Scanning with Laser Beam over the TiO2 Nanotubes Covered with Thin Chromium Layers towards the Activation of the Material under the Visible Light
Abstract
:1. Introduction
2. Methods
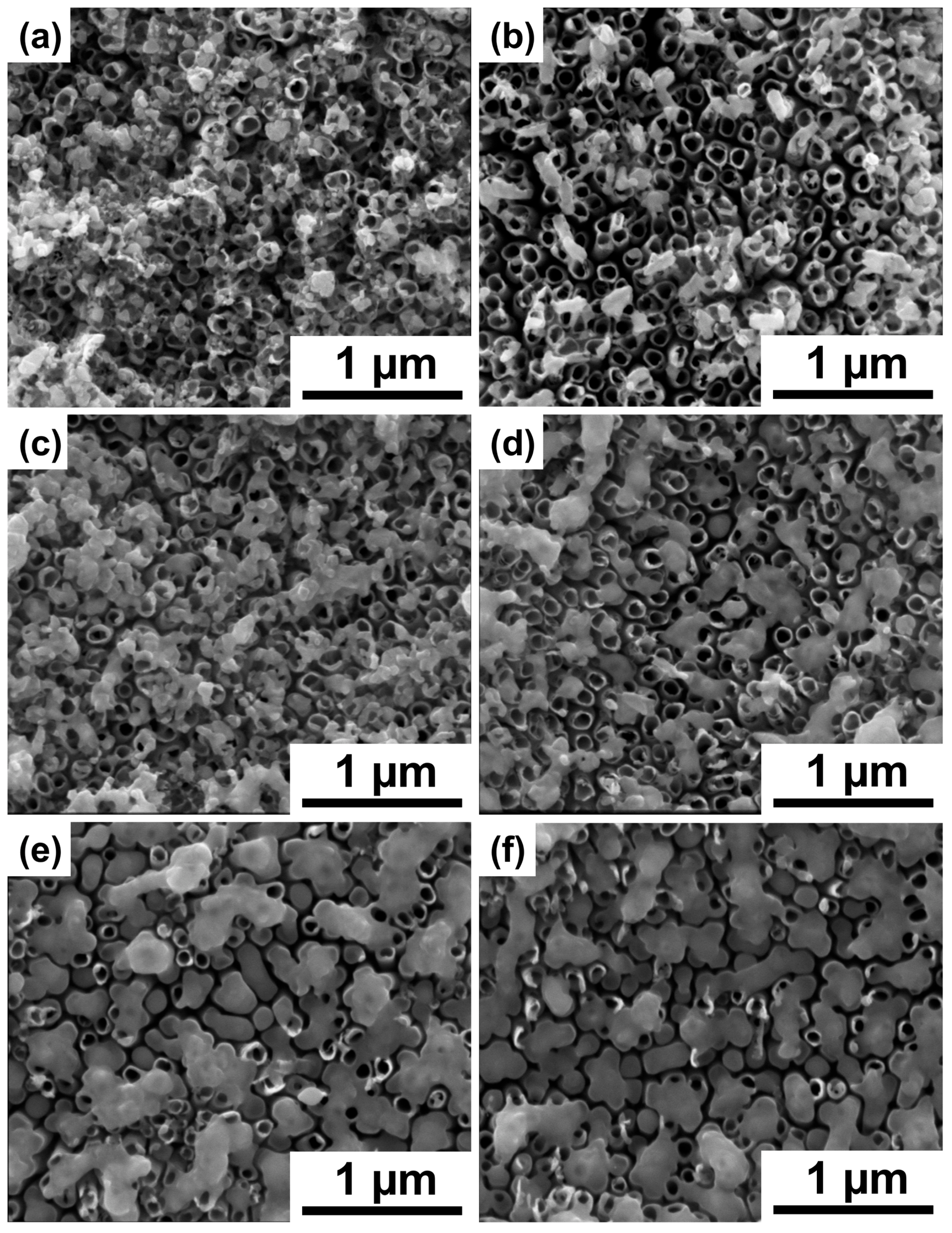
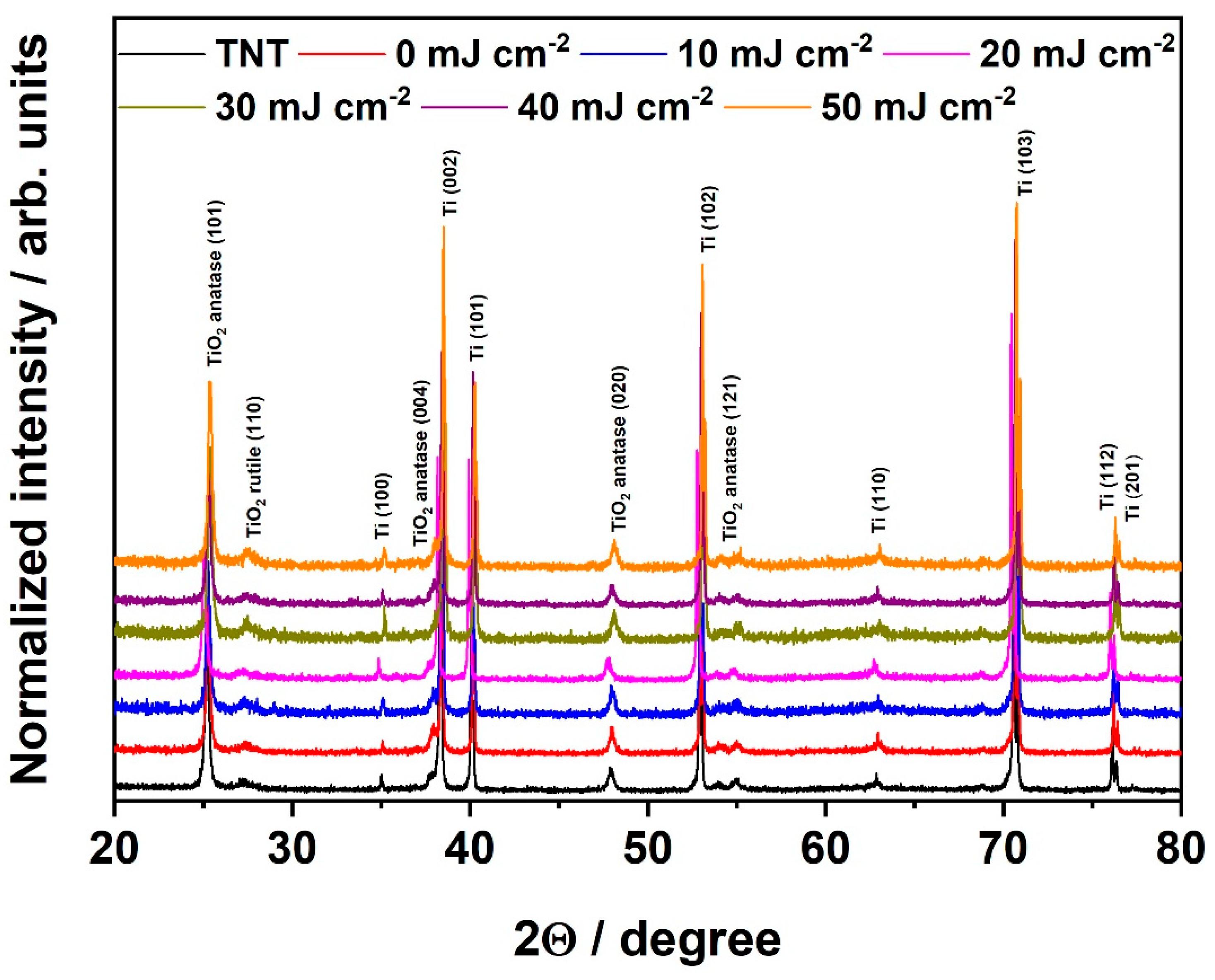
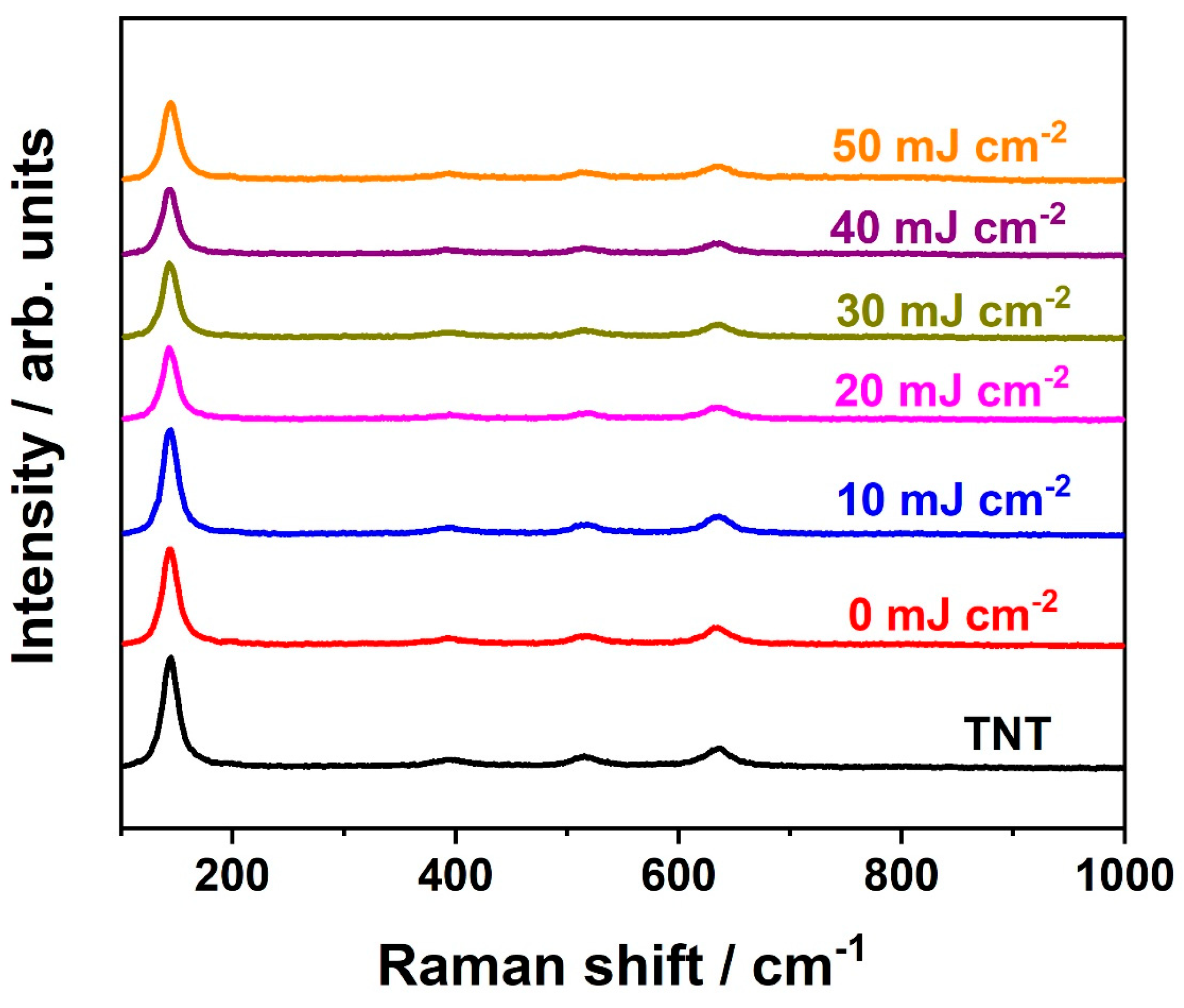
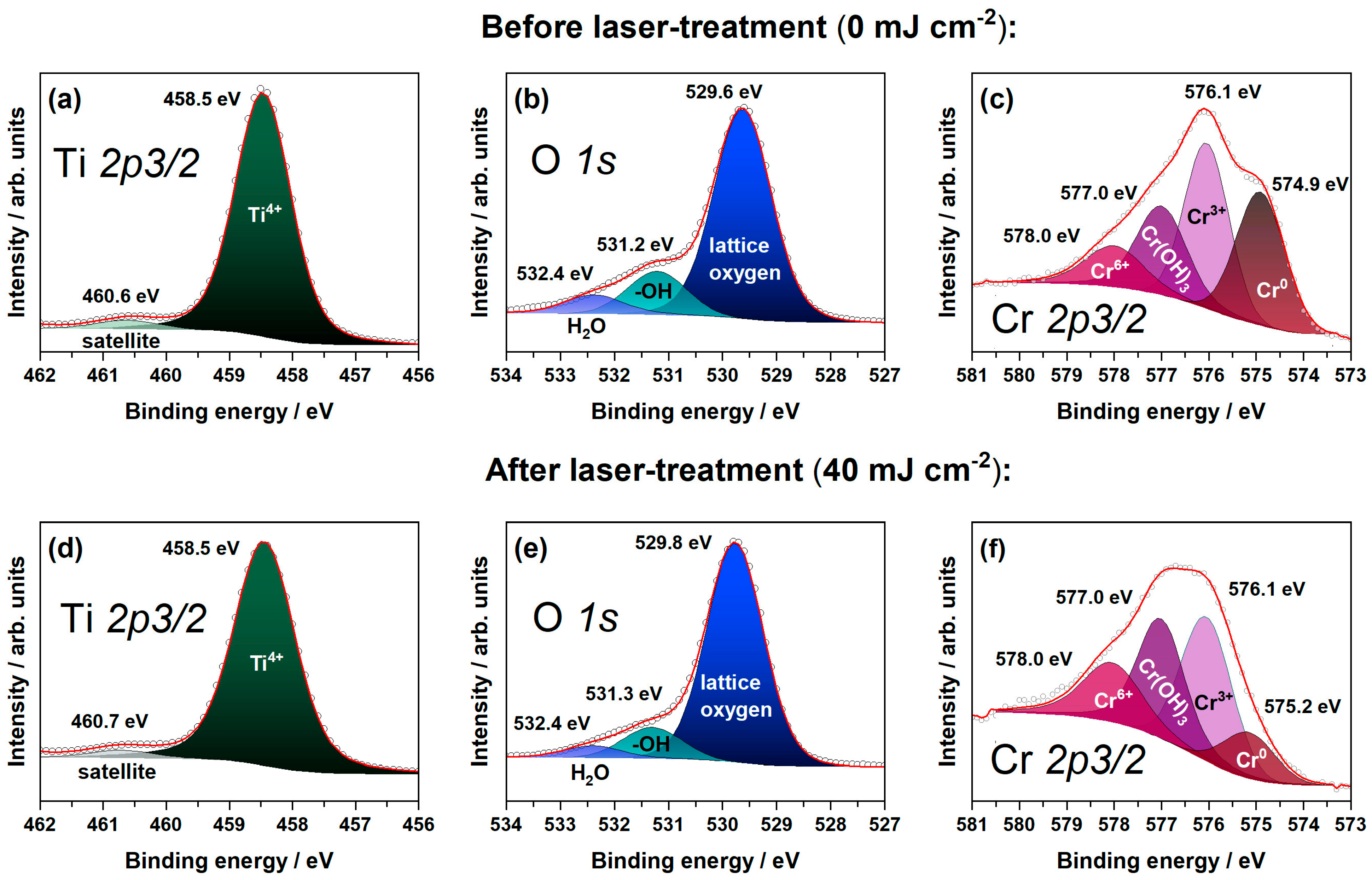
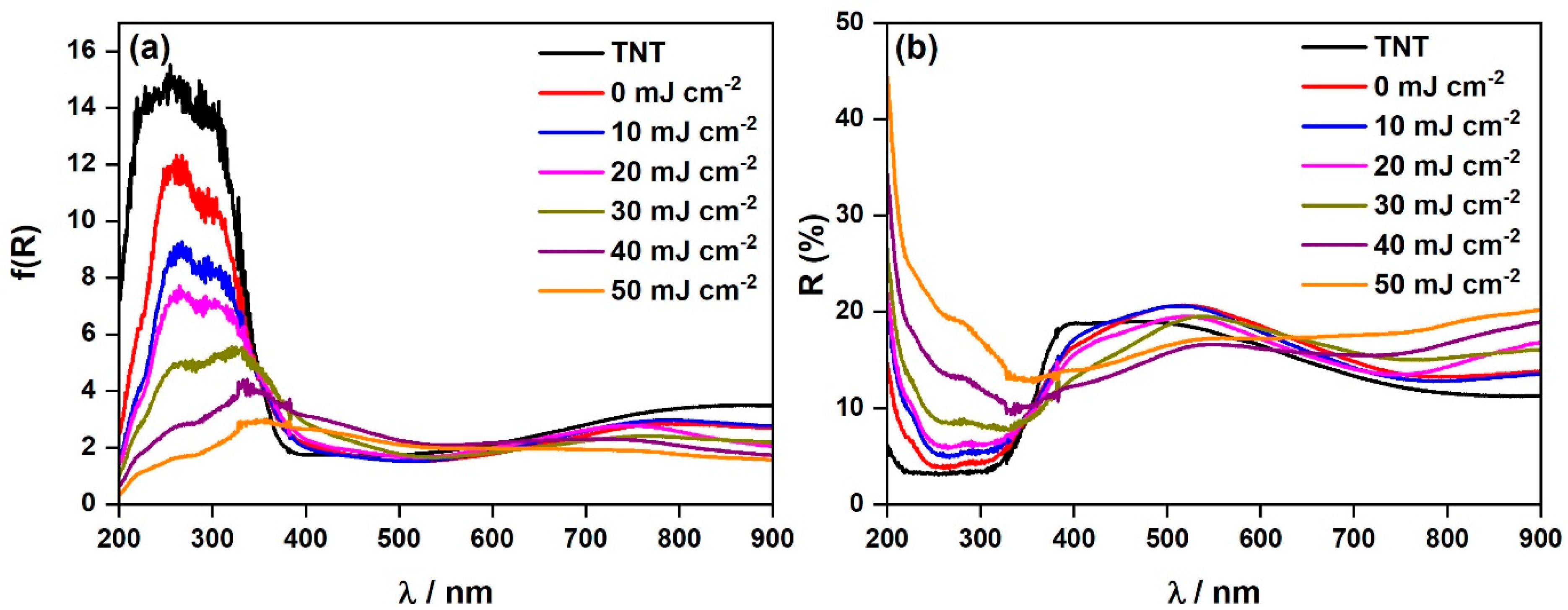
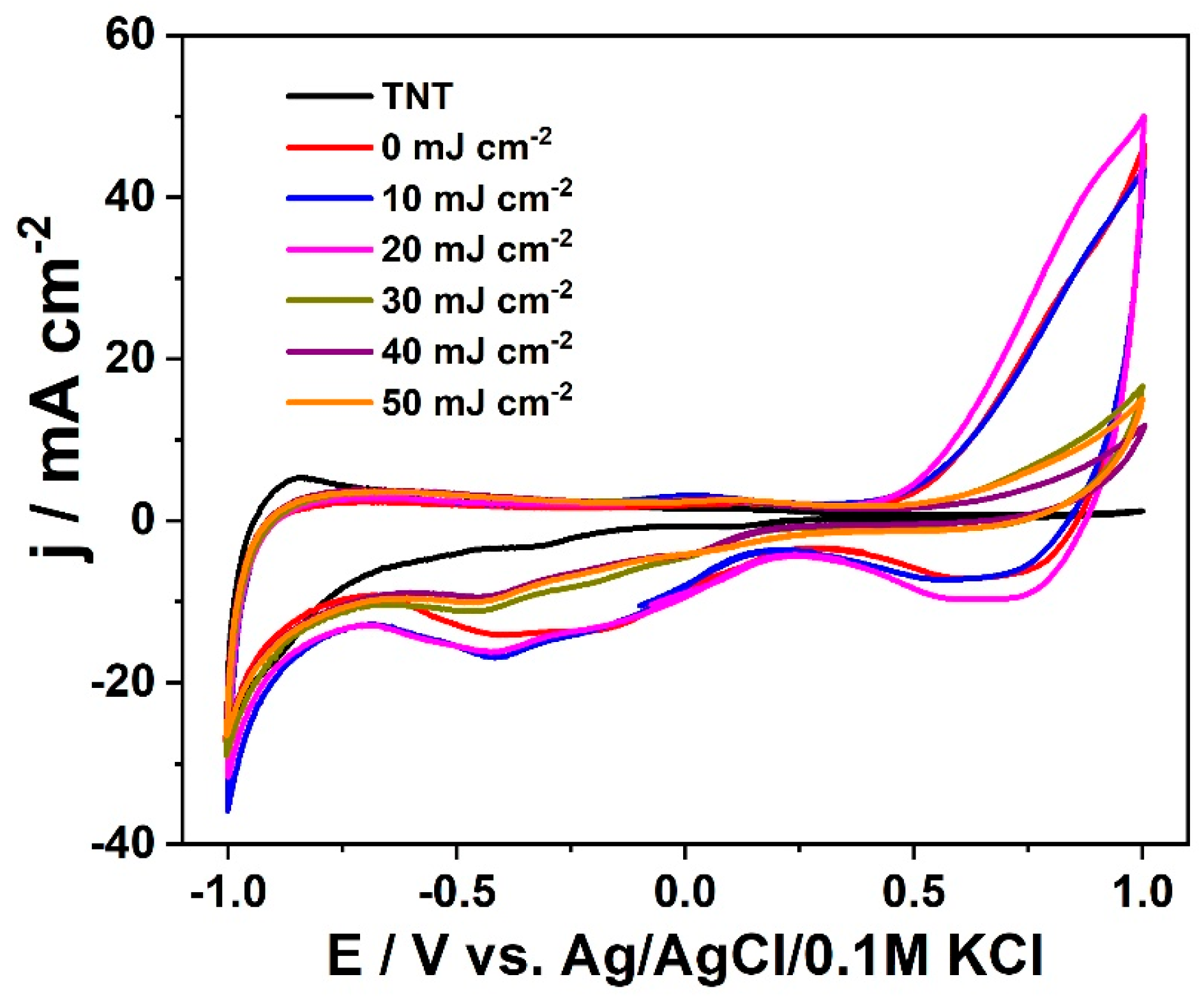
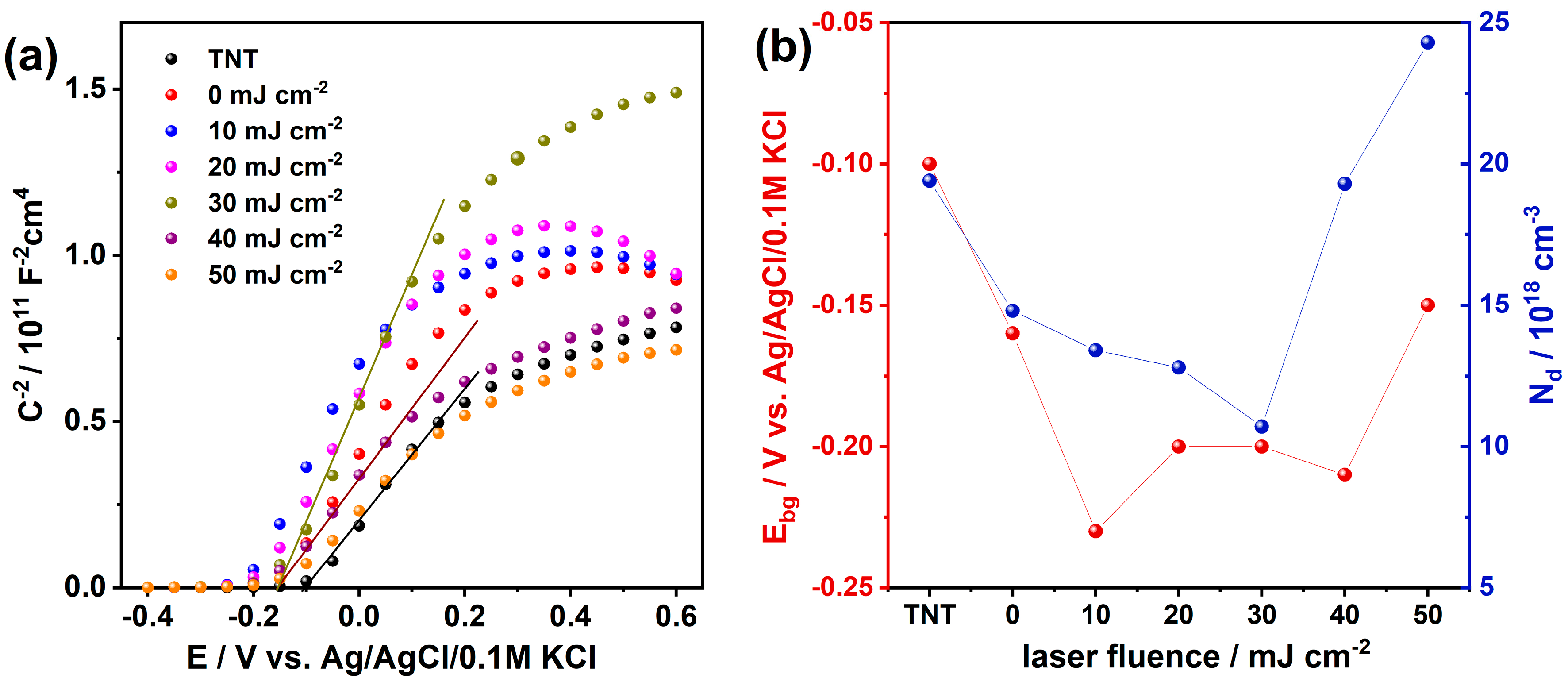
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Bai, J.; Li, Y.; Zeng, Q.; Li, J.; Zhou, B. BiVO4/TiO2(N2) Nanotubes Heterojunction Photoanode for Highly Efficient Photoelectrocatalytic Applications. Nano-Micro Lett. 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinchuk, V.A.; Kuzmin, A.V. The Effect of the Addition of TiO2 Nanoparticles to Coal-Water Fuel on Its Thermophysical Properties and Combustion Parameters. Fuel 2020, 267, 117220. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.-V.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Le, Q.V. Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Prado-Audelo, M.L.; García Kerdan, I.; Escutia-Guadarrama, L.; Reyna-González, J.M.; Magaña, J.J.; Leyva-Gómez, G. Nanoremediation: Nanomaterials and Nanotechnologies for Environmental Cleanup. Front. Environ. Sci. 2021, 9, 793765. [Google Scholar] [CrossRef]
- Al-Madanat, O.; AlSalka, Y.; Ramadan, W.; Bahnemann, D.W. TiO2 Photocatalysis for the Transformation of Aromatic Water Pollutants into Fuels. Catalysts 2021, 11, 317. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, P.; Yang, H.; Chen, J.; Xiao, F.; Guo, Y.; Liu, Y.; Zhang, W.; Huo, F.; Liu, B. Metal–Organic Frameworks as Promising Photosensitizers for Photoelectrochemical Water Splitting. Adv. Sci. 2016, 3, 1500243. [Google Scholar] [CrossRef]
- Kusior, A.; Wnuk, A.; Trenczek-Zajac, A.; Zakrzewska, K.; Radecka, M. TiO2 Nanostructures for Photoelectrochemical Cells (PECs). Int. J. Hydrogen Energy 2015, 40, 4936–4944. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Li, C.-H.; Li, S.-S.; Kuo, T.-R.; Tsai, C.-M.; Chen, T.-R.; Wang, Y.-C.; Chen, C.-W.; Chen, C.-C. Iron Pyrite/Titanium Dioxide Photoanode for Extended Near Infrared Light Harvesting in a Photoelectrochemical Cell. Sci. Rep. 2016, 6, 20397. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, C.; Yin, G.; Zhang, T.; Wang, W.; Ou, G.; Jin, H.; Chen, Z. A Three-Dimensional Branched TiO2 Photoanode with an Ultrathin Al 2O3 Passivation Layer and a NiOOH Cocatalyst toward Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 13301–13310. [Google Scholar] [CrossRef]
