Properties of Coatings Based on Calcium Phosphate and Their Effect on Cytocompatibility and Bioactivity of Titanium Nickelide
Abstract
:1. Introduction
2. Materials and Methods
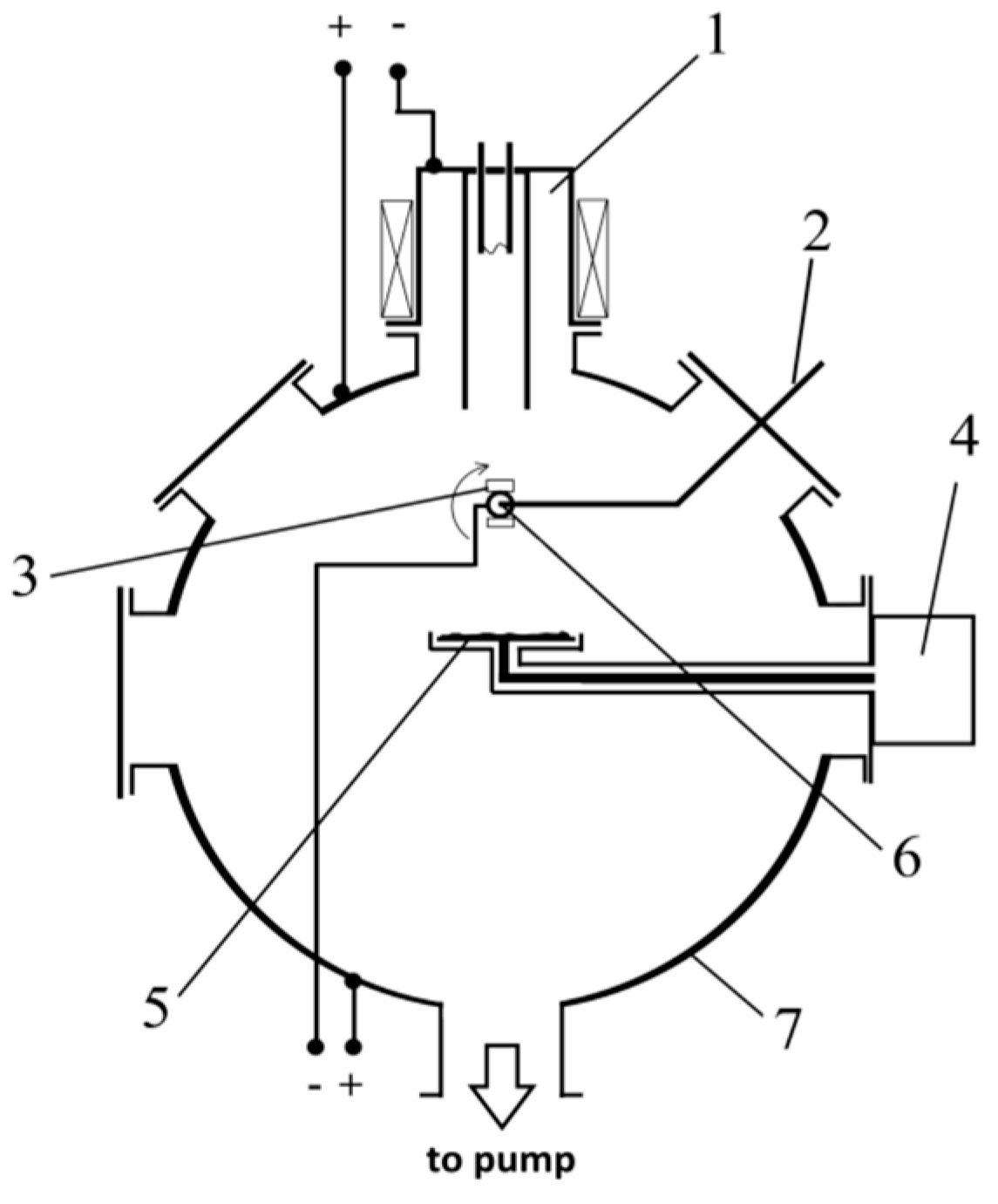
2.1. Deposition of Coating
2.2. Surface Characterization Methods
2.3. In Vitro Testing
3. Results and Discussion
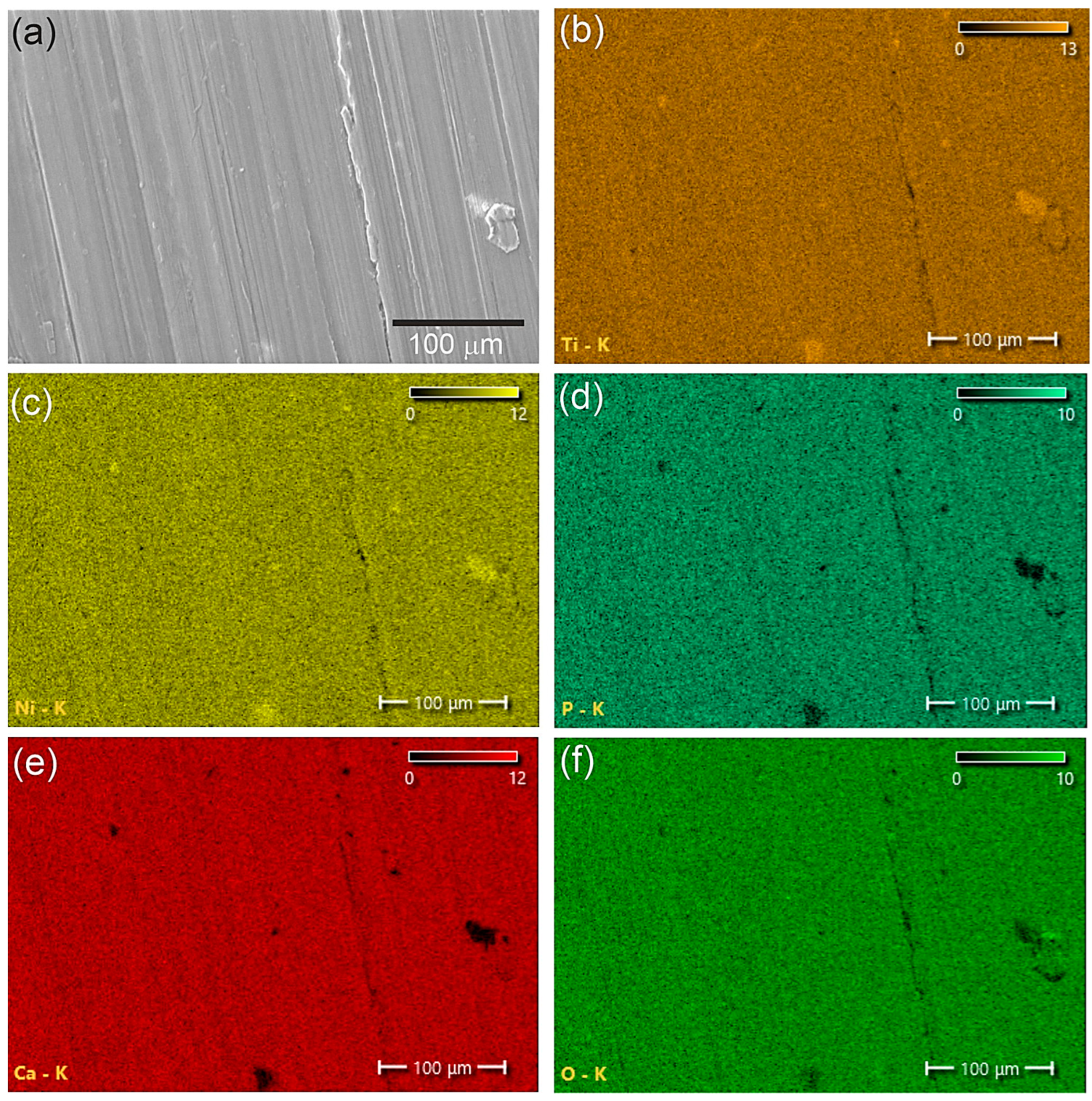
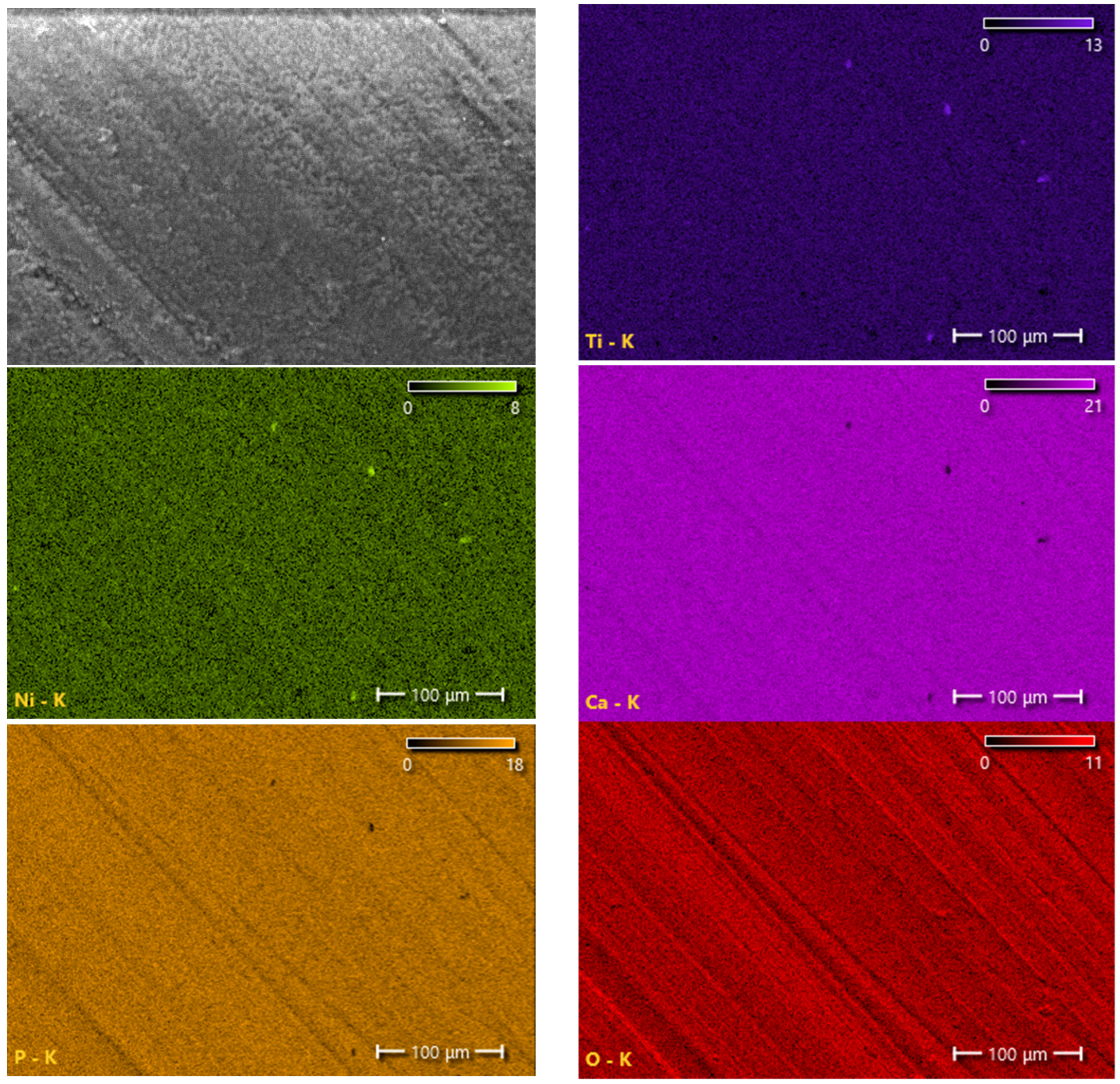
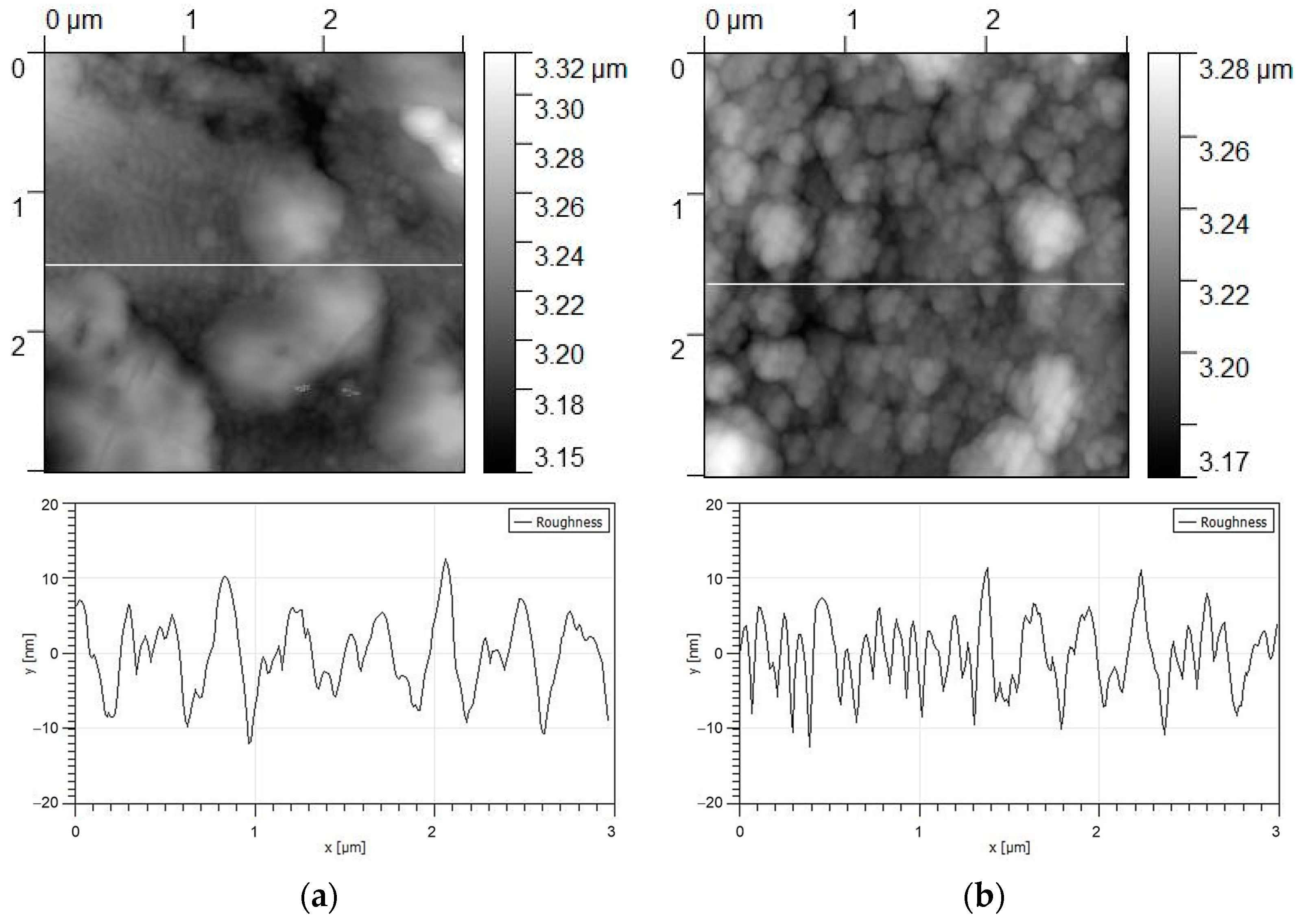
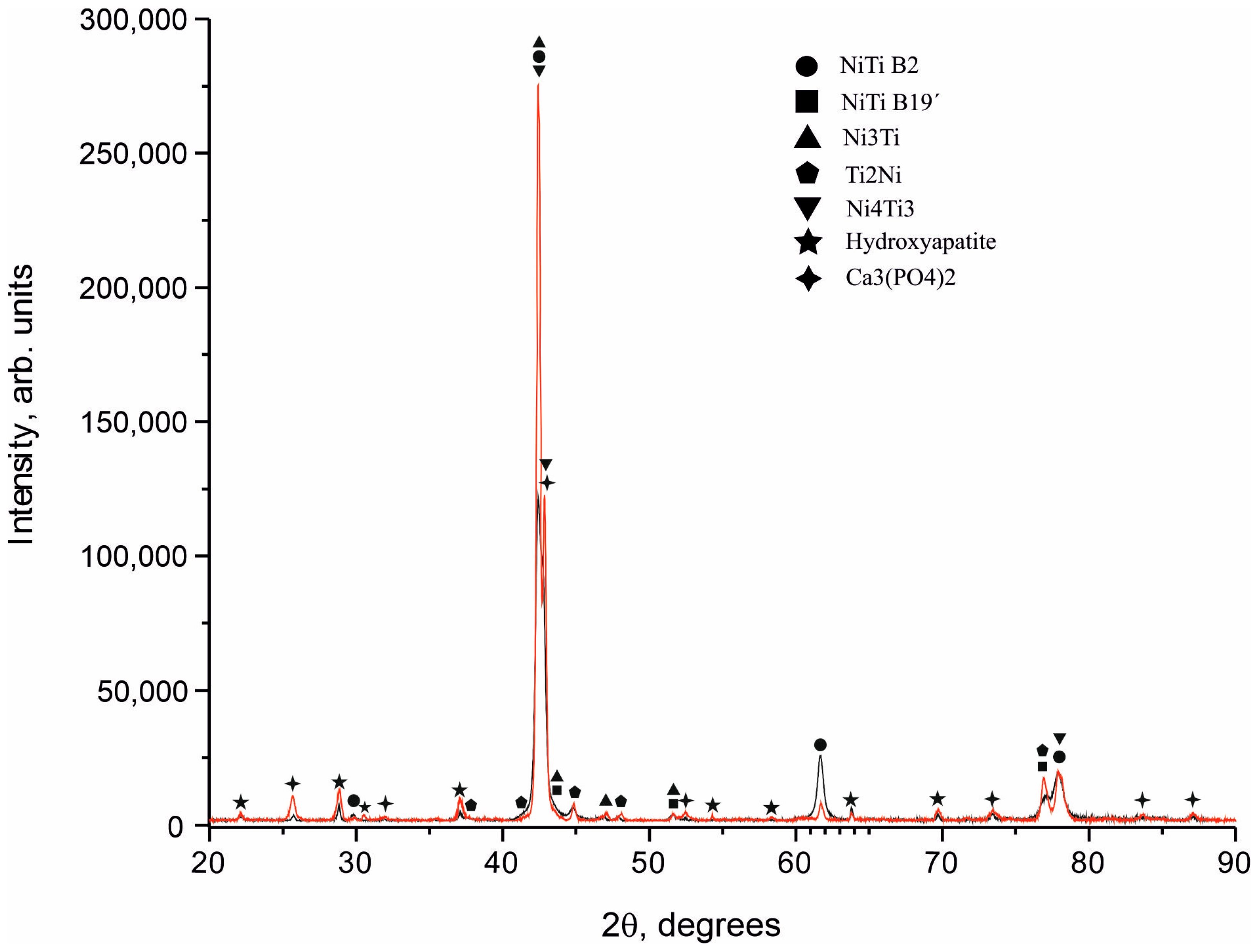
3.1. Structure and Phase Composition of Coatings
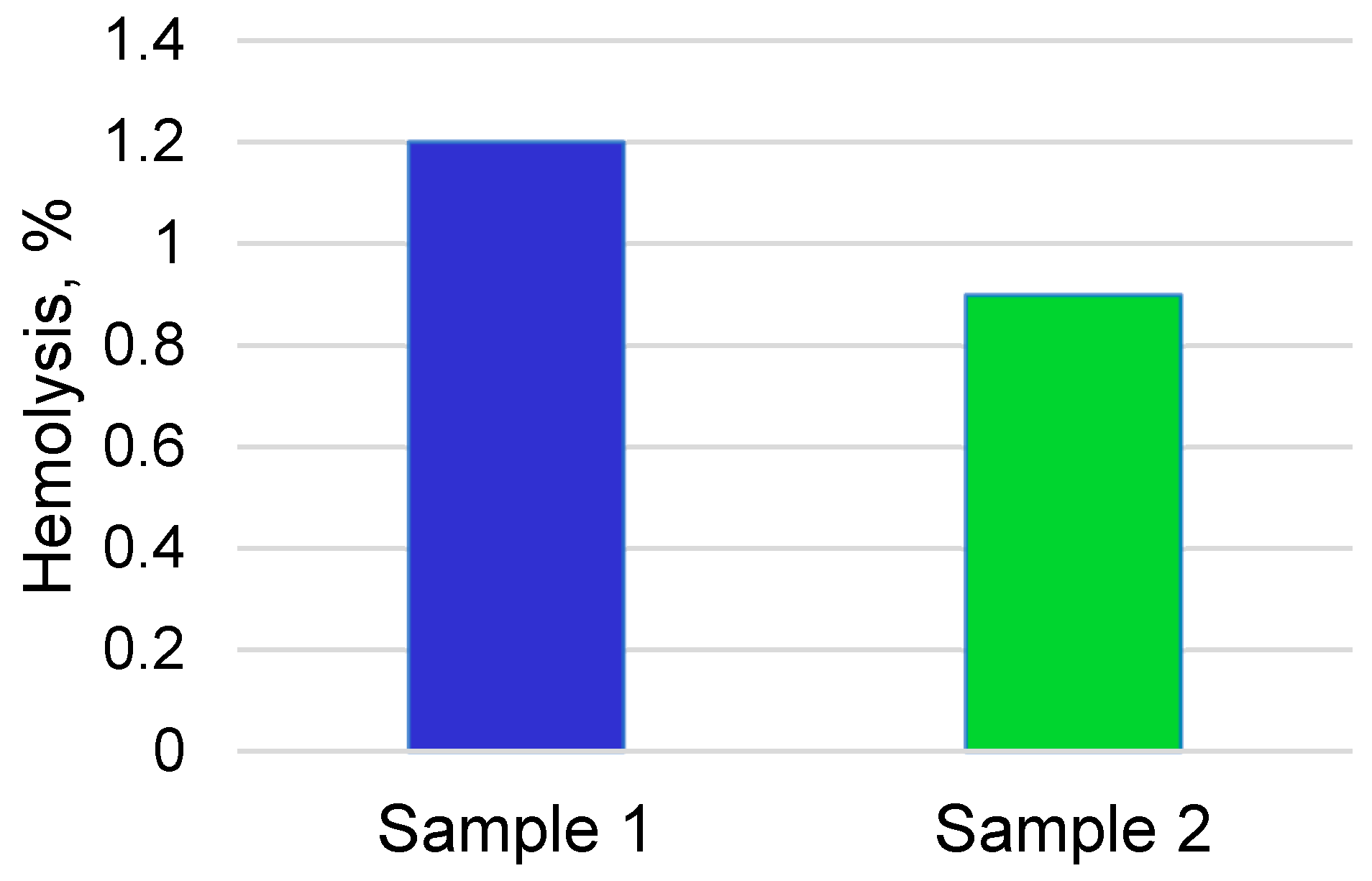
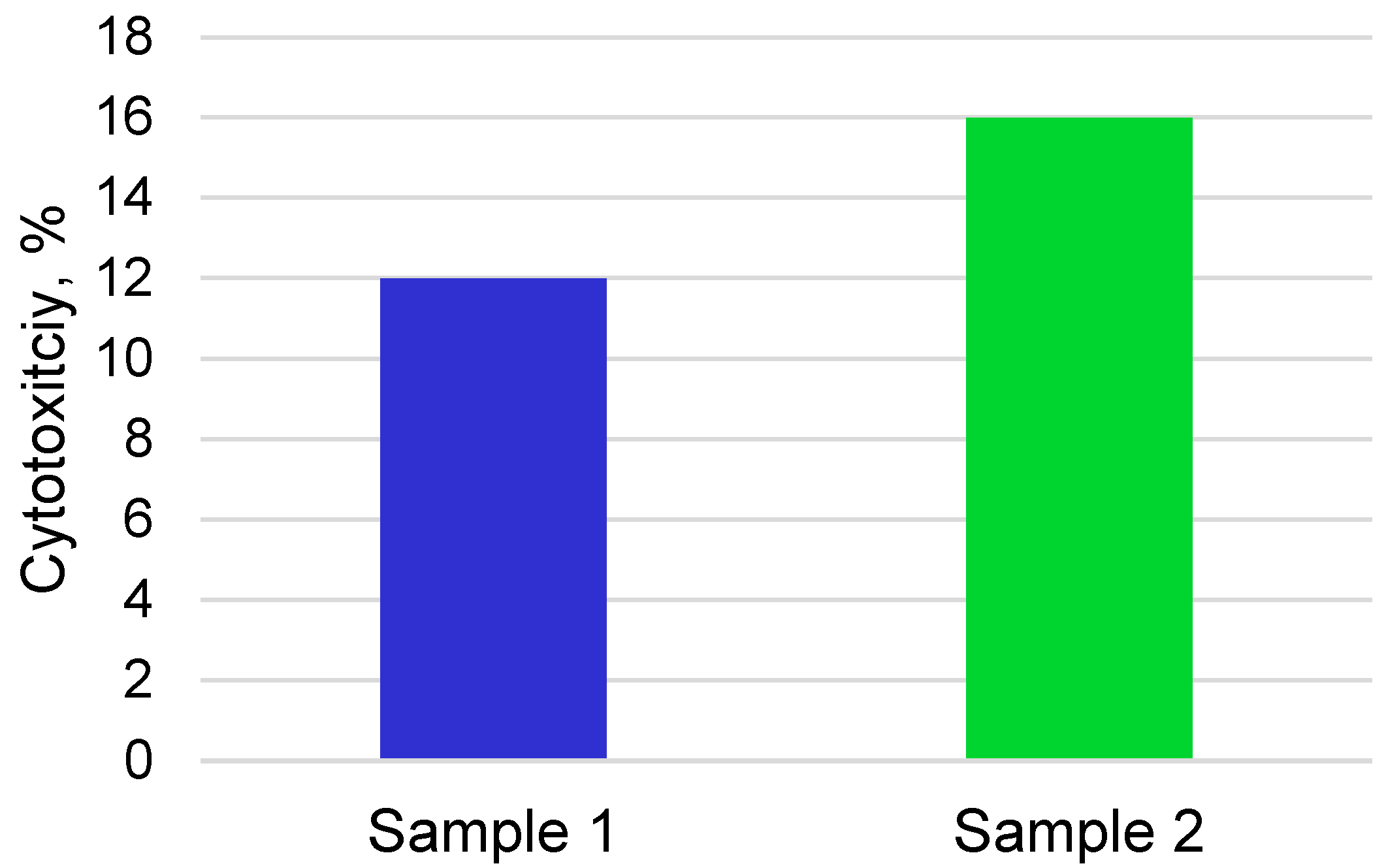
3.2. Cytocompatibility and Bioactivity of Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, R.; Seesala, V.S.; Sen, M.; Sengupta, S.; Dhara, S.; Saha, P.; Das, K.; Das, S. MWCNT reinforced bone like calcium phosphate—Hydroxyapatite composite coating developed through pulsed electrodeposition with varying amount of apatite phase and crystallinity to promote superior osteoconduction, cytocompatibility and corrosion protection performance compared to bare metallic implant surface. Surf. Coat. Technol. 2017, 325, 496–514. [Google Scholar]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate-based bioceramics. Materials 2013, 6, 3840–3942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.W.; Khor, K.A.; Cheang, P. In vitro studies of plasma-sprayed hydroxyapatite/Ti-6Al-4V composite coatings in simulated body fluid (SBF). Biomaterials 2003, 24, 1603–1611. [Google Scholar] [CrossRef]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H.; Narayan, R. Calcium phosphate based coatings on titanium and its alloys. J. Biomed. Mater. Res. 2008, 85B, 279–299. [Google Scholar] [CrossRef]
