Preliminary Study of Preheated Decarburized Activated Coal Gangue-Based Cemented Paste Backfill Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
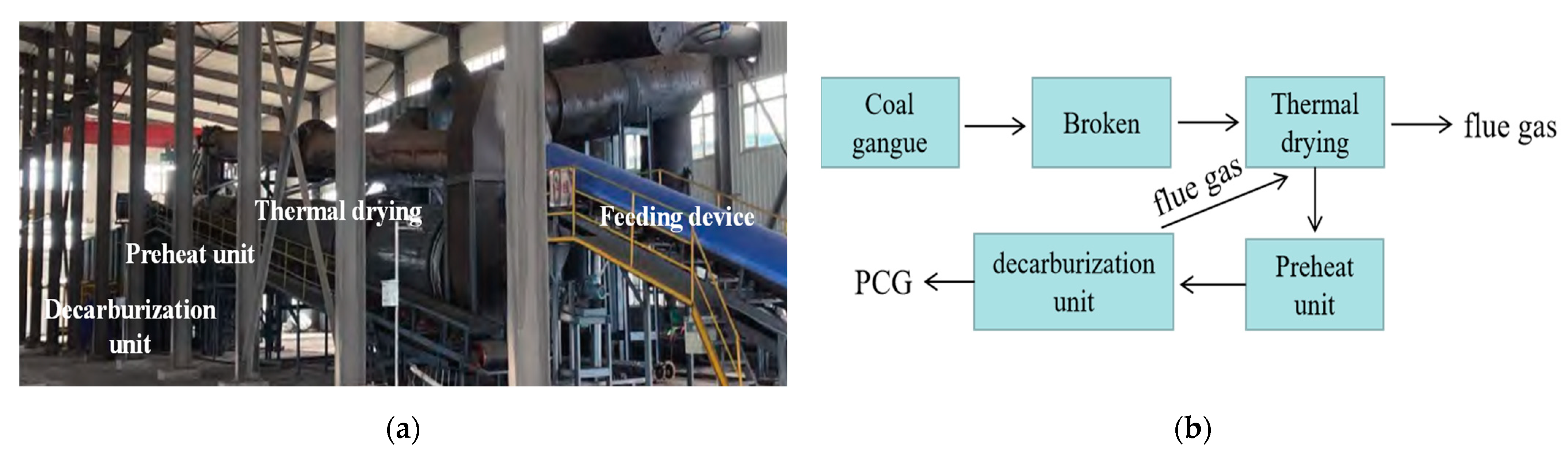
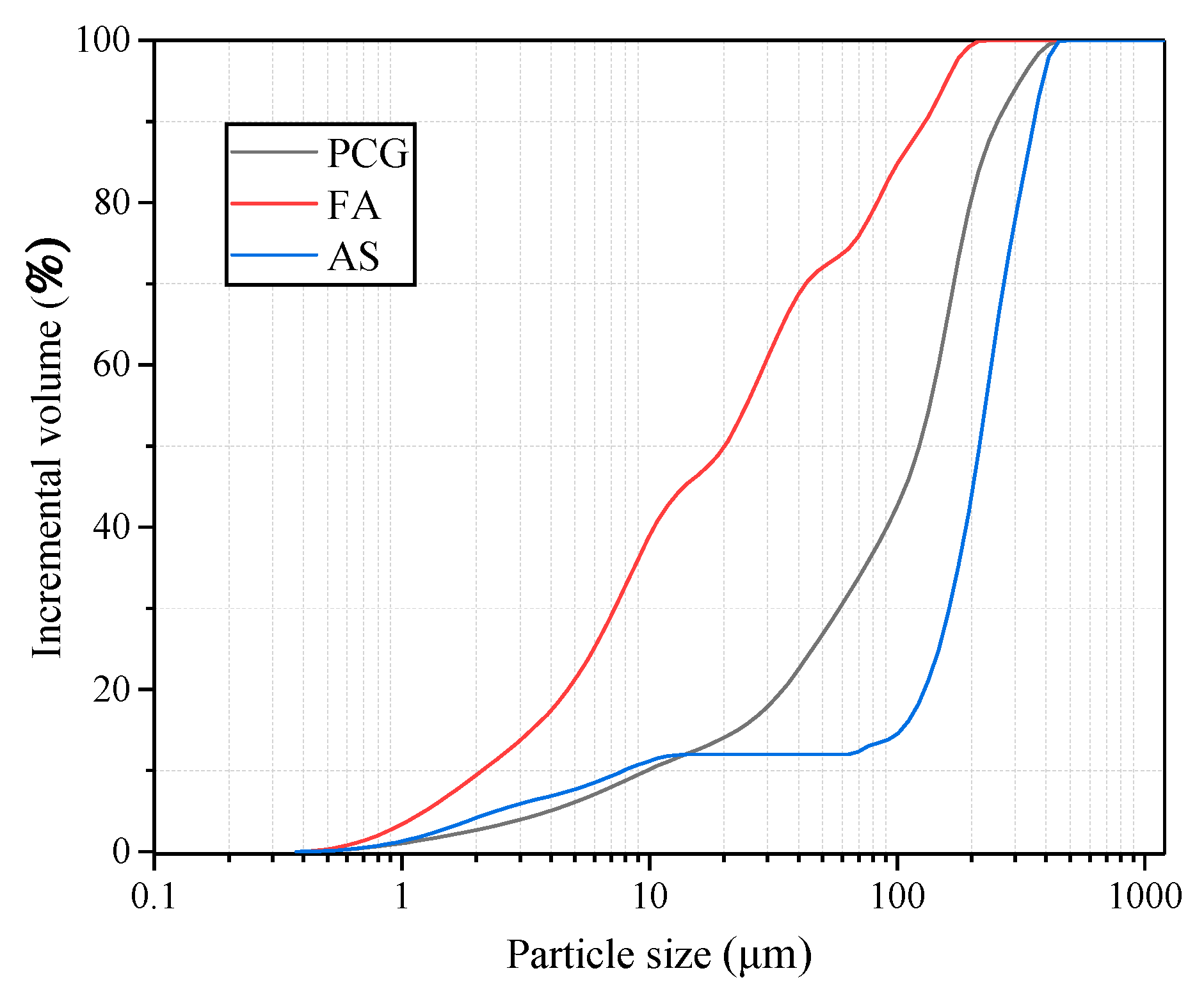
2.1.1. Preheated Decarburized Activated CG
2.1.2. Fly Ash
2.1.3. Aeolian Sand
2.1.4. Cement, Activator, and Water
2.2. Sample Preparation
2.3. Experimental Setup and Method
2.3.1. Mechanical Property Test
2.3.2. Microstructure Test
2.3.3. Leaching Toxicity Test
3. Results and Discussion
3.1. Mechanical Performance of PCCPB
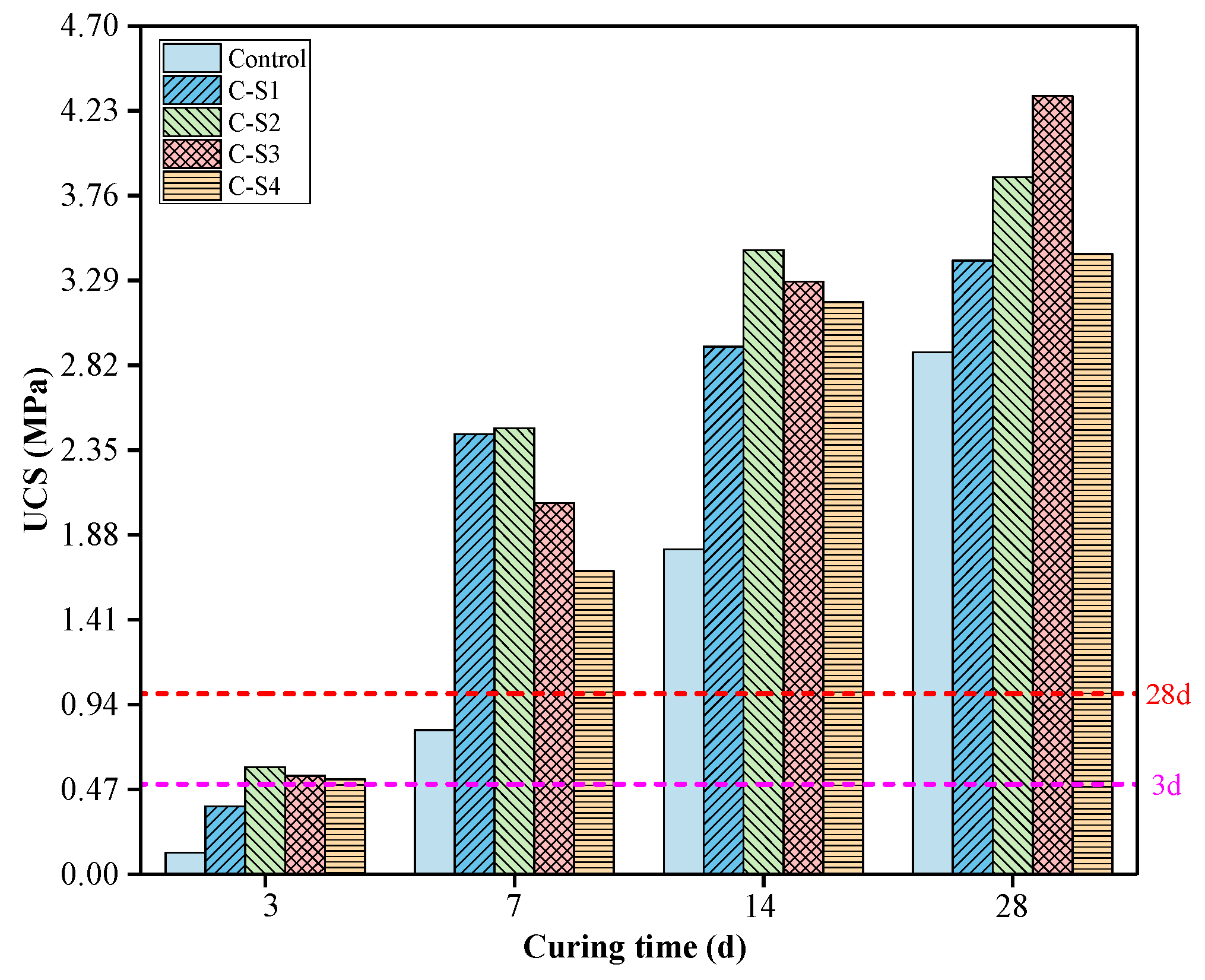
3.1.1. Analysis of UCS of PCCPB
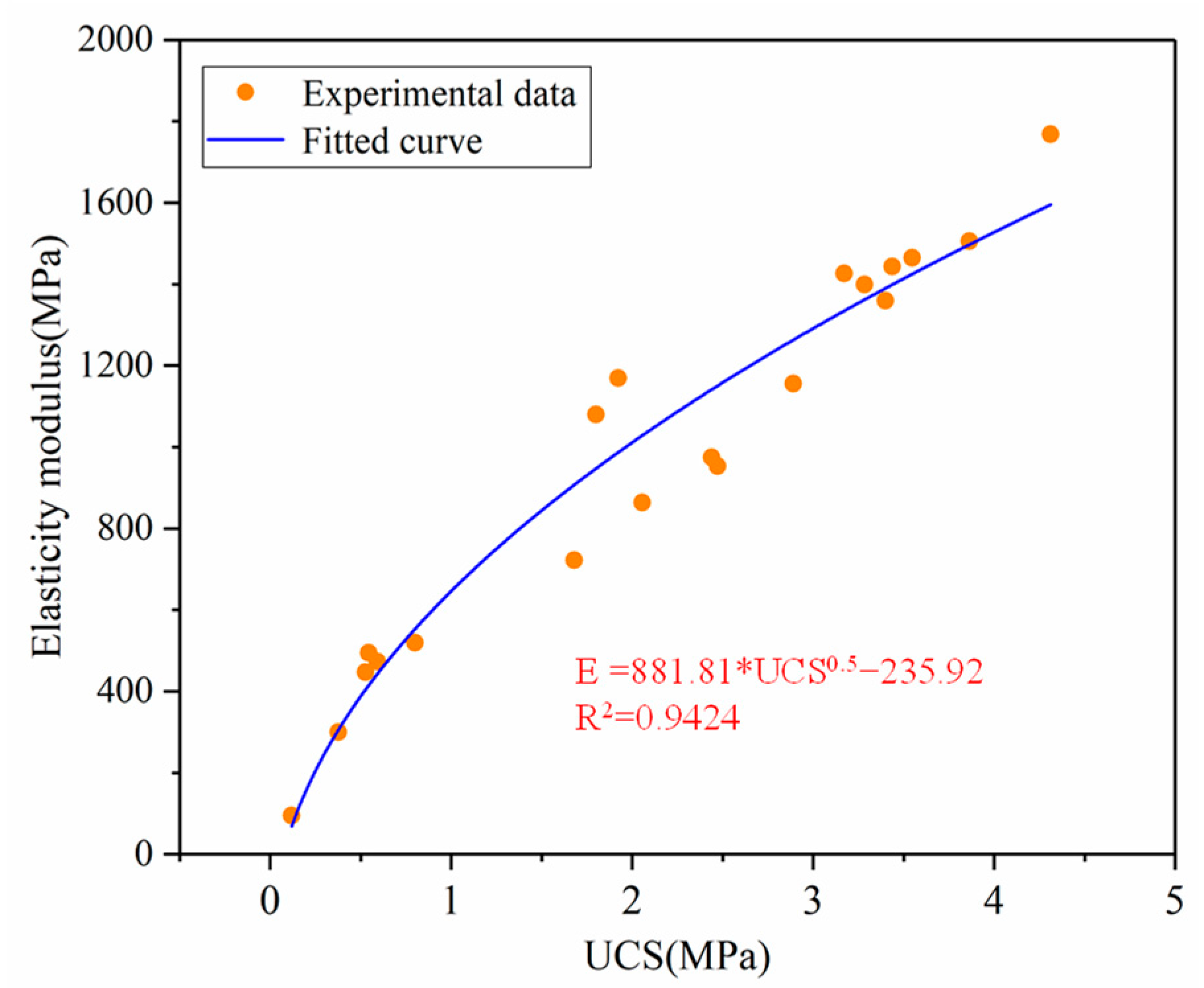
3.1.2. Analysis of Elastic Modulus of PCCPB
3.2. Microstructure of ARFGB
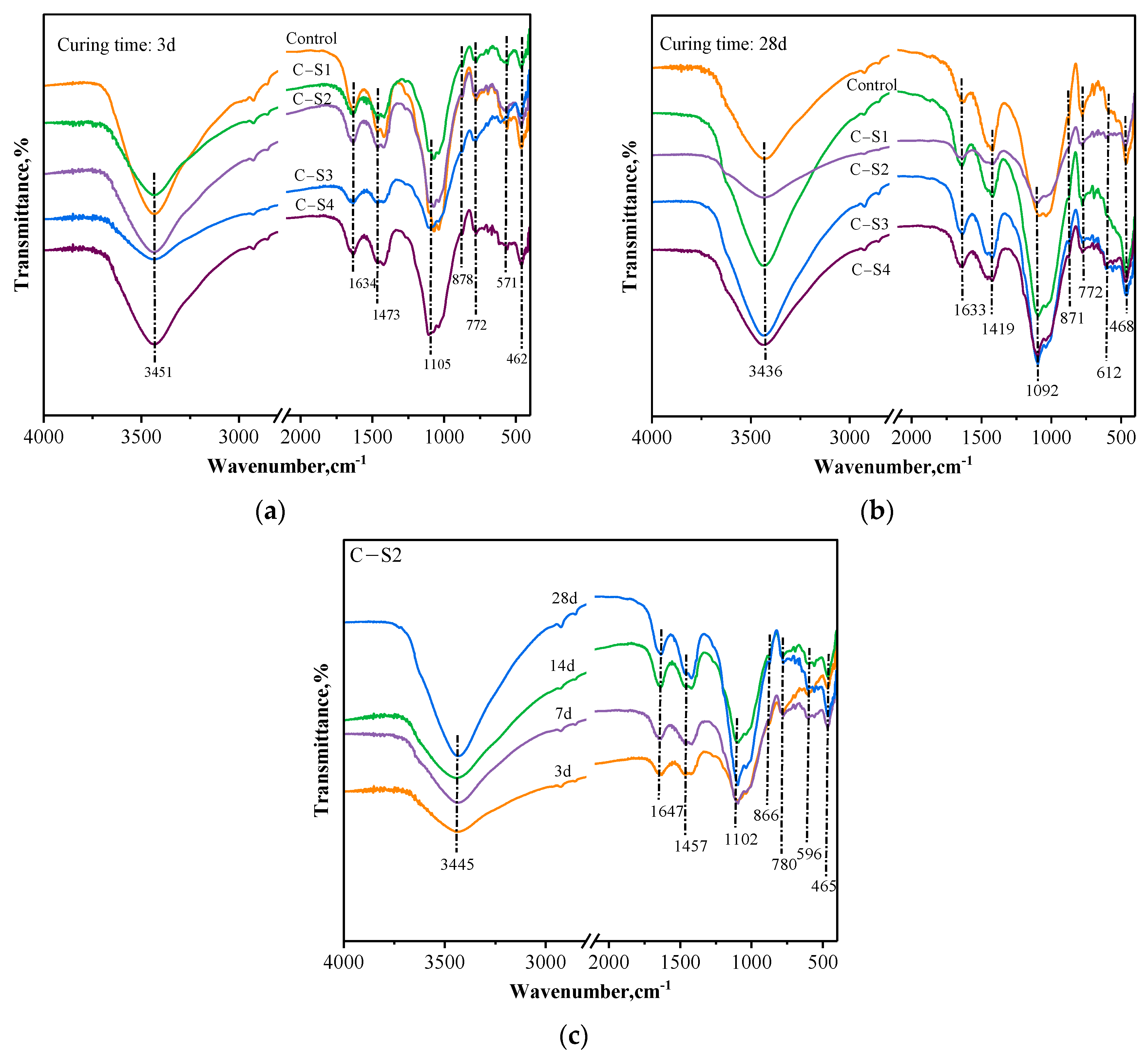
3.2.1. FTIR Results Analysis of PCCPB
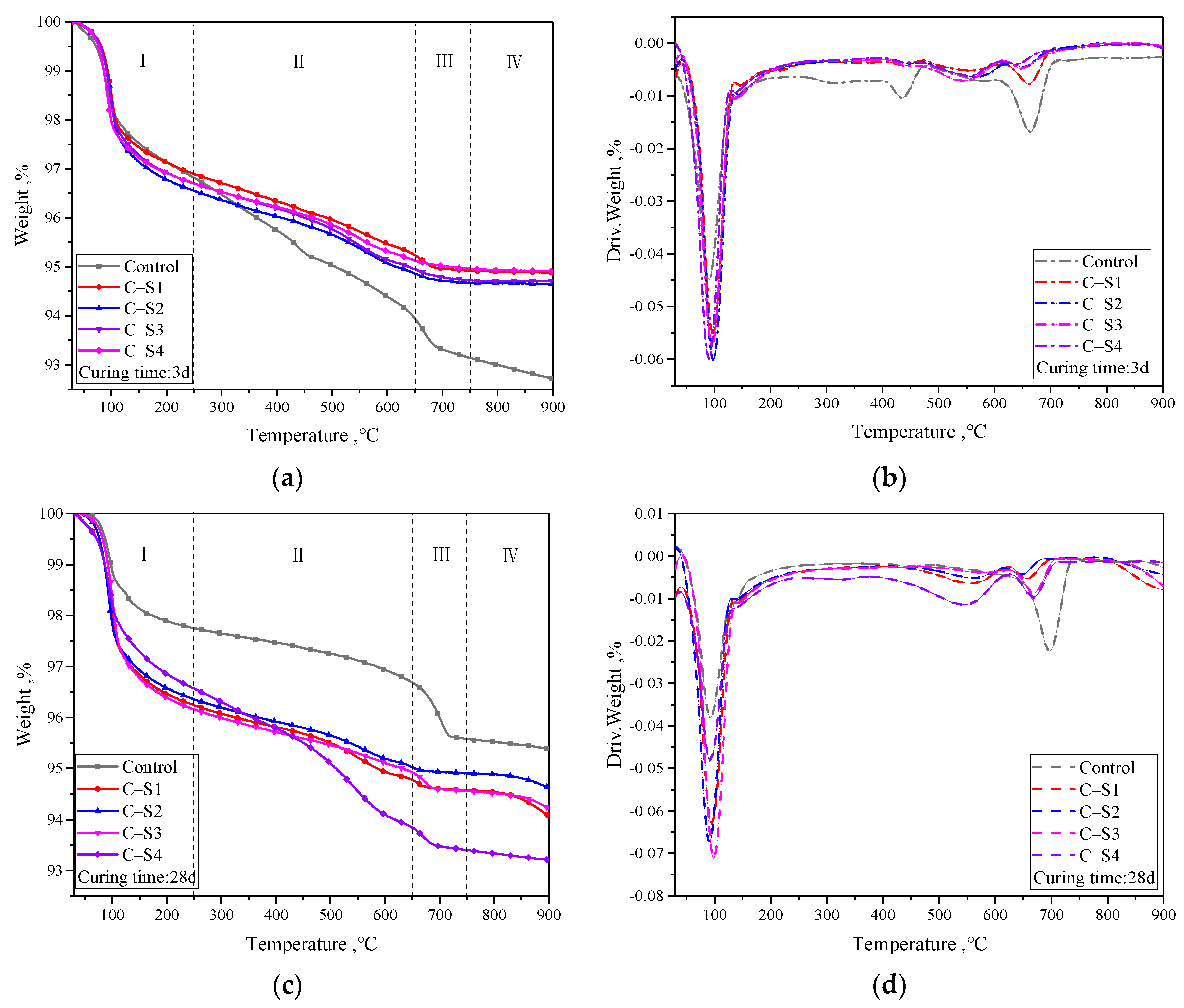
3.2.2. TG-DTG Results Analysis of PCCPB
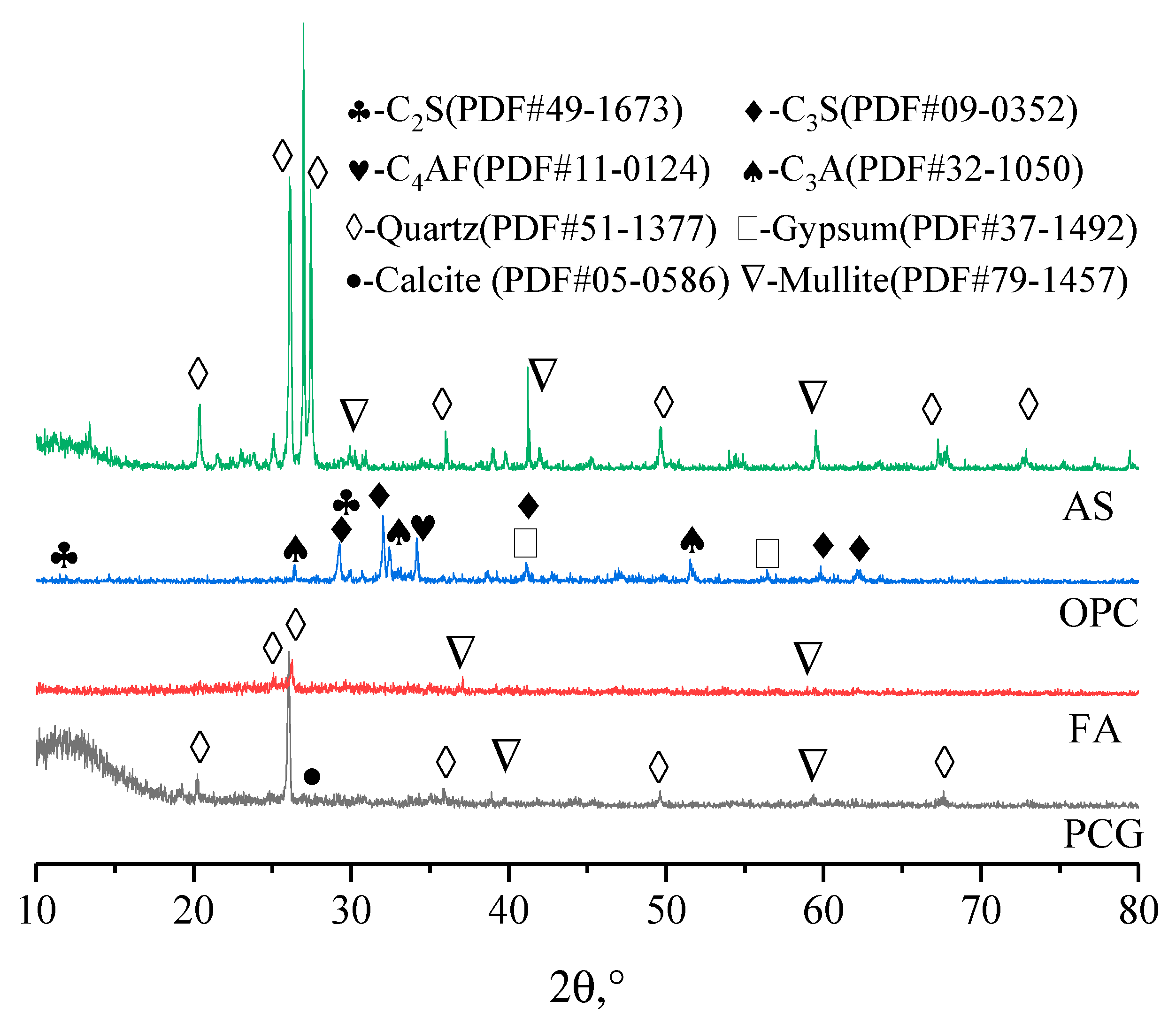
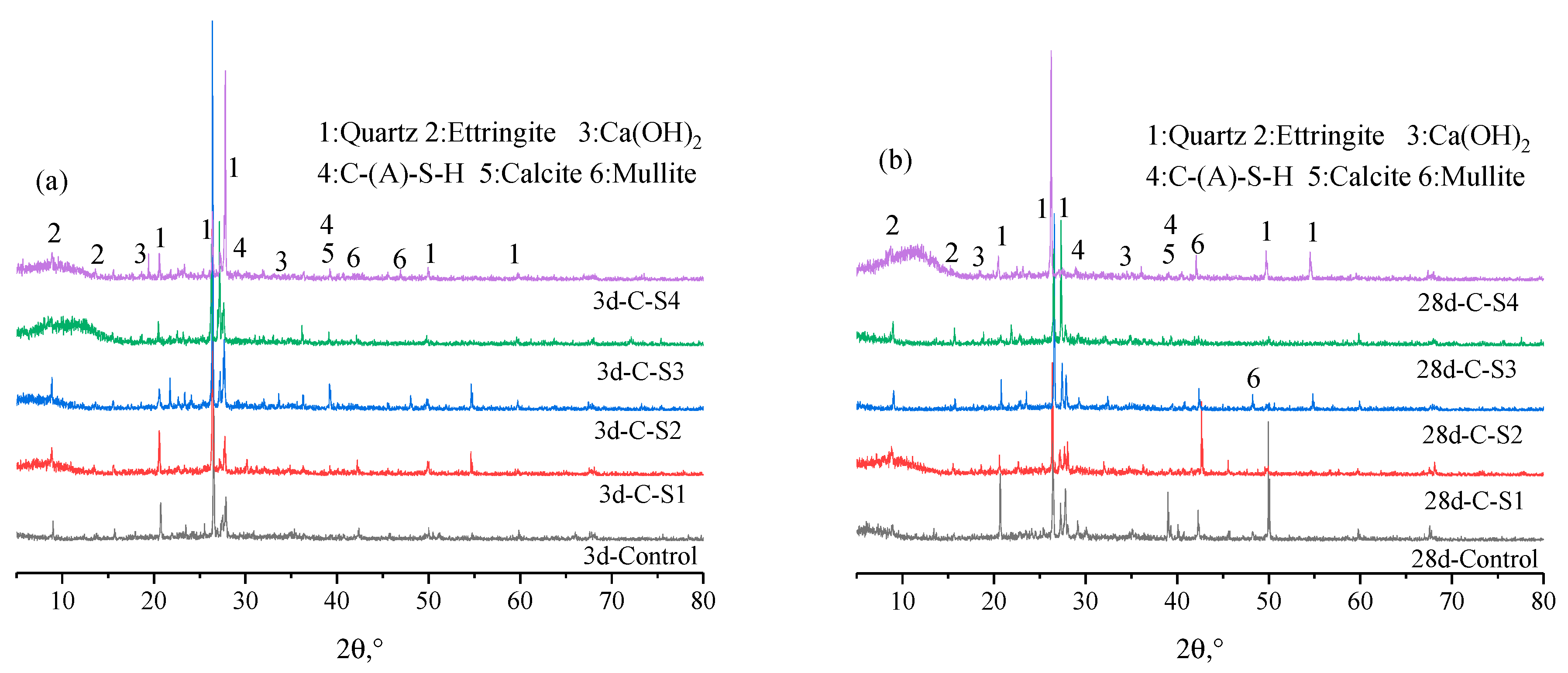
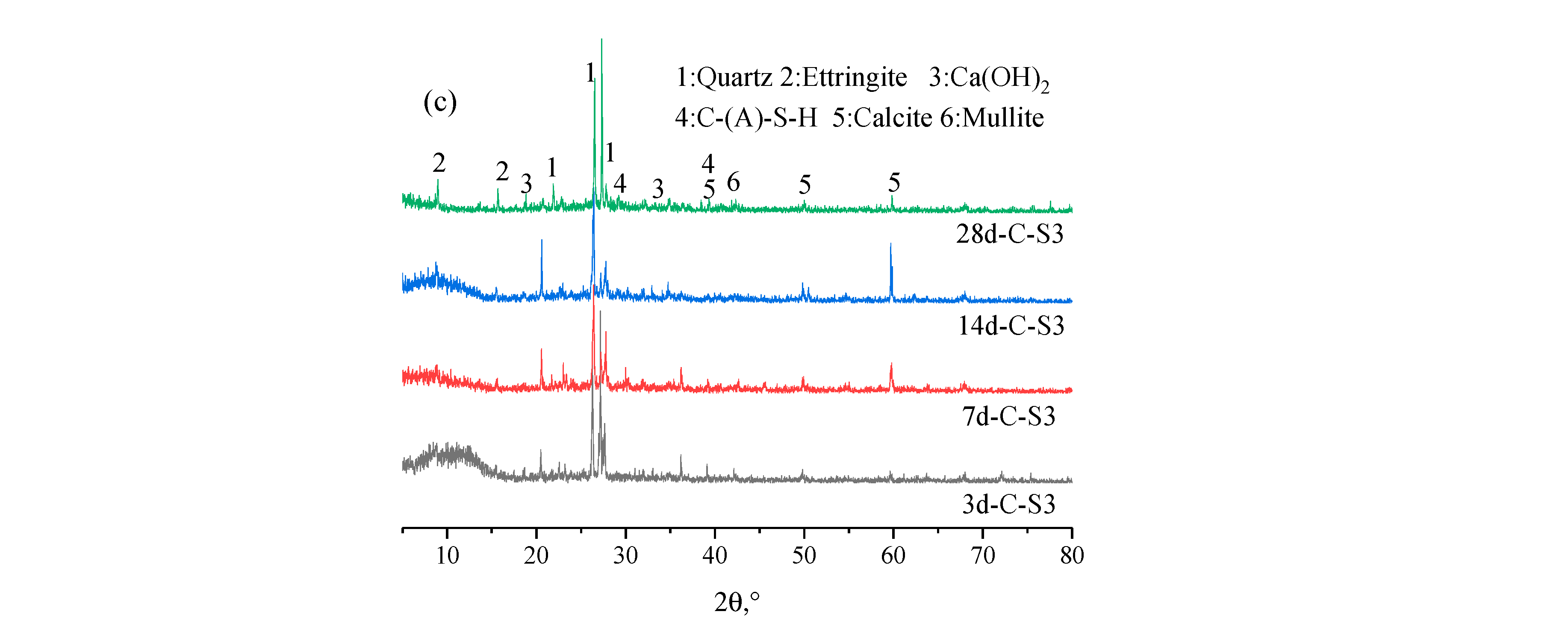
3.2.3. XRD Results Analysis of PCCPB
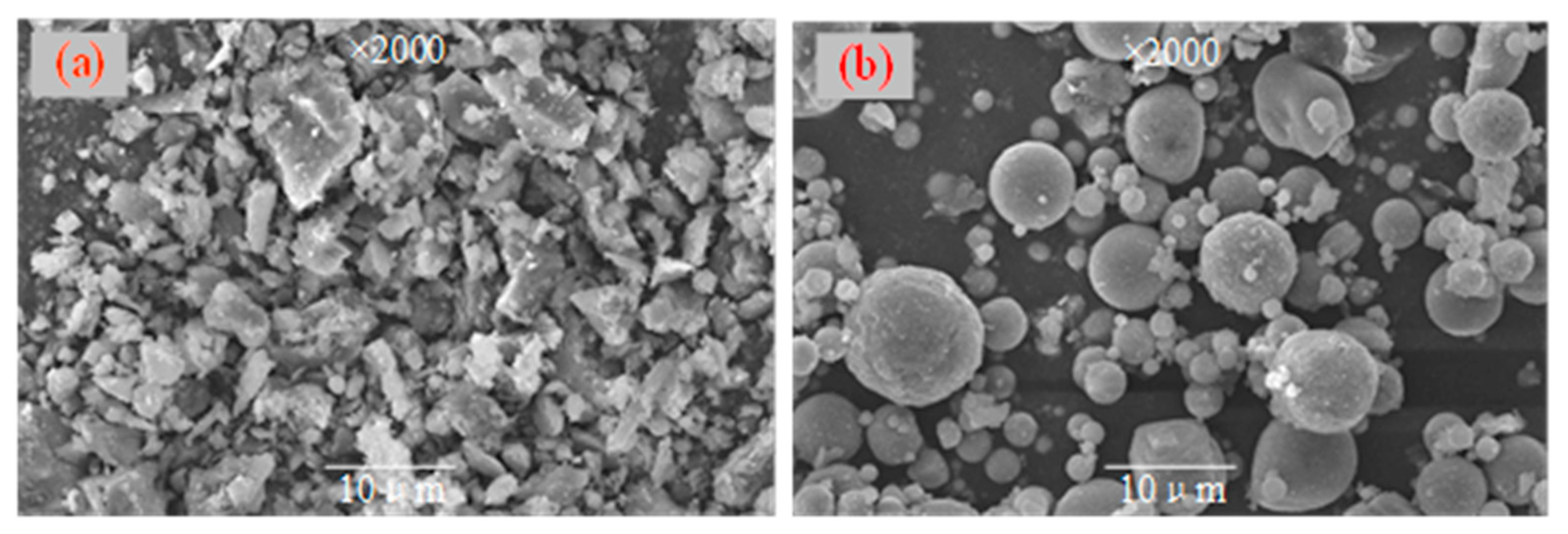
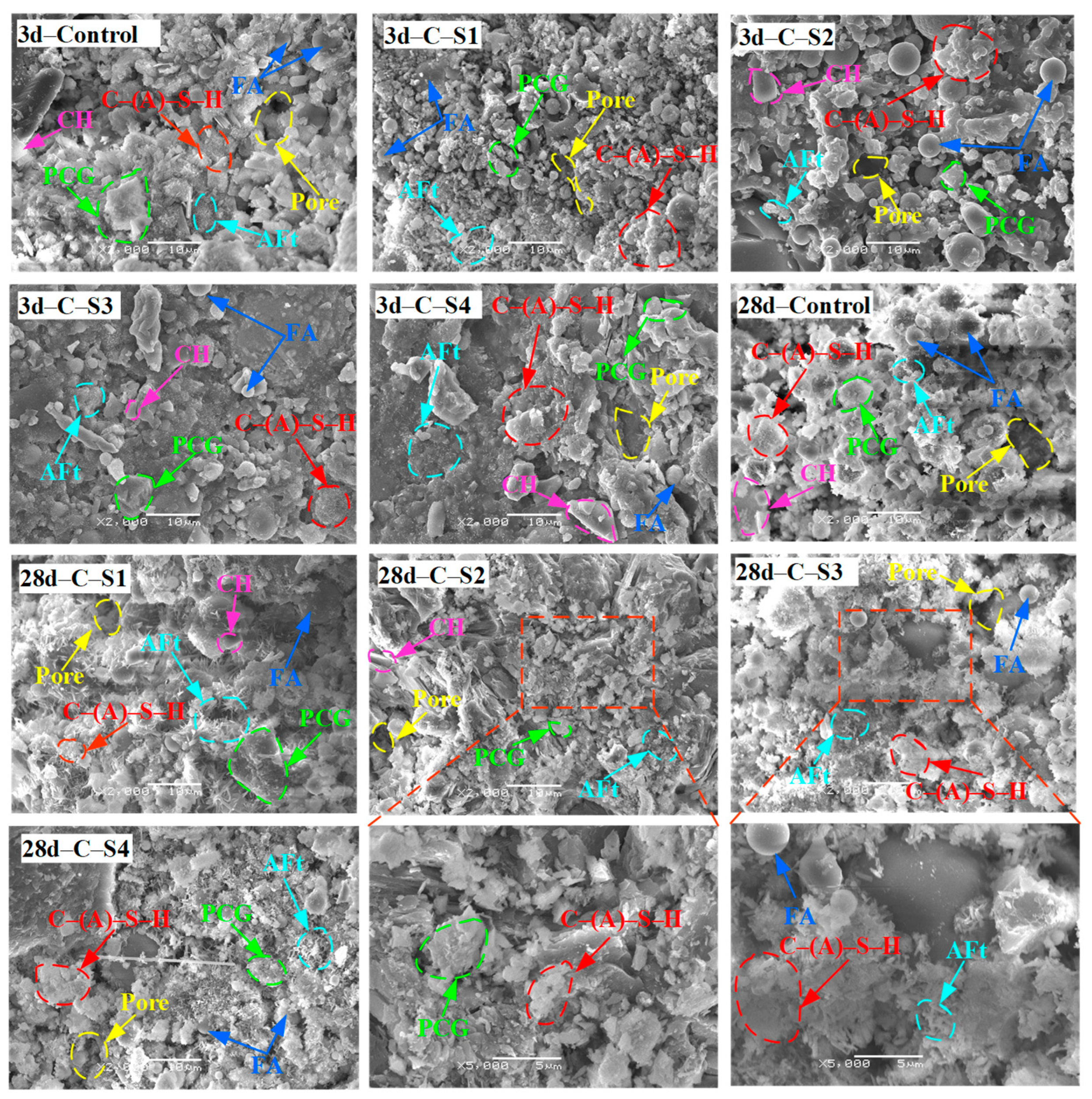
3.2.4. SEM Results Analysis of PCCPB
3.3. Leaching Toxicity Results Analysis of PCCPB
4. Conclusions
- (1)
- The UCS of C-S2, C-S3, and C-S4 can meet the requirements of backfill mining, among which the UCS of C-S3 is 0.545 and 4.312 MPa at 3 d and 28 d, respectively. Na2SO4 has an excitation effect on PCCPB at different curing times, and the UCS of PCCPB increases and then decreases with the increase in Na2SO4. The 3% Na2SO4 has the best excitation effect on the later strength (28 d) of PCCPB.
- (2)
- The main hydration products of PCCPB are C-(A)-S-H gel and calcium alumina (AFt), and the effect of different Na2SO4 dosages on the content and micromorphology of the gelling products of PCCPB is the main reason for changing its mechanical properties and leaching risk.
- (3)
- All groups’ (control group and CS1–CS4 group) leachate heavy metal ions meet the groundwater class III standard requirements, and PCCPB meets the environmental safety requirements. Based on the mechanical properties and leaching results, C-S3 was the best ratio.
- (4)
- Preheated decarburization (combustion temperature 900 °C, time 4 min) is an effective method of CG activation, and with PCG, FA, activator, low-dose cement, and water, preparation of the novel PCCPB is feasible.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, M.; Miao, X.; Xu, J. Green Mining of Coal Resources Harmonizing with Environment. J. China Coal Soc. 2007, 1, 1–7. [Google Scholar]
- Xiu, Z.; Wang, S.; Ji, Y.; Wang, F.; Ren, F.; Nguyen, V.T. Loading Rate Effect On the Uniaxial Compressive Strength (UCS) Behavior of Cemented Paste Backfill (CPB). Constr. Build. Mater. 2021, 271, 121526. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving Strength of Calcinated Coal Gangue Geopolymer Mortars Via Increasing Calcium Content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, H.; Wu, C.; Chen, H.; Sun, J.; Liu, J. Study On Compressive Strength and Durability of Alkali-Activated Coal Gangue-Slag Concrete and its Mechanism. Powder Technol. 2020, 368, 112–124. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X. Properties of Coal Gangue-Portland Cement Mixture with Carbonation. Fuel 2019, 245, 1–12. