Influence of Surface Characteristics of TiO2 Coatings on the Response of Gingival Cells: A Systematic Review of In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Focus Question
- -
- Population: Fibroblasts, epithelial cells and/or gingival tissue.
- -
- Intervention: TiO2 coatings with nanofeature characteristics.
- -
- Comparison: Non-coated controls.
- -
- Outcomes: Gingival cells and tissue response with a qualitative and/or quantitative evaluation.
- -
- Setting: In vitro studies.
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Selection Process
2.5. Data Extraction and Analysis
2.6. Quality Assessment of Individual Studies
3. Results
3.1. Study Selection
3.2. Quality Assessment of the Included Studies
3.3. Study Characteristics
3.3.1. Subgroup 1–Sol-Gel Derived TiO2 Coatings
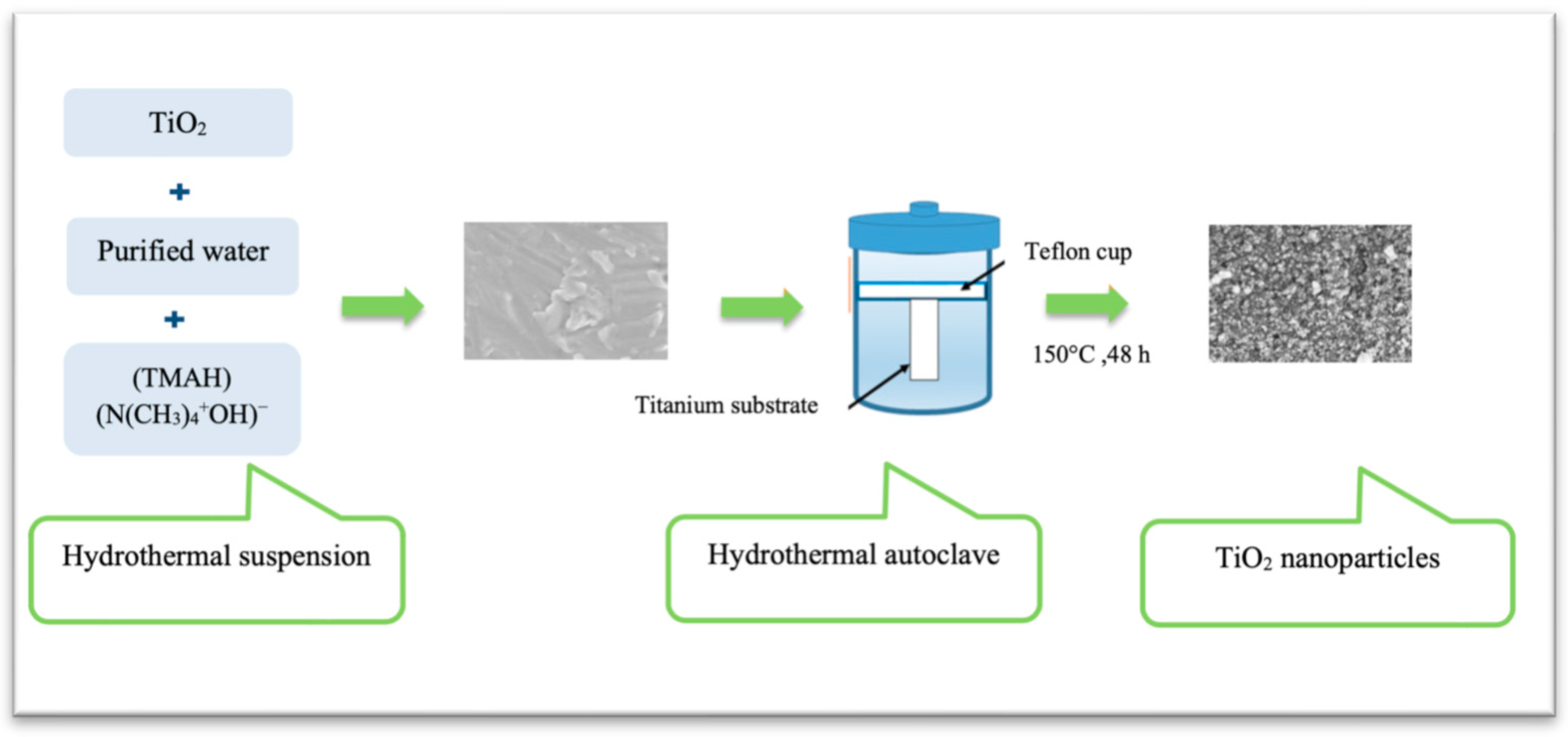
3.3.2. Subgroup 2–HT-Induced TiO2 Coatings and Acidic Treatment
3.3.3. Subgroup 3–TiO2 Coatings by Spray Coating and Deposition Techniques
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 628562016. [Google Scholar] [CrossRef] [PubMed]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Martín, I.; Sanz-Sánchez, I.; Carrillo de Albornoz, A.; Figuero, E.; Sanz, M. Effects of modified abutment characteristics on peri-implant soft tissue health: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Welander, M.; Abrahamsson, I.; Berglundh, T. The mucosal barrier at implant abutments of different materials. Clin. Oral Implant. Res. 2008, 19, 635–641. [Google Scholar]
- Canullo, L.; Annunziata, M.; Pesce, P.; Tommasato, G.; Nastri, L.; Guida, L. Influence of abutment material and modifications on peri-implant soft-tissue attachment: A systematic review and meta-analysis of histological animal studies. J. Prosthet. Dent. 2021, 125, 426–436. [Google Scholar] [CrossRef]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Larjava, H.; Koivisto, L.; Hakkinen, L.; Heino, J. Epithelial integrins with special reference to oral epithelia. J. Dent. Res. 2011, 90, 1367–1376. [Google Scholar] [CrossRef]
- Van den Borre, C.E.; Zigterman, B.G.R.; Mommaerts, M.Y.; Braem, A. How surface coatings on titanium implants affect keratinized tissue: A systematic review. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 17131723. [Google Scholar] [CrossRef]
- Narimatsu, I.; Atsuta, I.; Ayukawa, Y.; Oshiro, W.; Yasunami, N.; Furuhashi, A.; Koyano, K. Epithelial and Connective Tissue Sealing around Titanium Implants with Various Typical Surface Finishes. ACS Biomater. Sci. Eng. 2019, 5, 4976–4984. [Google Scholar] [CrossRef]
- Gulati, K.; Moon, H.-J.; Kumar, P.T.S.; Han, P.; Ivanovski, S. Anodized anisotropic titanium surfaces for enhanced guidance of gingival fibroblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110860. [Google Scholar] [CrossRef]
- Carossa, M.; Cavagnetto, D.; Mancini, F.; Mosca Balma, A.; Mussano, F. Plasma of Argon Treatment of the Implant Surface, Systematic Review of In Vitro Studies. Biomolecules 2022, 12, 1219. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J. Biomed. Mater. Res. A 2020, 108, 470–484. [Google Scholar] [CrossRef]
