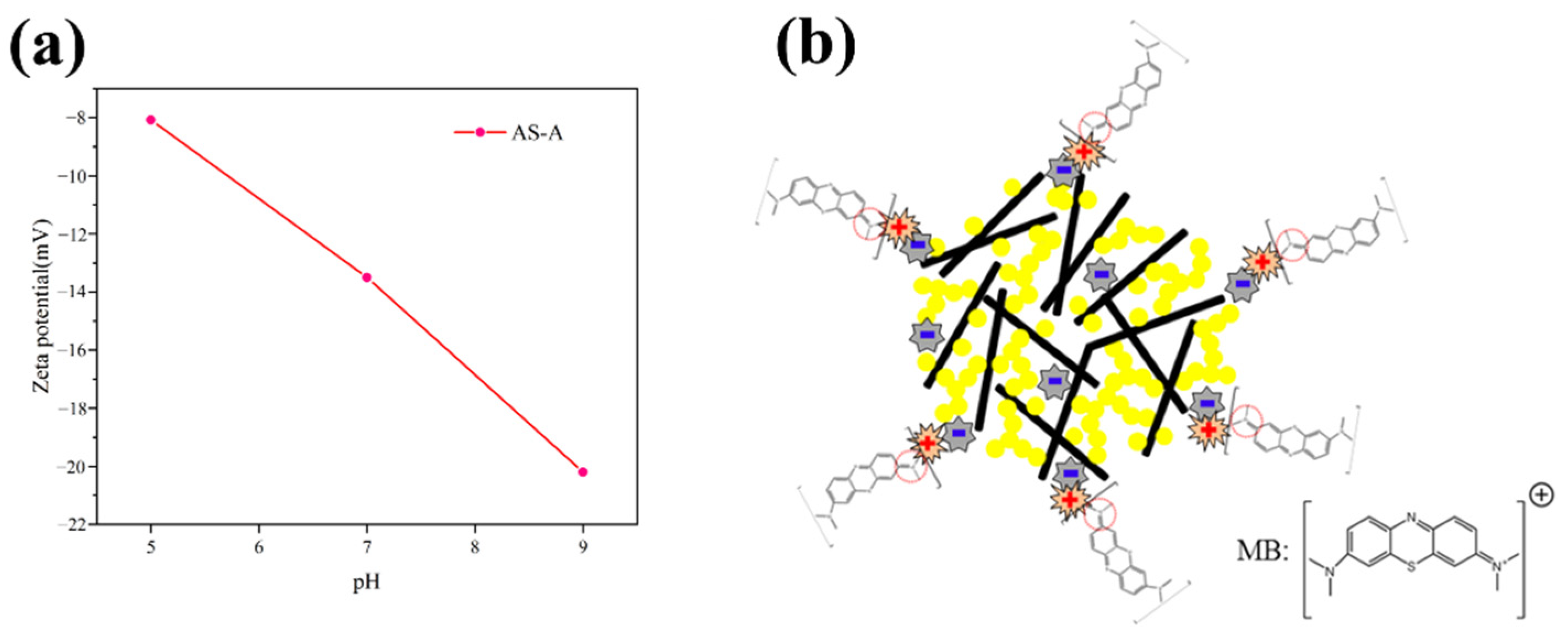
3.4.1. Effect of the Solution pH on MB Removal by AS-A
3.4.2. Effect of Adsorption Time on MB Removal
3.4.3. Effect of Initial Concentration on MB Removal
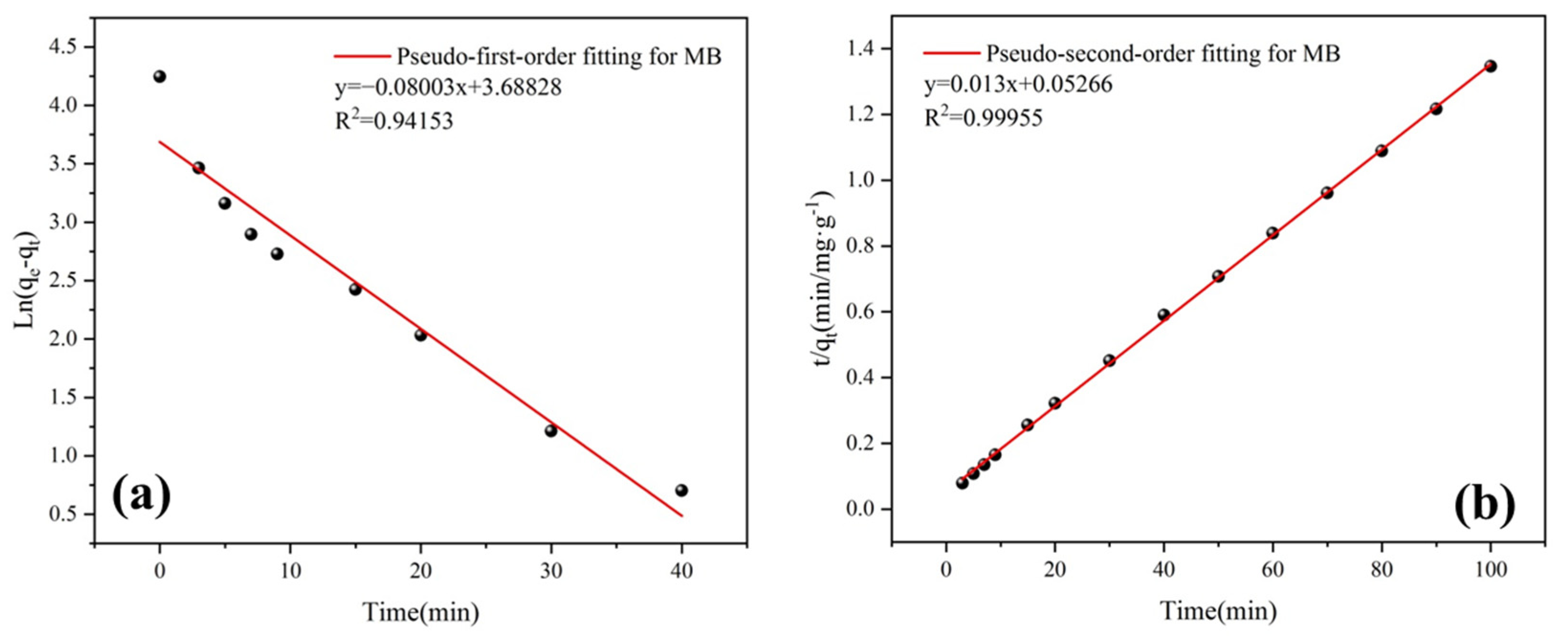
3.4.4. Adsorption Kinetics
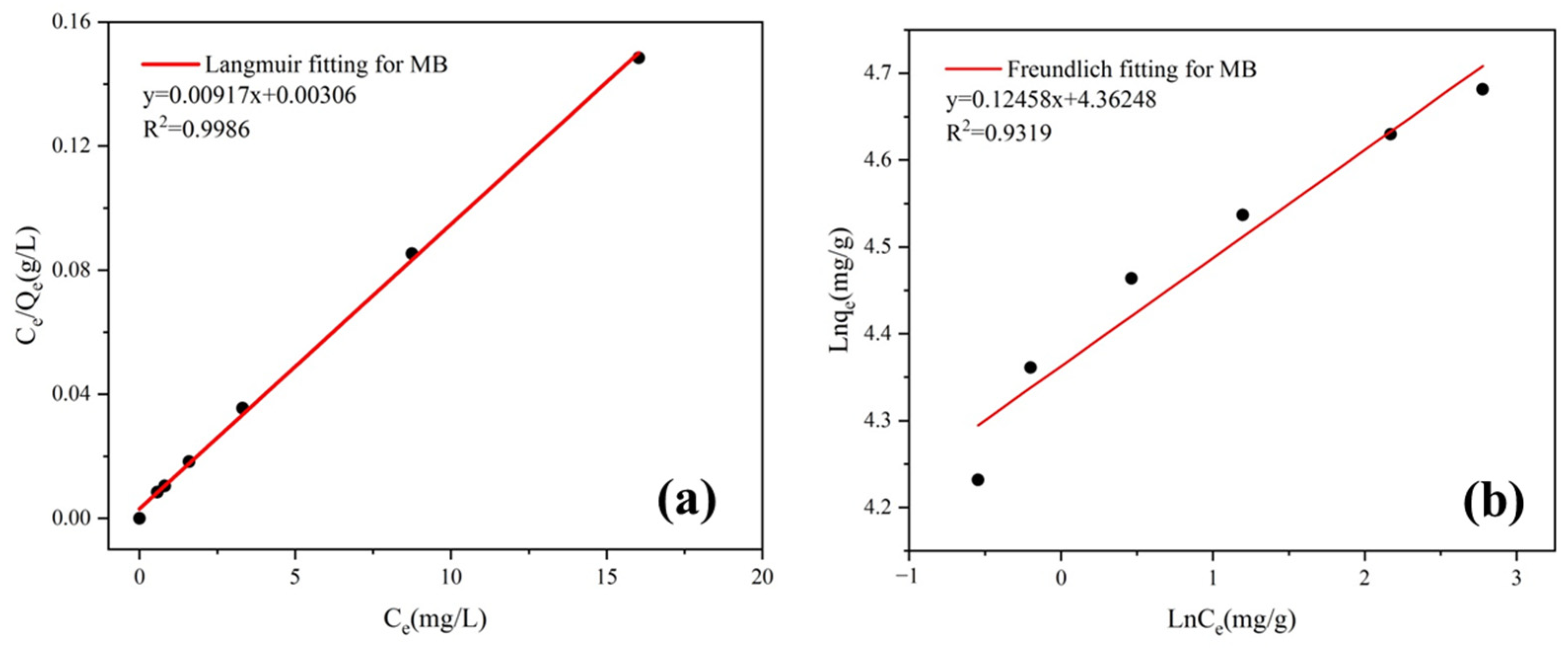
3.4.5. Adsorption Isotherms
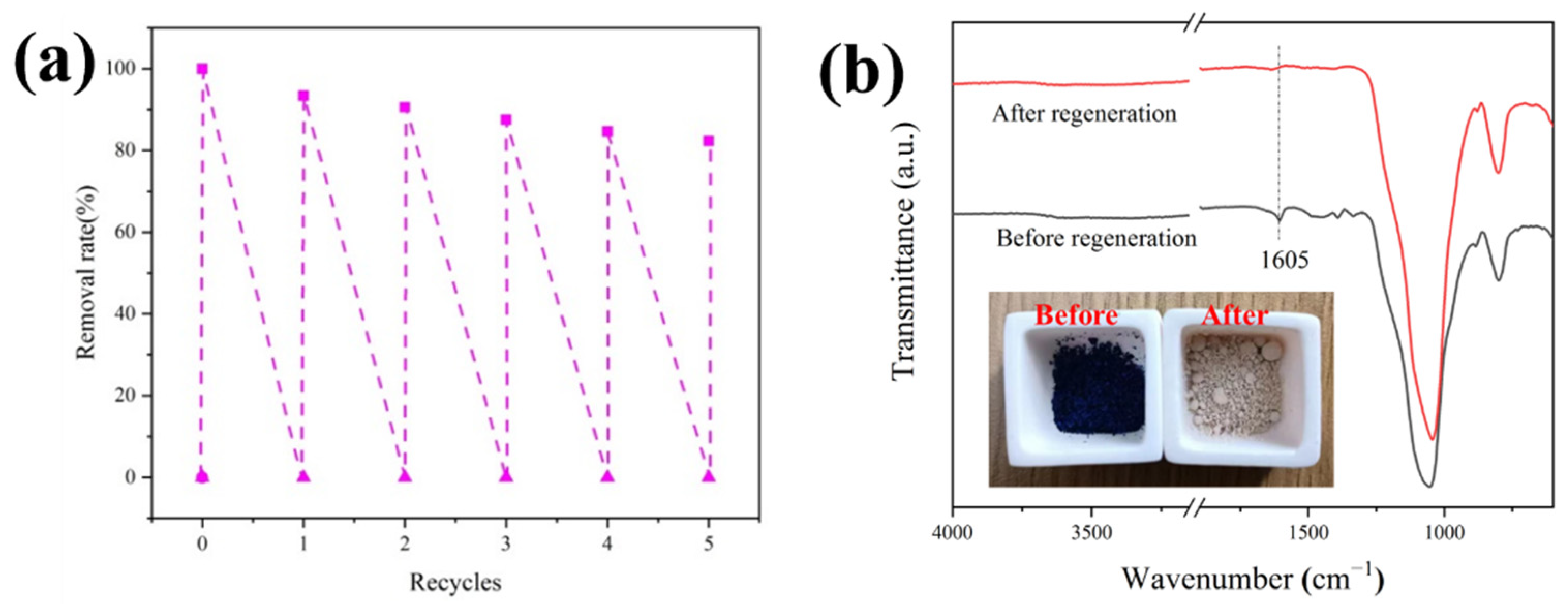
3.4.6. Recycling Studies
3.4.7. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.S.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef]
- Moradihamedani, P. Recent advances in dye removal from wastewater by membrane technology: A review. Polym. Bull. 2022, 79, 2603–2631. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Rafaqat, S.; Ali, N.; Torres, C.; Rittmann, B. Recent progress in treatment of dyes wastewater using microbial-electro-Fenton technology. RSC Adv. 2022, 12, 17104–17137. [Google Scholar] [CrossRef]
- Li, W.; Mu, B.N.; Yang, Y.Q. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef]
- Chang, Z.T.; Chen, Y.J.; Tang, S.X.; Yang, J.Y.; Chen, Y.; Chen, S.S.; Li, P.W.; Yang, Z.M. Construction of chitosan/polyacrylate/graphene oxide composite physical hydrogel by semi-dissolution/acidification/sol-gel transition method and its simultaneous cationic and anionic dye adsorption properties. Carbohydr. Polym. 2020, 229, 115431. [Google Scholar] [CrossRef] [PubMed]
- Krishna Kumar, A.S.; Warchol, J.; Matusik, J.; Tseng, W.-L.; Rajesh, N.; Bajda, T. Heavy metal and organic dye removal via a hybrid porous hexagonal boron nitride-based magnetic aerogel. NPJ Clean Water 2022, 5, 24. [Google Scholar] [CrossRef]
- Yao, C.; Dong, X.; Gao, G.; Sha, F.; Xu, D. Microstructure and Adsorption Properties of MTMS/TEOS Co-precursor Silica Aerogels Dried at Ambient Pressure. J. Non-Cryst. Solids 2021, 562, 120778. [Google Scholar] [CrossRef]