- Ducheyne, P.; Qiu, Q. Bioactive ceramics: The effect of surface reactivity on bone formation and bone cell function. Biomaterials 1999, 20, 2287–2303. [Google Scholar] [CrossRef]
- Radin, S.R.; Ducheyne, P. The effect of calcium phosphate ceramic composition and structure on in vitro behavior. II. Precipitation. J. Biomed. Mater. Res. 1993, 27, 35–45. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and β-tricalcium phosphate. Biomaterials 2005, 26, 3587–3596. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [Green Version]
- Mosaddad, S.A.; Yazdanian, M.; Tebyanian, H.; Tahmasebi, E.; Yazdanian, A.; Seifalian, A.; Tavakolizadeh, M. Fabrication and properties of developed collagen/strontium-doped Bioglass scaffolds for bone tissue engineering. J. Mater. Res. Technol. 2020, 9, 14799–14817. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity. Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012, 8, 963–977. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; Laboux, O.; Malard, O.; Weiss, P. Current state of the art of biphasic calcium phosphate bioceramics. J. Mater. Sci. Mater. Med. 2003, 14, 195–200. [Google Scholar] [CrossRef]
- Dorozhkina, E.I.; Dorozhkin, S.V. Mechanism of the solid-state transformation of a calcium-deficient hydroxyapatite (CDHA) into biphasic calcium phosphate (BCP) at elevated temperatures. Chem. Mater. 2002, 14, 4267–4272. [Google Scholar] [CrossRef]