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.; Hu, S.; Shao, H.; Liao, Q.; Fan, Y. Experimental Study of the Effects of Stacking Modes On the Spontaneous Combustion of Coal Gangue. Process Saf. Environ. Protect. 2018, 123, 39–47. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Li, M.; Zhou, N.; Song, W.; Wang, Z.; Qi, S. A Preliminary Study of Solid-Waste Coal Gangue Based Biomineralization as Eco-Friendly Underground Backfill Material: Material Preparation and Macro-Micro Analyses. Sci. Total Environ. 2021, 770, 145241. [Google Scholar] [CrossRef]
- Guo, Z.; Qiu, J.; Jiang, H.; Xing, J.; Sun, X.; Ma, Z. Flowability of Ultrafine-Tailings Cemented Paste Backfill Incorporating Superplasticizer: Insight From Water Film Thickness Theory. Powder Technol. 2021, 381, 509–517. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Spearing, A.J.S.; Miao, X.; Guo, S.; Sun, Q. Green Coal Mining Technique Integrating Mining-Dressing-Gas Draining-Backfilling-Mining. Int. J. Min. Sci. Technol. 2017, 27, 17–27. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Zhang, J.; Jiang, H.; Wang, Y.; Yang, K.; Tian, X.; Yuan, L. Integrated Green Mining Technology of “Coal Mining-Gangue Washing-Backfilling-Strata Control-System Monitoring”-Taking Tangshan Mine as a Case Study. Environ. Sci. Pollut. Res. Int. 2021, 29, 5798–5811. [Google Scholar] [CrossRef]
- Liu, C.; Deng, X.; Liu, J.; Hui, D. Mechanical Properties and Microstructures of Hypergolic and Calcined Coal Gangue Based Geopolymer Recycled Concrete. Constr. Build. Mater. 2019, 221, 691–708. [Google Scholar] [CrossRef]
- Bai, G.; Zhu, C.; Liu, C.; Liu, B. An Evaluation of the Recycled Aggregate Characteristics and the Recycled Aggregate Concrete Mechanical Properties. Constr. Build. Mater. 2020, 240, 117978. [Google Scholar] [CrossRef]
- Khudyakova, T.M.; Kolesnikova, O.G.; Zhanikulov, N.N.; Botabaev, N.E.; Kenzhibaeva, G.S.; Iztleuov, G.M.; Suigenbaeva, A.Z.; Kutzhanova, A.N.; Ashirbaev, H.A.; Kolesnikova, V.A. Low-Basicity Cement, Problems and Advantages of its Utilization. Refract. Ind. Ceram. 2021, 62, 369–374. [Google Scholar] [CrossRef]
- Kolesnikova, O.; Syrlybekkyzy, S.; Fediuk, R.; Yerzhanov, A.; Nadirov, R.; Utelbayeva, A.; Agabekova, A.; Latypova, M.; Chepelyan, L.; Volokitina, I.; et al. Thermodynamic Simulation of Environmental and Population Protection by Utilization of Technogenic Tailings of Enrichment. Materials 2022, 15, 6980. [Google Scholar] [CrossRef]
- Kolesnikova, O.; Vasilyeva, N.; Kolesnikov, A.; Zolkin, A. Optimization of Raw Mix Using Technogenic Waste to Produce Cement Clinker. Min. Inf. Anal. Bull. 2022, 60, 103–115. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, T. Reactivity Activation of Waste Coal Gangue and its Impact On the Properties of Cement-Based Materials—A Review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Li, C.; Wan, J.; Sun, H.; Li, L. Investigation On the Activation of Coal Gangue by a New Compound Method. J. Hazard. Mater. 2010, 179, 515–520. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhu, M.; Cheng, F.; Zhang, D. Decomposition of Key Minerals in Coal Gangues During Combustion in O2/N2 and O2/Co2 Atmospheres. Appl. Therm. Eng. 2018, 148, 977–983. [Google Scholar] [CrossRef]
- Xu, B.; Liu, Q.; Ai, B.; Ding, S.; Frost, R.L. Thermal Decomposition of Selected Coal Gangue. J. Therm. Anal. Calorim. 2018, 131, 1413–1422. [Google Scholar] [CrossRef]