- Jiang, X.; Kong, Y.; Zhao, Z.Y.; Shen, X.D. Spherical amine grafted silica aerogels for CO2 capture. RSC Adv. 2020, 10, 25911–25917. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.G.; Tang, Q.; Jiang, T.; Cheng, Y. Adsorption performance of hydrophobic/hydrophilic silica aerogel for low concentration organic pollutant in aqueous solution. Nanotechnol. Rev. 2019, 8, 266–274. [Google Scholar] [CrossRef]
- Wei, W.; Hu, H.H.; Ji, X.L.; Yan, Z.X.; Sun, W.; Xie, J.M. Selective adsorption of organic dyes by porous hydrophilic silica aerogels from aqueous system. Water Sci. Technol. 2018, 78, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Wu, Z.X.; Chen, H.F.; Du, Q.X.; Yu, L.; Zhang, R.Y.; Zhou, Y. A Facile Preparation of Ambient Pressure-Dried Hydrophilic Silica Aerogels and Their Application in Aqueous Dye Removal. Front. Mater. 2020, 7, 152. [Google Scholar] [CrossRef]
- Zu, G.Q.; Shimizu, T.; Kanamori, K.; Zhu, Y.; Maeno, A.; Kaji, H.; Shen, J.; Nakanishi, K. Transparent, Superflexible Doubly Cross-Linked Polyvinylpolymethylsiloxane Aerogel Superinsulators via Ambient Pressure Drying. ACS Nano 2018, 12, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.B.; Makki, A.; Hajjar, D.; Sarawade, P.B. Synthesis of light weight recron fiber-reinforced sodium silicate based silica aerogel blankets at an ambient pressure for thermal protection. J. Porous Mat. 2022, 29, 957–969. [Google Scholar] [CrossRef]
- Shi, W.; Ching, Y.C.; Chuah, C.H. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review. Int. J. Biol. Macromol. 2021, 170, 751–767. [Google Scholar] [CrossRef]
- Zong, S.K.; Wei, W.; Jiang, Z.F.; Yan, Z.X.; Zhu, J.J.; Xie, J.M. Characterization and comparison of uniform hydrophilic/hydrophobic transparent silica aerogel beads: Skeleton strength and surface modification. RSC Adv. 2015, 5, 55579–55587. [Google Scholar] [CrossRef]
- Tian, J.Q.; Shafi, S.; Tan, H.J.; Zhao, Y.P. Mechanical and thermal-insulating performance of silica aerogel enhanced jointly with glass fiber and fumed silica by a facile compressing technique. Chem. Phys. Lett. 2020, 739, 136950. [Google Scholar] [CrossRef]