- Li, Y.; Kong, F.; Weng, W. Preparation and characterization of novel biphasic calcium phosphate powders (α-TCP/HA) derived from carbonated amorphous calcium phosphates. J. Biomed. Mater. Res. Part B 2009, 89, 508–517. [Google Scholar] [CrossRef]
- Sureshbabu, S.; Komath, M.; Varma, H.K. In situ formation of hydroxyapatite–αlpha tricalcium phosphate biphasic ceramics with higher strength and bioactivity. J. Am. Ceram. Soc. 2012, 95, 915–924. [Google Scholar] [CrossRef]
- Radovanovic, Z.; Jokić, B.M.; Veljović, D.N.; Dimitrijević, S.; Kojić, V.V.; Petrović, R.; Janaćković, D. Antimicrobial activity and biocompatibility of Ag+-and Cu2+-doped biphasic hydroxyapatite/α-tricalcium phosphate obtained from hydrothermally synthesized Ag+-and Cu2+-doped hydroxyapatite. Appl. Surf. Sci. 2014, 307, 513–519. [Google Scholar] [CrossRef]
- Oishi, M.; Ohtsuki, C.; Kitamura, M.; Kamitakahara, M.; Ogata, S.; Miyazaki, T.; Tanihara, M. Fabrication and chemical durability of porous bodies consisting of biphasic tricalcium phosphates. Phosphorus Res. Bull. 2004, 17, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Kamitakahara, M.; Ohtsuki, C.; Oishi, M.; Ogata, S.; Miyazaki, T.; Tanihara, M. Preparation of porous biphasic tricalcium phosphate and its in vivo behavior. Key Eng. Mater. 2005, 284, 281–284. [Google Scholar] [CrossRef]
