Dual-Porosity (Ta0.2Nb0.2Ti0.2Zr0.2Hf0.2)C High-Entropy Ceramics with High Compressive Strength and Low Thermal Conductivity Prepared by Pressureless Sintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedure
2.3. Characterization
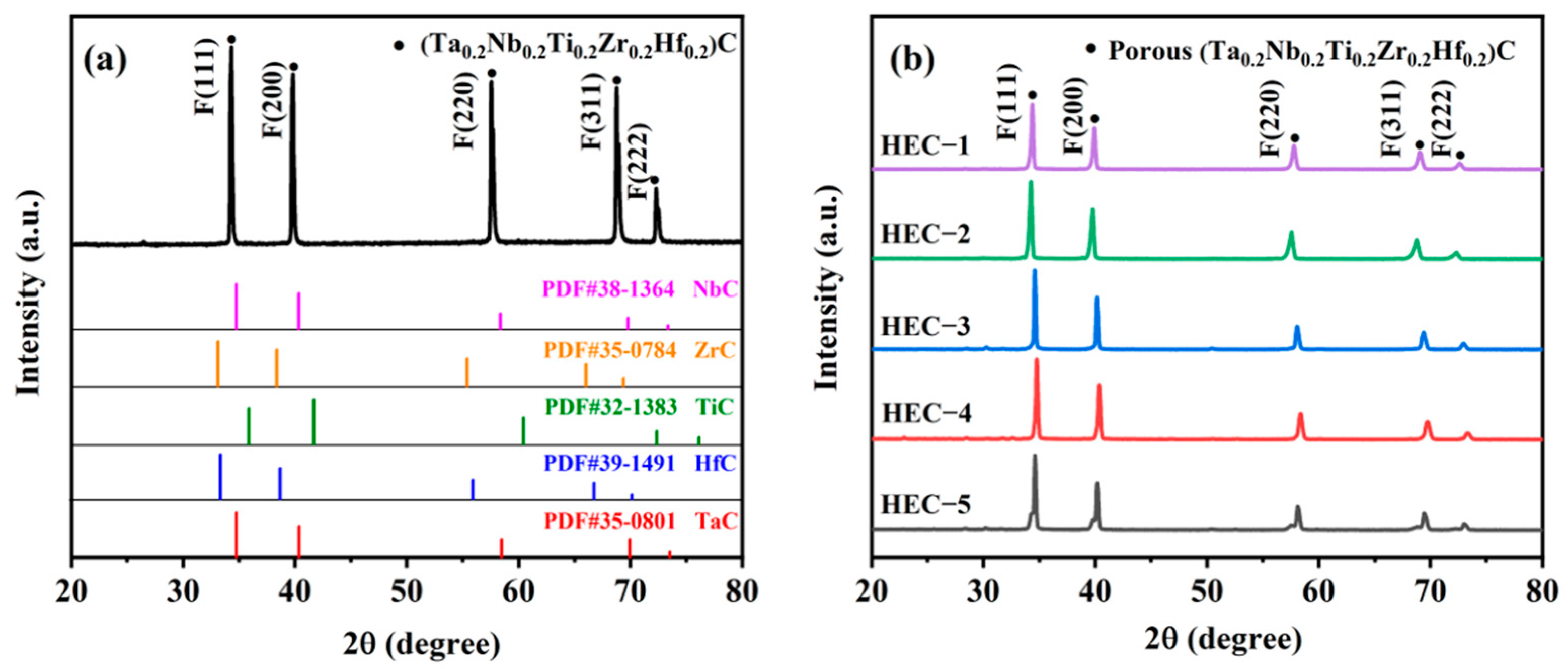