- Hao, R.; Li, X.; Xu, P.; Liu, Q. Thermal Activation and Structural Transformation Mechanism of Kaolinitic Coal Gangue From Jungar Coalfield, Inner Mongolia, China. Appl. Clay Sci. 2022, 223, 106508. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhang, Y.; Sun, J.; Hao, Z. The Thermal Activation Process of Coal Gangue Selected From Zhungeer in China. J. Therm. Anal. Calorim. 2016, 126, 1559–1566. [Google Scholar] [CrossRef]
- Song, X.; Gong, C.; Li, D. Study On Structural Characteristic and Mechanical Property of Coal Gangue in Activation Process. J. Chin. Ceram. Soc. 2004, 3, 358–363. [Google Scholar]
- Frías, M.; de Rojas, M.I.S.; García, R.; Valdés, A.J.; Medina, C. Effect of Activated Coal Mining Wastes On the Properties of Blended Cement. Cem. Concr. Compos. 2012, 34, 678–683. [Google Scholar] [CrossRef]
- Rabovitser, J.; Bryan, B.; Knight, R.; Nester, S.; Ake, T. Development and Testing of a Novel Coal Preheating Technology for Nox Reduction From Pulverized Coal-Fired Boilers. Gas 2003, 1, 4. [Google Scholar]
- Man, C.; Zhu, J.; Ouyang, Z.; Liu, J.; Lyu, Q. Experimental Study On Combustion Characteristics of Pulverized Coal Preheated in a Circulating Fluidized Bed. Fuel Process. Technol. 2018, 172, 72–78. [Google Scholar] [CrossRef]
- Pan, F.; Zhu, J.; Liu, J. Experimental Study and Numerical Simulation of Preheating Combustion in Circulating Fluidized Bed. Clean Coal Technol. 2021, 27, 180–188. [Google Scholar]
- Lyu, Q.; Wang, J.; Zhu, J. Experimental Study On Combustion Characteristics of Anthracite Pulverized Coal Preheated by Circulation Fluidized Bed. Boil. Technol. 2011, 42, 23–27. [Google Scholar]
- Zhu, J.; Ouyang, Z.; Lu, Q. An Experimental Study On Nox Emissions in Combustion of Pulverized Coal Preheated in a Circulating Fluidized Bed. Energy Fuels 2013, 27, 7724–7729. [Google Scholar] [CrossRef]
- Zhu, S.; Lyu, Q.; Zhu, J.; Liang, C. Experimental Study On No X Emissions of Pulverized Bituminous Coal Combustion Preheated by a Circulating Fluidized Bed. J. Energy Inst. 2018, 92, 247–256. [Google Scholar] [CrossRef]
- Lv, Z.; Xiong, X.; Yu, S.; Tan, H.; Xiang, B.; Huang, J.; Peng, J.; Li, P. Experimental Investigation On No Emission of Semi-Coke Under High Temperature Preheating Combustion Technology. Fuel 2021, 283, 119293. [Google Scholar] [CrossRef]
- Wang, S.; Gong, Y.; Niu, Y.; Hui, S. Study On No Formation During Preheating-Combustion Coupling of Pulverized Coal. Proc. CSEE 2020, 40, 2951–2959. [Google Scholar]
- Shi, Z.; Wang, G.; Wang, X.; Chen, Y.; Yu, W.; Miao, Y.; Peng, Y.; Tan, H. Study On Combustion Characteristics of Preheating Decarburization Process for Fine Slag in Coal Gasification. Clean Coal Technol. 2021, 27, 105–110. [Google Scholar]
- Shao, X.; Tian, C.; Li, C.; Fang, Z.; Zhao, B.; Xu, B.; Ning, J.; Li, L.; Tang, R. The Experimental Investigation On Mechanics and Damage Characteristics of the Aeolian Sand Paste-Like Backfill Materials Based On Acoustic Emission. Materials 2022, 15, 7235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Dong, C.; Ouyang, S.; Deng, X.; Du, E. Feasibility Study and Performance Optimization of Sand-Based Cemented Paste Backfill Materials. J. Clean Prod. 2020, 259, 120798. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, J.; Klein, B.; Zhou, N.; De Wit, B. Experimental Characterization of the Influence of Solid Components On the Rheological and Mechanical Properties of Cemented Paste Backfill. Int. J. Miner. Proc. 2017, 168, 116–125. [Google Scholar] [CrossRef]
