Seawater Biodegradable Poly(butylene succinate-co-adipate)—Wheat Bran Biocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Production
2.3. Thermal Characterization
2.4. Rheological Analysis
2.5. Fourier Transform Infrared Analysis
2.6. Scanning Electron Microscopy and Stereo Microscopy
2.7. Tensile Test
2.8. Biodegradability Test in Seawater
3. Results and Discussion
3.1. Thermal Analysis
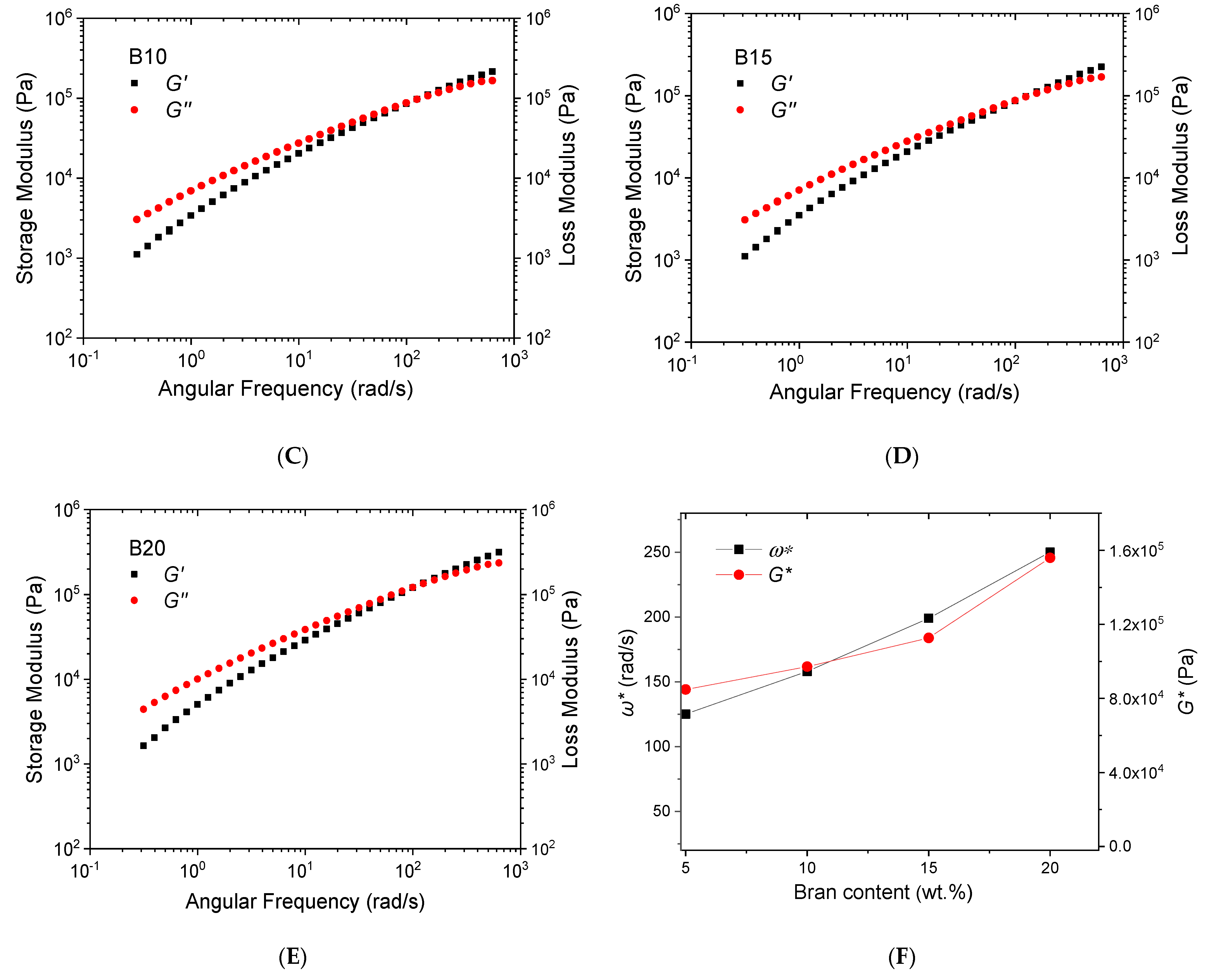
3.2. Rheological Analysis
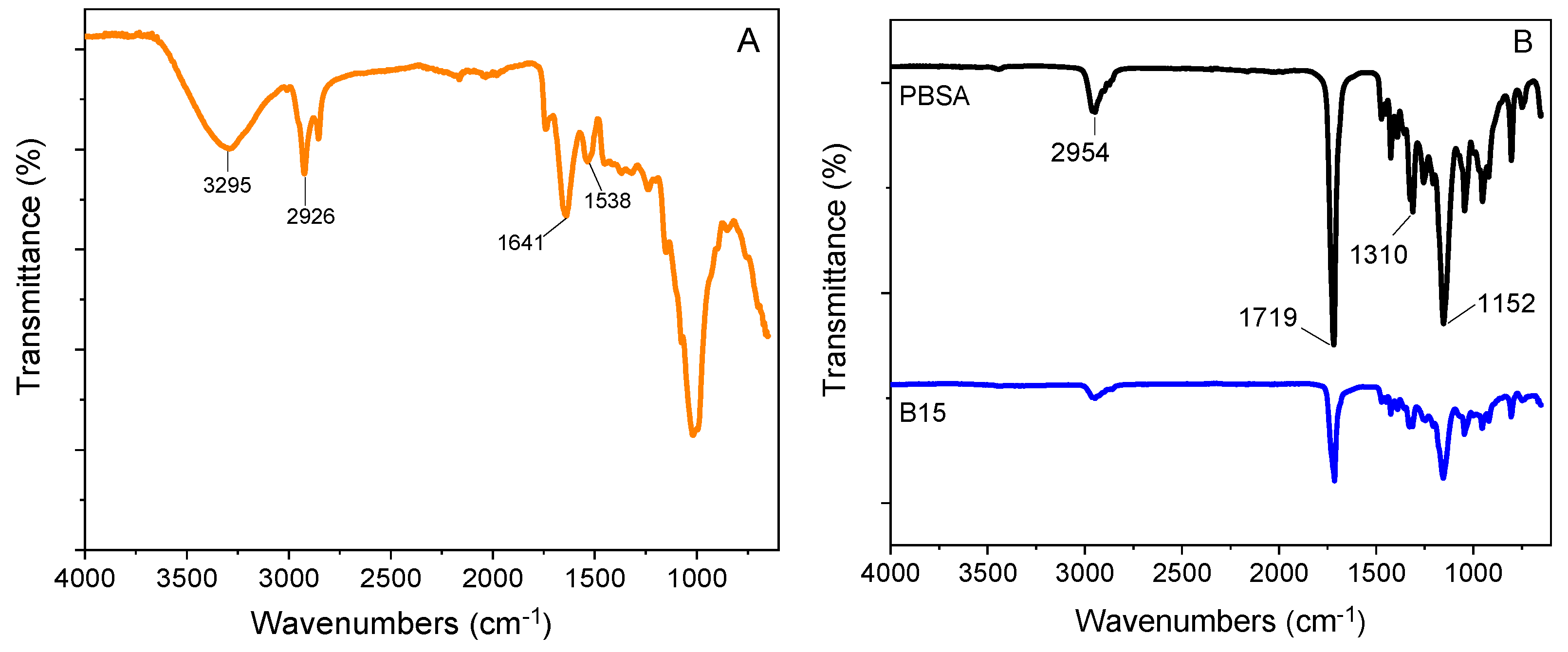
3.3. Fourier Transform Infrared Analysis
3.4. Morphological Analysis
3.5. Mechanical Properties
3.6. Biodegradation in Seawater
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.H.; Bhoi, P.R. An Overview of Non-Biodegradable Bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-Based Poly (Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- AlMaadeed, M.A.A.; Ponnamma, D.; El-Samak, A.A. Polymers to Improve the World and Lifestyle: Physical, Mechanical, and Chemical Needs. In Polymer Science and Innovative Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–19. [Google Scholar]
- Plastics—The Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 1 March 2023).
