Unusual Acid Sites in LSX Zeolite: Formation Features and Physico-Chemical Properties
Abstract
1. Introduction
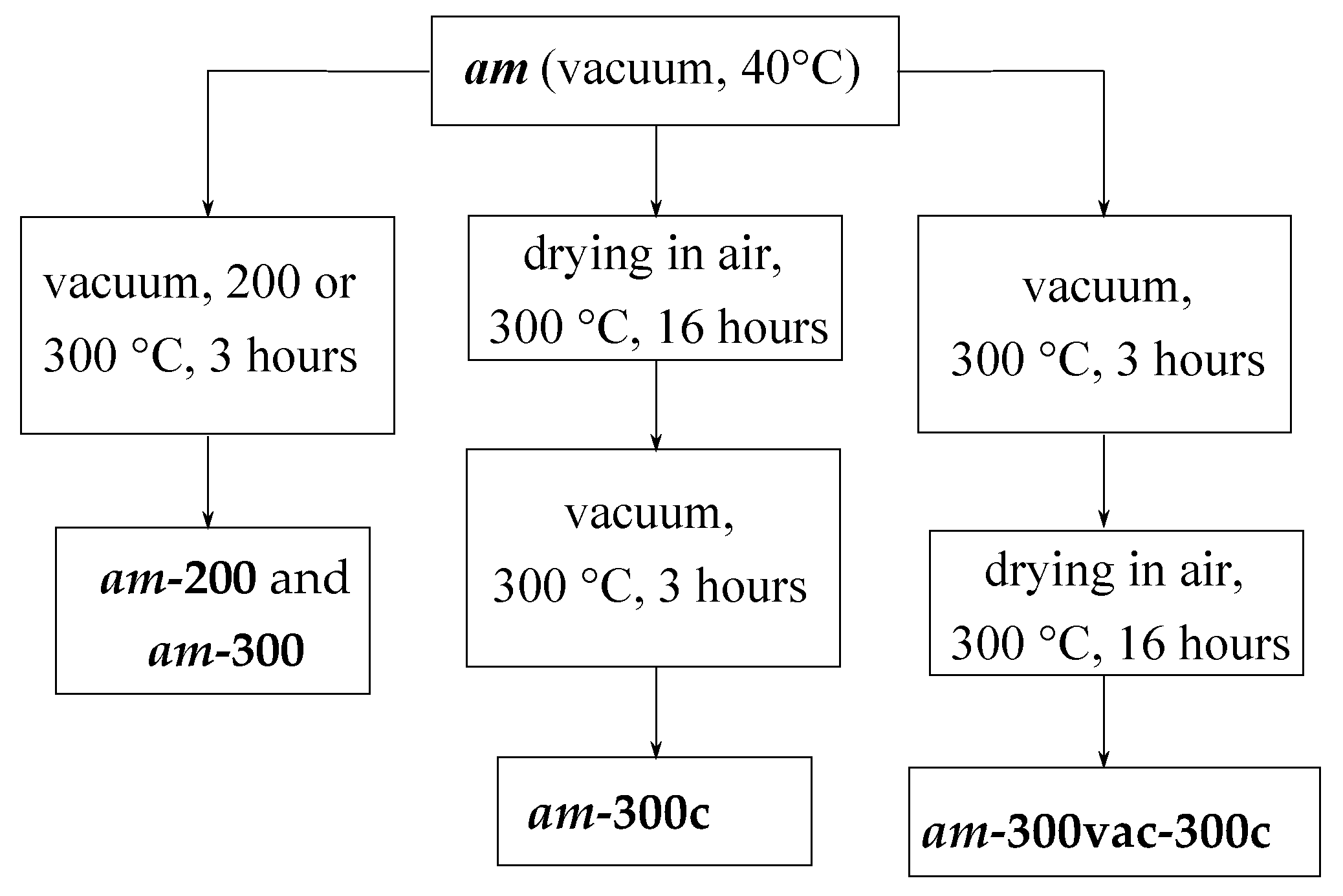
2. Materials and Methods
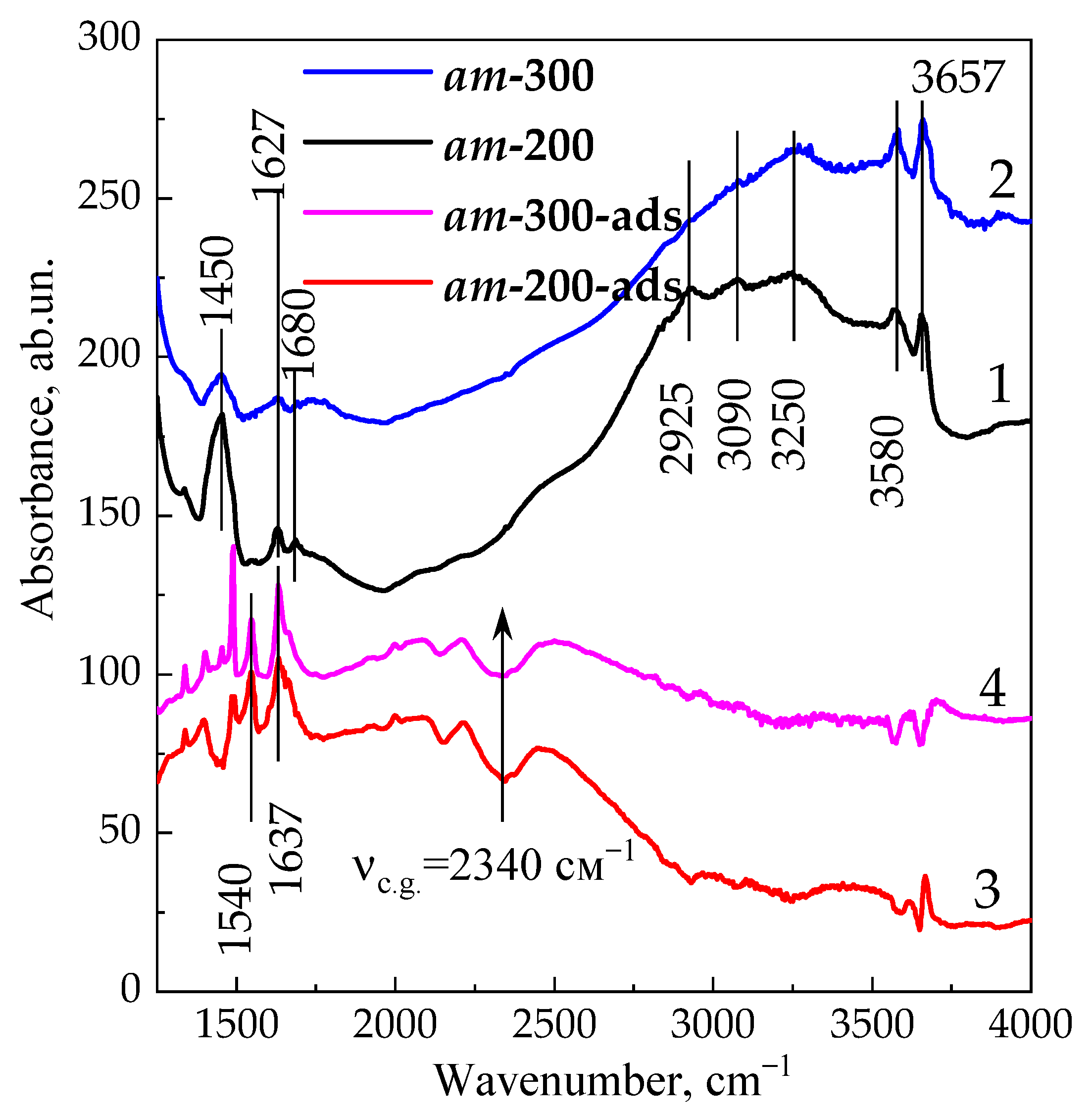
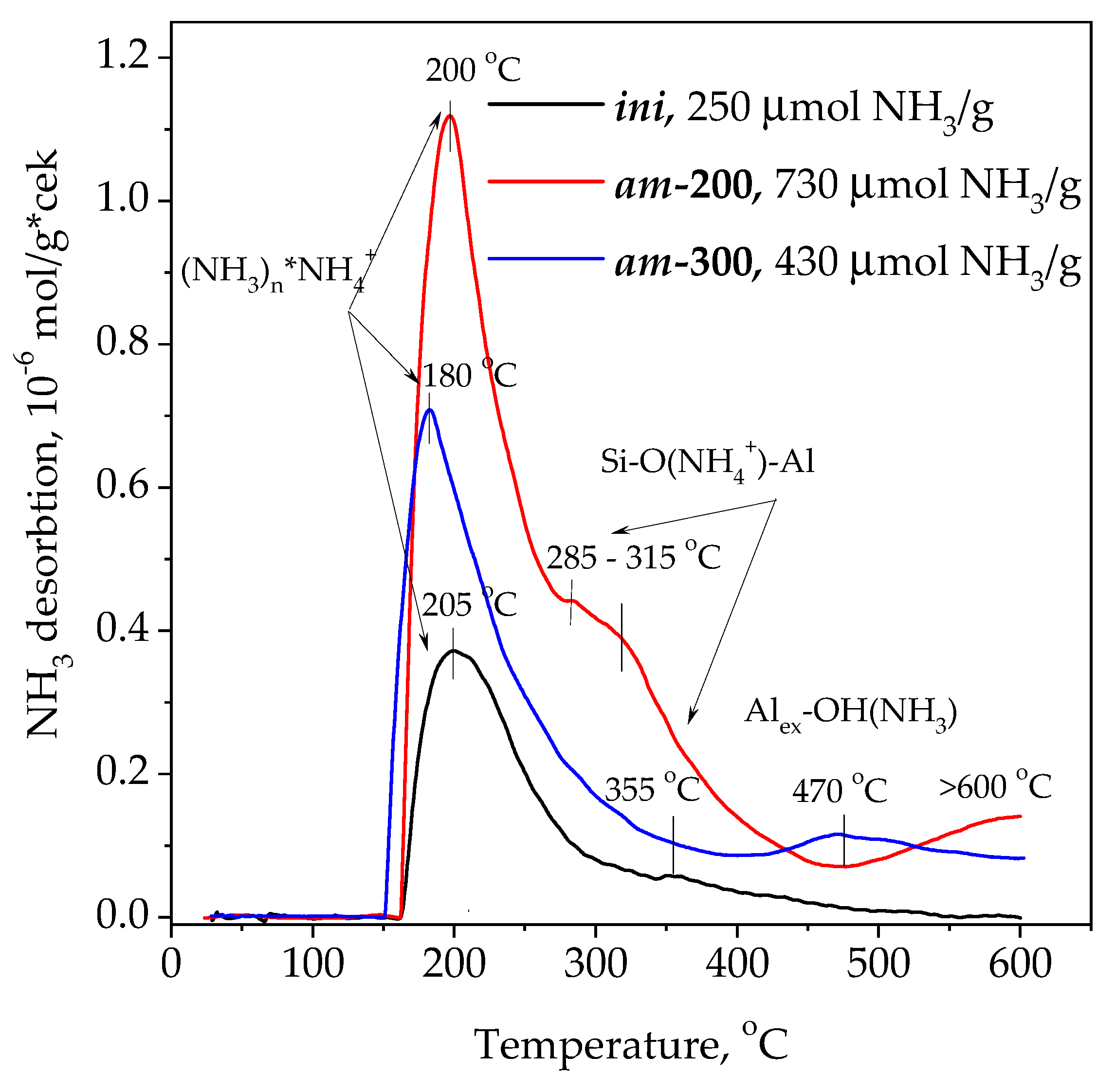
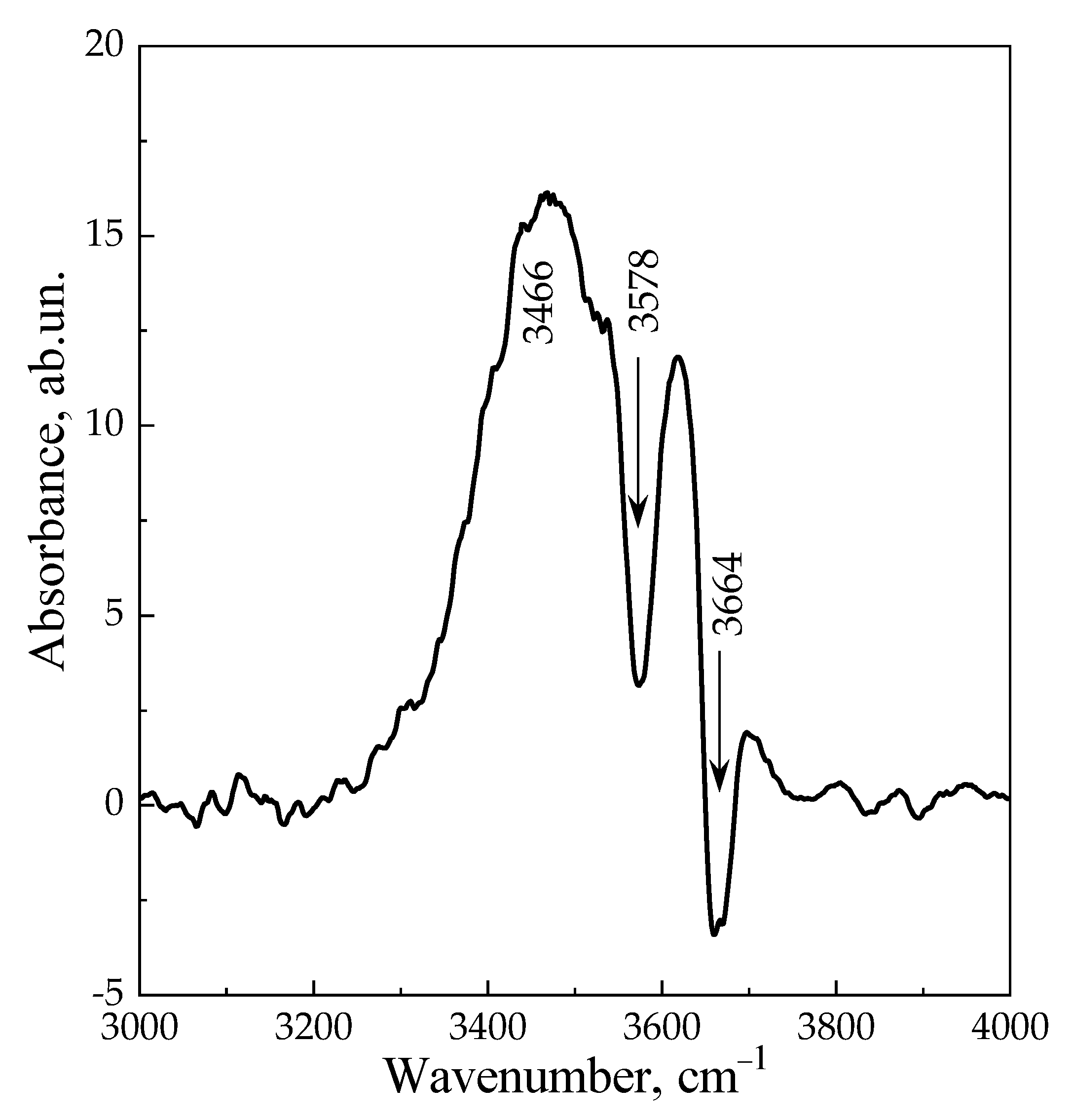
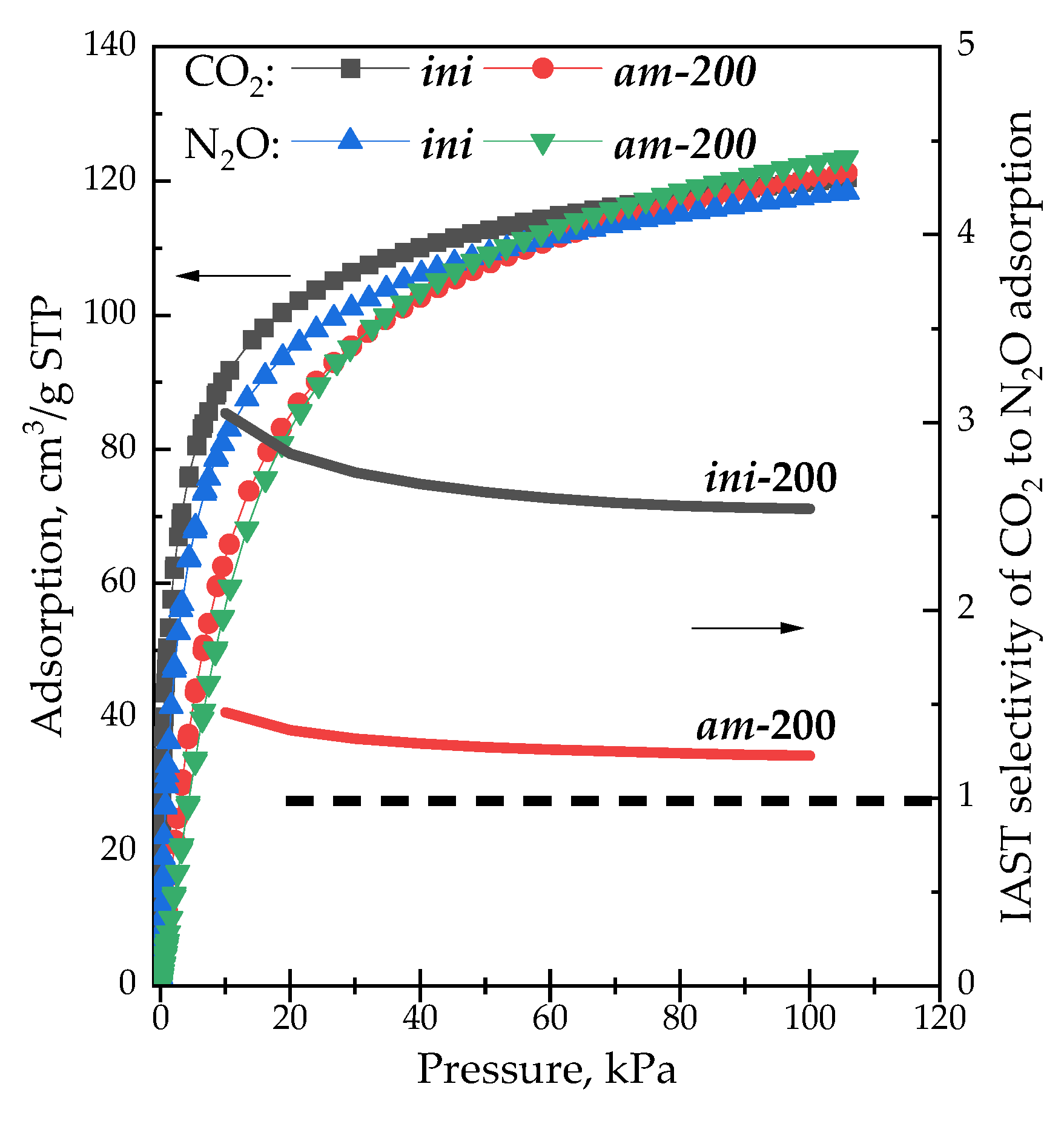
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kühl, G. Crystallization of low-silica faujasite (SiO2/Al2O3~2.0). Zeolites 1987, 7, 451–457. [Google Scholar] [CrossRef]
- Akolekar, D.; Chaffee, A.; Howe, R.F. The transformation of kaolin to low–silica X zeolite. Zeolites 1997, 19, 359–365. [Google Scholar] [CrossRef]
- Chao, C.C. Process for Separating Nitrogen from Mixtures Thereof with Less Polar Substances. U.S. Patent 4859217A, 22 August 1989. [Google Scholar]
- McKee, D.W. Separation of an Oxygen Nitrogen Mixture. U.S. Patent 3140933A, 14 July 1964. [Google Scholar]
- Fan, M.; Sun, J.; Bai, S.; Panezai, H. Size effects of extraframework monovalent cations on the thermal stability and nitrogen adsorption of LSX zeolite. Microporous Mesoporous Mater. 2015, 202, 44–49. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T. Limits for air separation by adsorption with LiX zeolite. Ind. Eng. Chem. Res. 1997, 36, 5358–5365. [Google Scholar] [CrossRef]
- Ackley, M.W.; Rege, S.U.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Takahashi, A.; Yang, R.T. New adsorbents for purification: Selective removal of aromatics. AIChE J. 2002, 48, 1457–1468. [Google Scholar] [CrossRef]
- Pavlova, I.N.; Travkina, O.S.; Kutepov, B.I.; Garieva, G.F. Activity of various cation–exchange forms of LSX and X zeolites in CO2 adsorption. Pet. Chem. 2021, 61, 925–931. [Google Scholar] [CrossRef]
- Hauchhum, L.; Mahanta, P. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. IJEEE 2014, 5, 329–356. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Y.; Li, Z.; Zhang, Q.; Yang, X.; Zhao, C.; Zhang, C.; Wang, H.; Yang, R.T. Insights into adsorption separation of N2/O2 mixture on FAU zeolites under plateau special conditions: A molecular simulation study. Sep. Purif. Technol. 2020, 251, 117405. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Lin, C.; Shang, H.; Yang, J.; Li, L.; Li, J. Effects of different alkali metal cations in FAU zeolites on the separation performance of CO2/N2O. Chem. Eng. J. 2022, 431, 134257. [Google Scholar] [CrossRef]