- Shao, X.; Sun, J.; Xin, J.; Zhao, B.; Sun, W.; Li, L.; Tang, R.; Tian, C.; Xu, B. Experimental Study On Mechanical Properties, Hydration Kinetics, and Hydration Product Characteristics of Aeolian Sand Paste-Like Materials. Constr. Build. Mater. 2021, 303, 124601. [Google Scholar] [CrossRef]
- HJ 557-2010; Institute of Solid Waste Pollution Control Technology, Solid Waste-Extraction Procedure for Leaching Toxicity-Horizontal Vibration Method. China Environmental Science Press: Beijing, China, 2010.
- Huang, Z.; Su, X.; Zhang, J.; Luo, D.; Chen, Y.; Li, H. Study On Leaching of Heavy Metals From Red Mud-Phosphogypsum Composite Materials. Inorg. Chem. Ind. 2022, 54, 133–140. [Google Scholar]
- Bhattacharyya, G.K.; Johnson, R.A. Statistical Concepts and Methods; Wiley & Sons: Hoboken, NJ, USA, 1977. [Google Scholar]
- Behera, S.K.; Ghosh, C.N.; Mishra, D.P.; Singh, P.; Mishra, K.; Buragohain, J.; Mandal, P.K. Strength Development and Microstructural Investigation of Lead-Zinc Mill Tailings Based Paste Backfill with Fly Ash as Alternative Binder. Cem. Concr. Compos. 2020, 109, 103553. [Google Scholar] [CrossRef]
- Qi, T.; Feng, G.; Guo, Y.; Zhang, Y.; Ren, A.; Kang, L.; Guo, J. Experimental Study On the Changes of Coal Paste Backfilling Material Performance During Hydration Process. J. Min. Saf. Eng. 2015, 32, 42–48. [Google Scholar]
- Shao, X.; Wang, L.; Li, X.; Fang, Z.; Zhao, B.; Tao, Y.; Liu, L.; Sun, W.; Sun, J. Study On Rheological and Mechanical Properties of Aeolian Sand-Fly Ash-Based Filling Slurry. Energies 2020, 13, 1266. [Google Scholar] [CrossRef]
- Liu, L.; Fang, Z.; Qi, C.; Zhang, B.; Guo, L.; Song, K. Experimental Investigation On the Relationship Between Pore Characteristics and Unconfined Compressive Strength of Cemented Paste Backfill. Constr. Build. Mater. 2018, 179, 254–264. [Google Scholar] [CrossRef]
- Liu, L.; Xin, J.; Qi, C.; Jia, H.; Song, K. Experimental Investigation of Mechanical, Hydration, Microstructure and Electrical Properties of Cemented Paste Backfill. Constr. Build. Mater. 2020, 263, 120137. [Google Scholar] [CrossRef]
- Phuong, T.B.; Yuko, O.; Kenji, K. Effect of Sodium Sulfate Activator On Compressive Strength and Hydration of Fly-Ash Cement Pastes. J. Mater. Civ. Eng. 2020, 32, 04020117. [Google Scholar]
- Li, W.; Fall, M. Sulphate Effect On the Early Age Strength and Self-Desiccation of Cemented Paste Backfill. Constr. Build. Mater. 2016, 106, 296–304. [Google Scholar] [CrossRef]
- Ercikdi, B.; Baki, H.; İzki, M. Effect of Desliming of Sulphide-Rich Mill Tailings On the Long-Term Strength of Cemented Paste Backfill. J. Environ. Manag. 2013, 115, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xin, J.; Feng, Y.; Zhang, B.; Song, K.I. Effect of the Cement–Tailing Ratio On the Hydration Products and Microstructure Characteristics of Cemented Paste Backfill. Arab. J. Sci. Eng. 2019, 44, 6547–6556. [Google Scholar] [CrossRef]
- Rao, Y.; Shao, Y.; Huang, Y.; Sun, X.; Li, Y.; Li, J. Effect of Superplasticizer On the Elastic Modulus of Super Fine Tailings Filling Body. Min. Res. Dev. 2016, 36, 31–35. [Google Scholar]