- Slosarczyk, A. Carbon Fiber-Silica Aerogel Composite with Enhanced Structural and Mechanical Properties Based on Water Glass and Ambient Pressure Drying. Nanomaterials 2021, 11, 258. [Google Scholar] [CrossRef]
- Linhares, T.; de Amorim, M.T.P.; Duraes, L. Silica aerogel composites with embedded fibres: A review on their preparation, properties and applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Zhu, J.D.; Zhao, F.X.; Xiong, R.J.; Peng, T.P.; Ma, Y.Y.; Hu, J.; Xie, L.; Jiang, C.W. Thermal insulation and flame retardancy of attapulgite reinforced gelatin-based composite aerogel with enhanced strength properties. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106040. [Google Scholar] [CrossRef]
- Finlay, K.A.; Gawryla, M.D.; Schiraldi, D.A. Effects of Fiber Reinforcement on Clay Aerogel Composites. Materials 2015, 8, 5440–5451. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, Q.P.; Li, H.L.; Ji, H.M.; Sun, X.H.; He, J. Effect of sepiolite fiber on the structure and properties of the sepiolite/silica aerogel composite. J. Sol-Gel Sci. Techn. 2013, 67, 646–653. [Google Scholar] [CrossRef]
- Cui, M.K.; Mu, P.; Shen, Y.Q.; Zhu, G.R.; Luo, L.; Li, J. Three-dimensional attapulgite with sandwich-like architecture used for multifunctional water remediation. Sep. Purif. Technol. 2020, 235, 116210. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Rao, A.V.; Kumar, R.; Ingale, S.V.; Wagh, P.B.; Gupta, S.C. Reduction of processing time by mechanical shaking of the ambient pressure dried TEOS based silica aerogel granules. J. Porous Mater. 2012, 19, 87–94. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.N.; Wang, J.G.; Tang, Y.; Zhang, Z. Selective adsorption of Pb2+ and Cu2+ on amino-modified attapulgite: Kinetic, thermal dynamic and DFT studies. J. Hazard. Mater. 