Study on Kinetics of Carbonization Reaction of Hardened Cement Paste Powder Based on Carbonization Degree
Abstract
1. Introduction
2. Experimental Methods
2.1. Preparation of HCP
2.2. Preparation and Carbonization of HCP Samples
2.2.1. Preparation of Test Blocks
2.2.2. Carbonization of the Test Block
2.3. Determination of Temperature in Carbonization Cauldron
2.4. Determination of Degree of Carbonation
3. Results and Discussion
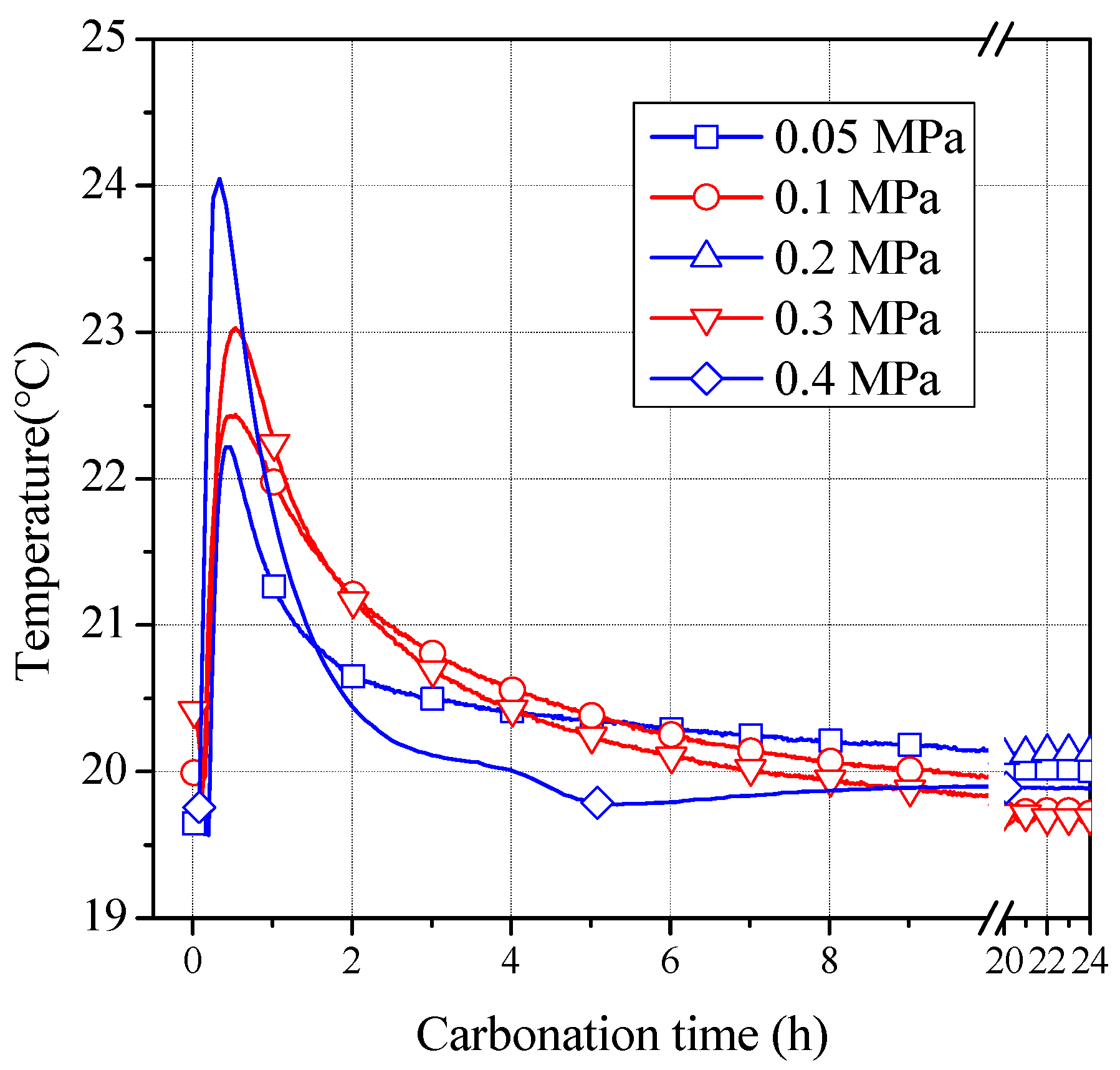
3.1. Change of Temperature during Carbonization Reaction
3.2. Change Law of Carbonization Degree with Carbonization Time
4. Kinetic Analysis of Carbonization of the HCP Test Block
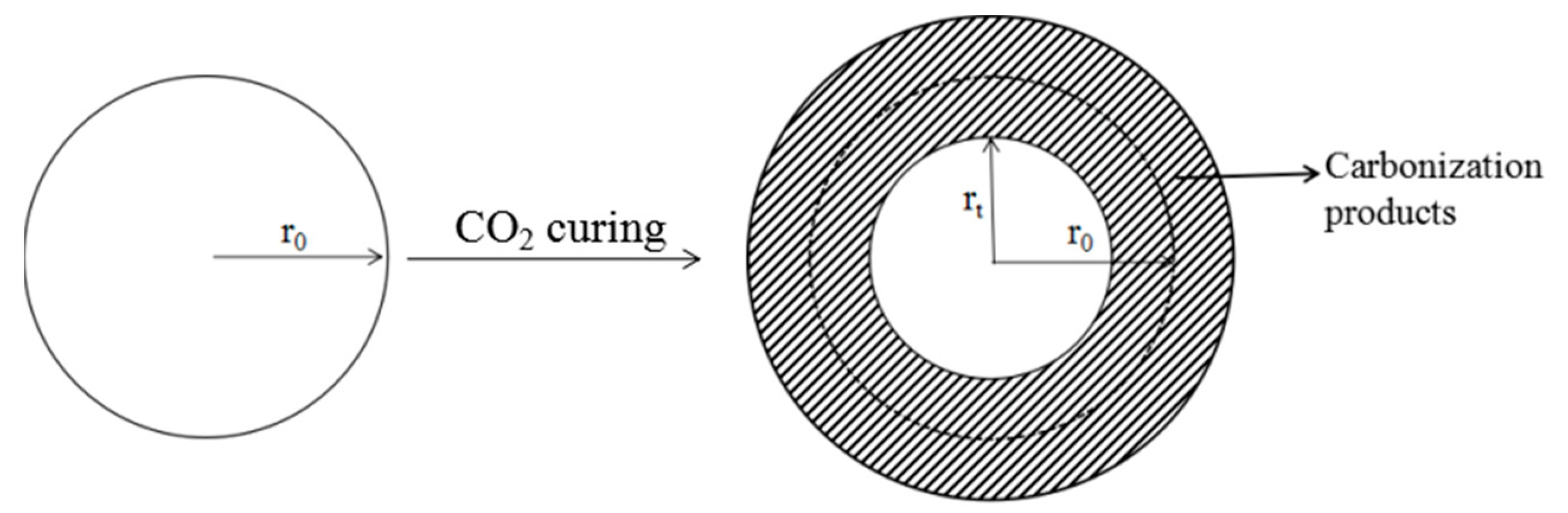
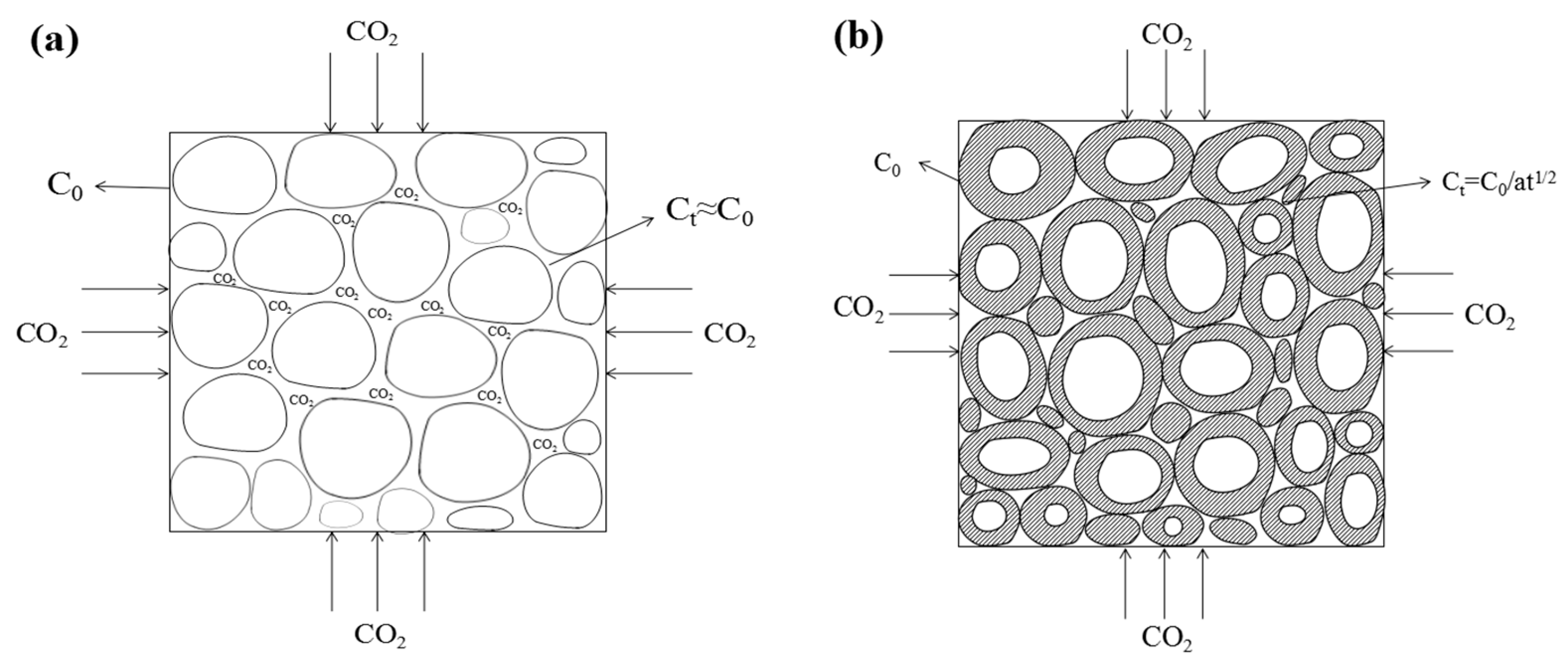
4.1. Carbonization Kinetics Model Based on Carbonization Degree
4.2. Fitting Analysis of Test Results and Kinetic Model
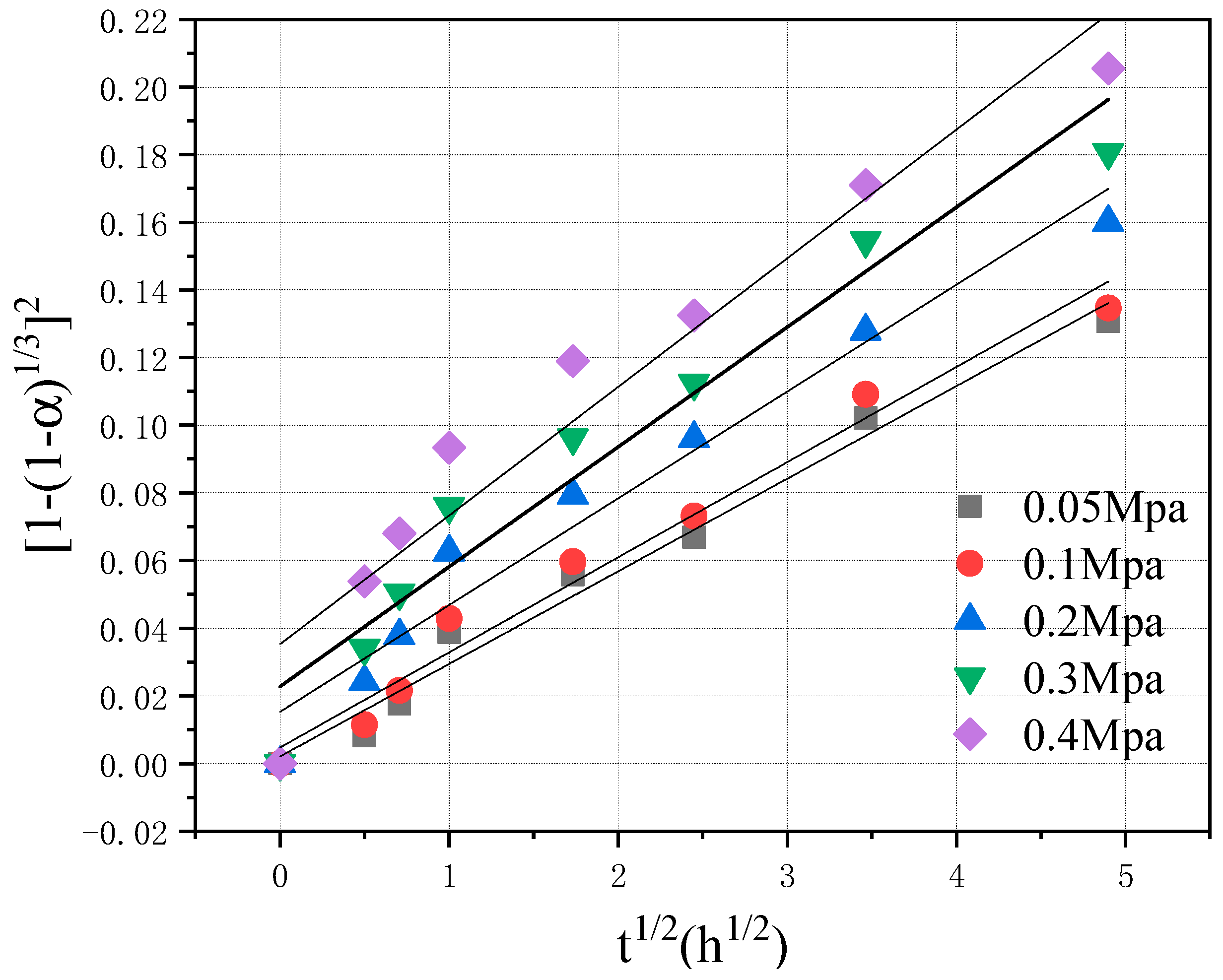
4.2.1. Analysis of Fitting Results
4.2.2. Effect of CO2 Pressure on k and B Values
4.3. Modification of the Kinetic Model of Carbonation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jonas, P. What Carbon Capture and Storage (CCS) is Expected to? Describing Potential Future of a CO2 Mitigation Technological System in the Seine Waterway Axis. Energy Procedia 2017, 114, 7333–7342. [Google Scholar] [CrossRef]