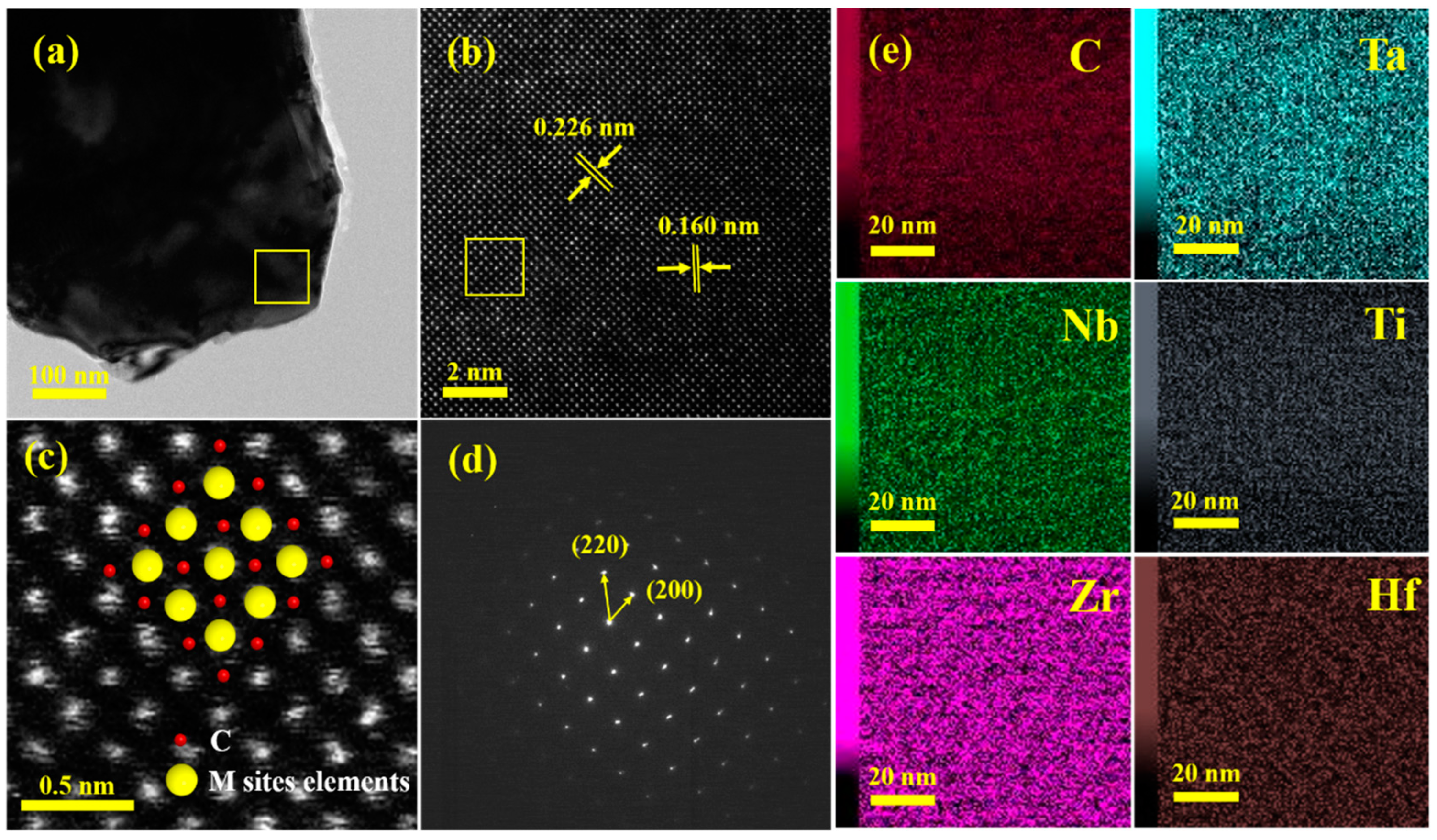
3. Results and Discussion
4. Conclusions
- (1)
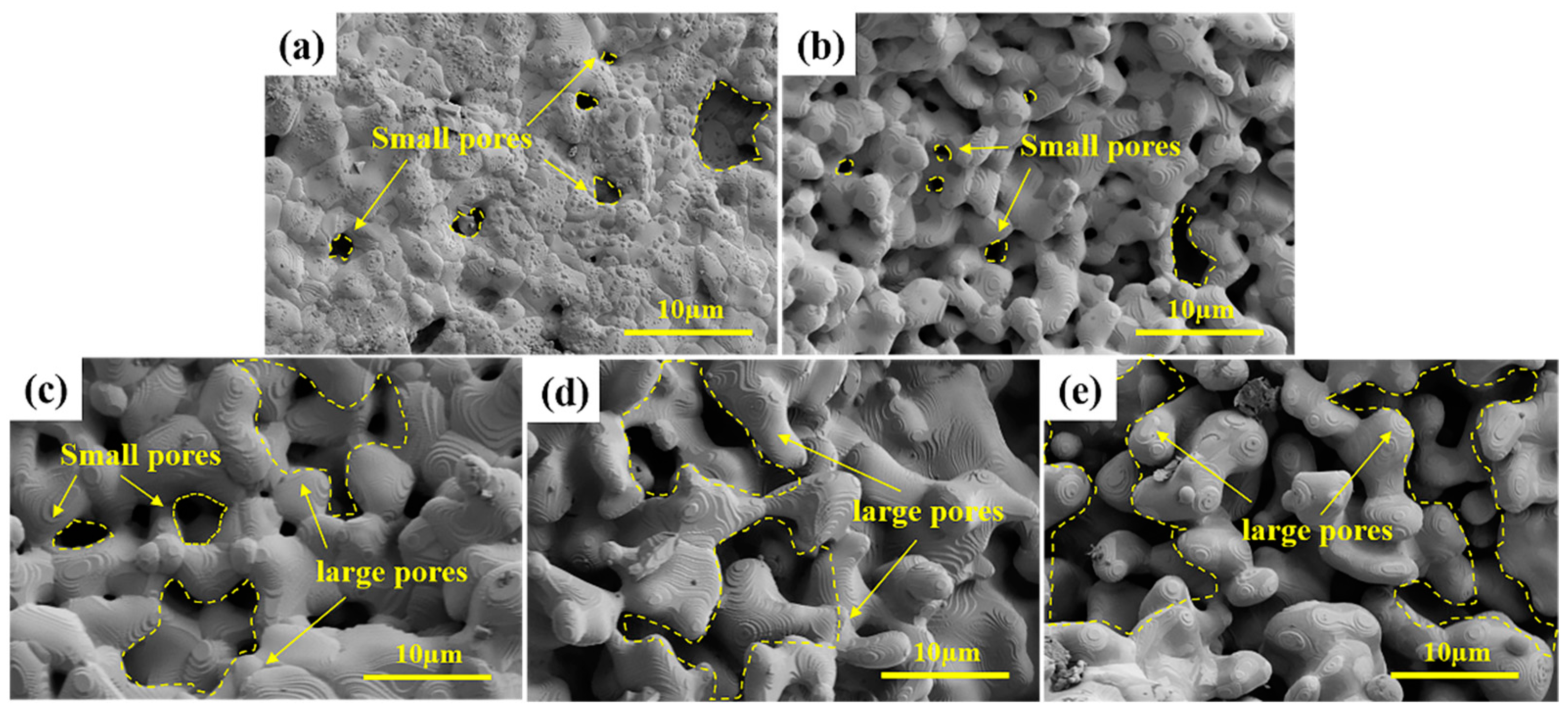
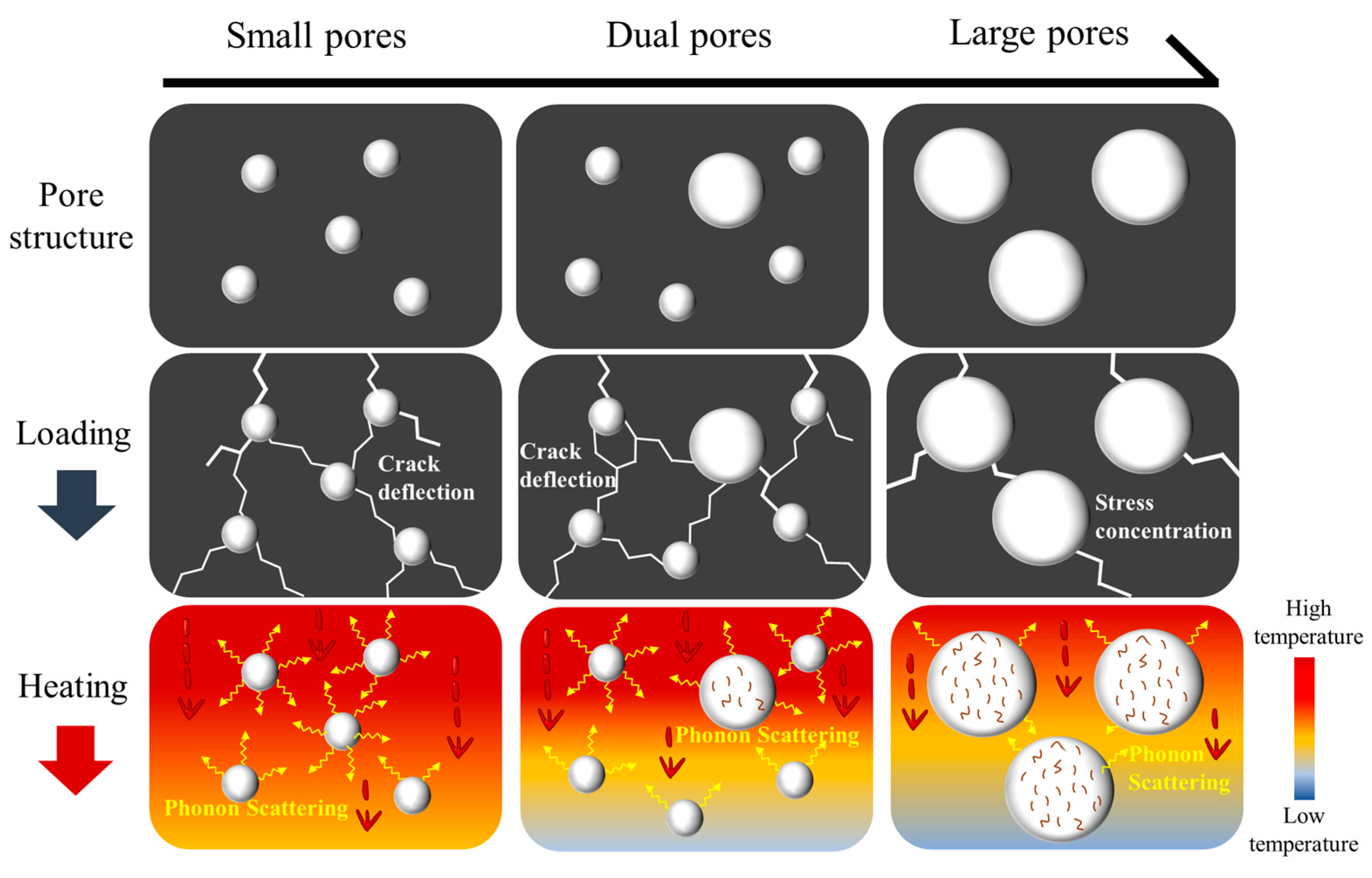
- The prepared porous HECs exhibited a dual-porosity structure. The carbothermal reaction and sintering of ceramics precursor led to the formation of small pores of sizes 0.4–3 μm. The SiO2 microspheres resulted in the generation of large pores in the range of 20–50 μm. With the increase in the number of SiO2 microspheres from 0 wt.% to 20 wt.%, the small pores disappeared gradually, and the large pores appeared.