- Jee, J.-G.; Lee, S.-J.; Kim, M.-B.; Lee, C.-H. Three-bed PVSA process for high–purity O2 generation from ambient air. AIChE J. 2005, 51, 2988–2999. [Google Scholar] [CrossRef]
- Ferreira, D.; Magalhăes, R.; Bessa, J.; Taveira, P.; Sousa, J.; Whitley, R.D.; Mendes, A. Study of AgLiLSX for single–stage high–purity oxygen production. Ind. Eng. Chem. Res. 2014, 53, 15508–15516. [Google Scholar] [CrossRef]
- Ferreira, D.; Boaventura, M.; Bárcia, P.; Whitley, R.D.; Mendes, A. Two–stage vacuum pressure swing adsorption using AgLiLSX zeolite for producing 99.5+% oxygen from air. Ind. Eng. Chem. Res. 2016, 55, 722–736. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.T. Hydrogen storage in low silica type X zeolites. J. Phys. Chem. B 2006, 110, 17175–17181. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. Hydrogen storage properties in low silica type X zeolites. Ind. Eng. Chem. Res. 2010, 49, 3634–3641. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Fermoso, J.A.; Sanna, A. Effect of Li-LSX-zeolite on the in-situ catalytic deoxygenation and denitrogenation of Isochrysis sp. microalgae pyrolysis vapours. Fuel Proc. Technol. 2018, 173, 253–261. [Google Scholar] [CrossRef]
- Lobo, R.F. Chapter 3. Introduction to the structural chemistry of zeolites. In Handbook of Zeolite Science and Technology; Auerbach, S.M., Carrado, K.A., Dutta, P.K., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 80–113. [Google Scholar]
- Dimitrijevich, R.; Lutz, W.; Ritzmann, A. Hydrothermal stability of zeolites: Determination of extra-framework species of H-Y faujasite-type steamed zeolite. J. Phys. Chem. Solids 2006, 67, 1741–1748. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, K.; Chen, B.; White, J.L.; Resasco, D.E. Factors that determine zeolite stability in hot liquid water. J. Am. Chem. Soc. 2015, 137, 11810–11819. [Google Scholar] [CrossRef]
