Low-Temperature Transient Liquid Phase Bonding Technology via Cu Porous-Sn58Bi Solid–Liquid System under Formic Acid Atmosphere
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Experiment and Analysis
- -
- F—Shear load. The unit is N;
- -
- S—Metal joint contact area. The unit is m2;
- -
- τ—Shear strength. The unit is MPa.
| Process Number | Temperature (°C) | Bonding Time (min) | Atmosphere | Pressure (MPa) | Catalytic |
|---|---|---|---|---|---|
| 1 | 250 | 5 | Air | No | - |
| 2 | 250 | 5 | FA | No | - |
| 3 | 250 | 10 | FA | No | - |
| 4 | 250 | 20 | FA | No | - |
| 5 | 250 | 5 | FA | 3 | - |
| 6 | 250 | 5 | FA | 5 | - |
| 7 | 250 | 5 | FA | 10 | - |
| 8 | 250 | 5 | FA | 20 | - |
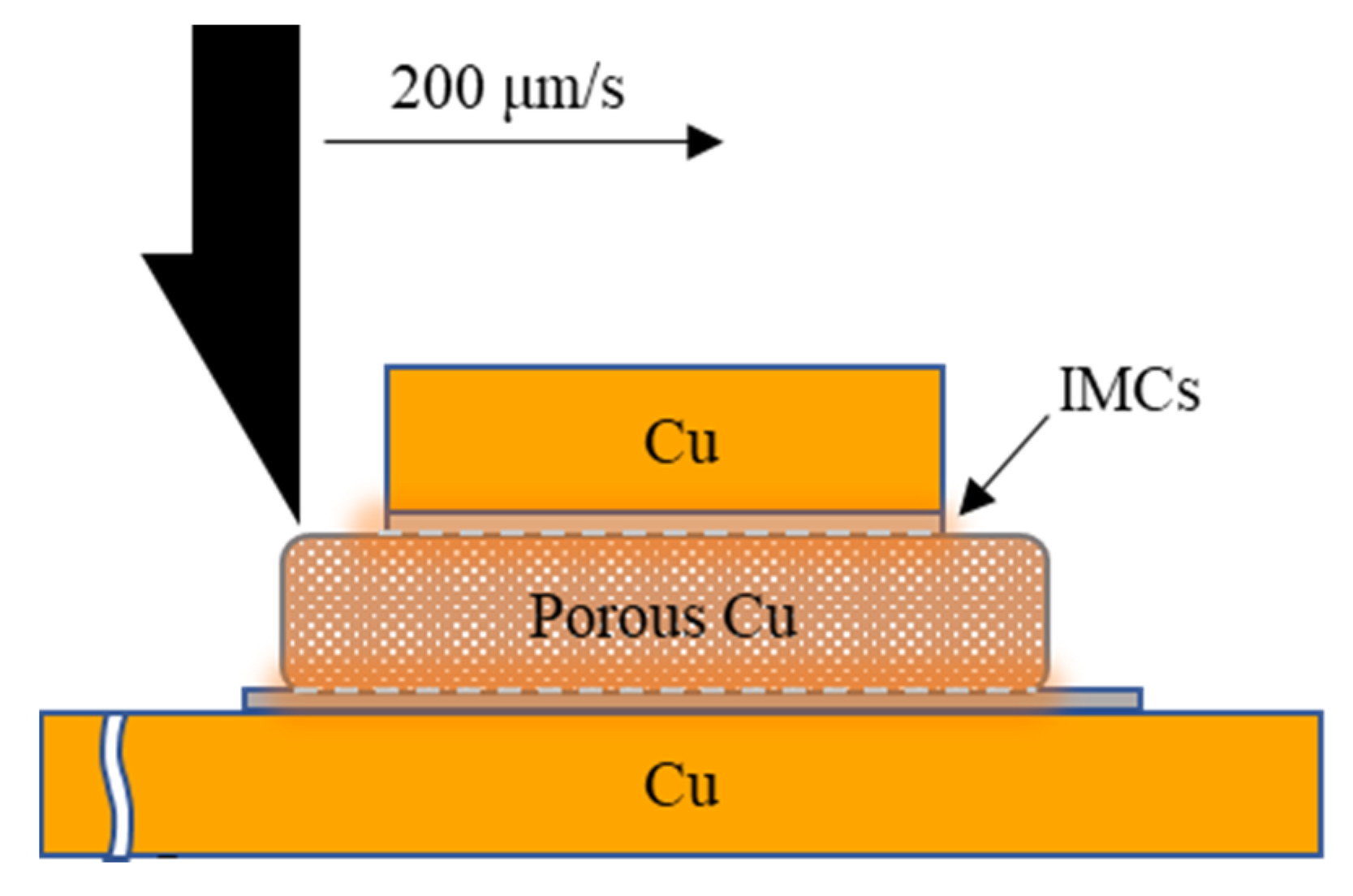
3. Results and Discussion
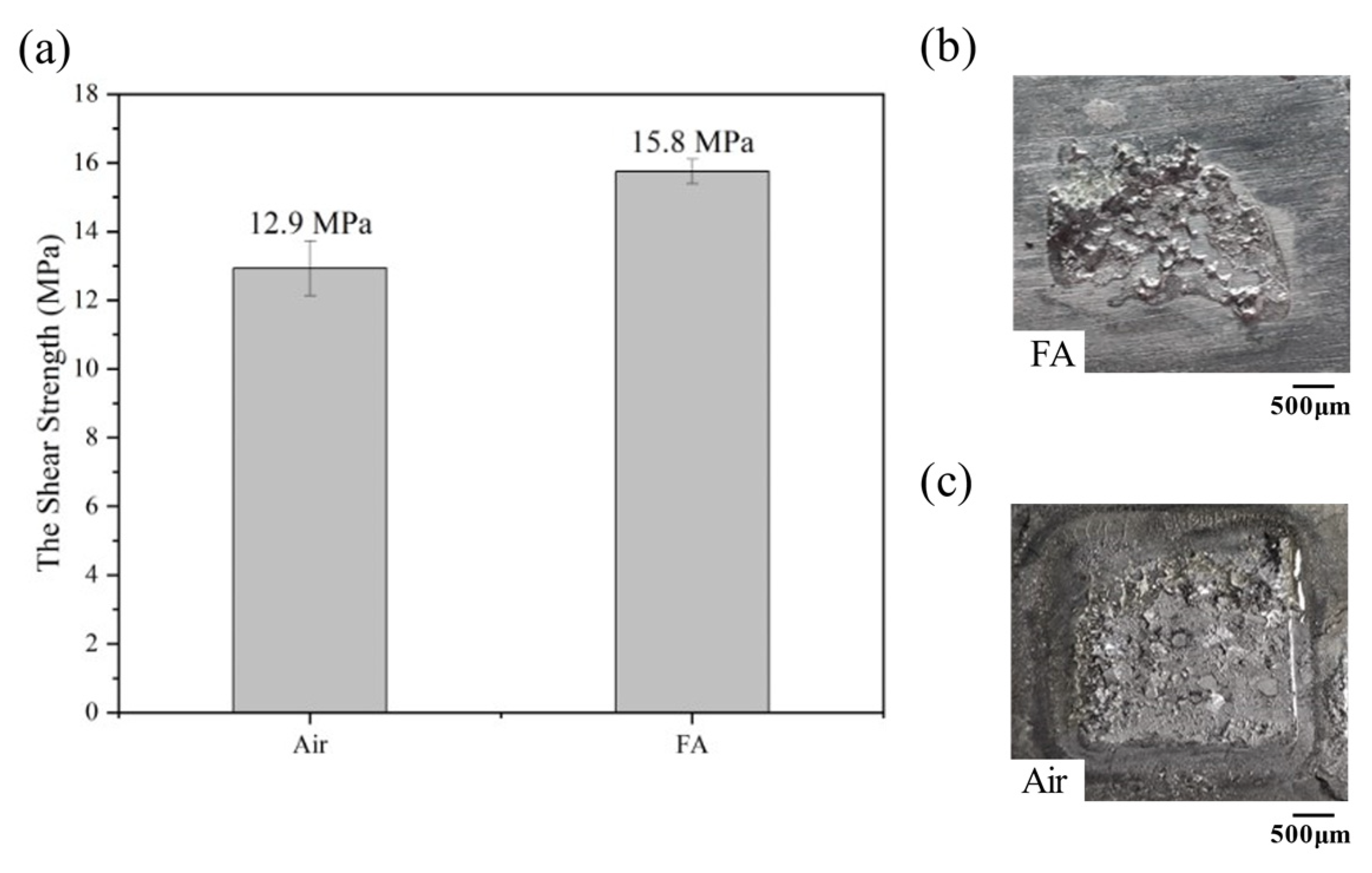
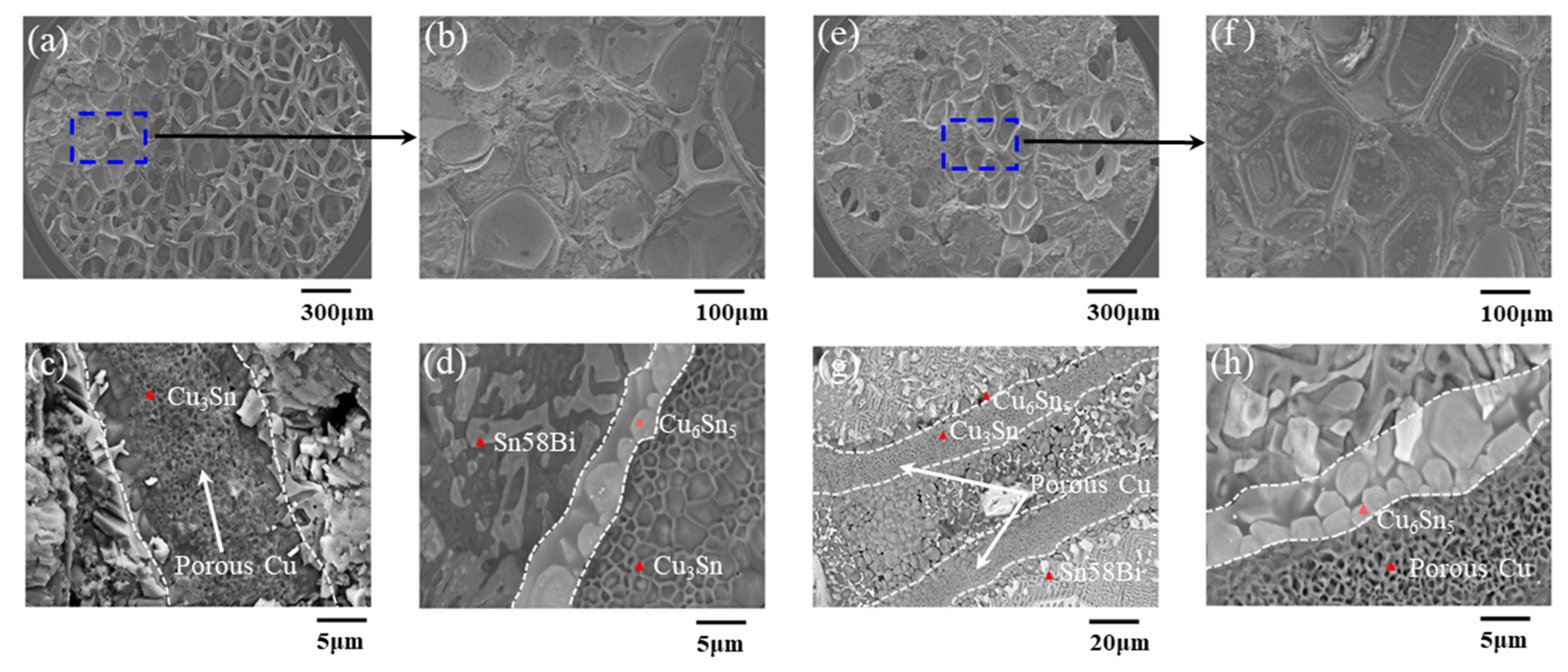
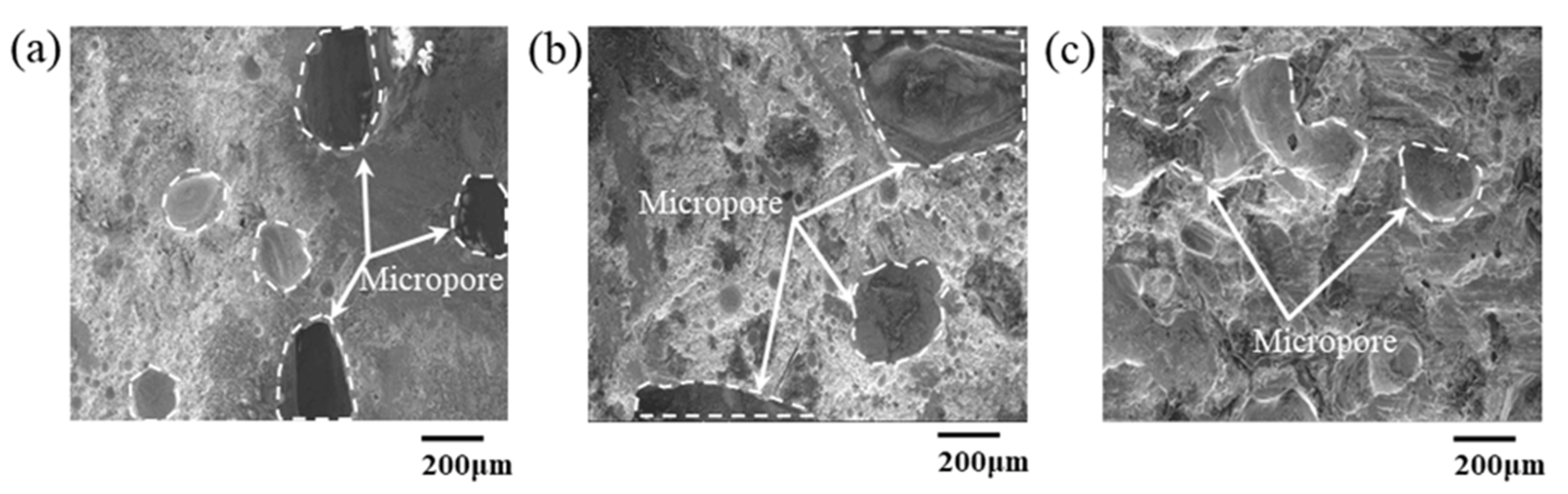
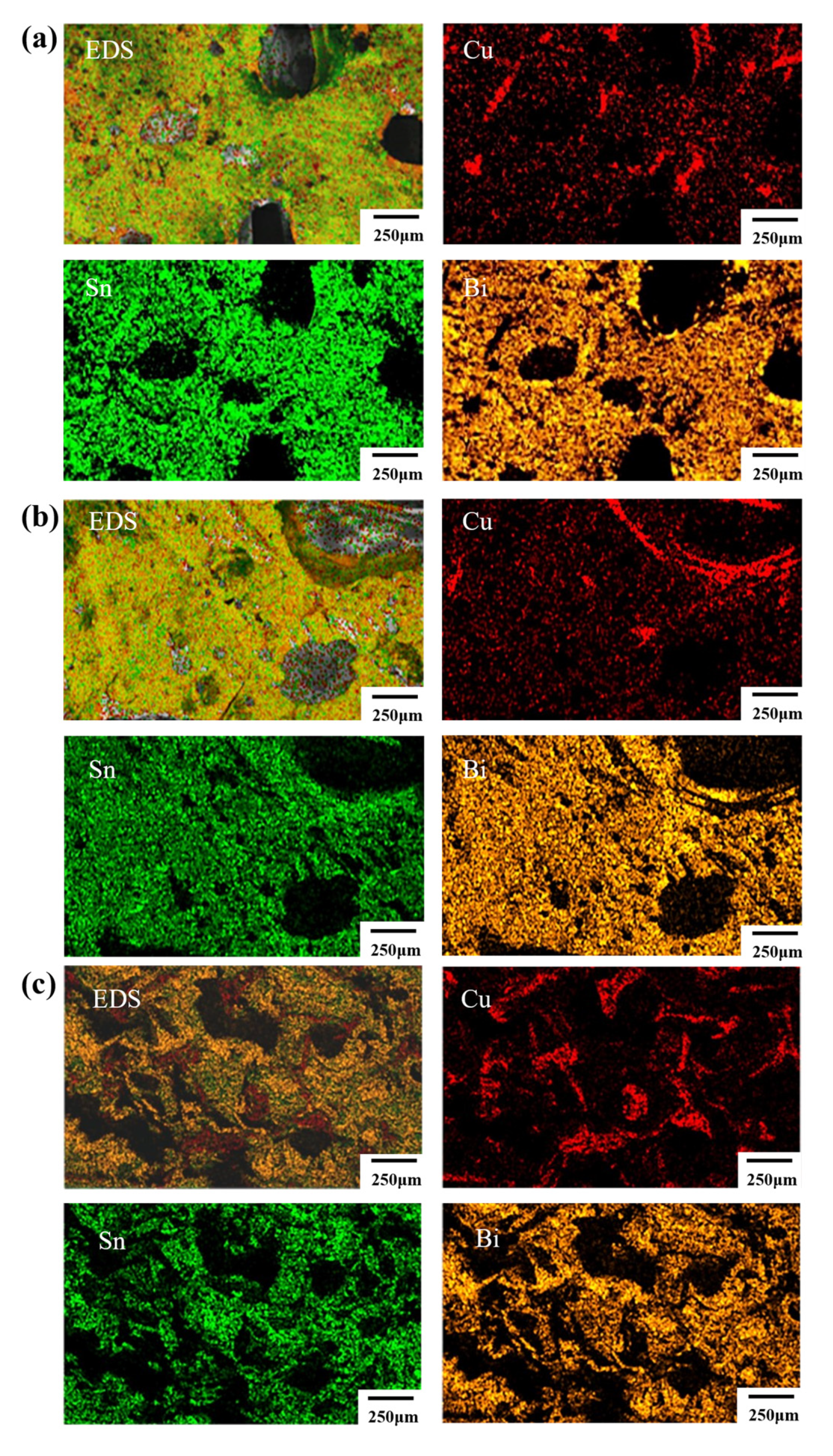
3.1. Effect of Bonding Atmosphere on the Shear Strength of Joints
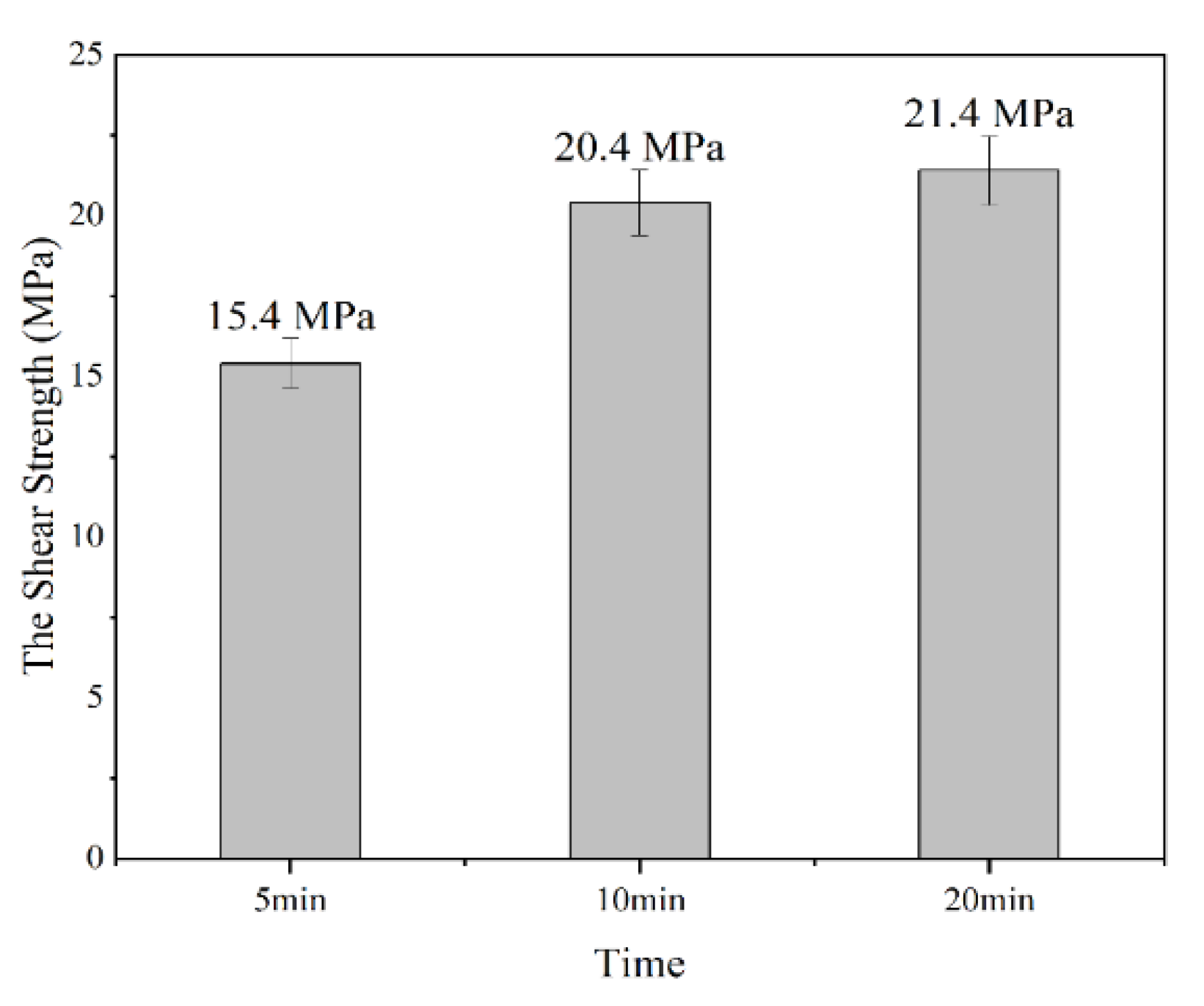
3.2. Effect of Bonding Time on the Shear Strength of Joints
3.3. Effect of Applied Pressure on Shear Strength of Joints
4. Conclusions
- (a)
- The FA could reduce the oxides of solder and Cu; thus, the interconnection between porous Cu and Cu substrate was fulfilled, and the shear strength was approximately 22.48% higher than that obtained under the air atmosphere.
- (b)
- The strength of metal joints increased with increases of bonding time significantly within 10 min due to the rapid infiltration and reaction between Sn58Bi solder and porous Cu layer with the help of FA. Beyond that, the shear strength increased slowly with the increase in bonding time, which might be the full and complete reaction between them within 10 min.
- (c)