2021, 404, 124140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.X.; Liu, P.; Wang, A.Q. High Clay-Content Attapulgite/Poly(acrylic acid) Nanocomposite Hydrogel via Surface-Initiated Redox Radical Polymerization with Modified Attapulgite Nanorods as Initiator and Cross-Linker. Ind. Eng. Chem. Res. 2014, 53, 2067–2071. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Zhong, A.G.; Jin, Y.X. Removal capacity and adsorption mechanism of heat-treated palygorskite clay for methylene blue. Chem. Eng. J. 2011, 174, 143–150. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Dang, J.Y.; Su, X.S.; Jin, H.C. Removal of total petroleum hydrocarbon from water using attapulgite fiber silica aerogel composite. Desalin. Water Treat. 2016, 57, 17463–17472. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Current status, opportunities, and challenges in fuel cell catalytic application of aerogels. Int. J. Energy Res. 2019, 43, 2447–2467. [Google Scholar] [CrossRef]
- Novak, Z.; Knez, Z. Diffusion of methanol-liquid CO2 and methanol-supercritical CO2 in silica aerogels. J. Non-Cryst. Solids 1997, 221, 163–169. [Google Scholar] [CrossRef]
- Pan, Y.L.; Cheng, X.D.; Zhou, T.; Gong, L.L.; Zhang, H.P. Spray freeze-dried monolithic silica aerogel based on water-glass with thermal superinsulating properties. Mater. Lett. 2018, 229, 265–268. [Google Scholar] [CrossRef]
- Li, J.; Lei, Y.; Xu, D.D.; Liu, F.H.; Li, J.W.; Sun, A.H.; Guo, J.J.; Xu, G.J. Improved mechanical and thermal insulation properties of monolithic attapulgite nanofiber/silica aerogel composites dried at ambient pressure. J. Sol-Gel Sci. Techn. 2017, 82, 702–711. [Google Scholar] [CrossRef]
- Ganbavle, V.V.; Kalekar, A.S.; Harale, N.S.; Patil, S.S.; Dhere, S.L. Rapid synthesis of ambient pressure dried tetraethoxysilane based silica aerogels. J. Sol-Gel Sci. Techn. 2021, 97, 5–10. [Google Scholar] [CrossRef]
- Cok, S.S.; Koc, F.; Gizli, N. Lightweight and highly hydrophobic silica aerogels dried in ambient pressure for an efficient oil/organic solvent adsorption. J. Hazard. Mater. 2021, 408, 124858. [Google Scholar]