- (2)
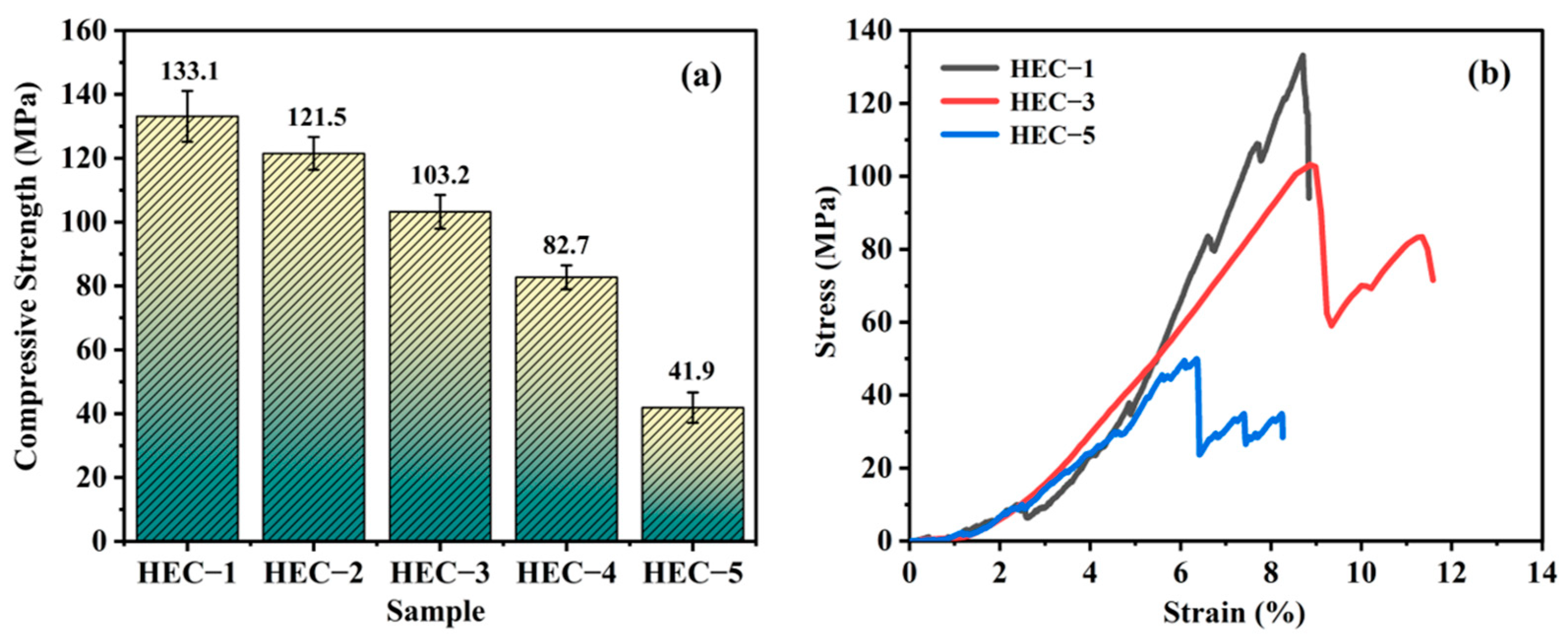
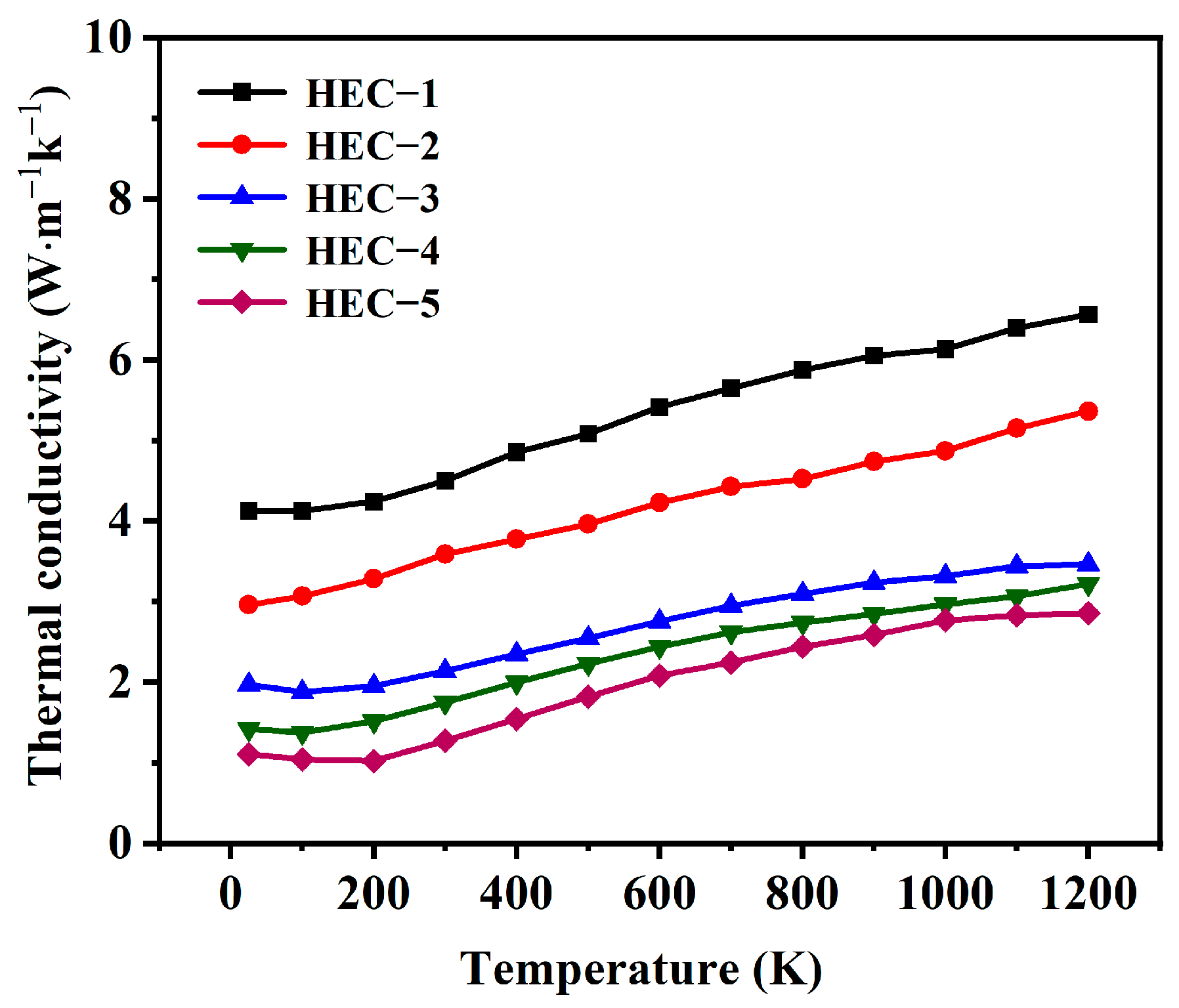
- The thermal conductivity of the porous HECs declined from 4.12 W·m−1 k−1 to 1.11 W·m−1 k−1 with the increase in porosity from 23.08% to 59.34%, while the compressive strength decreased from 133.1 MPa to 41.9 MPa. The obtained porous HECs showed competitive thermal insulation and excellent mechanical properties. The porous HECs are a promising family of materials for the TPS.
- (3)
- The unique properties of the porous HECs are ascribed to the high-entropy effects and dual-porosity structures. The severe lattice distortions in the HECs lead to the low intrinsic thermal conductivity by reducing phonons’ mean free path and increasing phonon scattering. Moreover, this results in high compressive strength by inhibiting the crack generations and propagations. The dual-porosity structure possesses several small pores in high-porosity ceramics. These small pores are more efficient in scattering phonon thermal conductivity and inhibiting crack propagations, which are beneficial to improving the thermal insulation and mechanical properties of the porous HECs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uyanna, O.; Najafi, H. Thermal protection systems for space vehicles: A review on technology development, current challenges and future prospects. Acta Astronaut. 2020, 176, 341–356. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Z.; Xiang, H.; Dai, F.Z.; Zhang, J.; Wang, S.; Liu, J.; Zhou, Y. Effect of reaction routes on the porosity and permeability of porous high entropy (Y0.2Yb0.2Sm0.2Nd0.2Eu0.2)B6 for transpiration cooling. J. Mater. Sci. Technol. 2020, 38, 80–85. [Google Scholar] [CrossRef]
- Miller, J.E.; Bohl, W.E.; Christiansen, E.L.; Davis, B.A. Ballistic performance of porous ceramic, thermal protection systems. Int. J. Impact. Eng. 2013, 56, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Golla, B.R.; Mukhopadhyay, A.; Basu, B.; Thimmappa, S.K. Review on Ultra High Temperature Boride Ceramics. Prog. Mater. Sci. 2020, 111, 100651. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E. Oxidation of ultra-high temperature transition metal diboride ceramics. Int. Mater. Rev. 2013, 57, 61–72. [Google Scholar] [CrossRef]
- Binner, J.; Porter, M.; Baker, B.; Zou, J.; Venkatachalam, V.; Diaz, V.R.; D’Angio, A.; Ramanujam, P.; Zhang, T.; Murthy, T.S.R.C. Selection, processing, properties and applications of ultra-high temperature ceramic matrix composites, UHTCMCs: A Review. Int. Mater. Rev. 2019, 65, 389–444. [Google Scholar] [CrossRef]
- Ni, D.; Cheng, Y.; Zhang, J.; Liu, J.X.; Zou, J.; Chen, B.; Wu, H.; Li, H.; Dong, S.; Han, J.; et al. Advances in ultra-high temperature ceramics, composites, and coatings. J. Adv. Ceram. 2021, 11, 1–56. [Google Scholar] [CrossRef]
- Tang, S.; Hu, C. Design, Preparation and Properties of Carbon Fibers Reinforced Ultra-high Temperature Ceramic Composites for Aerospace Applications: A Review. J. Mater. Sci. 2017, 33, 117–130. [Google Scholar] [CrossRef]