- Areva, S.; Paldan, H.; Peltola, T.; Närhi, T.; Jokinen, M.; Lindén, M. Use of sol-gel-derived titania coating for direct soft tissue attachment. J. Biomed. Mater. Res. A 2004, 70, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Fröjd, V.; Olsson, M.; Nannmark, U.; Emanuelsson, L.; Johansson, P.; Josefsson, Y.; Kangasniemi, I.; Peltola, T.; Tirri, T.; et al. Nanoporous TiO2 Thin Film on Titanium Oral Implants for Enhanced Human Soft Tissue Adhesion: A Light and Electron Microscopy Study. Clin. Implant. Dent. Relat. Res. 2011, 13, 184–196. [Google Scholar] [CrossRef]
- Ong, J.L.; Carnes, D.L.; Bessho, K. Evaluation of titanium plasma-sprayed and plasma- sprayedhydroxyapatite implants in vivo. Biomaterials 2004, 25, 4601–4606. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kim, J.K.; Kim, Y.D.; Choi, K.; Lee, K.H. In vivo behavior and mechanical stability of surface-modified titanium implants by plasma spray coating and chemical treatments. J. Biomed. Mater. Res. Part A 2004, 69, 279–285. [Google Scholar] [CrossRef]
- Jimbo, R.; Sawase, T.; Baba, K.; Kurogi, T.; Shibata, Y.; Atsuta, M. Enhanced initial cell responses to chemically modified anodized titanium. Clin. Implant. Dent. Relat. Res. 2008, 10, 55–61. [Google Scholar] [CrossRef]
- Peltola, T.; Patsi, M.; Rahiala, H.; Kangasniemi, I.; Yli-Urpo, A. Cal- calcium phosphate induction by sol-gel-derived titania coatings on titanium substrates in vitro. J. Biomed. Mater. Res. 1998, 41, 504–510. [Google Scholar] [CrossRef]
- Ueda, M.; Uchibayashi, Y.; Otsuka-Yao-Matsuo, S.; Okura, T. Hydrothermal synthesis of anatase-typeTiO2 films on Ti and Ti–Nb substrates. J. Alloys Compd. 2008, 459, 369–376. [Google Scholar] [CrossRef]
- Tomsia, A.P.; Lee, J.S.; Wegst, U.G.; Saiz, E. Nanotechnology for dental implants. Int. J. Oral. Maxillofac. Implant 2013, 28, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Lee, M.; Yeo, I.L. Three interfaces of the dental implant system and their clinical effects on hard and soft tissues. Mater. Horiz. 2022, 9, 1387–1411. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.A.; Shokoohi-Tabrizi, H.; Wehner, C.; Pippenger, B.E.; Wagner, R.S.; Ulm, C.; Moritz, A.; Chen, J.; Andrukhov, O. Impact of Implant Surface Material and Microscale Roughness on the Initial Attachment and Proliferation of Primary Human Gingival Fibroblasts. Biology 2021, 10, 356. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Lu, R.; Gao, S.; Ling, Y.; Chen, S. Responses of human gingival fibroblasts to superhydrophilic hydrogenated titanium dioxide nanotubes. Colloids Surf. B Biointerfaces 2021, 198, 111489. [Google Scholar] [CrossRef]
- Xu, R.; Hu, X.; Yu, X.; Wan, S.; Wu, F.; Ouyang, J.; Deng, F. Micro-/nano-topography of selective laser melting titanium enhances adhesion and proliferation and regulates adhesion-related gene expressions of human gingival fibroblasts and human gingival epithelial cells. Int. J. Nanomed. 2018, 13, 5045–5057. [Google Scholar] [CrossRef]
- Guida, L.; Oliva, A.; Basile, M.A.; Giordano, M.; Nastri, L.; Annunziata, M. Human gingival fibroblast functions are stimulated by oxidized nano-structured titanium surfaces. J. Dent. 2013, 41, 900–907. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Areid, N.; Riivari, S.; Abushahba, F.; Shahramian, K.; Närhi, T. Influence of Surface Characteristics of TiO2 Coatings on the Response of Gingival Cells: A Systematic Review of In Vitro Studies. Available online: https://doi.org/10.17605/OSF.IO/CFUVH (accessed on 24 February 2023).