- The applied pressure showed obvious influence on the shear strength of the joints, and it could be promoted to 34.2, 48.4, 62.2, and 71.2 MPa with the application pressure of 3, 5, 10, and 20 MPa, respectively, while the shear strength was only 15.4 MPa in the non-pressure case. The mechanism should be ascribed to the accelerated infiltration and reaction at the Sn58Bi solder/porous Cu interface and the compact structure at the interfacial zone upon pressure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T. Analysis on the application, development, and future prospects of Gallium Nitride (GaN). In Proceedings of the 2020 International Conference on Optoelectronic Materials and Devices, Guangzhou, China, 18–20 December 2020; SPIE: Bellingham, WA, USA, 2021; Volume 11767, pp. 200–205. [Google Scholar]
- Heeger, A.J. Semiconducting polymers: The third generation. Chem. Soc. Rev. 2010, 39, 2354–2371. [Google Scholar] [CrossRef]
- Deng, Z. Development of The Third Generation of Semiconductors with SiC and GaN as The Mainstay. Highlights Sci. Eng. Technol. 2022, 27, 436–442. [Google Scholar] [CrossRef]
- Wang, Y.; Lucia, O.; Zhang, Z.; Gao, S.; Guan, Y.; Xu, D. A review of high frequency power converters and related technologies. IEEE Open J. Ind. Electron. Soc. 2020, 1, 247–260. [Google Scholar] [CrossRef]
- Yadlapalli, R.T.; Kotapati, A.; Kandipati, R.; Balusu, S.R.; Koritala, C.S. Advancements in energy efficient GaN power devices and power modules for electric vehicle applications: A review. Int. J. Energy Res. 2021, 45, 12638–12664. [Google Scholar] [CrossRef]
- Wang, K.; Sheng, C. Application of GaN in 5G technology. J. Phys. Conf. Series. 2020, 1699, 12004. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.; Wang, R.; Cao, Y. Application of the third-generation power devices in aerospace power supply. In Proceedings of the Seventh Asia Pacific Conference on Optics Manufacture and 2021 International Forum of Young Scientists on Advanced Optical Manufacturing (APCOM and YSAOM 2021), Shanghai, China, 28–31 October 2021; SPIE: Bellingham, WA, USA, 2022; Volume 12166, pp. 2000–2009. [Google Scholar]