- Licheri, R.; Musa, C.; Locci, A.M.; Montinaro, S.; Orrù, R.; Cao, G.; Silvestroni, L.; Sciti, D.; Azzali, N.; Mercatelli, L.; et al. Ultra-high temperature porous graded ceramics for solar energy applications. J. Eur. Ceram. Soc. 2019, 39, 72–78. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, K.; Zhou, C.; Han, W.; Du, Z. Preparation and properties of high-porosity ZrB2-SiC ceramics by water-based freeze casting. J. Eur. Ceram. Soc. 2021, 41, 2239–2246. [Google Scholar] [CrossRef]
- Sani, E.; Mercatelli, L.; Sans, J.L.; Silvestroni, L.; Sciti, D. Porous and dense hafnium and zirconium ultra-high temperature ceramics for solar receivers. Opt. Mater. 2013, 36, 163–168. [Google Scholar] [CrossRef]
- Guo, Q.; Xiang, H.; Sun, X.; Wang, X.; Zhou, Y. Preparation of porous YB4 ceramics using a combination of in-situ borothermal reaction and high temperature partial sintering. J. Eur. Ceram. Soc. 2015, 35, 3411–3418. [Google Scholar] [CrossRef]
- Dollé, M.; Gosset, D.; Bogicevic, C.; Karolak, F.; Simeone, D.; Baldinozzi, G. Synthesis of nanosized zirconium carbide by a sol-gel route. J. Eur. Ceram. Soc. 2007, 27, 2061–2067. [Google Scholar] [CrossRef]
- Li, F.; Kang, Z.; Huang, X.; Wang, X.G.; Zhang, G.J. Preparation of zirconium carbide foam by direct foaming method. J. Eur. Ceram. Soc. 2014, 34, 3513–3520. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Chen, X.; Yang, L.; Hu, X.; Guo, A.; Xie, D.; Wang, W.; Du, H. Fabrication of mullite fiber porous ceramics with even structures by filtration freeze-drying. J. Ceram. Soc. Jpn. 2019, 127, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Schelm, K.; Dammler, K.; Betke, U.; Scheffler, M. Tailoring of the wetting behavior of alumina dispersions on polymer foams by methylcellulose addition: A route toward mechanically stable ceramic replica foams. Adv. Eng. Mater. 2019, 21, 1900635. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Zhang, M.; Xiu, Z.; Li, J.G.; Li, J.; Xie, M.; Chen, J.; Sun, X. High-strength macro-porous alumina ceramics with regularly arranged pores produced by gel-casting and sacrificial template methods. J. Mater. Sci. 2019, 54, 10119–10129. [Google Scholar] [CrossRef]
- Wang, S.; Yin, Y.; Chen, L.; Liu, X.; Jia, Q.; Zhang, S. Controllable preparation of porous ZrB2-SiC ceramics via using KCl space holders. Ceram. Int. 2021, 47, 33978–33987. [Google Scholar] [CrossRef]
- Yan, N.; Fu, Q.; Zhang, Y.; Li, K.; Xie, W.; Zhang, J.; Zhuang, L.; Shi, X. Preparation of pore-controllable zirconium carbide ceramics with tunable mechanical strength, thermal conductivity and sound absorption coefficient. Ceram. Int. 2020, 46, 19609–19616. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, F.; Cheng, L.; Zhou, J.; Wei, Y.; Cui, X. Formation of Ultra-High Temperature Ceramic Hollow Microspheres as Promising Lightweight Thermal Insulation Materials via a Molten Salt-Assisted Template Method. ACS. Appl. Mater. Interface 2021, 13, 37388–37397. [Google Scholar] [CrossRef]
- Dai, F.Z.; Wen, B.; Sun, Y.; Xiang, H.; Zhou, Y. Theoretical prediction on thermal and mechanical properties of high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C by deep learning potential. J. Mater. Sci. Technol. 2020, 43, 168–174. [Google Scholar] [CrossRef]