- Li, X.; Liu, C. Mechanical Properties and Damage Constitutive Model of High Water Material at Different Loading Rates. Adv. Eng. Mater. 2018, 20, 1701098. [Google Scholar] [CrossRef]
- Choe, G.; Kang, S.; Kang, H. Mechanical Properties of Concrete Containing Liquefied Red Mud Subjected to Uniaxial Compression Loads. Materials. 2020, 13, 854. [Google Scholar] [CrossRef]
- Andoni, A.; Delilaj, E.; Ylli, F.; Taraj, K.; Korpa, A.; Xhaxhiu, K.; Çomo, A. FTIR Spectroscopic Investigation of Alkali-Activated Fly Ash: Atest Study. Zaštita Mater. 2018, 59, 539–542. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; Palomo, A. Alkali Activation of Fly Ash: Effect of the SiO2/Na2O Ratio Part I: FTIR Study. Microporous Mesoporous Mat. 2007, 106, 180–191. [Google Scholar] [CrossRef]
- Nguyen, H.T. Evaluation On Formation of Aluminosilicate Network in Ternary-Blended Geopolymer Using Infrared Spectroscopy. Solid State Phenom. 2019, 296, 99–104. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Zhao, Y.; Liu, X. Hydration Characteristics and Environmental Friendly Performance of a Cementitious Material Composed of Calcium Silicate Slag. J. Hazard. Mater. 2016, 306, 67–76. [Google Scholar] [CrossRef]
- Xin, J.; Liu, L.; Xu, L.; Wang, J.; Yang, P.; Qu, H. A Preliminary Study of Aeolian Sand-Cement-Modified Gasification Slag-Paste Backfill: Fluidity, Microstructure, and Leaching Risks. Sci. Total Environ. 2022, 830, 154766. [Google Scholar] [CrossRef]
- Lecomte, I.; Henrist, C.; Liégeois, M.; Maseri, F.; Rulmont, A.; Cloots, R. (Micro)-Structural Comparison Between Geopolymers, Alkali-Activated Slag Cement and Portland Cement. J. Eur. Ceram. Soc. 2006, 26, 3789–3797. [Google Scholar] [CrossRef]
- Ouellet, S.; Bussière, B.; Aubertin, M.; Benzaazoua, M. Microstructural Evolution of Cemented Paste Backfill: Mercury Intrusion Porosimetry Test Results. Cem. Concr. Res. 2007, 37, 1654–1665. [Google Scholar] [CrossRef]
- Abdul-Hussain, N.; Fall, M. Unsaturated Hydraulic Properties of Cemented Tailings Backfill that Contains Sodium Silicate. Eng. Geol. 2011, 123, 288–301. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Li, Q.; Song, J. Hydration Properties of Portland Cement-Copper Tailing Powder Composite Binder. Constr. Build. Mater. 2020, 251, 118882. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-Efficient Cements: Potential Economically Viable Solutions for a Low-CO2 Cement-Based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Quanji, Z. Thixotropic Behavior of Cement-Based Materials: Effect of Clay and Cement Types. Master’s Thesis, Iowa State University, Ames, IA, USA, 2010. [Google Scholar]
- Zhang, S.; Niu, D.; Luo, D. Enhanced Hydration and Mechanical Properties of Cement-Based Materials with Steel Slag Modified by Water Glass. J. Mater. Res. Technol. 2022, 21, 1830–1842. [Google Scholar] [CrossRef]
- Feng, J.; Sun, J. A Comparison of the 10-Year Properties of Converter Steel Slag Activated by High Temperature and an Alkaline Activator. Constr. Build. Mater. 2020, 234, 116948. [Google Scholar] [CrossRef]
- Mota, B.; Matschei, T.; Scrivener, K. Impact of NaOH and Na2SO4 On the Kinetics and Microstructural Development of White Cement Hydration. Cem. Concr. Res. 2018, 108, 172–185. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, S.; Sun, Q.; Li, B.; Cai, C.; Xia, Y.; Wei, X.; Mu, Q. Preparation and Microstructure of Fly Ash Geopolymer Paste Backfill Material. J. Clean Prod. 2019, 225, 376–390. [Google Scholar] [CrossRef]