- Zhang, Y. The application of third generation semiconductor in power industry. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020; Volume 198, p. 04011. [Google Scholar]
- Shen, Y.A.; Lin, Y.X.; Ouyang, F.Y.; Nishikawa, H.; Tsai, M.-H. Thermomigration suppression in Sn3.5Ag solder joints by hot-end FeCoNiMn alloy. Intermetallics 2023, 154, 107821. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, H.; Yu, F.; Zhao, N. Quasi-in-situ observation on diffusion anisotropy dominated asymmetrical growth of Cu-Sn IMCs under temperature gradient. Acta Mater. 2021, 217, 117168. [Google Scholar] [CrossRef]
- Chen, C.; Suganuma, K. Microstructure and mechanical properties of sintered Ag particles with flake and spherical shape from nano to micro size. Mater. Des. 2019, 162, 311–321. [Google Scholar] [CrossRef]
- Gao, R.; He, S.; Shen, Y.A.; Nishikawa, H. Effect of substrates on fracture mechanism and process optimization of oxidation-reduction bonding with copper microparticles. J. Electron. Mater. 2019, 48, 2263–2271. [Google Scholar] [CrossRef]
- Chen, T.F.; Siow, K.S. Comparing the mechanical and thermal-electrical properties of sintered copper (Cu) and sintered silver (Ag) joints. J. Alloy. Compd. 2021, 866, 158783. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Q.; He, S.; Bian, J.; Jiu, J.; Chengxin, L.; Nishikawa, H. Large-area and low-cost Cu–Cu bonding with cold spray deposition, oxidation, and reduction processes under low-temperature conditions. J. Mater. Sci. Mater. Electron. 2021, 32, 20461–20473. [Google Scholar] [CrossRef]
- Masaru, Y. University-industry collaboration networks for the creation of innovation: A comparative analysis of the development of lead-free solders in Japan, Europe and the United States. In Proceedings of the Technology Management for the Global Future—PICMET 2006 Conference, 8–13 July 2006; IEEE: Piscataway, NJ, USA, 2006; Volume 1, pp. 368–386. [Google Scholar]