- Xiang, H.; Xing, Y.; Dai, F.Z.; Wang, H.; Su, L.; Miao, L.; Zhang, G.; Wang, Y.; Qi, X.; Yao, L.; et al. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Ye, B.; Wen, T.; Huang, K.; Wang, C.Z.; Chu, Y.H. First-principles study, fabrication and characterization of (Zr0.25Nb0.25Ti0.25V0.25)C high-entropy ceramics. J. Am. Ceram. Soc. 2019, 102, 4344–4352. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhang, J.; Zhang, F.; Niu, B.; Lei, L.; Wang, W. High-entropy carbide: A novel class of multicomponent ceramics. Ceram. Int. 2018, 44, 22014–22018. [Google Scholar] [CrossRef]
- Sarkar, A.; Breitung, B.; Hahn, H. High entropy oxides: The role of entropy, enthalpy and synergy. Scr. Mater. 2020, 187, 43–48. [Google Scholar] [CrossRef]
- Liu, D.; Liu, H.; Ning, S.; Ye, B.; Chu, Y.H. Synthesis of high-purity high-entropy metal diboride powders by boro/carbothermal reduction. J. Am. Ceram. Soc. 2019, 102, 7071–7076. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.G.; Brenner, D.W. High-Entropy Ultra-High-Temperature Borides and Carbides: A New Class of Materials for Extreme Environments. Annu. Rev. Mater. Res. 2021, 51, 165–185. [Google Scholar] [CrossRef]
- Gao, M.C.; Miracle, D.B.; Maurice, D.; Yan, X.; Zhang, Y.; Hawk, J.A. High-entropy functional materials. J. Mater. Res. 2018, 33, 3138–3155. [Google Scholar] [CrossRef] [Green Version]
- Ye, B.; Fan, C.; Han, Y.; Ma, M.; Chu, Y.H. Synthesis of high-entropy diboride nanopowders via molten salt-mediated magnesiothermic reduction. J. Am. Ceram. Soc. 2020, 103, 4738–4741. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.Z.; Liu, J.; Lei, Y.; Zhang, J.; Zhou, Y. High porosity and low thermal conductivity high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)C. J. Mater. Sci. Technol. 2019, 35, 1700–1705. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.Z.; Liu, J.; Zhou, Y. Porous high entropy (Zr0.2Hf0.2Ti0.2Nb0.2Ta0.2)B2: A novel strategy towards making ultrahigh temperature ceramics thermal insulating. J. Mater. Sci. Technol. 2019, 35, 2404–2408. [Google Scholar] [CrossRef]
- Yan, X.; Constantin, L.; Lu, Y.; Silvain, J.F.; Nastasi, M.; Cui, B. (Hf0.2 Zr0.2Ta0.2Nb0.2Ti0.2)C High-Entropy Ceramics with Low Thermal Conductivity. J. Am. Ceram. Soc. 2018, 101, 4486–4491. [Google Scholar] [CrossRef]
- Gild, J.; Kaufmann, K.; Vecchio, K.; Luo, J. Reactive flash spark plasma sintering of high-entropy ultrahigh temperature ceramics. Scr. Mater. 2019, 170, 106–110. [Google Scholar] [CrossRef]
- Backman, L.; Gild, J.; Luo, J.; Opila, E.J. Part II: Experimental Verification of Computationally Predicted Preferential Oxidation in Refractory High Entropy Ultra-high Temperature Ceramics. Acta Mater. 2020, 197, 81–90. [Google Scholar] [CrossRef]
- Castle, E.; Csanádi, T.; Grasso, S.; Dusza, J.; Reece, M. Processing and properties of high-entropy ultra-high temperature carbides. Sci. Rep. 2018, 8, 8609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Chen, L.; Xu, C.; Zhang, W.; Liu, Z.; Wang, Y. Microstructure and mechanical properties of (TiZrNbTaMo) C high-entropy ceramic. J. Mater. Sci. 2020, 39, 99–105. [Google Scholar]
- Li, R.; Luo, R.Y.; Lin, N.; Li, A.Q.; Zhang, X.C.; Tang, Y. A novel strategy for fabricating (Ti, Ta, Nb, Zr, W)(C, N) high-entropy ceramic reinforced with in situ synthesized W2C particles. Ceram. Int. 2022, 48, 32540–32545. [Google Scholar] [CrossRef]