- Almutairi, S.M.T.; Mezari, B.; Filonenko, G.A.; Magusin, P.C.M.M.; Rigutto, M.S.; Pidko, E.A.; Hensen, E.J.M. Influence of Extraframework Aluminum on the Brønsted Acidity and Catalytic Reactivity of Faujasite Zeolite. ChemCatChem 2013, 5, 452–466. [Google Scholar] [CrossRef]
- Guimon, C.; Zouiten, A.; Boreave, A.; Pfister-Guillouzo, G.; Schulz, P.; Fitoussi, F.; Quet, C. Surface and subsurface acidity of faujasite-type zeolites in relation to their composition: An XPS and TPD of ammonia study. J. Chem. Soc. Faraday Trans. 1994, 90, 3461–3467. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar]
- Kondo, J.N.; Nishitani, R.; Yoda, E.; Yokoi, T.; Tatsumi, T.; Domen, K. A comparative IR characterization of acidic sites of HY zeolite by pyridine and CO probes with silica-alumina and γ-alumina references. Phys. Chem. Chem. Phys. 2010, 12, 11576–11586. [Google Scholar] [CrossRef] [PubMed]
- Zholobenko, V.; Freitas, C.; Jendrlin, M.; Bazin, P.; Travert, A.; Thibault-Starzyk, F. Probing the acid sites of zeolites with pyridine: Quantitative AGIR measurements of the molar absorption coefficients. J. Catal. 2020, 385, 52–60. [Google Scholar] [CrossRef]
- Lakiss, L.; Vicente, A.; Gilson, J.-P.; Valtchev, V.; Mintova, S.; Vimont, A.; Bedard, R.; Abdo, S.; Bricker, J. Probing the Brønsted acidity of the external surface of faujasite-type zeolites. ChemPhysChem 2020, 21, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Janin, A.; Maache, M.; Lavalley, J.C.; Joly, J.F.; Raatz, F.; Szydlowski, N. FT ir study of the silanol groups in dealuminated HY zeolites: Nature of the extraframework debris. Zeolites 1991, 11, 391–396. [Google Scholar] [CrossRef]
- Vermeiren, W.; Gilson, J.-P. Impact of zeolites on the petroleum and petrochemical industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef]
- De Klerk, A. Zeolites as catalysts for fuels refining after indirect liquefaction processes. Molecules 2018, 23, 115. [Google Scholar] [CrossRef]