- Mokhtari, O.; Nishikawa, H. Transient liquid phase bonding of Sn–Bi solder with added Cu particles. J. Mater. Sci. Mater. Electron. 2016, 27, 4232–4244. [Google Scholar] [CrossRef]
- Pawar, K.; Dixit, P. Investigation of Cu-Sn-Cu transient liquid phase bonding for microsystems packaging. Mater. Manuf. Process. 2023, 38, 284–294. [Google Scholar] [CrossRef]
- Lis, A.; Leinenbach, C. Effect of process and service conditions on TLP-bonded components with (Ag,Ni–)Sn interlayer combinations. J. Electron. Mater. 2015, 44, 4576–4588. [Google Scholar] [CrossRef]
- Li, J.F.; Agyakwa, P.A.; Johnson, C.M. Interfacial reaction in Cu/Sn/Cu system during the transient liquid phase soldering process. Acta Mater. 2011, 59, 1198–1211. [Google Scholar] [CrossRef]
- Ghahferokhi, A.I.; Kasiri-Asgarani, M.; Ebrahimi-kahrizsangi, R.; Rafiei, M.; Bakhsheshi-Rad, H.R.; Amini, K.; Berto, F. Effect of bonding temperature and bonding time on microstructure of dissimilar transient liquid phase bonding of GTD111/BNi-2/IN718 system. J. Mater. Res. Technol. 2022, 21, 2178–2190. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, C.; Zhou, S.; Li, G.; Lu, T.; Tian, Y.; He, P. Low-temperature Co-hydroxylated Cu/SiO2 hybrid bonding strategy for a memory-centric chip architecture. ACS Appl. Mater. Interfaces 2021, 13, 38866–38876. [Google Scholar] [CrossRef]
- Paleu, C.C.; Munteanu, C.; Istrate, B.; Bhaumik, S.; Vizureanu, P.; Bălţatu, M.S.; Paleu, V. Microstructural analysis and tribological behavior of AMDRY 1371 (Mo–NiCrFeBSiC) atmospheric plasma spray deposited thin coatings. Coatings 2020, 10, 1186. [Google Scholar] [CrossRef]