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Rosli, N.A.; Ahmad, I. Mechanical Properties of Recycled Plastics. In Recent Developments in Plastic Recycling; Springer: Berlin/Heidelberg, Germany, 2021; pp. 239–258. [Google Scholar]
- Prajapati, R.; Kohli, K.; Maity, S.K.; Sharma, B.K. Recovery and Recycling of Polymeric and Plastic Materials. In Recent Developments in Plastic Recycling; Springer: Berlin/Heidelberg, Germany, 2021; pp. 15–41. [Google Scholar]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of Plastic Pollution in the Environment: A Review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef]
- Jin, H.; Gonzalez-Gutierrez, J.; Oblak, P.; Zupančič, B.; Emri, I. The Effect of Extensive Mechanical Recycling on the Properties of Low Density Polyethylene. Polym. Degrad. Stab. 2012, 97, 2262–2272. [Google Scholar] [CrossRef]
- Lange, J.-P. Managing Plastic Waste—Sorting, Recycling, Disposal, and Product Redesign. ACS Sustain. Chem. Eng. 2021, 9, 15722–15738. [Google Scholar] [CrossRef]
- Collias, D.I.; James, M.I.; Layman, J.M. Introduction—Circular Economy of Polymers and Recycling Technologies. In Circular Economy of Polymers: Topics in Recycling Technologies; ACS Publications: Washington, DC, USA, 2021; pp. 1–21. ISBN 1947-5918. [Google Scholar]
- RameshKumar, S.; Shaiju, P.; O’Connor, K.E. Bio-Based and Biodegradable Polymers-State-of-the-Art, Challenges and Emerging Trends. Curr. Opin. Green Sustain. Chem. 2020, 21, 75–81. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef]
- Banerjee, A.; Chatterjee, K.; Madras, G. Enzymatic Degradation of Polymers: A Brief Review. Mater. Sci. Technol. 2014, 30, 567–573. [Google Scholar] [CrossRef]
- Mangeon, C.; Renard, E.; Thevenieau, F.; Langlois, V. Networks Based on Biodegradable Polyesters: An Overview of the Chemical Ways of Crosslinking. Mater. Sci. Eng. C 2017, 80, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Hayase, N.; Yano, H.; Kudoh, E.; Tsutsumi, C.; Ushio, K.; Miyahara, Y.; Tanaka, S.; Nakagawa, K. Isolation and Characterization of Poly (Butylene Succinate-Co-Butylene Adipate)-Degrading Microorganism. J. Biosci. Bioeng. 2004, 97, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Debuissy, T.; Pollet, E.; Avérous, L. Synthesis and Characterization of Biobased Poly (Butylene Succinate-Ran-Butylene Adipate). Analysis of the Composition-Dependent Physicochemical Properties. Eur. Polym. J. 2017, 87, 84–98. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Thakur, V.K.; Barkane, A.; Beluns, S. Bio-Based Poly (Butylene Succinate): Recent Progress, Challenges and Future Opportunities. Eur. Polym. J. 2021, 161, 110855. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of Bioplastic Packaging Materials: An Overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef]
- Chien, H.-L.; Tsai, Y.-T.; Tseng, W.-S.; Wu, J.-A.; Kuo, S.-L.; Chang, S.-L.; Huang, S.-J.; Liu, C.-T. Biodegradation of PBSA Films by Elite Aspergillus Isolates and Farmland Soil. Polymers 2022, 14, 1320. [Google Scholar] [CrossRef]
- Koronis, G.; Silva, A.; Fontul, M. Green Composites: A Review of Adequate Materials for Automotive Applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B. Poly (Butylene Succinate) and Its Copolymers: Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable Aliphatic Polyesters. Part I. Properties and Biodegradation of Poly (Butylene Succinate-Co-Butylene Adipate). Polym. Degrad. Stab. 2006, 91, 367–376. [Google Scholar] [CrossRef]
- Ishioka, R.; Kitakuni, E.; Ichikawa, Y. Aliphatic Polyesters:“Bionolle.” In Biopolymers Online: Biology Chemistry Biotechnology Applications; Wiley Online Library: New York, NY, USA, 2005; Volume 4. [Google Scholar]
- Xu, J.; Guo, B.-H. Microbial Succinic Acid, Its Polymer Poly (Butylene Succinate), and Applications. In Plastics from Bacteria: Natural Functions and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 347–388. [Google Scholar]
- Fujimaki, T. Processability and Properties of Aliphatic Polyesters, ‘BIONOLLE’, Synthesized by Polycondensation Reaction. Polym. Degrad. Stab. 1998, 59, 209–214. [Google Scholar] [CrossRef]