- Rigutto, M.S.; van Veen, R.; Huve, L. Zeolites in Hydrocarbon Processing; Elsevier B.V.: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Kulprathipanja, S. Zeolites in Industrial Separation and Catalysis; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Ennaert, T.; Van Aelst, J.; Dijkmans, J.; De Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and challenges of zeolite chemistry in the catalystic conversion of biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Broach, R.W.; Wilson, W.T. Introduction. In Zeolites in Industrial Separation and Catalysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 1–26. [Google Scholar]
- Guisnet, M.; Gilson, J.-P. Zeolites for Cleaner Technologies; Imperial College Press: London, UK, 2003. [Google Scholar]
- Martínez, C.; Corma, A. Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 2011, 255, 1558–1580. [Google Scholar] [CrossRef]
- Verboekend, D.; Nuttens, N.; Locus, R.; Van Aelst, J.; Verolme, P.; Groen, J.C.; Pérez-Ramírez, J.; Sels, B.F. Synthesis, characterization and catalytic evaluation of hierarchical faujasite zeolites: Milestones, challenges, and future directions. Chem. Soc. Rev. 2016, 45, 3331–3352. [Google Scholar] [CrossRef]
- Verboekend, D.; Vilé, G.; Pérez-Ramírez, J. Hierarchical Y and USY zeolites designed by post-synthetic strategies. Adv. Funct. Mater. 2012, 22, 916–928. [Google Scholar] [CrossRef]
- Jia, X.; Khan, W.; Wu, Z.; Choi, J.; Yip, A.C.K. Modern synthesis strategies for hierarchical zeolites: Bottom-up versus top-down strategies. Adv. Powder Technol. 2019, 30, 467–484. [Google Scholar] [CrossRef]
- Verboekend, D.; Keller, T.C.; Mitchell, S.; Pérez-Ramírez, J. Hierarchical FAU- and LTA-type zeolites by post-synthetic design: A new generation of highly efficient base catalysts. Adv. Funct. Mater. 2013, 23, 1923–1934. [Google Scholar] [CrossRef]
- Silaghi, M.-C.; Chizallet, C.; Sauer, J.; Raybaud, P. Dealumination mechanisms of zeolites and extra-framework aluminium confinement. J. Catal. 2016, 339, 242–255. [Google Scholar] [CrossRef]
- Lutz, W.; Toufar, H.; Heidemann, H.; Salman, N.; Rüscher, C.H.; Gesing, T.M.; Buhl, J.-C.; Bertram, R. Sileceous extra-framework species in dealuminated Y zeolites generated by steaming. Microporous Mesoporous Mater. 2007, 104, 171–178. [Google Scholar] [CrossRef]