- Cok, S.S.; Gizli, N. Hydrophobic silica aerogels synthesized in ambient conditions by preserving the pore structure via two-step silylation. Ceram. Int. 2020, 46, 27789–27799. [Google Scholar] [CrossRef]
- Liu, Y.M.; Yan, W.Q.; Zhong, Y.; Wu, Z.W.; Shang, S.S.; Wu, X.D.; Shen, X.D.; Cui, S. Synthesis and characterization of amino-grafted attapulgite/graphene oxide nanocomposites and their adsorption for Pb(II) removal. J. Nanopart. Res. 2022, 24, 28. [Google Scholar] [CrossRef]
- Cai, Y.F.; Xue, J.Y.; Polya, D.A. A Fourier transform infrared spectroscopic study of Mg-rich, Mg-poor and acid leached palygorskites. Spectrochim. Acta A. 2007, 66, 282–288. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, A.S.; Wang, L.H.; Li, X.A.; Huang, W. Striving Toward Visible Light Photocatalytic Water Splitting Based on Natural Silicate Clay Mineral: The Interface Modification of Attapulgite at the Atomic-Molecular Level. ACS Sustain. Chem. Eng. 2016, 4, 4601–4607. [Google Scholar] [CrossRef]
- Shang, S.S.; Ye, X.; Jiang, X.; You, Q.; Zhong, Y.; Wu, X.D.; Cui, S. Preparation and characterization of cellulose/attapulgite composite aerogels with high strength and hydrophobicity. J. Non-Cryst. Solids 2021, 569, 120922. [Google Scholar] [CrossRef]
- Yu, H.J.; Liang, X.F.; Wang, J.X.; Wang, M.M.; Yang, S.Y. Preparation and characterization of hydrophobic silica aerogel sphere products by co-precursor method. Solid State Sci. 2015, 48, 155–162. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhong, K.; Liu, W.; Cui, S.; Zhong, Y.; Jiang, S.J. Preparation and oil adsorption properties of hydrophobic microcrystalline cellulose aerogel. Cellulose 2020, 27, 7663–7675. [Google Scholar] [CrossRef]