- Uchida, H.; Nakajima-Kambe, T.; Shigeno-Akutsu, Y.; Nomura, N.; Tokiwa, Y.; Nakahara, T. Properties of a Bacterium Which Degrades Solid Poly (Tetramethylene Succinate)-Co-Adipate, a Biodegradable Plastic. FEMS Microbiol. Lett. 2000, 189, 25–29. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer Biodegradation: Mechanisms and Estimation Techniques—A Review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-H.; Wang, X.-Q.; Zeng, J.; Yang, G.; Shi, F.-H.; Yan, Q. Biodegradation of Poly (Butylene Succinate-Co-Butylene Adipate) by Aspergillus Versicolor. Polym. Degrad. Stab. 2005, 90, 173–179. [Google Scholar] [CrossRef]
- Nishioka, M.; Tuzuki, T.; Wanajyo, Y.; Oonami, H.; Horiuchi, T. Biodegradation of BIONOLLE. In Studies in Polymer Science; Elsevier: Amsterdam, The Netherlands, 1994; Volume 12, pp. 584–590. ISBN 0922-5579. [Google Scholar]
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and Supramolecular Changes in Polybutylene Succinate (PBS) and Polybutylene Succinate Adipate (PBSA) Copolymer during Degradation in Various Environmental Conditions. Polymers 2018, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Yamano, N.; Kawasaki, N. Biodegradation in Seawater of Aliphatic Polyesters. Polym. Degrad. Stab. 2019, 166, 290–299. [Google Scholar] [CrossRef]
- Phosri, S.; Kunjiek, T.; Mukkhakang, C.; Suebthep, S.; Sinsup, W.; Phornsirigarn, S.; Charoeythornkhajhornchai, P. Biodegradability of Bioplastic Blown Film in a Marine Environment. Front. Mar. Sci. 2022, 9, 917397. [Google Scholar] [CrossRef]
- Lagrain, B.; Goderis, B.; Brijs, K.; Delcour, J.A. Molecular Basis of Processing Wheat Gluten toward Biobased Materials. Biomacromolecules 2010, 11, 533–541. [Google Scholar] [CrossRef]
- Elanchezhian, C.; Ramnath, B.V.; Ramakrishnan, G.; Rajendrakumar, M.; Naveenkumar, V.; Saravanakumar, M.K. Review on Mechanical Properties of Natural Fiber Composites. Mater. Today Proc. 2018, 5, 1785–1790. [Google Scholar] [CrossRef]
- Rahman, A.; Ulven, C.A.; Johnson, M.A.; Durant, C.; Hossain, K.G. Pretreatment of Wheat Bran for Suitable Reinforcement in Biocomposites. J. Renew. Mater. 2017, 5, 62–73. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Cinelli, P.; Fiori, S.; Coltelli, M.-B.; Lazzeri, A. Wheat Bran Addition as Potential Alternative to Control the Plasticizer Migration into PLA/PBSA Blends. J. Mater. Sci. 2022, 57, 14511–14527. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Cinelli, P.; Coltelli, M.-B.; Lazzeri, A. Essential Work of Fracture and Evaluation of the Interfacial Adhesion of Plasticized PLA/PBSA Blends with the Addition of Wheat Bran by-Product. Polymers 2022, 14, 615. [Google Scholar] [CrossRef] [PubMed]
- Tsenoglou, C.J.; Pavlidou, S.; Papaspyrides, C.D. Evaluation of Interfacial Relaxation Due to Water Absorption in Fiber–Polymer Composites. Compos. Sci. Technol. 2006, 66, 2855–2864. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Mokhena, T.C. A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS). Polymers 2021, 13, 1200. [Google Scholar] [CrossRef] [PubMed]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Artificial Ageing, Chemical Resistance, and Biodegradation of Biocomposites from Poly (Butylene Succinate) and Wheat Bran. Materials 2021, 14, 7580. [Google Scholar] [CrossRef] [PubMed]
- ISO 1183-1:2019; Plastics—Methods for Determining the Density of Non-Cellular Plastics—Part 1: Immersion Method, Liquid Pycnometer Method and Titration Method. ISO Copyright Office: Geneva, Switzerland, 2019.
- ISO 1133-1:2011; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics—Part 1: Standard Method. ISO Copyright Office: Geneva, Switzerland, 2011.
- Malz, F.; Arndt, J.-H.; Balko, J.; Barton, B.; Büsse, T.; Imhof, D.; Pfaendner, R.; Rode, K.; Brüll, R. Analysis of the Molecular Heterogeneity of Poly (Lactic Acid)/Poly (Butylene Succinate-Co-Adipate) Blends by Hyphenating Size Exclusion Chromatography with Nuclear Magnetic Resonance and Infrared Spectroscopy. J. Chromatogr. A 2021, 1638, 461819. [Google Scholar] [CrossRef]
- Mitsubishi Chemical Group. Available online: www.pttmcc.com/new/download/BioPBS_FD92PM_and_FD92PB_Technical_Data_Sheet_for_film.pdf (accessed on 30 January 2023).
- Bureepukdee, C.; Suttireungwong, S.; Seadan, M. A Study on Reactive Blending of (Poly Lactic Acid) and Poly (Butylene Succinate Co Adipate). In IOP Conference Series: Materials Science and Engineering, Proceedings of the 2015 Global Conference on Polymer and Composite Materials (PCM2015), Beijing, China, 16–18 May 2015; IOP Publishing: Bristol, UK, 2015; Volume 87, p. 12070. [Google Scholar]
- ASTM D1238; StandardTest Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. ASTM International: West Conshohocken, PA, USA, 2014.