- Williams, R. Electrochemical Reactions of Semiconductors. J. Vac. Sci. Technol. 1976, 13, 12–18. [Google Scholar] [CrossRef]
- Frank, S.N.; Bard, A.J. Semiconductor Electrodes. II. Electrochemistry at n-Type Titanium Dioxide Electrodes in Acetonitrile Solutions. J. Am. Chem. Soc. 1975, 97, 7427–7433. [Google Scholar] [CrossRef]
- Makimizu, Y.; Nguyen, N.T.; Tucek, J.; Ahn, H.; Yoo, J.; Poornajar, M.; Hwang, I.; Kment, S.; Schmuki, P. Activation of A-Fe2O3 for Photoelectrochemical Water Splitting Strongly Enhanced by Low Temperature Annealing in Low Oxygen Containing Ambient. Chem. Eur. J. 2020, 26, 2685–2692. [Google Scholar] [CrossRef] [Green Version]
- Kalanur, S.S.; Duy, L.T.; Seo, H. Recent Progress in Photoelectrochemical Water Splitting Activity of WO3 Photoanodes. Top. Catal. 2018, 61, 1043–1076. [Google Scholar] [CrossRef]
- Barawi, M.; Gomez-Mendoza, M.; Oropeza, F.E.; Gorni, G.; Villar-Garcia, I.J.; Giménez, S.; de la Peña O’Shea, V.A.; García-Tecedor, M. Laser-Reduced BiVO4 for Enhanced Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2022, 14, 33200–33210. [Google Scholar] [CrossRef]
- Arifin, K.; Yunus, R.M.; Minggu, L.J.; Kassim, M.B. Improvement of TiO2 Nanotubes for Photoelectrochemical Water Splitting: Review. Int. J. Hydrogen Energy 2021, 46, 4998–5024. [Google Scholar] [CrossRef]
- Abdullah, M.; Kamarudin, S.K. Titanium Dioxide Nanotubes (TNT) in Energy and Environmental Applications: An Overview. Renew. Sustain. Energy Rev. 2017, 76, 212–225. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile?—Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, V.K.; Mohapatra, S.K.; Misra, M. Stability of TiO₂ Nanotubes Arrays in Photoelectrochemical Studies. Int. J. Hydrogen Energy 2008, 33, 5369–5374. [Google Scholar] [CrossRef]
- Momeni, M.M.; Motalebian, M. Chromium-Doped Titanium Oxide Nanotubes Grown via One-Step Anodization for Efficient Photocathodic Protection of Stainless Steel. Surf. Coat. Technol. 2021, 420, 127304. [Google Scholar] [CrossRef]
- Momeni, M.M.; Mahvari, M.; Ghayeb, Y. Photoelectrochemical Properties of Iron-Cobalt WTiO2 Nanotube Photoanodes for Water Splitting and Photocathodic Protection of Stainless Steel. J. Electroanal. Chem. 2019, 832, 7–23. [Google Scholar] [CrossRef]