- Barcelo, L.; Kline, J.; Walenta, G.; Gartner, E. Cement and carbon emissions. Mater. Struct. 2014, 47, 1055–1065. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Miller, S.; John, V.; Pacca, S.; Horvath, A. Carbon dioxide reduction potential in the global cement industry by 2050. Cem. Concr. Res. 2018, 114, 115–124. [Google Scholar] [CrossRef]
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef]
- Zhu, C.; Lou, Y.; Shen, X.; Xu, H.; Yang, J. Influence of CaO Content on the Fly Ash-Lime System Hydrothermal Synthesis Reaction Under Autoclave Curing. Front. Phys. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Zhu, C.; Wan, Y.; Wang, L.; Ye, Y.; Yu, H.; Yang, J. Strength Characteristics and Microstructure Analysis of Alkali-Activated Slag–Fly Ash Cementitious Material. Materials 2022, 15, 6169. [Google Scholar] [CrossRef]
- Berger, R.L.; Young, J.F.; Leung, K. Acceleration of Hydration of Calcium Silicates by Carbon Dioxide Treatment. Nat. Phys. Sci. 1972, 240, 16–18. [Google Scholar] [CrossRef]
- Klemm, W.A.; Berger, R.L. Accelerated curing of cementitious systems by carbon dioxide: Part I. Portland cement. Cem. Concr. Res. 1972, 2, 567–576. [Google Scholar] [CrossRef]
- Berger, R.L.; Klemm, W.A. Accelerated curing of cementitious systems by carbon dioxide: Part II. Hydraulic calcium silicates and aluminates. Cem. Concr. Res. 1972, 2, 647–652. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Jang, J.G.; Kim, G.M.; Kim, H.J.; Lee, H.K. Review on recent advances in CO2 utilization and sequestration technologies in cement-based materials. Constr. Build. Mater. 2016, 127, 762–773. [Google Scholar] [CrossRef]