- Du, B.; Liu, H.; Chu, Y.H. Fabrication and characterization of polymer-derived high-entropy carbide ceramic powders. J. Am. Ceram. Soc. 2020, 103, 4063–4068. [Google Scholar] [CrossRef]
- Wang, J.; Carson, J.K.; North, M.F.; Cleland, D.J. A new approach to modelling the effective thermal conductivity of heterogeneous materials. Int. J. Heat. Mass. Transf. 2006, 49, 3075–3083. [Google Scholar] [CrossRef]
- Zhu, F.; Cui, S.; Gu, B. Fractal analysis for effective thermal conductivity of random fibrous porous materials. Phys. Lett. A 2010, 374, 4411–4414. [Google Scholar] [CrossRef]
- Wang, J.; Carson, J.K.; North, M.F.; Cleland, D.J. A new structural model of effective thermal conductivity for heterogeneous materials with co-continuous phases. Int. J. Heat. Mass. Transf. 2008, 51, 2389–2397. [Google Scholar] [CrossRef]
- Carson, J.K.; Lovatt, S.J.; Tanner, D.J.; Cleland, A.C. Thermal conductivity bounds for isotropic, porous materials. Int. J. Heat. Mass. Transf. 2005, 48, 2150–2158. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, H.; Dai, F.Z.; Liu, J.; Zhou, Y. Low thermal conductivity and high porosity ZrC and HfC ceramics prepared by in-situ reduction reaction/partial sintering method for ultrahigh temperature applications. J. Mater. Sci. Technol. 2019, 35, 2778–2784. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, S.; Li, W.; Chen, Z. Fabrication and characterization of ZrC foam by melt infiltration. J. Alloy. Compd. 2017, 695, 2295–2300. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.X.; Huang, X.; Bao, W.; Zhang, G.J.; Wang, H. Carbothermal Conversion of Self-Supporting Organic/Inorganic Interpenetrating Networks to Porous Metal Boride Monoliths. J. Am. Ceram. Soc. 2019, 102, 5746–5762. [Google Scholar] [CrossRef]
- Tandon, R.; Dumm, H.P.; Corral, E.L.; Loehman, R.E.; Kotula, P.G. Ultra High Temperature Ceramics for Hypersonic Vehicle Applications; Sandia National Laboratories (SNL): Albuquerque, NM, USA, 2006. [Google Scholar]
- Kunhao, X.; Yang, S.; Yong, H.; Jialin, S. Research of (TBA)-Based Gel-Casting Process and (Water)-Based Foaming-Gel Process for Porous Alumina Ceramics Preparation. Rare Metal. Mater. Eng. 2011, 40, 345–348. [Google Scholar]
- Wang, S.; Liu, M.; Liu, X.; Jia, Q.; Zhang, S. Carbothermal reduction synthesis of high porosity and low thermal conductivity ZrC-SiC ceramics via an one-step sintering technique. J. Eur. Ceram. Soc. 2022, 42, 4465–4471. [Google Scholar] [CrossRef]
- Shao, Z.; Wu, Z.; Sun, L.; Liang, X.; Luo, Z.; Chen, H.; Li, J.; Wang, J. High entropy ultra-high temperature ceramic thermal insulator (Zr1/5Hf1/5Nb1/5Ta1/5Ti1/5)C with controlled microstructure and outstanding properties. J. Mater. Sci. Technol. 2022, 119, 190–199. [Google Scholar] [CrossRef]
- Chen, G.; Li, C.; Li, H.; Wang, L.; Chen, K.; An, L. Porous (Ce0.2Zr0.2Ti0.2Sn0.2Ca0.2)O2-δ high-entropy ceramics with both high strength and low thermal conductivity. J. Eur. Ceram. Soc. 2021, 41, 309–314. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, Z.; Wang, Y.; Xu, B. Highly porous (La1/5Nd1/5Sm1/5Gd1/5Yb1/5)2Zr2O7 ceramics with ultra-low thermal conductivity. Ceram. Int. 2022, 48, 26400–26407. [Google Scholar] [CrossRef]