- Crenn, M.J.; Dubot, P.; Mimran, E.; Fromentin, O.; Lebon, N.; Peyre, P. Influence of Anodized Titanium Surfaces on the Behavior of Gingival Cells in Contact with: A Systematic Review of In Vitro Studies. Crystals 2021, 11, 1566. [Google Scholar] [CrossRef]
- Corvino, E.; Pesce, P.; Mura, R.; Marcano, E.; Canullo, L. Influence of Modified Titanium Abutment Surface on Peri-implant Soft Tissue Behavior: A Systematic Review of in vitro Studies. Int. J. Oral Maxillofac. Implant. 2020, 35, 503–519. [Google Scholar] [CrossRef]
- Riivari, S.; Närvä, E.; Kangasniemi, I.; Willberg, J.; Närhi, T. Epithelial cell attachment and adhesion protein expression on novel in sol TiO2 coated zirconia and titanium alloy surfaces. J. Biomed. Mater. Res. 2022, 110, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Areid, N.; Willberg, J.; Kangasniemi, I.; Närhi, T.O. Organotypic in vitro block culture model to investigate tissue-implant interface. An experimental study on pig mandible. J. Mater. Sci. Mater. Med. 2021, 32, 136. [Google Scholar] [CrossRef]
- Shahramian, K.; Gasik, M.; Kangasniemi, I.; Walboomers, X.F.; Willberg, J.; Abdulmajeed, A.; Närhi, T. Zirconia implants with improved attachment to the gingival tissue. J. Periodontol. 2020, 91, 213–1224. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ayukawa, Y.; Furuhashi, A.; Kamo, M.; Ikeda, J.; Atsuta, I.; Haraguchi, T.; Koyano, K. Effect of Hydrothermal Treatment with Distilled Water on Titanium Alloy for Epithelial Cellular Attachment. Materials 2019, 12, 748. [Google Scholar] [CrossRef] [PubMed]
- Riivari, S.; Shahramian, K.; Kangasniemi, I.; Willberg, J.; Närhi, T.O. TiO2 -Modified Zirconia Surface Improves Epithelial Cell Attachment. Int. J. Oral. Maxillofac. Implant. 2019, 34, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Masa, R.; Deák, Á.; Braunitzer, G.; Tóth, Z.; Kopniczky, J.; Pelsőczi-Kovács, I.; Ungvári, K.; Dékány, I.; Turzó, K. TiO2/Ag-TiO2 Nanohybrid Films are Cytocompatible with Primary Epithelial Cells of Human Origin: An In Vitro Study. J. Nanosci. Nanotechnol. 2018, 18, 3916–3924. [Google Scholar] [CrossRef] [PubMed]
- Areid, N.; Peltola, A.; Kangasniemi, I.; Ballo, A.; Närhi, T.O. Effect of ultraviolet light treatment on surface hydrophilicity and human gingival fibroblast response on nano-structured titanium surfaces. Clin. Exp. Dent. Res. 2018, 4, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Shahramian, K.; Leminen, H.; Meretoja, V.; Linderbäck, P.; Kangasniemi, I.; Lassila, L.; Abdulmajeed, A.; Närhi, T. Sol-gel derived bioactive coating on zirconia: Effect on flexural strength and cell proliferation. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2401–2407. [Google Scholar] [CrossRef]
- Vignesh; Nayar, S.; Bhuminathan; Mahadevan; Santhosh, S. Comparative evaluation of the three different surface treatments—Conventional, laser and Nano technology methods in enhancing the surface characteristics of commercially pure titanium discs and their effects on cell adhesion: An in vitro study. J. Pharm. Bioallied. Sci. 2015, 7, 87–91. [Google Scholar]