- Mohammadi, T.; Sharifi, S.; Ghayeb, Y.; Sharifi, T.; Momeni, M.M. Photoelectrochemical Water Splitting and H2 Generation Enhancement Using an Effective Surface Modification of W-Doped TiO2 Nanotubes (WT) with Co-Deposition of Transition Metal Ions. Sustainability 2022, 14, 13251. [Google Scholar] [CrossRef]
- Haryński, Ł.; Olejnik, A.; Karczewski, J.; Ryl, J.; Grochowska, K.; Siuzdak, K. Linking Optical and Electronic Properties to Photoresponse of Heterojunctions Based on Titania Nanotubes and Chromium, Molybdenum, and Tungsten Oxides. Opt. Mater. 2022, 134, 113183. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Gomi, H.; Sakata, H. Electrical and Optical Properties of Cr₂O₃ Films Prepared by Chemical Vapour Deposition. Phys. Status Solidi A 1996, 155, 417–425. [Google Scholar] [CrossRef]
- Shafeeq, K.M.; Rizwana, E.; Kishor, C.H.R.; Aneesh, P.M. Growth and Characterization of α-MoO₃ Thin Films Grown by Spray Pyrolysis Technique. AIP Conf. Proc. 2019, 2082, 050010. [Google Scholar] [CrossRef]
- Gullapalli, S.K.; Vemuri, R.S.; Ramana, C.V. Structural Transformation Induced Changes in the Optical Properties of Nanocrystalline Tungsten Oxide Thin Films. Appl. Phys. Lett. 2010, 96, 171903. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Ceraj-Cerić, M. P-Type and N-Type Behavior of Chromium Oxide as a Function of the Applied Potential. J. Electrochem. Soc. 1987, 134, 2193–2197. [Google Scholar] [CrossRef]
- Peelaers, H.; Chabinyc, M.L.; Van de Walle, C.G. Controlling N-Type Doping in MoO3. Chem. Mater. 2017, 29, 2563–2567. [Google Scholar] [CrossRef]
- Gillet, M.; Aguir, K.; Gillet, E.; Schirbaum, K. The Structure and Electrical Conductivity of Vacuum-Annealed WO₃ Thin Films. Thin Solid Film. 2004, 467, 239–246. [Google Scholar] [CrossRef]
- Haryński, Ł.; Karczewski, J.; Ryl, J.; Grochowska, K.; Siuzdak, K. Rapid Development of the Photoresponse and Oxygen Evolution of TiO2 Nanotubes Sputtered with Cr Thin Films Realized via Laser Annealing. J. Alloys Compd. 2021, 877, 160316. [Google Scholar] [CrossRef]
- Haryński, Ł.; Grochowska, K.; Karczewski, J.; Ryl, J.; Siuzdak, K. Scalable Route towards Superior Photoresponse of UV-Laser-Treated TiO₂ Nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 3225–3235. [Google Scholar] [CrossRef]