- Hejna, A.; Barczewski, M.; Andrzejewski, J.; Kosmela, P.; Piasecki, A.; Szostak, M.; Kuang, T. Rotational Molding of Linear Low-Density Polyethylene Composites Filled with Wheat Bran. Polymers 2020, 12, 1004. [Google Scholar] [CrossRef]
- Gigante, V.; Cinelli, P.; Righetti, M.C.; Sandroni, M.; Polacco, G.; Seggiani, M.; Lazzeri, A. On the Use of Biobased Waxes to Tune Thermal and Mechanical Properties of Polyhydroxyalkanoates–Bran Biocomposites. Polymers 2020, 12, 2615. [Google Scholar] [CrossRef]
- Seggiani, M.; Gigante, V.; Cinelli, P.; Coltelli, M.-B.; Sandroni, M.; Anguillesi, I.; Lazzeri, A. Processing and Mechanical Performances of Poly (Butylene Succinate–Co–Adipate)(PBSA) and Raw Hydrolyzed Collagen (HC) Thermoplastic Blends. Polym. Test. 2019, 77, 105900. [Google Scholar] [CrossRef]
- Makhatha, M.E.; Sinha Ray, S.; Hato, J.; Luyt, A.S. Thermal and Thermomechanical Properties of Poly (Butylene Succinate) Nanocomposites. J. Nanosci. Nanotechnol. 2008, 8, 1679–1689. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Analysis of Selected Properties of Injection Moulded Sustainable Biocomposites from Poly (Butylene Succinate) and Wheat Bran. Materials 2021, 14, 7049. [Google Scholar] [CrossRef]
- Srubar, W.V., III; Pilla, S.; Wright, Z.C.; Ryan, C.A.; Greene, J.P.; Frank, C.W.; Billington, S.L. Mechanisms and Impact of Fiber–Matrix Compatibilization Techniques on the Material Characterization of PHBV/Oak Wood Flour Engineered Biobased Composites. Compos. Sci. Technol. 2012, 72, 708–715. [Google Scholar] [CrossRef]
- Móczó, J.; Pukánszky, B. Particulate Fillers in Thermoplastics, Fillers for Polymer Applications; Rothon, R., Ed.; Springer: Chester, UK, 2017; Volume 51, p. 93. [Google Scholar]
- Rueda, M.M. Rheology and Processing of Highly Filled Materials. Ph.D. Thesis, Université de Lyon, Lyon, France, 2017. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Cherian, B.M.; Pothan, L.A.; Nguyen-Chung, T.; Mennig, G.; Kottaisamy, M.; Thomas, S. A Novel Method for the Synthesis of Cellulose Nanofibril Whiskers from Banana Fibers and Characterization. J. Agric. Food Chem. 2008, 56, 5617–5627. [Google Scholar] [CrossRef] [PubMed]