- Hoshi, N.; Negishi, H.; Okada, S.; Nonami, T.; Kimoto, K. Response of human fibroblasts to implant surface coated with titanium dioxide photocatalytic films. J. Prosthodont. Res. 2010, 4, 185–191. [Google Scholar] [CrossRef]
- Meretoja, V.V.; Rossi, S.; Peltola, T.; Pelliniemi, L.J.; Närhi, T.O. Adhesion and proliferation of human fibroblasts on sol-gel coated titania. J. Biomed. Mater. Res. A 2010, 95, 269–275. [Google Scholar] [CrossRef]
- Pera, F.; Menini, M.; Alovisi, M.; Crupi, A.; Ambrogio, G.; Asero, S.; Marchetti, C.; Canepa, C.; Merlini, L.; Pesce, P.; et al. Can Abutment with Novel Superlattice CrN/NbN Coatings Influence Peri-Implant Tissue Health and Implant Survival Rate Compared to Machined Abutment? 6-Month Results from a Multi-Center Split-Mouth Randomized Control Trial. Materials 2022, 16, 246. [Google Scholar] [CrossRef]
- Mendonca, G.; Mendonca, D.B.; Aragao, F.J.; Cooper, L.F. Advancing dental implant surface technology from micron to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Huttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Rossi, S.; Moritz, N.; Tirri, T.; Peltola, T.; Areva, S.; Jokinen, M.; Happonen, R.P.; Närhi, T. Comparison between sol-gel-derived anatase- and rutile-structured TiO2 coatings in soft-tissue environment. J. Biomed. Mater. Res. A 2007, 82, 965–974. [Google Scholar] [CrossRef]
- Paldan, H.; Areva, S.; Tirri, T.; Peltola, T.; Lindholm, T.C.; Lassila, L.; Pelliniemi, L.J.; Happonen, R.P.; Närhi, T.O. Soft tissue attachment on sol- gel-treated titanium implants in vivo. J. Mater. Sci. Mater. Med. 2008, 19, 1283–1290. [Google Scholar] [CrossRef]
- Yilmaz, Ö.; Young, P.A.; Lamont, R.J.; Kenny, G.E. Gingival epithelial cell signaling and cytoskeletal responses to Porphyromonas gingivalis invasion. Microbiology 2003, 149, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Kinumatsu, T.; Hashimoto, S.; Muramatsu, T.; Sasaki, H.; Jung, H.S.; Yamada, S.; Shimono, M. Involvement of laminin and integrins in adhesion and migration of junctional epithelium cells. J. Periodontal. Res. 2009, 44, 13–20. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Goto, T.; Kido, M.A.; Kagiya, T.; Mino, S.; Shimono, M.; Tanaka, T. Ultrastructural localization of laminin-5 (gamma2 chain) in the rat peri-implant oral mucosa around a titanium-dental implant by immuno-electron microscopy. Biomaterials 2005, 26, 6280–6287. [Google Scholar] [CrossRef]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Mino, S.; Goto, T.; Kido, M.A.; Terada, Y.; Tanaka, T. Changes in the distribution of laminin-5 during peri-implant epithelium formation after immediate titanium implantation in rats. Biomaterials 2005, 26, 1751–1760. [Google Scholar]
- Roffel, S.; Wu, G.; Nedeljkovic, I.; Meyer, M.; Razafiarison, T.; Gibbs, S. Evaluation of a novel oral mucosa in vitro implantation model for analysis of molecular interactions with dental abutment surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Neilands, J.; Davies, J.R.; Ekestubbe, A.; Friberg, B. A randomized, controlled, clinical study on a new titanium oxide abutment surface for improved healing and soft tissue health. Clin. Implant Dent. Relat. Res. 2019, 21, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Tirri, T.; Paldan, H.; Kuntsi-Vaattovaara, H.; Tulamo, R.; Närhi, T. Peri-implant tissue response to TiO2 surface modified implants. Clin. Oral Implant. Res. 2008, 19, 348–355. [Google Scholar] [CrossRef] [PubMed]