- Heard, C.J.; Grajciar, L.; Uhlík, F.; Shamzhy, M.; Opanasenko, M.; Čejka, J.; Nachtigall, P. Zeolite (In)stability under aqueous or steaming conditions. Adv. Mater. 2020, 32, 2003264. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Choi, M. Controlled decationization of X zeolite: Mesopore generation within zeolite crystallites for bulky molecular adsorption and transformation. J. Mater. Chem. A 2013, 1, 12096–12102. [Google Scholar] [CrossRef]
- Mousavi, H.; Darian, J.T.; Mokhtarani, B. Enhanced nitrogen adsorption capacity on Ca2+ ion-exchanged hierarchical X zeolite. Sep. Purif. Technol. 2021, 264, 118442. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Fermoso, J.; Sanna, A. Stability of Li-LSX Zeolite in the Catalytic Pyrolysis of Non-Treated and Acid Pre-Treated Isochrysis sp. Microalgae. Energies 2020, 13, 959. [Google Scholar] [CrossRef]
- Li, X.; Han, H.; Xu, W.; Hwang, S.-J.; Lu, P.; Bhan, A.; Tsapatsis, M. Enhanced reactivity of accessible proton in sodalite cages of faujasite zeolite. Angew. Chem. Int. Ed. 2022, 61, e202111180. [Google Scholar]
- Li, X.; Han, H.; Xu, W.; Hwang, S.-J.; Shi, Z.; Lu, P.; Bhan, A.; Tsapatsis, M. Acid catalysis over low-silica faujasite zeolites. J. Am. Chem. Soc. 2022, 144, 9324–9329. [Google Scholar] [CrossRef]
- Epiepang, F.E.; Yang, X.; Li, J.; Yang, R.T. Mixed-cation LiCa-LSX zeolite with minimum lithium for air separation. AIChE J. 2018, 64, 406–415. [Google Scholar] [CrossRef]
- Epiepang, F.E.; Yang, X.; Li, J.; Wei, Y.; Liu, Y.; Yang, R.T. Air separation sorbents: Mixed-cation zeolites with minimum lithium and silver. Chem. Eng. Sci. 2019, 198, 43–51. [Google Scholar] [CrossRef]
- Jasra, R.V.; Choudary, N.V.; Bhat, S.G.T. Correlation of sorption behavior of nitrogen, oxygen and argon with cation locations in zeolite X. Ind. Eng. Chem. Res. 1996, 35, 4221–4229. [Google Scholar] [CrossRef]
- Mofarahi, M.; Gholipour, F. Gas adsorption separation of CO2/CH4 system using zeolite 5A. Microporous Mesoporous Mater. 2014, 200, 1–10. [Google Scholar] [CrossRef]
- ASTM D3906–03; Standard Test Method for Determination of Relative X-ray Diffraction Intensities of Faujasite-Type Zeolite-Containing Materials. ASTM International: West Conshohocken, PA, USA, 2003.