- Shen, Y.A.; Chen, S.W.; Chen, H.Z.; Chang, C.M.; Ouyang, Y.H. Extremely thin interlayer of multi-element intermetallic compound between Sn-based solders and FeCoNiMn high-entropy alloy. Appl. Surf. Sci. 2021, 558, 149945. [Google Scholar] [CrossRef]
- He, S.; Gao, R.; Shen, Y.A.; Li, J.; Nishikawa, H. Wettability, interfacial reactions, and impact strength of Sn–3.0 Ag–0.5 Cu solder/ENIG substrate used for fluxless soldering under formic acid atmosphere. J. Mater. Sci. 2020, 55, 3107–3117. [Google Scholar] [CrossRef]
- Chen BW, J.; Mavrikakis, M. Formic acid: A hydrogen-bonding cocatalyst for formate decomposition. ACS Catal. 2020, 10, 10812–10825. [Google Scholar] [CrossRef]
- He, S.; Shen, Y.A.; Xiong, B.; Huo, F.; Li, J.; Ge, J.; Pan, Z.; Li, W.; Hu, C.; Nishikawa, H. Behavior of Sn-3.0 Ag-0.5 Cu solder/Cu fluxless soldering via Sn steaming under formic acid atmosphere. J. Mater. Res. Technol. 2022, 21, 2352–2361. [Google Scholar] [CrossRef]
- Nasir Bashir, M.; Saad, H.M.; Rizwan, M.; Bingöl, S.; Channa, I.A.; Gul, M.; Haseeb, A.S.M.A.; Naher, S. Effect of cobalt nanoparticles on mechanical properties of Sn–58Bi solder joint. J. Mater. Sci. Mater. Electron. 2022, 33, 22573–22579. [Google Scholar] [CrossRef]
- Bashir, M.N.; Saad, H.M.; Rizwan, M.; Quazi, M.M.; Ali, M.M.; Ahmed, A.; Zaidi, A.A.; Soudagar, M.E.M.; Haseeb, A.S.M.A.; Naher, S. Effects of tin particles addition on structural and mechanical properties of eutectic Sn–58Bi solder joint. J. Mater. Sci. Mater. Electron. 2022, 33, 22499–22507. [Google Scholar] [CrossRef]
- Bashir, M.N.; Haseeb, A.; Naher, S.; Ali, M.M.; Bashir, M.B.A.; Zaidi, A.A.; Jamshaid, M.; Javed, I. Effects of cobalt nanoparticle on microstructure of Sn58Bi solder joint. J. Mater. Sci. Mater. Electron. 2023, 34, 248. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Qian, G.; Zhou, J.; Xue, F. Effect of Sb content on properties of Sn—Bi solders. Trans. Nonferrous Met. Soc. China 2014, 24, 184–191. [Google Scholar] [CrossRef]