- Wang, R.B.; Weng, W.J.; Deng, X.L.; Cheng, K.; Liu, X.G.; Du, P.Y.; Shen, G.; Han, G.R. Dissolution behavior of submicron biphasic tricalcium phosphate powders. Key Eng. Mater. 2006, 309, 223–226. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Djurado, D.; Pouget, S.; Blacha-Grzechnik, A.; Kalemba-Rec, I.; Simka, W. Analysis of the calcium phosphate-based hybrid layer formed on a Ti-6Al-7Nb alloy to enhance the ossseointegration process. Materials 2020, 13, 5468. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.T.; Park, I.S.; Lee, M.H.; Bae, T.S. Enhanced biocompatibility of a pre-calcified nanotubular TiO2 layer on Ti-6Al-7Nb alloy. Surf. Coat. Technol. 2013, 236, 127–134. [Google Scholar] [CrossRef]
- Nguyen, T.D.T.; Moon, S.H.; Oh, T.J.; Park, I.S.; Lee, M.H.; Bae, T.S. The effect of APH treatment on surface bonding and osseointegration of Ti-6Al-7Nb implants: An in vitro and in vivo study. J. Biomed. Mater. Res. Part B 2015, 103, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Sygnatowicz, M.; Tiwari, A. Controlled synthesis of hydroxyapatite-based coatings for biomedical applications. Mater. Sci. Eng. C 2009, 29, 1071–1076. [Google Scholar] [CrossRef]
- Saphronov, V.; Shishkovsky, I. Laser annealing for gas-dynamical spraying of HA coating on a titanium surface. Crystals 2015, 5, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Sattar, T.; Manzoor, T.; Khalid, F.A.; Akmal, M.; Saeed, G. Improved in vitro bioactivity and electrochemical behavior of hydroxyapatite-coated NiTi shape memory alloy. J. Mater. Sci. 2009, 54, 7300–7306. [Google Scholar] [CrossRef]