- Rost, C.M.; Borman, T.; Hossain, M.D.; Lim, M.; Quiambao-Tomko, K.F.; Tomko, J.A.; Brenner, D.W.; Maria, J.P.; Hopkins, P.E. Electron and phonon thermal conductivity in high entropy carbides with variable carbon content. Acta Mater. 2020, 196, 231–239. [Google Scholar] [CrossRef]
- Dusza, J.; Csanádi, T.; Medve, D.; Sedlák, R.; Vojtko, M.; Ivor, M.; Ünsal, H.; Tatarko, P.; Tatarková, M.; Šajgalík, P. Nanoindentation and tribology of a (Hf-Ta-Zr-Nb-Ti)C high-entropy carbide. J. Eur. Ceram. Soc. 2021, 41, 5417–5426. [Google Scholar] [CrossRef]
- Pia, G.; Sanna, U. An intermingled fractal units model to evaluate pore size distribution influence on thermal conductivity values in porous materials. Appl. Therm. Eng. 2014, 65, 330–336. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, Y.; Xu, C.; Du, X.; Yang, Y. A thermal conductivity study of double-pore distributed powdered silica aerogels. Int. J. Heat. Mass. Transf. 2017, 108, 1297–1304. [Google Scholar] [CrossRef]
- Ackermann, S.; Scheffe, J.R.; Duss, J. Morphological characterization and effective thermal conductivity of dual-scale reticulated porous structures. Materials 2014, 7, 7173–7195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deo, O.; Neithalath, N. Compressive response of pervious concretes proportioned for desired porosities. Constr. Build. Mater. 2011, 25, 4181–4189. [Google Scholar] [CrossRef]
- Jin, S.; Zhou, J.; Zhao, X. Quantitative relationship between pore size distribution and compressive strength of cementitious materials. Constr. Build. Mater. 2021, 273, 121727. [Google Scholar] [CrossRef]
- Schafföner, S.; Fruhstorfer, J.; Ludwig, S.; Aneziris, C.G. Cyclic cold isostatic pressing and improved particle packing of coarse grained oxide ceramics for refractory applications. Ceram. Int. 2018, 44, 9027–9036. [Google Scholar] [CrossRef]




| Samples | Porosity (%) | Sintered Density (g·cm−3) | Compressive Strength (MPa) |
|---|---|---|---|
| HEC-1 | 23.08 | 7.29 | 133.1 |
| HEC-2 | 28.67 | 6.74 | 121.5 |
| HEC-3 | 36.92 | 5.76 | 103.2 |
| HEC-4 | 47.23 | 4.72 | 82.7 |
| HEC-5 | 59.34 | 3.97 | 41.9 |
| Samples | α (mm2 S−1) | Cp (J·g−1 k−1) | k (W·m−1 k−1) |
|---|---|---|---|
| HEC-1 | 2.68 | 0.21 | 4.12 |
| HEC-2 | 1.94 | 0.22 | 2.96 |
| HEC-3 | 1.36 | 0.25 | 1.97 |
| HEC-4 | 1.07 | 0.28 | 1.42 |
| HEC-5 | 0.92 | 0.30 | 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Li, C.; Ouyang, H.; Gao, R.; Shen, T.; Huang, J. Dual-Porosity (Ta0.2Nb0.2Ti0.2Zr0.2Hf0.2)C High-Entropy Ceramics with High Compressive Strength and Low Thermal Conductivity Prepared by Pressureless Sintering. Materials 2023, 16, 2495. https://doi.org/10.3390/ma16062495
Yang Q, Li C, Ouyang H, Gao R, Shen T, Huang J. Dual-Porosity (Ta0.2Nb0.2Ti0.2Zr0.2Hf0.2)C High-Entropy Ceramics with High Compressive Strength and Low Thermal Conductivity Prepared by Pressureless Sintering. Materials. 2023; 16(6):2495. https://doi.org/10.3390/ma16062495
Chicago/Turabian StyleYang, Qian, Cuiyan Li, Haibo Ouyang, Ruinan Gao, Tianzhan Shen, and Jianfeng Huang. 2023. "Dual-Porosity (Ta0.2Nb0.2Ti0.2Zr0.2Hf0.2)C High-Entropy Ceramics with High Compressive Strength and Low Thermal Conductivity Prepared by Pressureless Sintering" Materials 16, no. 6: 2495. https://doi.org/10.3390/ma16062495