- Zhou, T.; Cheng, X.; Pan, Y.; Li, C.; Gong, L.; Zhang, H. Mechanical performance and thermal stability of glass fiber reinforced silica aerogel composites based on co-precursor method by freeze drying. Appl. Surf. Sci. 2018, 437, 321–328. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Shao, G.N.; Quang, D.V.; Kim, H.T. Effect of various structure directing agents on the physicochemical properties of the silica aerogels prepared at an ambient pressure. Appl. Surf. Sci. 2013, 287, 84–90. [Google Scholar] [CrossRef]
- He, S.; Huang, Y.; Chen, G.; Feng, M.; Dai, H.; Yuan, B.; Chen, X. Effect of heat treatment on hydrophobic silica aerogel. J. Hazard. Mater. 2019, 362, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Shang, S.; Zhao, Y.; Cui, S.; Zhong, Y.; Huang, L. Ultra-efficient adsorption of copper ions in chitosan–montmorillonite composite aerogel at wastewater treatment. Cellulose 2021, 28, 7201–7212. [Google Scholar] [CrossRef]
- Hu, M.Q.; Yan, X.L.; Hu, X.Y.; Feng, R.; Zhou, M. Synthesis of silver decorated silica nanoparticles with rough surfaces as adsorbent and catalyst for methylene blue removal. J. Sol-Gel Sci. Techn. 2019, 89, 754–763. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Li, L.F.; Yang, Y.Z.; Liu, X.G. Magnetic porous carbon microspheres synthesized by simultaneous activation and magnetization for removing methylene blue. J. Porous Mat. 2017, 24, 341–353. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Javanbakht, V. Fabrication of Zn-based magnetic zeolitic imidazolate framework bionanocomposite using basil seed mucilage for removal of azo cationic and anionic dyes from aqueous solution. J. Biol. Macromol. 2021, 167, 1076–1090. [Google Scholar] [CrossRef]
- Li, P.H.; Yang, C.; Xu, X.W.; Miao, C.; He, T.J.; Jiang, B.; Wu, W.J. Preparation of Bio-Based Aerogel and Its Adsorption Properties for Organic Dyes. Gels 2022, 8, 755. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; De Silva, K.K.H.; Hara, M.; Yoshimura, M. Facile synthesis of carbon nanotubes and cellulose nanofiber incorporated graphene aerogels for selective organic dye adsorption. Appl. Surf. Sci. 2022, 600, 154098. [Google Scholar] [CrossRef]