- Qiu, D.; Yang, L.; Yin, Y.; Wang, A. Preparation and characterization of hydroxyapatite/titania composite coating on NiTi alloy by electrochemical deposition. Surf. Coat. Technol. 2011, 205, 3280–3284. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Khalili, V.; Khalil-Allafi, J.; Javidi, M. Hydroxyapatite coating on NiTi shape memory alloy by electrophoretic deposition process. Surf. Coat. Technol. 2012, 208, 57–63. [Google Scholar] [CrossRef]
- Zhang, J.X.; Guan, R.F.; Zhang, X.P. Synthesis and characterization of sol-gel hydroxyapatite coatings deposited on porous NiTi alloys. J. Alloys Compd. 2011, 509, 4643–4648. [Google Scholar] [CrossRef]
- Marashi-Najafi, F.; Khalil-Allafi, J.; Etminanfar, M.R. Biocompatibility of hydroxyapatite coatings deposited by pulse electrodeposition technique on the Nitinol superelastic alloy. Mater. Sci. Eng. C 2017, 76, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Baigonakova, G.A.; Marchenko, E.S.; Kovaleva, M.A.; Chudinova, E.A.; Volinsky, A.A.; Zhang, Y. Thickness Effects on the Martensite Transformations and Mechanical Properties of Nanocrystalline NiTi Wires. Nanomaterials 2022, 12, 4442. [Google Scholar] [CrossRef]
- Marchenko, E.; Monogenov, A.; Klopotov, A.; Baigonakova, G.; Chudinova, E.; Vorozhtsov, A.; Sokolov, S. Phase composition, microstructure, multiple shape memory effect of TiNi50−xVx (x = 1; 2; 4 at.%) system alloys. Materials 2022, 15, 8359. [Google Scholar] [CrossRef]
- Chen, M.F.; Yang, X.J.; Hu, R.X.; Cui, Z.D.; Man, H.C. Bioactive NiTi shape memory alloy used as bone bonding implants. Mater. Sci. Eng. C 2004, 24, 497–502. [Google Scholar] [CrossRef]