- Kong, X.; Lu, Z.; Zhang, C. Recent Development On Understanding Cement Hydration Mechanism and Effects of Chemical Admixtures On Cement Hydration. J. Chin. Ceram. Soc. 2017, 45, 274–281. [Google Scholar]
- Justnes, H.; Sellevold, E.J.; Lundevall, G. High Strength Concrete Binders. Part a: Reactivity and Composition of Cement Pastes with and without Condensed Silica Fume. In Proceedings of the 4th International Conference on Fly Ash, Silica Fume, Slag and Natural Pozzolana in Concrete, Istanbul, Turkey, 3–8 May 1992. [Google Scholar]
- Kipkemboi, B.; Zhao, T.; Miyazawa, S.; Sakai, E.; Hirao, H. Effect of C3S Content of Clinker On Properties of Fly Ash Cement Concrete. Constr. Build. Mater. 2020, 240, 117840. [Google Scholar] [CrossRef]
- Liu, L.; Ruan, S.; Qi, C.; Zhang, B.; Tu, B.; Yang, Q.; Song, K.I.I.L. Co-Disposal of Magnesium Slag and High-Calcium Fly Ash as Cementitious Materials in Backfill. J. Clean Prod. 2021, 279, 123684. [Google Scholar] [CrossRef]
- Razali, N.N.; Sukardi, M.A.; Sopyan, I.; Mel, M.; Salleh, H.M.; Rahman, M.M. The Effects of Excess Calcium On the Handling and Mechanical Properties of Hydrothermal Derived Calcium Phosphate Bone Cement. IOP Conf. Ser. Mater. Sci. Eng. 2018, 290, 012053. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Zhang, S.; Guo, Z.; Ma, Z.; Sun, X.; Xing, J. Effect of Sodium Sulfate On the Hydration and Mechanical Properties of Lime-Slag Based Eco-Friendly Binders. Constr. Build. Mater. 2020, 250, 118603. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. Naoh-Activated Ground Fly Ash Geopolymer Cured at Ambient Temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Salih, M.A.; Ali, A.A.A.; Farzadnia, N. Characterization of Mechanical and Microstructural Properties of Palm Oil Fuel Ash Geopolymer Cement Paste. Constr. Build. Mater. 2014, 6, 592–603. [Google Scholar] [CrossRef]
- Ouyang, S.; Huang, Y.; Zhou, N.; Li, J.; Gao, H.; Guo, Y. Experiment On Hydration Exothermic Characteristics and Hydration Mechanism of Sand-Based Cemented Paste Backfill Materials. Constr. Build. Mater. 2022, 318, 125870. [Google Scholar] [CrossRef]
- Roy, D.M.; Ldorn, G.M. Hydration, Structure, and Properties of Blast Furnace Slag Cements, Mortars, and Concrete. J. Proc. 1982, 79, 444–457. [Google Scholar]
- Rachid, H.S.; Walid, M.; Mahfoud, B.; Richard, L.; Keith, T.; Agnes, Z.; NorEdine, A. Mechanical Properties and Microstructure of Low Carbon Binders Manufactured From Calcined Canal Sediments and Ground Granulated Blast Furnace Slag (GGBS). Sustainability 2021, 13, 9057. [Google Scholar]
- Poon, C.; Azhar, S.; Anson, M.; Wong, Y. Strength and Durability Recovery of Fire-Damaged Concrete After Post-Fire-Curing. Cem. Concr. Res. 2001, 31, 1307–1318. [Google Scholar] [CrossRef]
- Tang, P.; Chen, W.; Xuan, D.; Cheng, H.; Poon, C.S.; Tsang, D.C.W. Immobilization of Hazardous Municipal Solid Waste Incineration Fly Ash by Novel Alternative Binders Derived From Cementitious Waste. J. Hazard. Mater. 2020, 393, 122386. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, J.; Su, Q.; Wang, Q.; Sun, H. Mechanical Properties of Geopolymer Recycled Aggregate Concrete. J. Shenyang Jianzhu Univ. Nat. Sci. 2021, 37, 138–146. [Google Scholar]
- Ding, Z.; Hong, X.; Zhu, J.; Tian, B.; Fang, Y. Alkali-Activated Red Mud-Slag Cementitious Materials. J. Chin. Electron Microsc. Soc. 2018, 37, 145–153. [Google Scholar]