| #1 | “Dental implant” OR “healing abutment” OR abutment * OR “dental abutment” OR “oral implant” OR “prosthetic abutment” |
| #2 | “Titanium dioxide” OR TiO2 * OR “titanium oxide” OR titanium dioxide coat * OR titanium oxide coat * OR surface modification* OR modified surface* OR nanotube * OR nanostructure * OR nanoporous * |
| #3 | “peri-implant soft tissue” OR gingiva * OR fibroblast * OR “human gingival fibroblast” OR “Gingival epithelial cell” OR keratinocyte * OR mucosa * OR “tissue-implant interface” OR “peri-implant tissue” |
| #4 | titanium* OR “Ti6Al4V” OR Zircon * |
| #5 | #1 AND #2 AND #3 AND #4 |
| Author (Year) | Reporting Quality | Methodologic Quality | References |
|---|---|---|---|
| Riivari et al., 2022 | 83 | 90 | [31] |
| Areid et al., 2021 | 85 | 80 | [32] |
| Shahramian et al., 2020 | 79 | 88 | [33] |
| Sakamoto et al., 2019 | 87 | 90 | [34] |
| Riivari et al., 2019 | 88 | 87.5 | [35] |
| Masa et al., 2018 | 85.5 | 85 | [36] |
| Areid et al., 2018 | 85.5 | 88 | [37] |
| Shahramian. et al., 2017 | 68 | 85 | [38] |
| Vignesh et al., 2015 | 84 | 86.5 | [39] |
| Hoshi et al., 2010 | 89.5 | 97 | [40] |
| Meretoja et al., 2010 | 74 | 85 | [41] |
| Author/Year | Material type | Tio2 Coating Technique | Control Group | Cell Line/Tissue | Analyzed Functions, Methodology, Cell Density, Duration | Number of Replicates |
|---|---|---|---|---|---|---|
| Riivari et al., 2022 [31] | Zirconia grade 5 Titanium alloy (Ti-6Al-4V) | A novel in sol TiO2-polycondensation coating | Non-coated zirconia Non-coated polished titanium | hGKs from biopsies |
| n = 3 |
| Areid et al., 2021 [32] | Titanium alloy (Ti-6Al-4V) | HT-induced-TiO2 coating | Non-coated polished titanium | Tissue culture model using mandibular pig block including gingival soft tissues. |
| n = 2 |
| Shahramian et al., 2020 [33] | Zirconia | Sol-gel derived TiO2 coating | Non-coated zirconia | Porcine gingival tissue culture model |
| n = 2/time-point |
| Sakamoto et al., 2019 [34] | Titanium alloy (Ti-6Al-4V) | HT-treatment | Non-coated polished titanium | GE1 mouse-derived gingival epithelial cell line |
| n = 6 |
| Riivari et al., 2019 [35] | Zirconia | Sol-gel derived TiO2 coating | Non-coated zirconia | hGKs from biopsies |
| n = 4 |
| Masa et al., 2018 [36] | Titanium grade IV | TiO2 nanohybrid films using spray coating technique | Non-coated polished titanium | Primary HGECs Passage: at least three times |
| n = 4 |
| Areid et al., 2018 [37] | Titanium alloy (Ti-6Al-4V) | HT- induced-TiO2 coating | Non-coated polished titanium | HGFs from biopsies Passages: 8 and 10 |
| n = 6 n = 4 |
| Shahramian et al., 2017 [38] | Zirconia | Sol-gel derived TiO2 coating | Non-coated zirconia | HGFs from biopsies |
| n = 4 |
| Vignesh et al., 2015 [39] | Titanium grade II | Pulse laser deposition | Non-coated machined titanium | L929 murine fibroblasts | Cell attachment and growth by SEM (5 × 103 cells/ well) at 48 h | NS |
| Hoshi et al., 2010 [40] | Titanium grade II | Chemical treatment using peroxotitanium acid solution | Non-coated polished titanium | HPLFs from biopsies Passages: 6 and 8 |