- Mel’gunov, M.S.; Ayupov, A.B. Direct method for evaluation of BET adsorbed monolayer capacity. Microporous Mesoporous Mater. 2017, 243, 147–153. [Google Scholar] [CrossRef]
- Gurvich, L.; Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; p. 124. [Google Scholar]
- Evans, R. Chapter 3. Density functionals in the theory of nonuniform fluids. In Fundamentals of Inhomogeneous Fluids; Henderson, D., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1992; pp. 85–177. [Google Scholar]
- Paukshtis, E.A. Infrared Spectroscopy in Heterogeneous Acid–Base Catalysis; Nauka: Novosibirsk, Russia, 1992. [Google Scholar]
- Malyshev, M.E.; Paukshtis, E.A.; Malysheva, L.V.; Toktarev, A.V.; Vostrikova, L.A. N2 and CO as probe molecules for determining the properties of acid sites on the surface of zeolites. Kinet. Catal. 2005, 46, 100–106. [Google Scholar] [CrossRef]
- Paukshtis, E.A.; Yurchenko, E.N. Study of the acid–base properties of heterogeneous catalysts by infrared spectroscopy. Russ. Chem. Rev. 1983, 52, 242–258. [Google Scholar] [CrossRef]
- Kim, J.-B. Li+- and H+-exchanged low-silica X zeolite as selective nitrogen adsorbent for air separation. Bull. Korean Chem. Soc. 2003, 24, 1814–1818. [Google Scholar]
- Khaleghian-Moghadam, R.; Seyedeyn-Azad, F. A study on the thermal behavior of low–silica type X zeolite ion–exchanged with alkaline earth cations. Microporous Mesoporous Mater. 2009, 120, 285–293. [Google Scholar] [CrossRef]
- Basaldella, E.I.; Tara, J.C. Synthesis of LSX zeolite in the NaK system: Influence of the NaK ratio. Zeolites 1995, 15, 243–246. [Google Scholar] [CrossRef]
- Chu, P.; Dwyer, F.G. The deammonation reaction of ammonium X zeolite. J. Catal. 1980, 61, 454–460. [Google Scholar] [CrossRef]
- Medrud, R.C. Petroleum Catalysts. In Industrial Applications of X-ray Diffraction; Chung, F.H., Smith, D.K., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2000; p. 276. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kühl, G.H.; Schweizer, A.E. Structural stability of sodium ammonium zeolite X. J. Catal. 1975, 38, 469–476. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Available online: www.iza-structure.org (accessed on 1 November 2022).
- White, J.L.; Jelli, A.N.; André, J.M.; Fripiat, J.J. Perturbation of OH–groups in decationated Y–zeolites by physically adsorbed gases. Trans. Faraday Soc. 1967, 63, 461–475. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Uytterhoeven, J.B. Assignement of hydroxyl bands in the infrared spectra of zeolites X and Y. Part 1.—Na–H–zeolites. J. Chem. Soc. Faraday Trans. 1973, 69, 359–372. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Uytterhoeven, J.B. Assignement of hydroxyl bands in the infrared spectra of zeolites X and Y. Part 2.—After different pretreatments. J. Chem. Soc. Faraday Trans. 1973, 69, 373–386. [Google Scholar] [CrossRef]
- Dwyer, J.; Dewing, J.; Thompson, N.E.; O’Malley, P.J.; Karim, K. The high–frequency hydroxy region in H–Y zeolites; A comment on a previous communication and corrigendum. J. Chem. Soc. Chem. Commun. 1989, 13, 843–844. [Google Scholar] [CrossRef]
- Gil, B.; Broclawik, E.; Datka, J.; Klinowski, J. Acidic hydroxyl groups in zeolites X and Y: A correlation between infrared and solid–state NMR spectra. J. Phys. Chem. 1994, 98, 930–933. [Google Scholar] [CrossRef]
- Guilleux, M.F.; Delafosse, D. Spectroscopic study of surface properties of various NH4–exchanged X zeolites. J. Chem. Soc. Faraday Trans. 1975, 71, 1777–1783. [Google Scholar] [CrossRef]
- Bertsch, L.; Habgood, H.W. An infrared spectroscopic study of the adsorption of water and carbon dioxide by Linde molecular sieve X. J. Phys. Chem. 1963, 67, 1621–1628. [Google Scholar] [CrossRef]
- Jacobs, P.A.; van Cauwelaert, F.H.; Vansant, E.F. Surface probing of synthetic faujasites by adsorption of carbon dioxide. Part 2 Infra–red study of carbon dioxide adsorbed on X zeolites exchanged with mono- and bi-valent ions. J. Chem. Soc. Faraday Trans. 1973, 69, 2130–2139. [Google Scholar] [CrossRef]
- Rege, S.U.; Yang, R.T. A novel FTIR method for studying mixed gas adsorption at low concentrations: H2O and CO2 on NaX zeolite and γ–alumina. Chem. Eng. Sci. 2001, 56, 3781–3796. [Google Scholar] [CrossRef]
- Ivanova, E.N.; Averin, A.A.; Alekhina, M.B.; Sokolova, N.P.; Kon’kova, T.V. Thermal activation of type X zeolites in the presence of carbon dioxide. Prot. Met. Phys. Chem. Surf. 2016, 52, 267–272. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; Poduval, D.G.; Ligthart, D.A.J.M.; van Veen, J.A.R.; Rigutto, M.S. Quantification of Strong Brønsted Acid Sites in Aluminosilicates. J. Phys. Chem. C 2010, 114, 8363–8374. [Google Scholar] [CrossRef]
- Gόra-Marek, K.; Datka, J. IR studies of OH–groups in mesoporous aluminosilicates. Appl. Catal. A 2006, 302, 104–109. [Google Scholar] [CrossRef]