- Liu, J.; Li, Z.; Zhang, M.; Wang, S.; Hai, R. Mechanical Property and Polymerization Mechanism of Red Mud Geopolymer Cement. J. Build. Mater. 2022, 25, 178–183. [Google Scholar]
- Tang, R.; Zhao, B.; Li, C.; Xin, J.; Xu, B.; Tian, C.; Ning, J.; Li, L.; Shao, X. Experimental Study On the Effect of Fly Ash with Ammonium Salt Content On the Properties of Cemented Paste Backfill. Constr. Build. Mater. 2023, 369, 130513. [Google Scholar] [CrossRef]
- Türkel, S. Long-Term Compressive Strength and some Other Properties of Controlled Low Strength Materials Made with Pozzolanic Cement and Class C Fly Ash. J. Hazard. Mater. 2006, 137, 261–266. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Ding, S.; Zhang, H.; Xia, F.; Wang, J.; Zhou, M. Utilization of Red Mud in Geopolymer-Based Pervious Concrete with Function of Adsorption of Heavy Metal Ions. J. Clean Prod. 2019, 207, 789–800. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S.; Li, Q.; Li, N.; Yuan, B.; Chen, W.; Brouwers, H.J.H.; Yu, Q. Uptake of Heavy Metal Ions in Layered Double Hydroxides and Applications in Cementitious Materials: Experimental Evidence and First-Principle Study. Constr. Build. Mater. 2019, 222, 96–107. [Google Scholar] [CrossRef]
- Peng, D.; Wang, Y.; Liu, X.; Tang, B.; Zhang, N. Preparation, Characterization, and Application of an Eco-Friendly Sand-Fixing Material Largely Utilizing Coal-Based Solid Waste. J. Hazard. Mater. 2019, 373, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhi, T.; Liu, L.; Mi, J.; Zhang, M.; Tian, C.; Si, Z.; Liu, X.; Mu, Y. Solidification/Stabilization of Chromium Slag in Red Mud-Based Geopolymer. Constr. Build. Mater. 2022, 316, 125813. [Google Scholar] [CrossRef]
- Bah, A.; Jin, J.; Ramos, A.O.; Bao, Y.; Ma, M.; Li, F. Arsenic(V) Immobilization in Fly Ash and Mine Tailing-Based Geopolymers: Performance and Mechanism Insight. Chemosphere 2022, 306, 135636. [Google Scholar] [CrossRef]



| Chemical Composition | OPC | AS | PCG | FA |
|---|---|---|---|---|
| Al2O3 | 5.53% | 10.30% | 20.41% | 17.14% |
| SiO2 | 22.36% | 67.80% | 53.55% | 41.01% |
| CaO | 65.08% | 5.30% | 3.76% | 14.03% |
| Fe2O3 | 3.46% | 5.80% | 8.99% | 14.47% |
| K2O | 0.62% | 7.50% | 1.62% | 5.36% |
| Mg2O | 1.27% | 1.78% | 3.65% | 4.31% |
| TiO2 | 0.59% | 0.35% | 0.73% | 1.60% |
| SO3 | / | / | 1.27% | 0.711% |
| Others | 1.09% | 1.17% | 5.62% | 0.83% |
| Group Number | Concentration (CO) a, % | Cement Dosage (CD) b, wt.% | AS Dosage (AD) d, wt.% | FA/PCG | FA+PCG Content (FC) c, wt.% | Activator Content (AC) e, wt.% | Activator Type |
|---|---|---|---|---|---|---|---|
| Control | 78% | 3% | 50 | 7/3 | 47 | / | / |
| C-S1 f | 46 | 1 | Na2SO4 | ||||
| C-S2 | 45 | 2 | |||||
| C-S3 | 44 | 3 | |||||
| C-S4 | 43 | 4 |
| Sample | UCS, MPa | R, % | UCS, MPa | R,% | UCS, MPa | R,% | UCS, MPa | R, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 14 d | 28d | |||||||||||||
| No. of Sample (n): 3 | No. of Sample (n): 3 | No. of Sample (n): 3 | No. of Sample (n): 3 | |||||||||||||
| Mean | SD | CV | Mean | SD | CV | Mean | SD | CV | Mean | SD | CV | |||||
| Control | 0.119 | 0.005 | 4.12 | / | 0.799 | 0.037 | 4.68 | / | 1.8 | 0.086 | 4.79 | / | 2.891 | 0.085 | 2.96 | / |
| C-S1 | 0.376 | 0.015 | 3.99 | 215.97 | 2.438 | 0.052 | 2.13 | 205.13 | 2.924 | 0.109 | 3.72 | 62.44 | 3.4 | 0.075 | 2.22 | 17.61 |