- Khalili, V.; Khalil-Allafi, J.; Frenzel, J.; Eggeler, G. Bioactivity and electrochemical behavior of hydroxyapatite-silicon-multi walled carbon nano-tubes composite coatings synthesized by EPD on NiTi alloys in simulated body fluid. Mater. Sci. Eng. C 2017, 71, 473–482. [Google Scholar] [CrossRef]
- Spence, G.; Patel, N.; Brooks, R.; Rushton, N. Carbonate substituted hydroxyapatite: Resorption by osteoclasts modifies the osteoblastic response. J. Biomed. Mater. Res. Part A 2009, 90, 217–224. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.H.; Liu, B.S.; Hsu, S.H.; Chen, Y.S.; Tsai, C.C. Biocompatibility and biodegradation of a bone composite containing tricalcium phosphate and genipin crosslinked gelatin. J. Biomed. Mater. Res. A 2004, 69, 709–717. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Ohtsuki, C.; Miyazaki, T. Behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J. Biomater. Appl. 2008, 23, 197–212. [Google Scholar] [CrossRef]
- Barradas, J.; Ferreira, J.M.F. Biphasic calcium phosphate coatings for biomedical applications A review. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar]
- Gurav, S.S.; Mishra, A.J.; Mali, S.S.; Joshi, S.V. Comparative study of hydroxyapatite and β-tricalcium phosphate coatings on titanium substrates. J. Mater. Sci. Mater. Med. 2017, 28, 7. [Google Scholar]
- Zhu, X.D.; Zhang, H.J.; Fan, H.S.; Li, W.; Zhang, X.D. Effect of phase composition and microstructure of calcium phosphate ceramic particles on protein adsorption. Acta Biomater. 2010, 6, 1536–1541. [Google Scholar] [CrossRef]