- Paixao, J.L.; Gomes, L.F.; Reyes, R.V.; Garcia, A.; Spinelli, J.E.; Silva, B.L. Microstructure characterization and tensile properties of directionally solidified Sn-52 wt% Bi-1 wt% Sb and Sn-52 wt% Bi-2 wt% Sb alloys. Mater. Charact. 2020, 166, 110445. [Google Scholar] [CrossRef]
- Chantaramanee, S.; Sungkhaphaitoon, P. Effects of antimony on the microstructure, thermal properties, mechanical performance, and interfacial behavior of Sn–0.7 Cu–0.05 Ni–xSb/Cu solder joints. J. Mater. Sci. Mater. Electron. 2021, 32, 27607–27624. [Google Scholar] [CrossRef]
- Fan, X.Y.; Ke, F.S.; Wei, G.Z.; Huang, L.; Sun, S.G. Sn–Co alloy anode using porous Cu as current collector for lithium ion battery. J. Alloys Compd. 2009, 476, 70–73. [Google Scholar] [CrossRef]
- Liu, B.; Cai, C.; Yang, B.; Chen, K.; Long, Y.; Wang, Q.; Wang, S.; Chen, G.; Li, H.; Hu, J.; et al. Intermediate enrichment effect of porous Cu catalyst for CO2 electroreduction to C2 fuels. Electrochim. Acta 2021, 388, 138552. [Google Scholar] [CrossRef]
- Shigetou, A.; Itoh, T.; Matsuo, M.; Hayasaka, N.; Okumura, K.; Suga, T. Bumpless interconnect through ultrafine Cu electrodes by means of surface-activated bonding (SAB) method. IEEE Trans. Adv. Packag. 2006, 29, 218–226. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Ji, H.; Li, M. Highly conductive Cu–Cu joint formation by low-temperature sintering of formic acid-treated Cu nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 33289–33298. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Howlader MM, R.; Itoh, T.; Suga, T. Room temperature Cu–Cu direct bonding using surface activated bonding method. J. Vac. Sci. Technol. A Vac. Surf. Film. 2003, 21, 449–453. [Google Scholar] [CrossRef]