| n = 9 |
| Meretoja et al., 2010 [41] | Titanium grade II | Sol-gel derived TiO2 coating | Non-coated polished titanium | HGFs from biopsies |
| n = 4 |
| Author/Year | Surface Morphology | Surface Roughness | Water Contact Angle (WCA) | Analyzed Functions and Duration Time | Results Compared to the Control Surface |
|---|---|---|---|---|---|
| Sol-gel derived TiO2 coatings | |||||
| Riivari et al., 2022 [31] | Smooth surfaces with small, nanostructure particles similar in size and shape on zirconia and titanium-coated surfaces | NS | NS | Cell adhesion at 1, 3, 6 and 24 h | Cell adhesion was greater on TiO2-coated zirconia compared to non-coated surface at 24 h. |
| Cell proliferation at 1, 3 and 7 d | Cell proliferation was higher on coated zirconia at 1 d and on coated titanium at 3 and 7 d compared to non-coated surfaces. | ||||
| Gene expression at 3 d |
| ||||
| Cell spreading, actin cytoskeleton and focal adhesion proteins at 24 h |
| ||||
| Shahramian et al., 2020 [33] | Smooth surface with some cracks on the superficial layer of coating (Data from Shahramian et al., 2017, that used the same material) | TiO2-coated zirconia: Sa = 34.2 nm Control: Sa = 533.8 nm. (Data from Shahramian et al., 2017, that used the same material) | TiO2-coated zirconia: 53.0° ± 4.8° Non-coated: 74.1° ± 6.9° (data from their previous work, Riivari et al., 2019) | Biomechanical analysis at 7 and 14 d |
|
| Gene expression at 7 and 14 d |
| ||||
| Riivari et al., 2019 [35] | NS | NS | TiO2-coated zirconia: 53.0° ± 4.8° Non-coated: 74.1° ± 6.9° | Cell adhesion at 1, 3, 6 and 24 h | Cell adhesion was higher on TiO2-coated zirconia compared to non-coated surface at 24 h. |
| Cell proliferation at 1, 3 and 7 d | Cell proliferation on TiO2-coated zirconia was higher compared to non-coated surface at 3 and 7 d | ||||
| Cell morphology by SEM | More cells with more uniform cell layers on coated zirconia | ||||
| Shahramian et al., 2017 [38] | Smooth surface with some cracks on the superficial layer of coating | TiO2-coated zirconia: Sa = 34.2 nm Control: Sa = 533.8 nm | TiO2-coated zirconia: 53.0° ± 4.8° Non-coated: 74.1° ± 6.9° | Cell proliferation at 1, 4, 7 and 12 d. |
|
| Meretoja et al., 2010 [41] | Uniform surface with extensive cracking | Sol-gel derived TiO2 coated surface: Sa = 0.255 μm Sq = 0.322 μm | NS | Initial cell attachment at 1, 3, 6 and 24 h |
|
| Cell adhesion at 6 h |
| ||||
| Cell proliferation at 1, 3, 5, 7 and 10 d |
| ||||
| Cell morphology at 6 h, 1, 3 and 7 d and Confocal fluorescence microscopy at 3 d |
| ||||
| Ultrastructural analysis at 7 d | A continuous layer of two to three cells thickness covered both surfaces. | ||||
| HT-induced TiO2 coatings and acidic treatment | |||||
| Areid et al., 2021 [32] | Titanium nanoparticles with a diameter of 20–50 nm (Data from Areid et al., 2018, that used the same material) | NS | HT-induced TiO2: 31.1° ± 2.5° NC control: 50.3° ± 4.5° (Data from Areid et al., 2018, that used the same material) | Histological analysis at 7 and 14 d |
|
| Gene expression at 14 d |
| ||||