- Morrison, J.; Jauffret, G.; Galvez-Martos, J.L.; Glasser, F.P. Magnesium-based cements for CO2 capture and utilisation. Cem. Concr. Res. 2016, 85, 183–191. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Early age carbonation curing for precast reinforced concretes. Constr. Build. Mater. 2016, 113, 134–143. [Google Scholar] [CrossRef]
- Junior, A.N.; Filho, R.; Fairbairn, E.; Dweck, J. The effects of the early carbonation curing on the mechanical and porosity properties of high initial strength Portland cement pastes. Constr. Build. Mater. 2015, 77, 448–454. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem. Concr. Res. 2016, 82, 50–57. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Effects of accelerated carbonation on the microstructure of Portland cement pastes containing reactive MgO. Cem. Concr. Res. 2012, 42, 769–777. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M. Effects of carbonation treatment on the properties of hydrated fly ash-MgO-Portland cement blends. Constr. Build. Mater. 2015, 96, 147–154. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Durability of concrete pipes subjected to combined steam and carbonation curing. Constr. Build. Mater. 2011, 25, 3345–3355. [Google Scholar] [CrossRef]
- Fang, X.; Zhan, B.; Chi, S.P. Enhancement of recycled aggregates and concrete by combined treatment of spraying Ca2+ rich wastewater and flow-through carbonation. Constr. Build. Mater. 2021, 277, 122202. [Google Scholar] [CrossRef]
- Liu, S.; Shen, P.; Xuan, D.; Li, L.; Sojobi, A.; Zhan, B.; Chi, S.P. A comparison of liquid-solid and gas-solid accelerated carbonation for enhancement of recycled concrete aggregate. Cem. Concr. Compos. 2021, 118, 103988. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.; Shi, C. CO2 curing for improving the properties of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2013, 42, 1–8. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.; Shi, C. Materials characteristics affecting CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 67, 50–59. [Google Scholar] [CrossRef]
- Pan, G.; Zhan, M.; Fu, M.; Wang, Y.P.; Liu, X.J. Effect of CO2 curing on demolition recycled fine aggregates enhanced by calcium hydroxide pre-soaking. Constr. Build. Mater. 2017, 154, 810–818. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Li, Y.; Pan, X.; Chi, S.; Xie, Z. Influence of carbonated recycled concrete aggregate on properties of cement mortar. Constr. Build. Mater. 2015, 98, 1–7. [Google Scholar] [CrossRef]
- Branch, J.L.; Kosson, D.S.; Garrabrants, A.C.; He, P.J. The impact of carbonation on the microstructure and solubility of major constituents in microconcrete materials with varying alkalinities due to fly ash replacement of ordinary Portland cement. Cem. Concr. Res. 2016, 89, 297–309. [Google Scholar] [CrossRef]