- Cappello, M.; Rossi, D.; Filippi, S.; Cinelli, P.; Seggiani, M. Wood Residue-Derived Biochar as a Low-Cost, Lubricating Filler in Poly (Butylene Succinate-Co-Adipate) Biocomposites. Materials 2023, 16, 570. [Google Scholar] [CrossRef]
- Babu, C.R.; Ketanapalli, H.; Beebi, S.K.; Kolluru, V.C. Wheat Bran-Composition and Nutritional Quality: A Review. Adv. Biotechnol. Microbiol. 2018, 9, 555754. [Google Scholar]
- Huang, T.-H.; Chen, C.-S.; Chang, S.-W. Microcrack Patterns Control the Mechanical Strength in the Biocomposites. Mater. Des. 2018, 140, 505–515. [Google Scholar] [CrossRef]

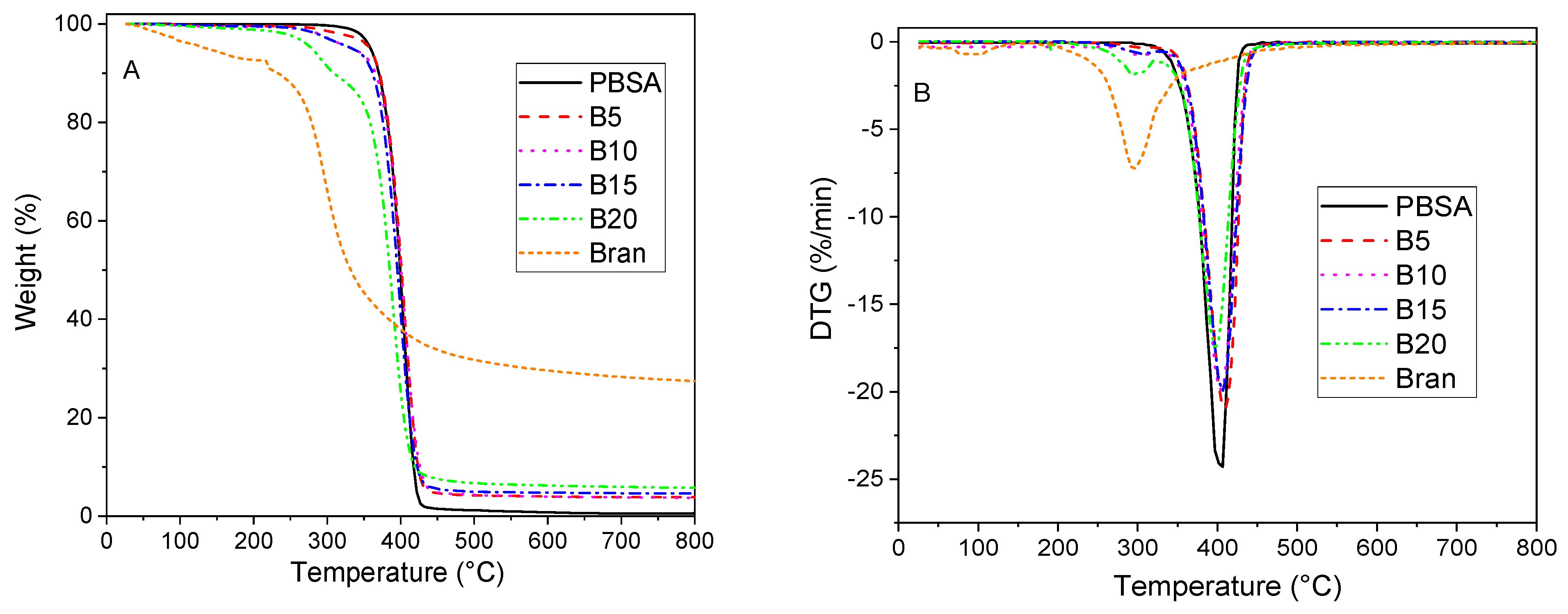

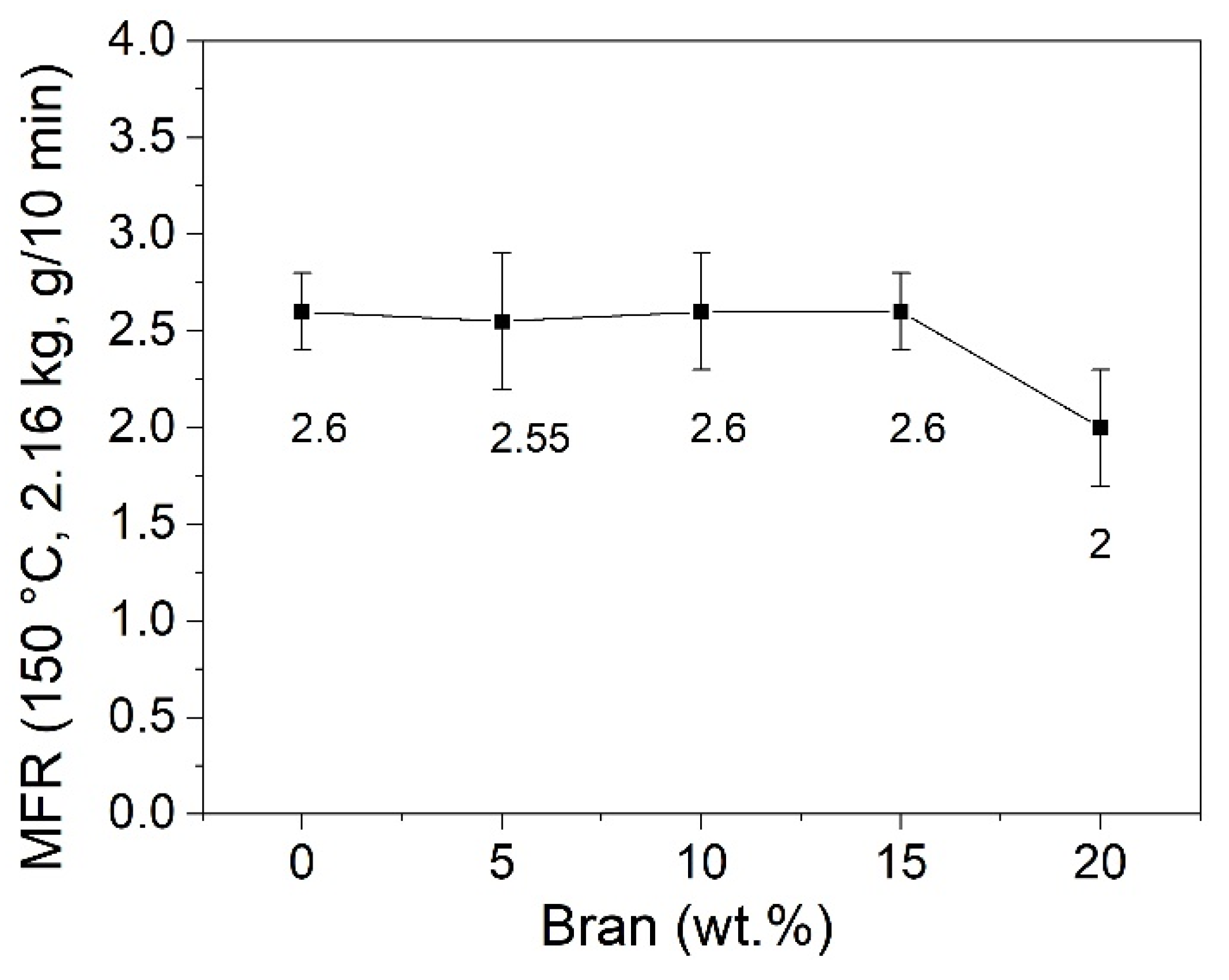
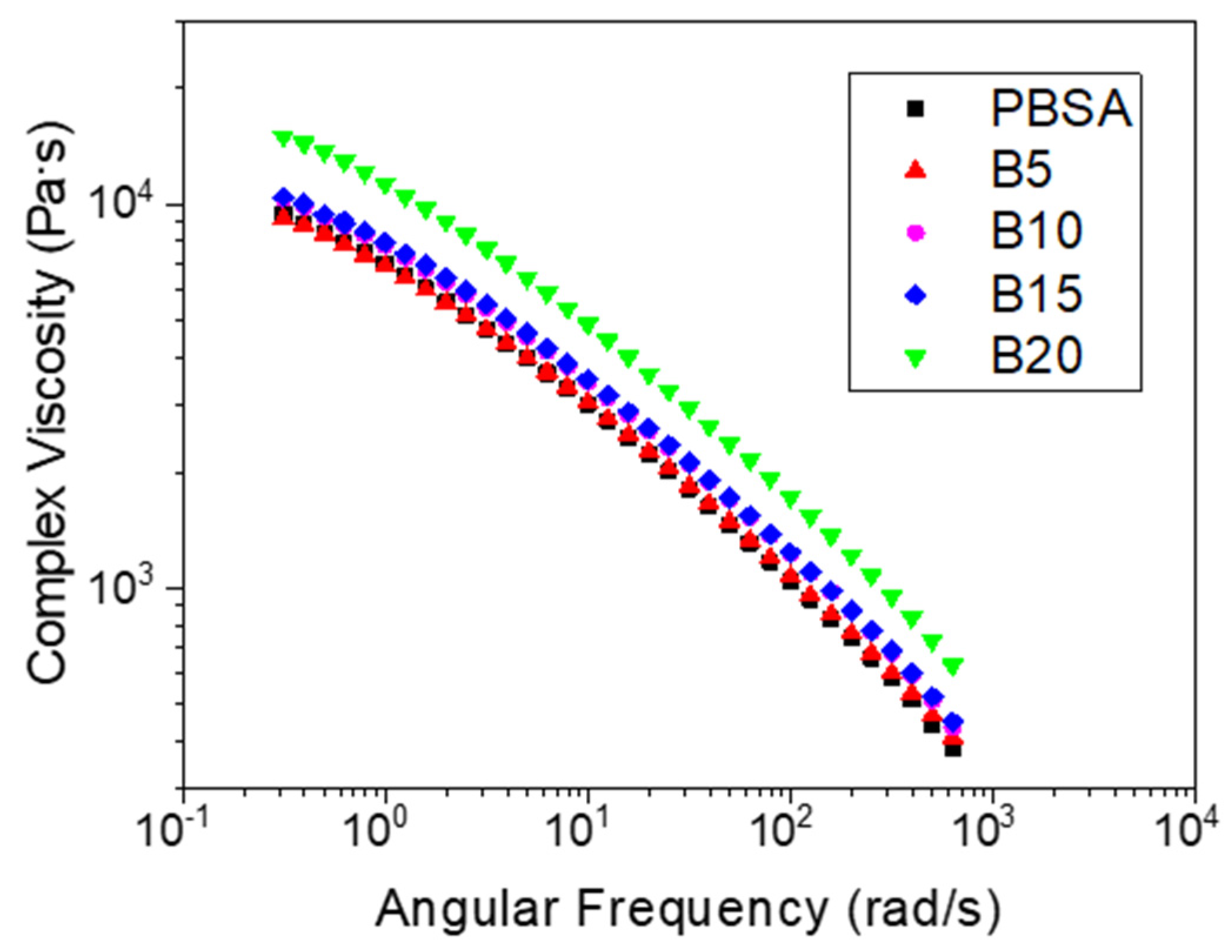
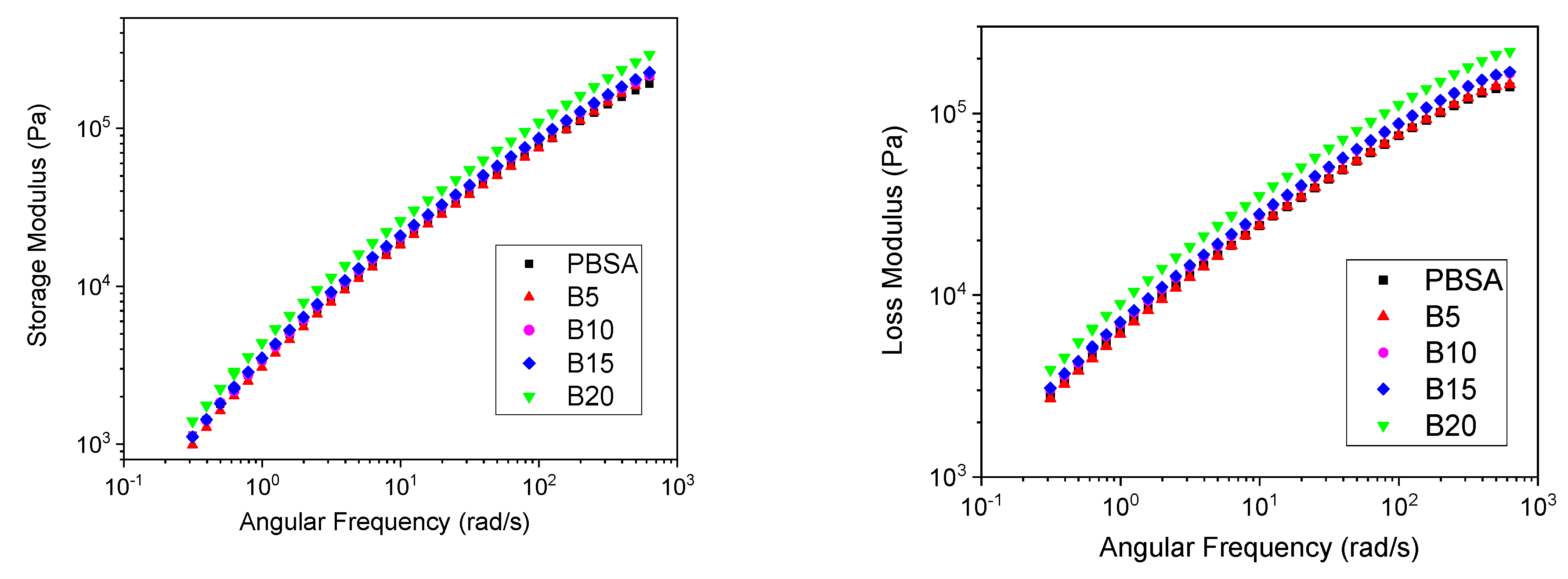




| Sample | Tm1 (°C) | Tm2 (°C) | ΔHm* (J/g) | Xc (%) |
|---|---|---|---|---|
| PBSA | - | 85.5 | 45.6 | 32.1 |
| B5 | 77.7 | 86.6 | 30.5 | 22.6 |
| B10 | 78.7 | 87.8 | 30.4 | 23.8 |
| B15 | 77.8 | 86.8 | 35.2 | 29.2 |
| B20 | 74.7 | 86.2 | 24.9 | 21.9 |