- Brown, M.A.; Vila, F.; Sterrer, M.; Thürmer, S.; Winter, B.; Ammann, M.; Rehr, J.J.; van Bokhoven, J.A. Electronic Structures of Formic Acid (HCOOH) and Formate (HCOO−) in Aqueous Solutions. J. Phys. Chem. Lett. 2012, 3, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Lu, H.; Bi, Y.; Huang, J.; He, S. Shear Strength of Joint Formed Using Sn-58Bi/Cu Porous Structure via TLP Bonding. In Proceedings of the MEMAT 2022—2nd International Conference on Mechanical Engineering, Intelligent Manufacturing and Automation Technology, Guilin, China, 7–9 January 2022; VDE: Frankfurt, Germany, 2022; pp. 1–4. [Google Scholar]
- Yang, W.; Shintani, H.; Akaike, M.; Suga, T. Low temperature Cu-Cu direct bonding using formic acid vapor pretreatment. In Proceedings of the 2011 IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 2079–2083. [Google Scholar]
- Pan, J.; Toleno, B.J.; Chou, T.-C.; Dee, W.J. The effect of reflow profile on SnPb and SnAgCu solder joint shear strength. Solder. Surf. Mt. Technol. 2006, 18, 48–56. [Google Scholar] [CrossRef]
- Nai SM, L.; Wei, J.; Gupta, M. Interfacial intermetallic growth and shear strength of lead-free composite solder joints. J. Alloys Compd. 2009, 473, 100–106. [Google Scholar]
- Gao, R.; He, S.; Li, J.; Shen, Y.-A.; Nishikawa, H. Interfacial transformation of preoxidized Cu microparticles in a formic-acid atmosphere for pressureless Cu–Cu bonding. J. Mater. Sci. Mater. Electron. 2020, 31, 14635–14644. [Google Scholar] [CrossRef]
- Liu, X.; Nishikawa, H. Low-pressure Cu-Cu bonding using in-situ surface-modified microscale Cu particles for power device packaging. Scr. Mater. 2016, 120, 80–84. [Google Scholar] [CrossRef]
- Hou, M.M.; Eagar, T.W. Low temperature transient liquid phase (LTTLP) bonding for Au/Cu and Cu/Cu interconnections. J. Electron. Packag. 1992, 114, 443–447. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, B.; Xue, Y.; Zhou, M.; Cao, R.; Zeng, X. Improvement on the mechanical properties of eutectic Sn58Bi alloy with porous Cu addition during isothermal aging. Mater. Res. Express 2021, 8, 76302. [Google Scholar] [CrossRef]