- Xu, Y.; Melia, M.A.; Tsui, L.; Fitz-Gerald, J.M.; Zangari, G. Laser-Induced Surface Modification at Anatase TiO₂ Nanotube Array Photoanodes for Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2017, 121, 17121–17128. [Google Scholar] [CrossRef]
- Haryński, Ł.; Olejnik, A.; Grochowska, K.; Siuzdak, K. A Facile Method for Tauc Exponent and Corresponding Electronic Transitions Determination in Semiconductors Directly from UV–Vis Spectroscopy Data. Opt. Mater. 2022, 127, 112205. [Google Scholar] [CrossRef]
- Park, B.H.; Li, L.S.; Gibbons, B.J.; Huang, J.Y.; Jia, Q.X. Photovoltaic Response and Dielectric Properties of Epitaxial Anatase-TiO2 Films Grown on Conductive La0.5Sr0.5CoO3 Electrodes. Appl. Phys. Lett. 2001, 79, 2797–2799. [Google Scholar] [CrossRef]
- Slimen, H.; Houas, A.; Nogier, J.P. Elaboration of Stable Anatase TiO2 through Activated Carbon Addition with High Photocatalytic Activity under Visible Light. J. Photochem. Photobiol. A Chem. 2011, 221, 13–21. [Google Scholar] [CrossRef]
- Haryński, Ł.; Grochowska, K.; Kupracz, P.; Karczewski, J.; Coy, E.; Siuzdak, K. The In-Depth Studies of Pulsed UV Laser-Modified TiO2 Nanotubes: The Influence of Geometry, Crystallinity, and Processing Parameters. Nanomaterials 2020, 10, 430. [Google Scholar] [CrossRef] [Green Version]
- Sopha, H.; Mirza, I.; Turčičova, H.; Pavlinak, D.; Michalicka, J.; Krbal, M.; Rodriguez-Pereira, J.; Hromadko, L.; Novák, O.; Mužík, J.; et al. Laser-Induced Crystallization of Anodic TiO2 Nanotube Layers. RSC Adv. 2020, 10, 22137–22145. [Google Scholar] [CrossRef]
- Haryński, Ł.; Grochowska, K.; Karczewski, J.; Ryl, J.; Rysz, J.; Siuzdak, K. Free-Standing TiO₂ Nanotubes Decorated with Spherical Nickel Nanoparticles as a Cost-Efficient Electrocatalyst for Oxygen Evolution Reaction. RSC Adv. 2021, 11, 219–228. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Albu, S.P.; Tsuchiya, H.; Fujimoto, S.; Schmuki, P. TiO2 Nanotubes—Annealing Effects on Detailed Morphology and Structure. Eur. J. Inorg. Chem. 2010, 2010, 4351–4356. [Google Scholar] [CrossRef]
- Grochowska, K.; Nedyalkov, N.; Karczewski, J.; Haryński, Ł.; Śliwiński, G.; Siuzdak, K. Anodic Titania Nanotubes Decorated with Gold Nanoparticles Produced by Laser-Induced Dewetting of Thin Metallic Films. Sci. Rep. 2020, 10, 20506. [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Mao, S.S. Black TiO2 for Solar Hydrogen Conversion. J. Mater. 2017, 3, 96–111. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G.G. The Formation and Detection Techniques of Oxygen Vacancies in Titanium Oxide-Based Nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, N.; Kumar, P.; Goswami, A.; Perdicakis, B.; Shankar, K.; Sadrzadeh, M. Robust Polymer Nanocomposite Membranes Incorporating Discrete TiO2 Nanotubes for Water Treatment. Nanomaterials 2019, 9, 1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farsinezhad, S.; Sharma, H.; Shankar, K. Interfacial Band Alignment for Photocatalytic Charge Separation in TiO2 Nanotube Arrays Coated with CuPt Nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 29723–29733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-X.; Liang, Y.; Luo, J.-W.; Jia, A.-P.; Wang, Y.-J.; Lu, J.-Q.; Luo, M.-F. Morphological Effects of Ordered Cr2O3 Nanorods and Cr2O3 Nanoparticles on Fluorination of 2-Chloro-1,1,1-Trifluoroethane. J. Mater. Sci. 2016, 51, 6488–6496. [Google Scholar] [CrossRef]
- Akshay, V.R.; Arun, B.; Mandal, G.; Vasundhara, M. Visible Range Optical Absorption, Urbach Energy Estimation and Paramagnetic Response in Cr-Doped TiO2 Nanocrystals Derived by a Sol–Gel Method. Phys. Chem. Chem. Phys. 2019, 21, 12991–13004. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J. Coupling Interface Constructions of NiO–Cr2O3 Heterostructures for Efficient Electrocatalytic Oxygen Evolution. Electrochim. Acta 2019, 320, 134577. [Google Scholar] [CrossRef]
- Nemanič, V.; Kovač, J.; Žumer, M.; Zavašnik, J. Impact of Surface Oxide on Hydrogen Permeability of Chromium Membranes. Int. J. Hydrogen Energy 2022, 48, 9723–9733. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Brown, C.; Mycroft, J.R.; Davidson, R.D.; McIntyre, N.S. X-Ray Photoelectron Spectroscopy Studies of Chromium Compounds. Surf. Interface Anal. 2004, 36, 1550–1563. [Google Scholar] [CrossRef]
- Song, X.; Li, W.; Liu, X.; Wu, Y.; He, D.; Ke, Z.; Cheng, L.; Jiang, C.; Wang, G.; Xiao, X.; et al. Oxygen Vacancies Enable the Visible Light Photoactivity of Chromium-Implanted TiO2 Nanowires. J. Energy Chem. 2021, 55, 154–161. [Google Scholar] [CrossRef]
- Shirato, N.; Strader, J.; Kumar, A.; Vincent, A.; Zhang, P.; Karakoti, A.; Nacchimuthu, P.; Cho, H.-J.; Seal, S.; Kalyanaraman, R. Thickness Dependent Self Limiting 1-D Tin Oxidenanowire Arrays by Nanosecond Pulsed Laser Irradiation. Nanoscale 2011, 3, 1090–1101. [Google Scholar] [CrossRef] [Green Version]