- Ruan, C.; Ma, Y.; Shi, G.; He, C.; Du, C.; Jin, X.; Liu, X.; He, S.; Huang, Y. Self-assembly cellulose nanocrystals/SiO2 composite aerogel under freeze-drying: Adsorption towards dye contaminant. Appl. Surf. Sci. 2022, 592, 153280. [Google Scholar] [CrossRef]
- Canete, S.J.; Zhang, Z.; Kong, L.; Schlegel, V.L.; Plantz, B.A.; Dowben, P.A.; Lai, R.Y. Application of synchrotron FTIR microspectroscopy for determination of spatial distribution of methylene blue conjugated onto a SAM via “click” chemistry. Chem. Commun. 2011, 47, 11918–11920. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K.; Wei, W.; Jiang, Z.F.; Lu, J.W.; Zhu, J.J.; Xie, J.M. Removal of cationic dyes from aqueous solution by adsorption onto hydrophobic/hydrophilic silica aerogel. Colloid. Surf. A 2016, 509, 539–549. [Google Scholar] [CrossRef]

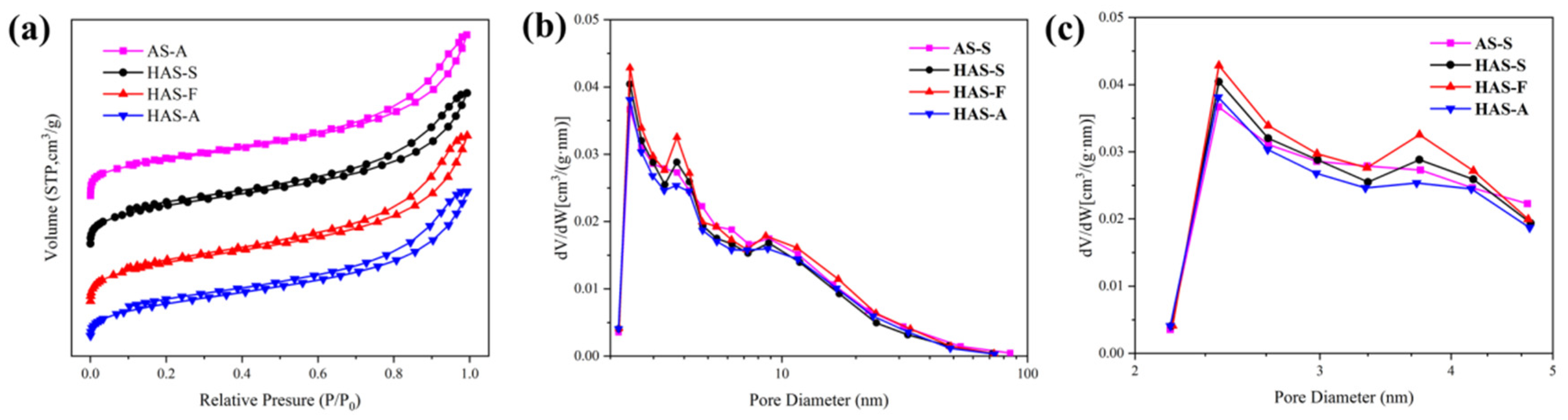


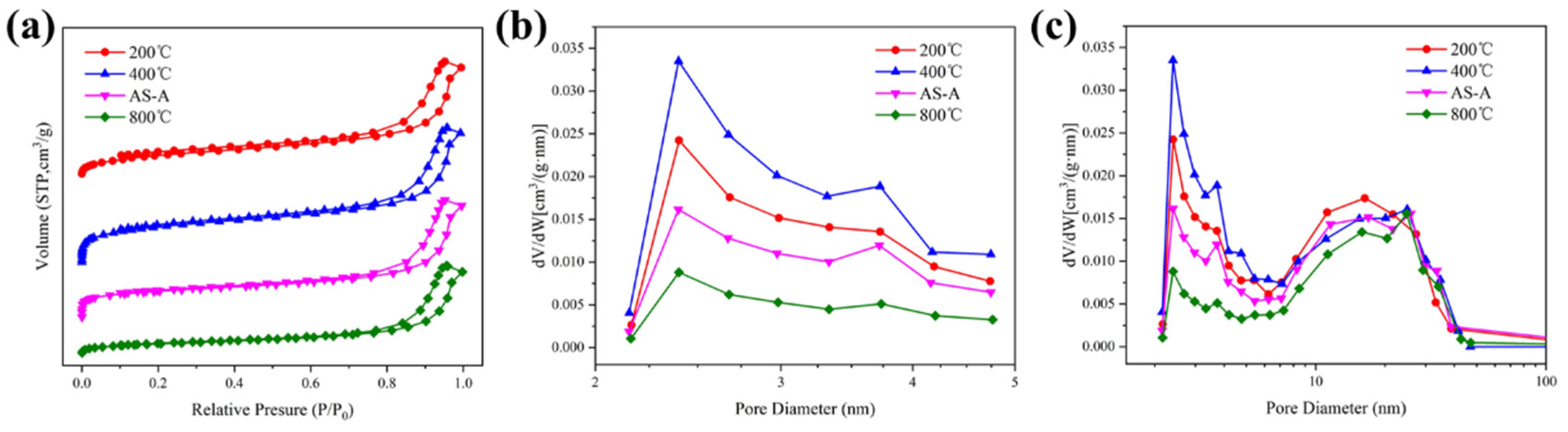
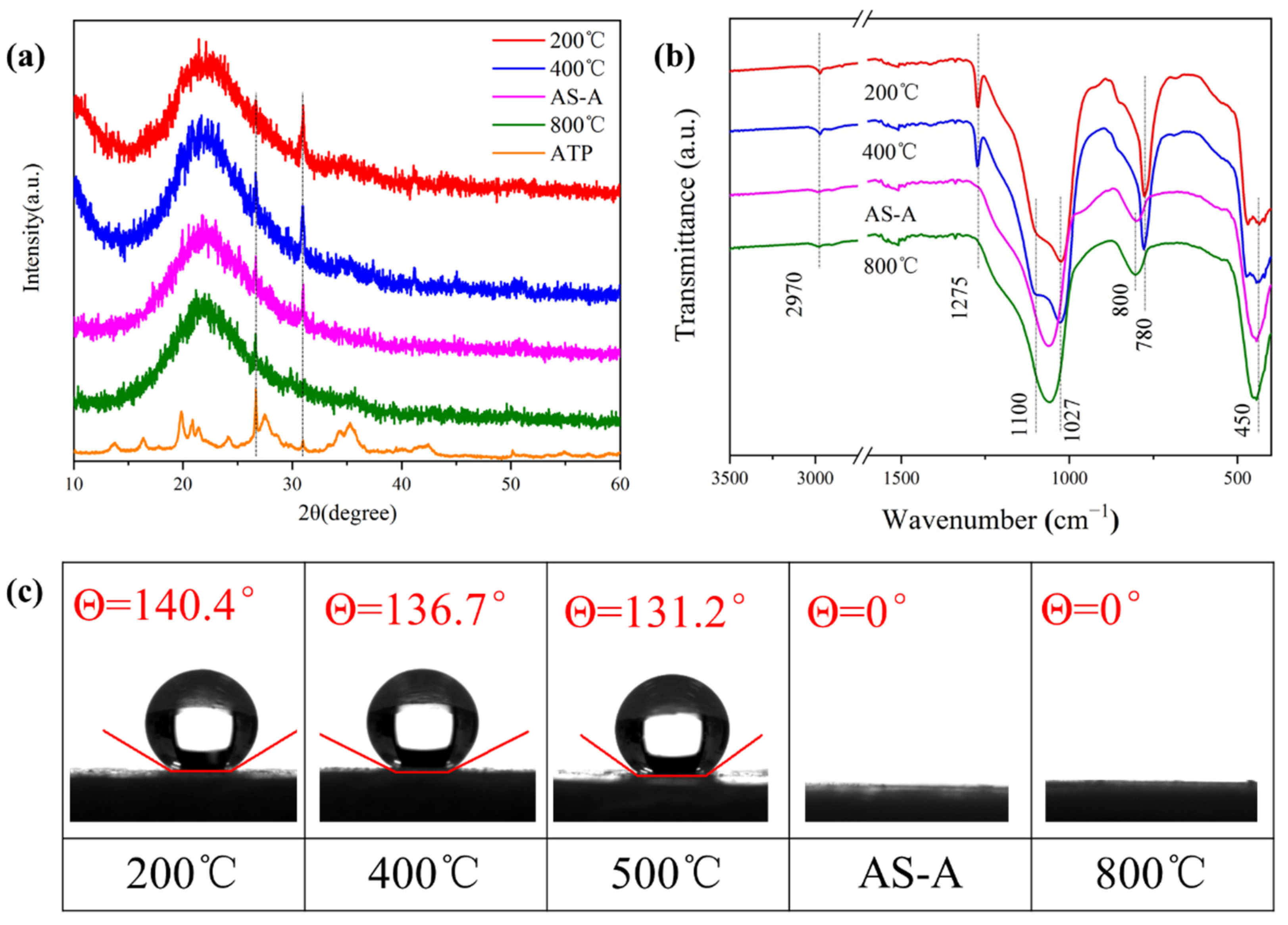
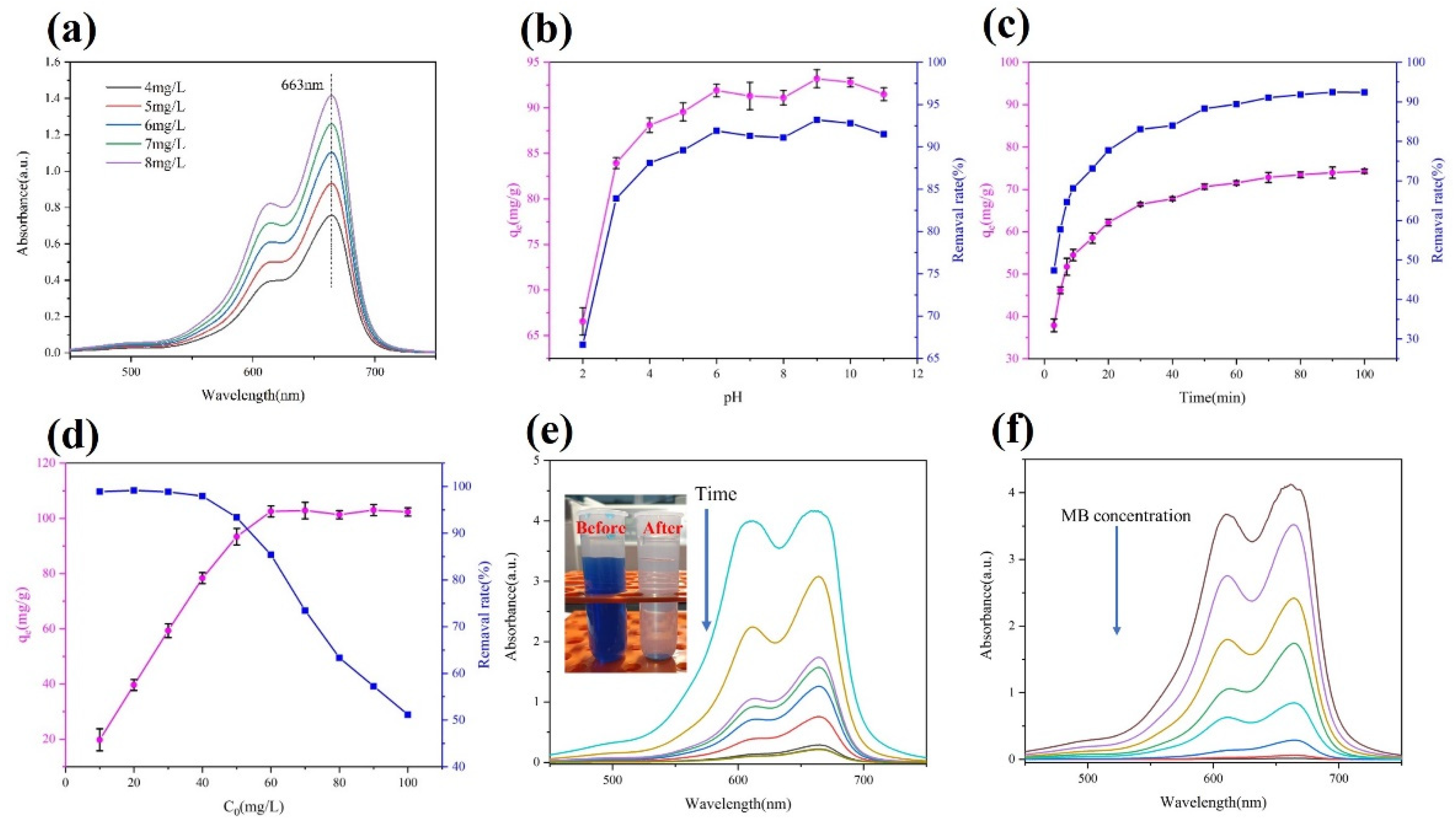
| Sample | ρ (g/cm3) | SBET (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| ATP | 2.41 | 160.5 | 8.6 | 0.27 |
| AS-S | 0.46 | 248.7 | 8.3 | 0.41 |
| HAS-S | 0.53 | 267.4 | 7.9 | 0.37 |
| HAS-F | 0.56 | 241.7 | 8.1 | 0.35 |
| HAS-A | 0.65 | 218.5 | 6.8 | 0.33 |
| Sample | C (wt%) | O (wt%) | Si (wt%) | Metal (wt%) |
|---|---|---|---|---|
| AS-S | 23.8 | 42.9 | 24.6 | 8.7 |
| HAS-S | 40.1 | 40.8 | 17.2 | 2.3 |
| Sample | SBET (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| 200 °C | 231.5 | 11.3 | 0.40 |
| 400 °C | 337.7 | 10.6 | 0.43 |
| AS-A | 246.6 | 12.4 | 0.40 |
| 800 °C | 107.1 | 13.9 | 0.33 |
| Pseudo-First-Order Model | Pseudo-Second-Order Mode | ||||
|---|---|---|---|---|---|
| qe (mg/g) | k1 | R2 | qe (mg/g) | k2 | R2 |
| 39.98 | 0.08003 | 0.94153 | 76.90 | 0.00321 | 0.99955 |
| Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|
| qmax (mg/g) | kL | R2 | kF | 1/n | R2 |
| 109.05 | 2.997 | 0.9986 | 78.45 | 0.12458 | 0.9319 |