- Angell, C.L.; Schaffer, P.C. Infrared spectroscopic investigations of zeolites and adsorbed molecules. I. Structural OH–groups. J. Phys. Chem. 1965, 69, 3463–3470. [Google Scholar] [CrossRef]
- Ramdas, S.; Thomas, J.M.; Klinowski, J.; Fyfe, C.A.; Hartman, J.S. Ordering of aluminium and silicon in synthetic faujasites. Nature 1981, 292, 228–230. [Google Scholar] [CrossRef]
- Thuadaij, P.; Nuntiya, A. Effect of the SiO2/Al2O3 ratio on the synthesis of Na–X zeolite from Mae Moh fly ash. Sci. Asia 2012, 38, 295–300. [Google Scholar] [CrossRef]
- Lippmaa, E.; Mägi, M.; Samoson, A.; Tarmak, M.; Engelhardt, G. Investigation of the structure of zeolites by solid–state high–resolution 29Si NMR spectroscopy. J. Am. Chem. Soc. 1981, 103, 4992–4996. [Google Scholar] [CrossRef]
- Cattanach, J.; Wu, E.L.; Venuto, P.B. Stoichiometry of thermochemical transformations of NH4Y zeolite. J. Catal. 1968, 11, 342–347. [Google Scholar] [CrossRef]
- Hidalgo, C.V.; Itoh, H.; Hattori, T.; Niwa, M.; Murakami, Y. Measurement of the acidity of various zeolites by Temperature–Programmed Desorption of ammonia. J. Catal. 1984, 85, 362–369. [Google Scholar] [CrossRef]
- Lohse, U.; Parlitz, B. Y zeolite acidity dependence on Si/Al ratio. J. Phys. Chem. 1989, 93, 3677–3683. [Google Scholar] [CrossRef]
- Niwa, M.; Suzuki, K.; Katada, N.; Kanougi, T.; Atoguchi, T. Ammonia IRMS-TPD study on the distribution of acid sites in mordenite. J. Phys. Chem. B 2005, 109, 18749–18757. [Google Scholar] [CrossRef]
- Niwa, M.; Nishikawa, S.; Katada, N. IRMS-TPD of ammonia for characterization of acid sites in β-zeolite. Microporous Mesoporous Mater. 2005, 82, 105–112. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared adsorbtion bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Gould, N.S.; Xu, B. Quantification of acid sites densities on zeolites in the presence of solvents via determination of extinction coefficients of adsorbed pyridine. J. Catal. 2018, 358, 80–88. [Google Scholar] [CrossRef]
- Odinokov, S.E.; Mashkovsky, A.A.; Glazunov, V.P.; Iogansen, A.V.; Rassadin, B.V. Spectral manifestations of intermolecular and interionic hydrogen bonding in adducts of various acids with pyridine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1976, 32, 1355–1363. [Google Scholar] [CrossRef]
- Glazunov, V.P.; Odinokov, S.E. Infrared spectra of pyridinium salts in solution II. Fermi resonance and structure of νNH bands. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1982, 38, 409–415. [Google Scholar] [CrossRef]
- Melikova, S.M.; Rutkowski, K.S.; Gurinov, A.A.; Denisov, G.S.; Rospenk, M.; Shederovich, I.G. FTIR study of the hydrogen bond symmetry in protonated homodimers of pyridine and collidine in solution. J. Mol. Struct. 2012, 27, 39–44. [Google Scholar] [CrossRef]
- Garrone, E.; Delgado, M.R.; Bonelli, B.; Arean, C.O. Probing gas adsorbtion in zeolites by variable–temperature IR spectroscopy: An overview of current research. Molecules 2017, 22, 1557. [Google Scholar] [CrossRef]
- Arean, C.O.; Manoilova, O.V.; Tsyganenko, A.A.; Palomino, G.T.; Mentruit, M.P.; Geobaldo, F.; Garrone, E. Thermodynamics of hydrogen bonding between CO and the supercage Brønsted acid sites of the H–Y zeolite studies from variable–temperature IR spectrometry. Eur. J. Inorg. Chem. 2001, 2001, 1739–1743. [Google Scholar]
- Szymansky, H.A. Infrared structural studies of zeolite frameworks. In Molecular Sieve Zeolites—I; Flanigen, M., Khatami, H., Eds.; American Chemical Society: Washington, DC, USA, 1974; pp. 201–229. [Google Scholar]
- Knözinger, H.; Huber, S. IR spectroscopy of small and weakly interacting molecular probes for acidic and basic zeolites. J. Chem. Soc. Faraday Trans. 1998, 94, 2047–2059. [Google Scholar] [CrossRef]
- Grigor’eva, N.G.; Filippova, N.A.; Agliullin, M.R.; Kutepov, B.I.; Narender, N. Crystalline and amorphous aluminosilicates with different pore structures for the synthesis of pyridines. J. Chem. Res. 2017, 41, 253–261. [Google Scholar] [CrossRef]
- Gackowski, M.; Tarach, K.; Kuterasiński, Ł.; Podobiński, J.; Jarczewski, S.; Kuśtrowski, P.; Datka, J. Hierarchical zeolite Y obtained by desislication: Porosity, acidity and catalytic properties. Microporous Mesoporous Mater. 2018, 263, 282–288. [Google Scholar] [CrossRef]
- Barthomeuf, D. Zeolite acidity dependence on structure and chemical environment. Correlations with catalysis. Mater. Chem. Phys. 1984, 17, 49–71. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Leuven, K.U. Acid zeolites: An attempt to develop unifying concepts. Catal. Rev. Sci. Eng. 1982, 24, 415–440. [Google Scholar] [CrossRef]
- Kawakami, H.; Yoshida, S.; Yonezawa, T. A quantum–chemical approach to the generation of solid acidity in composite metal oxides. J. Chem. Soc. Faraday Trans. 1984, 80, 205–217. [Google Scholar] [CrossRef]