- Kotsedi, L.; Furlan, V.; Bharadwaj, V.; Kaviyarasu, K.; Sotillo, B.; Mtshali, C.B.; Matinise, N.; Demir, A.G.; Previtali, B.; Ramponi, R.; et al. Chromium Oxide Formation on Nanosecond and Femtosecond Laser Irradiated Thin Chromium Films. Opt. Mater. 2019, 95, 109206. [Google Scholar] [CrossRef] [Green Version]
- Xie, B.; Sun, M.; Xu, B.; Wang, C.; Jiang, H.; Li, D.; Li, Y. Oxidation of Stainless Steel in Vacuum and Evolution of Surface Oxide Scales during Hot-Compression Bonding. Corros. Sci. 2019, 147, 41–52. [Google Scholar] [CrossRef]
- Veiko, V.P.; Poleshchuk, A.G. Laser-Induced Local Oxidation of Thin Metal Films: Physical Fundamentals and Applications. In Fundamentals of Laser-Assisted Micro- and Nanotechnologies; Veiko, V.P., Konov, V.I., Eds.; Springer Series in Materials Science; Springer International Publishing: Cham, Switzerland, 2014; Volume 195, pp. 149–171. [Google Scholar] [CrossRef]
- Abouhaswa, A.S. Physical Properties of Anatase TiO2 Nanocrystallites: Based Photoanodes Doped with Cr2O3. Opt. Quantum Electron. 2020, 52, 144. [Google Scholar] [CrossRef]
- Roy, P.; Kim, D.; Lee, K.; Spiecker, E.; Schmuki, P. TiO2 Nanotubes and Their Application in Dye-Sensitized Solar Cells. Nanoscale 2010, 2, 45–59. [Google Scholar] [CrossRef]
- Yaghoubi, H.; Li, Z.; Chen, Y.; Ngo, T.H.; Bhethanabotla, V.R.; Joseph, B.; Ma, S.; Schlaf, R.; Takshi, A. Toward a Visible Light-Driven Photocatalyst: The Effect of Midgap-States-Induced Energy Gap of Undoped TiO₂ Nanoparticles. ACS Catal. 2015, 5, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Peighambardoust, N.S.; Khameneh Asl, S.; Mohammadpour, R.; Asl, S.K. Band-Gap Narrowing and Electrochemical Properties in N-Doped and Reduced Anodic TiO2 Nanotube Arrays. Electrochim. Acta 2018, 270, 245–255. [Google Scholar] [CrossRef]
- Freitas, R.G.; Santanna, M.A.; Pereira, E.C. Dependence of TiO2 Nanotube Microstructural and Electronic Properties on Water Splitting. J. Power Sources 2014, 251, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Xu, M.; Shen, Z.; Yu, J.C. A Nanostructured Chromium(III) Oxide/Tungsten(vi) Oxide p–n Junction Photoanode toward Enhanced Efficiency for Water Oxidation. J. Mater. Chem. A 2015, 3, 14046–14053. [Google Scholar] [CrossRef]
- Pishkar, N.; Jedi-soltanabadi, Z.; Ghoranneviss, M. Reduction in the Band Gap of Anodic TiO2 Nanotube Arrays by H2 Plasma Treatment. Results Phys. 2018, 10, 466–468. [Google Scholar] [CrossRef]