- Sevelsted, T.F.; Skibsted, J. Carbonation of C-S-H and C-A-S-H samples studied by 13C, 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2015, 71, 56–65. [Google Scholar] [CrossRef]
- Zhu, C.; Fang, Y.; Wei, H. Carbonation-cementation of recycled hardened cement paste powder. Constr. Build. Mater. 2018, 192, 224–232. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Experimental investigation and mathematical modeling of the concrete carbonation problem. Chem. Eng. Sci. 1991, 46, 1333–1338. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental modeling and experimental investigation of concrete carbonation. ACI Mater. J. 1991, 88, 363–373. [Google Scholar] [CrossRef]
- Papadakis, V.G. Physical and cemical characteristics affecting the durability of concrete. ACI Mater. J. 1991, 88, 186–196. [Google Scholar] [CrossRef]
- Shi, C.J.; Zou, Q.; He, F. Study on CO2 curing kinetics of concrete. J. Chin. Ceram. Soc. 2010, 38, 1179–1184. [Google Scholar]
- Shi, C.; Liu, M.; He, P.; Ou, Z.H. Factors affecting kinetics of CO2 curing of concrete. J. Sustain. Cem. Based Mater. 2012, 10, 24–33. [Google Scholar] [CrossRef]

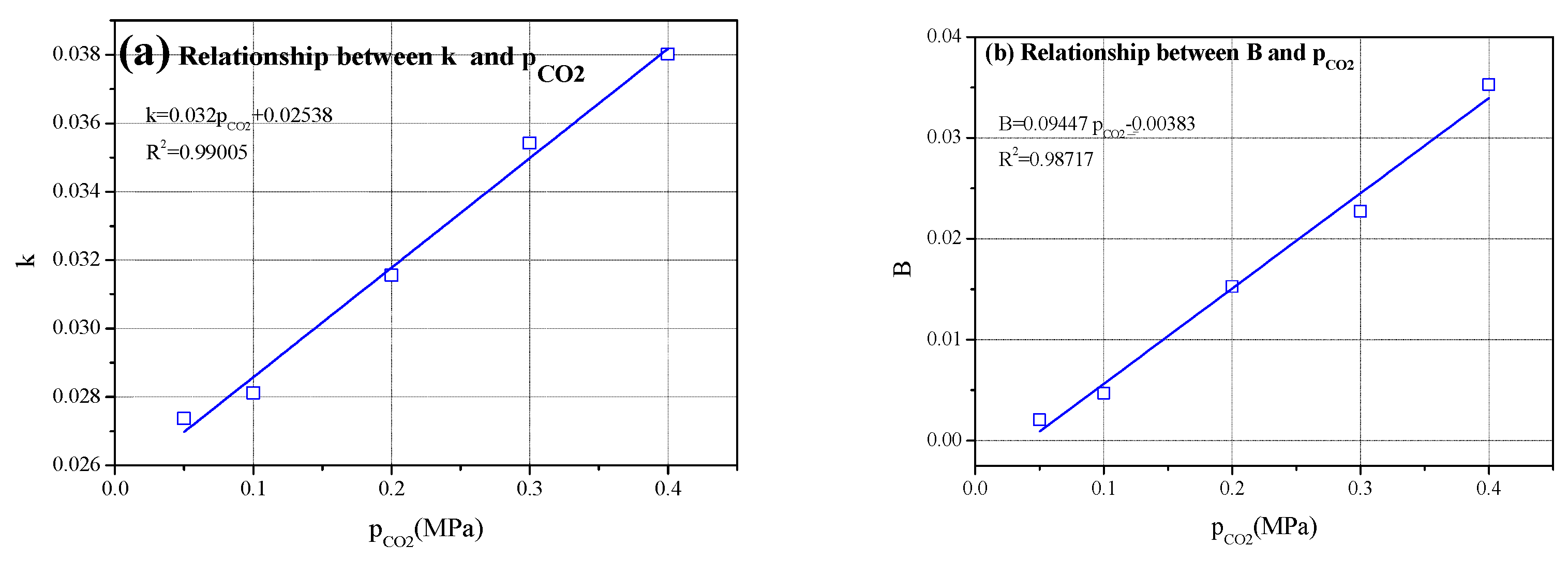


| CO2 Pressure/MPa | y = kx + B | Standard Error | R2 | ||
|---|---|---|---|---|---|
| Slope k | Intercept B | k | B | ||
| 0.05 | 0.02737 | 0.00207 | 0.0015 | 0.00364 | 0.97922 |