- Senchenya, I.N.; Kazansky, V.B.; Beran, S. Quantum chemical study of the effect of the structural characteristics of zeolites on the properties of their bridging hydroxyl groups. Part 2. J. Phys. Chem. 1986, 90, 4857–4859. [Google Scholar] [CrossRef]
- Chen, D.T.; Sharma, S.B.; Filimonov, I.; Dumesic, J.A. Microcalorimetric studies of zeolite acidity. Catal. Lett. 1992, 12, 201–211. [Google Scholar] [CrossRef]
- Mintova, S.; Valtchev, V.; Onfroy, T.; Marichal, C.; Knözinger, H.; Bein, T. Variation of the Si/Al ratio in nanosized zeolite Beta crystals. Microporous Mesoporous Mater. 2006, 90, 237–245. [Google Scholar] [CrossRef]
- Martens, J.A.; Souverijns, W.; Van Rhijn, W.; Jacobs, P.; Ertl, G.; Knözinger, H.; Weitkamp, J. (Eds.) Handbook of Heterogeneous Catalysis; Wiley-WCH: Weinheim, Germany, 1997; Volume 1, p. 324. [Google Scholar]
- Kazansky, V.B.; Ertl, G.; Knözinger, H.; Weitkamp, J. (Eds.) Handbook of Heterogeneous Catalysis; Wiley-WCH: Weinheim, Germany, 1997; Volume 2, p. 740. [Google Scholar]
- Cairon, O.; Chevreau, T.; Lavalley, J.-C. Brønsted acidity of extraframework debris in steamed Y zeolites from the FTIR study of CO adsorption. J. Chem. Soc. Faraday Trans. 1998, 94, 3039–3047. [Google Scholar] [CrossRef]
- Dik, P.P.; Danilova, I.G.; Golubev, I.S.; Kazakov, M.O.; Nadeina, K.A.; Budukva, S.V.; Pereima, V.Y.; Klimov, O.V.; Prosvirin, I.P.; Gerasimov, E.Y.; et al. Hydrocracking of vacuum gas oil over NiMo/zeolite-Al2O3: Influence of zeolite properties. Fuel 2019, 237, 178–190. [Google Scholar] [CrossRef]
- Daniell, W.; Topsøe, N.-Y.; Knözinger, H. An FTIR study of the surface acidity of USY zeolites: Comparison of CO, CD3CN, and C5H5N probe molecules. Langmuir 2001, 17, 6233–6239. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, S.; Raja, D.; Khusni, N.B.; Liu, J.; Zhang, J.; Abdulridha, S.; Xiang, H.; Jiang, S.; Guan, Y.; et al. On the effect of mesoporosity of FAU Y zeolites in the liquid-phase catalysis. Microporous Mesoporous Mater. 2019, 278, 297–306. [Google Scholar] [CrossRef]
- Selli, E.; Forni, L. Comparison between the surface acidity of solid catalysts determined by TPD and FTIR analysis of pre-adsorbed pyridine. Microporous Mesoporous Mater. 1999, 31, 129–140. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Ganjkhanlou, Y.; Kubička, D.; Bulánek, R.; Čejka, J. Characterization of potassium-modified FAU zeolites and their performance in aldol condensation of furfural and acetone. Appl. Catal. A Gen. 2018, 549, 8–18. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Puértolas, B.; Oliveira, C.C.S.; Manayil, J.C.; Barbero, B.; Isaacs, M.; Michailof, C.; Heracleous, E.; Pérez-Ramírez, J.; Lee, A.F.; et al. On the influence of Si:Al ratio and hierarchical porosity of FAU zeolites in solid acid catalysed esterification pretreatment of bio-oil. Biomass Convers. Biorefin. 2017, 7, 331–342. [Google Scholar] [CrossRef]
- Xu, B.; Bordiga, S.; Prins, R.; van Bokhoven, J.A. Effect of framework Si/Al ratio and extra-framework aluminum on the catalytic activity of Y zeolite. Appl. Catal. A Gen. 2007, 333, 245–253. [Google Scholar] [CrossRef]
- Anson, A.; Lin, C.C.H.; Kuznicki, S.M.; Sawada, J.A. Adsorption of carbon dioxide, ethane, and methane on titanosilicate type molecular sieves. Chem. Eng. Sci. 2009, 64, 3683–3687. [Google Scholar] [CrossRef]






| Sample | K | Na | Al | Si | Si/Al | NH4 2 |
|---|---|---|---|---|---|---|
| ini | 24 | 72 | 96 | 96 | 1 | 0 |
| am | 12 | 32 | 96 | 96 | 1 | 52 |
| Sample | As, m2/g | Vmic, cm3/g | Vmeso, cm3/g | VƩ, cm3/g | D, nm |
|---|---|---|---|---|---|
| ini-200 | 697 | 0.27 | 0 | 0.27 | 1.1 |
| ini-300 | 707 | 0.28 | 0 | 0.28 | 1.1 |
| am-200 | 787 | 0.29 | 0.03 | 0.34 | 1.2; 2.5 |
| am-300 | 738 | 0.27 | 0.05 | 0.32 | 1.2; 2.5 |
| am-300vac-300c | 440 | 0.15 | 0.07 | 0.21 | 2.5 |
| am-300c | 245 | 0.05 | 0.15 | 0.21 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonova, A.A.; Yashnik, S.A.; Paukshtis, E.A.; Mel’gunov, M.S. Unusual Acid Sites in LSX Zeolite: Formation Features and Physico-Chemical Properties. Materials 2023, 16, 2308. https://doi.org/10.3390/ma16062308
Leonova AA, Yashnik SA, Paukshtis EA, Mel’gunov MS. Unusual Acid Sites in LSX Zeolite: Formation Features and Physico-Chemical Properties. Materials. 2023; 16(6):2308. https://doi.org/10.3390/ma16062308
Chicago/Turabian StyleLeonova, Aleksandra A., Svetlana A. Yashnik, Evgeny A. Paukshtis, and Maksim S. Mel’gunov. 2023. "Unusual Acid Sites in LSX Zeolite: Formation Features and Physico-Chemical Properties" Materials 16, no. 6: 2308. https://doi.org/10.3390/ma16062308
APA StyleLeonova, A. A., Yashnik, S. A., Paukshtis, E. A., & Mel’gunov, M. S. (2023). Unusual Acid Sites in LSX Zeolite: Formation Features and Physico-Chemical Properties. Materials, 16(6), 2308. https://doi.org/10.3390/ma16062308