| C-S2 | 0.592 | 0.031 | 5.28 | 397.48 | 2.471 | 0.064 | 2.61 | 209.26 | 3.458 | 0.036 | 1.05 | 92.11 | 3.863 | 0.127 | 3.30 | 33.62 |
| C-S3 | 0.545 | 0.008 | 1.44 | 357.98 | 2.057 | 0.038 | 1.83 | 157.45 | 3.284 | 0.066 | 2.02 | 82.44 | 4.312 | 0.095 | 2.20 | 49.15 |
| C-S4 | 0.527 | 0.013 | 2.56 | 342.86 | 1.68 | 0.095 | 5.67 | 110.26 | 3.171 | 0.089 | 2.80 | 76.17 | 3.437 | 0.073 | 2.13 | 18.89 |
| Temperature Range, °C | Weight Change (3 d, 28 d), % | ||||
|---|---|---|---|---|---|
| Control | C-S1 | C-S2 | C-S3 | C-S4 | |
| I (30–250) | −2.25, −3.19 | −3.11, −3.76 | −3.45, −3.64 | −3.3, −3.84 | −3.31, −3.43 |
| II (250–650) | −2.87, −1.06 | −1.66, −1.47 | −1.68, −1.34 | −1.75, −1.24 | −1.57, −2.72 |
| III (650–750) | −0.80, −1.11 | −0.31, −0.20 | −0.19, −0.12 | −0.22, −0.35 | −0.16, −0.45 |
| IV (750–900) | −0.42, −0.20 | −0.04, −0.51 | −0.03, −0.27 | −0.03, −0.35 | −0.06, −0.12 |
| Control, mg/L | C-S1, mg/L | C-S2, mg/L | C-S3, mg/L | C-S4, mg/L | Limit, mg/L | |
|---|---|---|---|---|---|---|
| Mn | ND 1.2 × 10−4 | ND 1.2 × 10−4 | ND 1.2 × 10−4 | ND 1.2 × 10−4 | ND 1.2 × 10−4 | 0.1 |
| Zn | ND 6.7 × 10−4 | ND 6.7 × 10−4 | ND 6.7 × 10−4 | ND 6.7 × 10−4 | ND 6.7 × 10−4 | 1 |
| As | 7.5 × 10−3 | 6.8 × 10−3 | 6.2 × 10−3 | 4.7 × 10−3 | 7.0 × 10−3 | 0.01 |
| Cd | ND 5.0 × 10−5 | ND 5.0 × 10−5 | ND 5.0 × 10−5 | ND 5.0 × 10−5 | ND 5.0 × 10−5 | 0.005 |
| Hg | 1.8 × 10−4 | 1.4 × 10−4 | 1.1 × 10−4 | 8.1 × 10−5 | 1.3 × 10−4 | 0.001 |
| Pb | ND 9.0 × 10−5 | ND 9.0 × 10−5 | ND 9.0 × 10−5 | ND 9.0 × 10−5 | ND 9.0 × 10−5 | 0.01 |
| Cr | 0.01 | 8.4 × 10−3 | 6.2 × 10−3 | 4.3 × 10−3 | 5.2 × 10−3 | 0.05 |
| Cu | ND 8.0 × 10−5 | ND 8.0 × 10−5 | ND 8.0 × 10−5 | ND 8.0 × 10−5 | ND 8.0 × 10−5 | 1 |
| Ba | ND 2.0 × 10−4 | ND 2.0 × 10−4 | ND 2.0 × 10−4 | ND 2.0 × 10−4 | ND 2.0 × 10−4 | 0.7 |
| Ni | ND 6.0 × 10−5 | ND 6.0 × 10−5 | ND 6.0 × 10−5 | ND 6.0 × 10−5 | ND 6.0 × 10−5 | 0.02 |
| Ag | ND 4.0 × 10−5 | ND 4.0 × 10−5 | ND 4.0 × 10−5 | ND 4.0 × 10−5 | ND 4.0 × 10−5 | 0.05 |
| Se | 2.3 × 10−3 | 1.8 × 10−3 | 1.5 × 10−3 | 1.1 × 10−3 | 1.6 × 10−3 | 0.01 |
| Mo | 0.02 | 0.017 | 0.014 | 0.012 | 0.013 | 0.07 |
| Sb | 1.9 × 10−3 | 1.6 × 10−3 | 1.4 × 10−3 | 1.1 × 10−3 | 1.3 × 10−3 | 0.005 |
| Co | ND 3.0 × 10−5 | 1ND 3.0 × 10−5 | ND 3.0 × 10−5 | ND 3.0 × 10−5 | ND 3.0 × 10−5 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Zhao, B.; Tian, C.; Xu, B.; Li, L.; Shao, X.; Ren, W. Preliminary Study of Preheated Decarburized Activated Coal Gangue-Based Cemented Paste Backfill Material. Materials 2023, 16, 2354. https://doi.org/10.3390/ma16062354
Tang R, Zhao B, Tian C, Xu B, Li L, Shao X, Ren W. Preliminary Study of Preheated Decarburized Activated Coal Gangue-Based Cemented Paste Backfill Material. Materials. 2023; 16(6):2354. https://doi.org/10.3390/ma16062354
Chicago/Turabian StyleTang, Renlong, Bingchao Zhao, Chuang Tian, Baowa Xu, Longqing Li, Xiaoping Shao, and Wuang Ren. 2023. "Preliminary Study of Preheated Decarburized Activated Coal Gangue-Based Cemented Paste Backfill Material" Materials 16, no. 6: 2354. https://doi.org/10.3390/ma16062354
APA StyleTang, R., Zhao, B., Tian, C., Xu, B., Li, L., Shao, X., & Ren, W. (2023). Preliminary Study of Preheated Decarburized Activated Coal Gangue-Based Cemented Paste Backfill Material. Materials, 16(6), 2354. https://doi.org/10.3390/ma16062354