- Zhou, M.; Glushenkov, A.M.; Kartachova, O.; Li, Y.; Chen, Y. Titanium Dioxide Nanotube Films for Electrochemical Supercapacitors: Biocompatibility and Operation in an Electrolyte Based on a Physiological Fluid. J. Electrochem. Soc. 2015, 162, A5065–A5069. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Mora-Seró, I.; Garcia-Belmonte, G.; Bisquert, J. Cyclic Voltammetry Studies of Nanoporous Semiconductors. Capacitive and Reactive Properties of Nanocrystalline TiO2 Electrodes in Aqueous Electrolyte. J. Phys. Chem. B 2003, 107, 758–768. [Google Scholar] [CrossRef]
- Oliva, F.Y.; Avalle, L.B.; Cámara, O.R. Electrochemical Behaviour of Human Serum Albumin–TiO2 Nanocrystalline Electrodes Studied as a Function of PH. J. Electroanal. Chem. 2002, 534, 19–29. [Google Scholar] [CrossRef]
- Macak, J.M.; Gong, B.G.; Hueppe, M.; Schmuki, P. Filling of TiO2 Nanotubes by Self-Doping and Electrodeposition. Adv. Mater. 2007, 19, 3027–3031. [Google Scholar] [CrossRef]
- Pelouchova, H.; Janda, P.; Weber, J.; Kavan, L. Charge Transfer Reductive Doping of Single Crystal TiO2 Anatase. J. Electroanal. Chem. 2004, 566, 73–83. [Google Scholar] [CrossRef]
- Sab, N.; Claes, P.; Glibert, J. Chemical and Electrochemical Behaviour in Molten Hydroxides. VII. Chromium Species in Sodium Hydroxide. Electrochim. Acta 1998, 43, 2089–2100. [Google Scholar] [CrossRef]
- Cramer, S.D.; Covino, B.S. (Eds.) Electrochemical Series. In Corrosion: Materials; ASM International: Singapore, 2005; pp. 665–671. [Google Scholar] [CrossRef] [Green Version]
- Radecka, M.; Rekas, M.; Trenczek-Zajac, A.; Zakrzewska, K. Importance of the Band Gap Energy and Flat Band Potential for Application of Modified TiO2 Photoanodes in Water Photolysis. J. Power Sources 2008, 181, 46–55. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, Y.; Peng, S.; Wang, X.; Zhou, L. Modification of Zr-Doped Titania Nanotube Arrays by Urea Pyrolysis for Enhanced Visible-Light Photoelectrochemical H2 Generation. Electrochim. Acta 2013, 87, 794–800. [Google Scholar] [CrossRef]
- Siuzdak, K.; Szkoda, M.; Sawczak, M.; Lisowska-Oleksiak, A.; Karczewski, J.; Ryl, J. Enhanced Photoelectrochemical and Photocatalytic Performance of Iodine-Doped Titania Nanotube Arrays. RSC Adv. 2015, 5, 50379–50391. [Google Scholar] [CrossRef]
- He, Z.-K.; Lu, Y.; Zhao, J.; Zhao, J.; Gao, Z.; Song, Y.-Y. Engineering Carrier Density at TiO2 Nanotube Metasurface with Hole Reservoir for Enhanced Photo-Electrocatalysis. Appl. Surf. Sci. 2023, 613, 155974. [Google Scholar] [CrossRef]