| Sakamoto et al., 2019 [34] | HT treatment changed the surface crystal structure into an anatase type of TiO2 without an apparent change in surface topography. | HT treated: Ra = 0.072 ± 0.010 μm Rt = 0.95 ± 0.24 μm Control: Ra = 0.070 ± 0.008 μm Rt = 1.03 ± 0.23 μm | HT treated: 8.0° ± 1.6° Control: 78.9° ± 4.8° | Amount of Adsorbed Ln at1 h | The amount of adsorbed Ln was greater on the HT surface than that on the control surface. |
| Initial cell attachment at 1 h | No difference | ||||
| Cell proliferation at 1, 3 and 7 d |
| ||||
| Cell adhesion strength at 1 d | The cell adhesion ratio was greater on the HT surface at 1 d. | ||||
| Gene Expression at 1 d | A stronger signal of ITGβ4 was observed on HT coated surface. | ||||
| Areid et al., 2018 [37] | Nanoparticles with a diameter of 20–50 nm | NS | HT-induced TiO2: 31.1° ± 2.5° NC control: 50.3° ± 4.5° Sol-gel derived TiO2: 35.3° ± 4.3° | Cell adhesion resistance against enzymatic detachment at 6 h | The detachment percentage was lower on coated surfaces than on non-coated surface. |
| Cell proliferation at 1, 3, 7 and 10 d. |
| ||||
| Cell morphology at 6 h and 1, 3, 7 and 10 d | More cells with an elongated shape were observed on coated surfaces. | ||||
| Hoshi et al., 2010 [40] | Smooth surface texture with anatase structure | TiO2-coated 228.3 ± 22.1 nm non-coated 275.7 ± 23.5 nm | (Diagram without associated values) TiO2-coated: Hydrophilic or super-hydrophilic Non-coated: Hydrophobic | Initial cell spreading and morphology at 12 h and 3 d |
|
| Cell proliferation 3 and 7 d |
| ||||
| TiO2 coatings by spray coating and deposition techniques | |||||
| Masa et al., 2018 [36] | TiO2-coated showed an amorphous surface pattern. Characteristic grains appeared on the silver-containing coated surfaces. | TiO2: Ra = 1.79 ± 0.13 μm polished Ra = 0.13 ± 0.01 μm | NS | Cell adhesion at 24 h |
|
| Cell proliferation at 3 and 7 d | No difference | ||||
| Cell morphology at 1, 3 and 7 d |
| ||||
| Vignesh et al., 2015 [39] | Spherical nanoparticles with 20 nm covered with pits 1.5 μm depth 3–5 μm diameter | NS | NS | Cell attachment and growth at 48 h | TiO2 nanoparticle-coated surface showed better cell response and attachment than the control group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Areid, N.; Riivari, S.; Abushahba, F.; Shahramian, K.; Närhi, T. Influence of Surface Characteristics of TiO2 Coatings on the Response of Gingival Cells: A Systematic Review of In Vitro Studies. Materials 2023, 16, 2533. https://doi.org/10.3390/ma16062533
Areid N, Riivari S, Abushahba F, Shahramian K, Närhi T. Influence of Surface Characteristics of TiO2 Coatings on the Response of Gingival Cells: A Systematic Review of In Vitro Studies. Materials. 2023; 16(6):2533. https://doi.org/10.3390/ma16062533
Chicago/Turabian StyleAreid, Nagat, Sini Riivari, Faleh Abushahba, Khalil Shahramian, and Timo Närhi. 2023. "Influence of Surface Characteristics of TiO2 Coatings on the Response of Gingival Cells: A Systematic Review of In Vitro Studies" Materials 16, no. 6: 2533. https://doi.org/10.3390/ma16062533
APA StyleAreid, N., Riivari, S., Abushahba, F., Shahramian, K., & Närhi, T. (2023). Influence of Surface Characteristics of TiO2 Coatings on the Response of Gingival Cells: A Systematic Review of In Vitro Studies. Materials, 16(6), 2533. https://doi.org/10.3390/ma16062533