| Adsorbent | Treatment Method | SBET (m2/g) | qmax (mg/g) | Reference |
|---|---|---|---|---|
| SiO2(AG) | Heat treated at 500 °C for 3 h | 902.0 | 9.53 | Yi et al. 2019 [10] |
| HSA | Heat treated at 600 °C for 3 h | 888.7 | 51.16 | Wei et al. 2018 [11] |
| SAs | Mg2+ soaked | 468.2 | 40.40 | Yang et al. 2020 [12] |
| Attapulgite | Heat treated at 700 °C for 4 h | - | 78.11 | Chen et al. 2011 [27] |
| Ag/SiO2 | Ag decorated | 208.0 | 55.00 | Hu et al. 2019 [46] |
| MPCMs | Heat treated at 700 °C for 30 min | 480.3 | 56.44 | Zhang et al. 2021 [47] |
| ZIF-8 | Fe decorated | 329.9 | 9.09 | Mahmoodi et al. 2021 [48] |
| CaCO3@STA/PAM/TOCN | - | 39.2 | 101.01 | Li et al. 2022 [49] |
| CCGA | - | 225.2 | 96.10 | Shimizu et al. 2022 [50] |
| CNCS/SiO2 aerogel | - | 440.7 | 190.85 | Ruan et al. 2022 [51] |
| AS-A | Heat treated at 600 °C for 3 h | 246.6 | 102.50 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Wang, S.; Zhong, Y.; You, Q.; Gao, J.; Cui, S.; Shen, X. Spherical Attapulgite/Silica Aerogels Fabricated via Different Drying Methods with Excellent Adsorption Performance. Materials 2023, 16, 2292. https://doi.org/10.3390/ma16062292
Zhu Z, Wang S, Zhong Y, You Q, Gao J, Cui S, Shen X. Spherical Attapulgite/Silica Aerogels Fabricated via Different Drying Methods with Excellent Adsorption Performance. Materials. 2023; 16(6):2292. https://doi.org/10.3390/ma16062292
Chicago/Turabian StyleZhu, Zhixiang, Shengyuan Wang, Ya Zhong, Qi You, Jun Gao, Sheng Cui, and Xiaodong Shen. 2023. "Spherical Attapulgite/Silica Aerogels Fabricated via Different Drying Methods with Excellent Adsorption Performance" Materials 16, no. 6: 2292. https://doi.org/10.3390/ma16062292
APA StyleZhu, Z., Wang, S., Zhong, Y., You, Q., Gao, J., Cui, S., & Shen, X. (2023). Spherical Attapulgite/Silica Aerogels Fabricated via Different Drying Methods with Excellent Adsorption Performance. Materials, 16(6), 2292. https://doi.org/10.3390/ma16062292