- Hardee, K.L.; Bard, A.J. Semiconductor Electrodes: X. Photoelectrochemical Behavior of Several Polycrystalline Metal Oxide Electrodes in Aqueous Solutions. J. Electrochem. Soc. 1977, 124, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Li, D.; Chi, H.; Zhao, Y.; Wang, J.; Li, D.; Pang, S.; Fu, P.; Shi, J.; Li, C. Unveiling the Hydration Structure of Ferrihydrite for Hole Storage in Photoelectrochemical Water Oxidation. Angew. Chem. Int. Ed. 2021, 60, 6691–6698. [Google Scholar] [CrossRef]
- Tafalla, D.; Salvador, P. Kinetic Approach to the Photocurrent Transients in Water Photoelectrolysis at N-TiO₂ Electrodes. J. Electrochem. Soc. 1990, 137, 1810–1815. [Google Scholar] [CrossRef]
- Ding, J.; Ming, J.; Lu, D.; Wu, W.; Liu, M.; Zhao, X.; Li, C.; Yang, M.; Fang, P. Study of the Enhanced Visible-Light-Sensitive Photocatalytic Activity of Cr2O3-Loaded Titanate Nanosheets for Cr(VI) Degradation and H2 Generation. Catal. Sci. Technol. 2017, 7, 2283–2297. [Google Scholar] [CrossRef]
- Shaislamov, U.; Yang, B.; Park, K. Enhanced Photocatalytic Properties of TiO2 Nanotube Arrays with Cr2O3 Nanoparticles under Visible Light. J. Korean Phys. Soc. 2012, 61, 759–763. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochowska, K.; Haryński, Ł.; Karczewski, J.; Jurak, K.; Siuzdak, K. Scanning with Laser Beam over the TiO2 Nanotubes Covered with Thin Chromium Layers towards the Activation of the Material under the Visible Light. Materials 2023, 16, 2572. https://doi.org/10.3390/ma16072572
Grochowska K, Haryński Ł, Karczewski J, Jurak K, Siuzdak K. Scanning with Laser Beam over the TiO2 Nanotubes Covered with Thin Chromium Layers towards the Activation of the Material under the Visible Light. Materials. 2023; 16(7):2572. https://doi.org/10.3390/ma16072572
Chicago/Turabian StyleGrochowska, Katarzyna, Łukasz Haryński, Jakub Karczewski, Kacper Jurak, and Katarzyna Siuzdak. 2023. "Scanning with Laser Beam over the TiO2 Nanotubes Covered with Thin Chromium Layers towards the Activation of the Material under the Visible Light" Materials 16, no. 7: 2572. https://doi.org/10.3390/ma16072572
APA StyleGrochowska, K., Haryński, Ł., Karczewski, J., Jurak, K., & Siuzdak, K. (2023). Scanning with Laser Beam over the TiO2 Nanotubes Covered with Thin Chromium Layers towards the Activation of the Material under the Visible Light. Materials, 16(7), 2572. https://doi.org/10.3390/ma16072572