| 0.1 | 0.02812 | 0.00472 | 0.00169 | 0.00409 | 0.97522 |
| 0.2 | 0.03156 | 0.01526 | 0.00251 | 0.00606 | 0.95743 |
| 0.3 | 0.03542 | 0.02274 | 0.00342 | 0.00826 | 0.93834 |
| 0.4 | 0.03802 | 0.03528 | 0.00442 | 0.01068 | 0.91264 |
| Carbonization Pressure /MPa | 0–1 h | 1–24 h | ||
|---|---|---|---|---|
| R2 | R2 | |||
| 0.05 | 0.99716 | 0.98540 | ||
| 0.1 | 0.99972 | 0.98252 | ||
| 0.2 | 0.97926 | 0.99548 | ||
| 0.3 | 0.95968 | 0.97626 | ||
| 0.4 | 0.90693 | 0.99016 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Jiang, Y.; Shang, Q.; Ye, Y.; Yang, J. Study on Kinetics of Carbonization Reaction of Hardened Cement Paste Powder Based on Carbonization Degree. Materials 2023, 16, 2584. https://doi.org/10.3390/ma16072584
Zhu C, Jiang Y, Shang Q, Ye Y, Yang J. Study on Kinetics of Carbonization Reaction of Hardened Cement Paste Powder Based on Carbonization Degree. Materials. 2023; 16(7):2584. https://doi.org/10.3390/ma16072584
Chicago/Turabian StyleZhu, Chenhui, Yibo Jiang, Qizhi Shang, Yuchen Ye, and Jie Yang. 2023. "Study on Kinetics of Carbonization Reaction of Hardened Cement Paste Powder Based on Carbonization Degree" Materials 16, no. 7: 2584. https://doi.org/10.3390/ma16072584
APA StyleZhu, C., Jiang, Y., Shang, Q., Ye, Y., & Yang, J. (2023). Study on Kinetics of Carbonization Reaction of Hardened Cement Paste Powder Based on Carbonization Degree. Materials, 16(7), 2584. https://doi.org/10.3390/ma16072584