- Lobo, S.E.; Livingston, A.T. Biphasic calcium phosphate ceramics for bone regeneration and tissue engineering applications. Materials 2010, 3, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Daculsi, G.; Baroth, S.; LeGeros, R. 20 years of biphasic calcium phosphate bioceramics development and applications. Adv. Bioceram. Porous Ceram. II 2010, 45–58. [Google Scholar]
- Arinzeh, T.L.; Tran, T.; Mcalary, J.; Daculsi, G. A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials 2005, 26, 3631–3638. [Google Scholar] [CrossRef]
- He, F.; Ren, W.; Tian, X.; Liu, W.; Wu, S.; Chen, X. Comparative study on in vivo response of porous calcium carbonate composite ceramic and biphasic calcium phosphate ceramic. Mater. Sci. Eng. C 2016, 64, 117–123. [Google Scholar] [CrossRef]
- Rahnejat, B.; Nemati, N.H.; Khatiboleslam, S.S.; Shokrgozar, M.A. Promoting osteoblast proliferation and differentiation on functionalized and laser treated titanium substrate using hydroxyapatite/β-tricalcium phos-phate/silver nanoparticles. Mater. Chem. Phys. 2023, 293, 126885. [Google Scholar] [CrossRef]



| № | Name of Indicator | Specification Requirements in GB 1889–2004/FCC | Analysis Result |
|---|---|---|---|
| 1. | Appearance | Homogeneous, white crystalline powder, tasteless and odorless | Conforms |
| 2. | Mass fraction of 2-water disubstituted potassium phosphate (CaHPO4.2H2O), %, not less than | 98.5 | 99.58 |