- Shao, H.; Wu, A.; Bao, Y.; Zhao, Y.; Zou, G. Interfacial reaction and mechanical properties for Cu/Sn/Ag system low temperature transient liquid phase bonding. J. Mater. Sci. Mater. Electron. 2016, 27, 4839–4848. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, L.; Zhang, Y.; Chen, M.-H.; Chen, C.-P. Interfacial reaction, shear behavior and microhardness of Cu-Sn TLP bonding joints bearing CuZnAl powder for 3D packaging. J. Manuf. Process. 2021, 68, 1672–1682. [Google Scholar] [CrossRef]
- Jiang, H.; Robertson, S.; Liang, S.; Zhou, Z.; Zhao, L.; Liu, C. Microstructural and mechanical characteristics of Cu-Sn intermetallic compound interconnects formed by TLPB with Cu-Sn nanocomposite. Mater. Today Commun. 2022, 33, 104623. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Xiong, B.; Xu, F.; Chen, B.; Cui, Y.; Hu, C.; Yue, G.; Shen, Y.-A. Low-Temperature Transient Liquid Phase Bonding Technology via Cu Porous-Sn58Bi Solid–Liquid System under Formic Acid Atmosphere. Materials 2023, 16, 2389. https://doi.org/10.3390/ma16062389
He S, Xiong B, Xu F, Chen B, Cui Y, Hu C, Yue G, Shen Y-A. Low-Temperature Transient Liquid Phase Bonding Technology via Cu Porous-Sn58Bi Solid–Liquid System under Formic Acid Atmosphere. Materials. 2023; 16(6):2389. https://doi.org/10.3390/ma16062389
Chicago/Turabian StyleHe, Siliang, Bifu Xiong, Fangyi Xu, Biyang Chen, Yinhua Cui, Chuan Hu, Gao Yue, and Yu-An Shen. 2023. "Low-Temperature Transient Liquid Phase Bonding Technology via Cu Porous-Sn58Bi Solid–Liquid System under Formic Acid Atmosphere" Materials 16, no. 6: 2389. https://doi.org/10.3390/ma16062389
APA StyleHe, S., Xiong, B., Xu, F., Chen, B., Cui, Y., Hu, C., Yue, G., & Shen, Y.-A. (2023). Low-Temperature Transient Liquid Phase Bonding Technology via Cu Porous-Sn58Bi Solid–Liquid System under Formic Acid Atmosphere. Materials, 16(6), 2389. https://doi.org/10.3390/ma16062389