| 3. | Content CaO, % | 31.4–32.9 | 32.4 |
| 4. | Degree of whiteness (WG), %, not less than | 95 | 96.12 |
| 5. | Dispersion | ||
| (Sieve 325 mix), %, not less | 99.0 | 99.6 | |
| (Sieve 200 mix), %, not less | 99.95 | 99.96 | |
| 6. | Mass fraction of fluorides, mg/kg, no more than | 50 | 9 |
| 7. | Mass fraction of chlorides, mg/kg, no more than | 300 | <100 |
| 8. | Mass fraction of losses during annealing, %, within | 24.5–26.5 | 26.1 |
| Sample Number | Deposition Time, h | Thickness, µm | Deposition Rate, µm/h |
|---|---|---|---|
| 1 | 1 | 0.5 | 0.5 |
| 2 | 4 | 2 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchenko, E.S.; Baigonakova, G.A.; Dubovikov, K.M.; Kokorev, O.V.; Gordienko, I.I.; Chudinova, E.A. Properties of Coatings Based on Calcium Phosphate and Their Effect on Cytocompatibility and Bioactivity of Titanium Nickelide. Materials 2023, 16, 2581. https://doi.org/10.3390/ma16072581
Marchenko ES, Baigonakova GA, Dubovikov KM, Kokorev OV, Gordienko II, Chudinova EA. Properties of Coatings Based on Calcium Phosphate and Their Effect on Cytocompatibility and Bioactivity of Titanium Nickelide. Materials. 2023; 16(7):2581. https://doi.org/10.3390/ma16072581
Chicago/Turabian StyleMarchenko, Ekaterina S., Gulsharat A. Baigonakova, Kirill M. Dubovikov, Oleg V. Kokorev, Ivan I. Gordienko, and Ekaterina A. Chudinova. 2023. "Properties of Coatings Based on Calcium Phosphate and Their Effect on Cytocompatibility and Bioactivity of Titanium Nickelide" Materials 16, no. 7: 2581. https://doi.org/10.3390/ma16072581