| Sample | Young’s Modulus (GPa) | Yield Strength (MPa) | Elongation at Yield (%) | Stress at Break (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| PBSA | 0.29 ± 0.05 | 17.50 ± 1.10 | 14.50 ± 0.30 | 21.10 ± 0.50 | 400.00 ± 53.00 |
| B5 | 0.34 ± 0.01 | 17.60 ± 0.93 | 16.60 ± 2.32 | 23.70 ± 2.36 | 306.50 ± 14.27 |
| B10 | 0.39 ± 0.03 | 18.41 ± 1.87 | 19.22 ± 2.91 | 21.95 ± 1.91 | 200.50 ± 30.07 |
| B15 | 0.37 ± 0.06 | 17.09 ± 0.75 | 16.53 ± 1.49 | 21.08 ± 1.93 | 207.00 ± 14.74 |
| B20 | 0.44 ± 0.02 | * | * | 16.67 ± 1.43 | 72.13 ± 22.24 |
| Time (Months) | Young’s Modulus (GPa) | Yield Strength (MPa) | Elongation at Yield (%) | Stress at Break (MPa) | Elongation at Break (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBSA | B15 | PBSA | B15 | PBSA | B15 | PBSA | B15 | PBSA | B15 | |
| 0 | 0.29 ± 0.05 | 0.37 ± 0.06 | 17.50 ± 1.10 | 17.09 ± 0.75 | 14.50 ± 0.30 | 16.53 ± 1.49 | 21.00 ± 0.50 | 21.08 ± 1.93 | 400.00 ± 53.00 | 207.00 ± 14.74 |
| 3 | 0.20 ± 0.04 | 0.20 ± 0.09 | 20.52 ± 1.54 | 19.01 ± 0.75 | 22.11 ± 0.32 | 17.99 ± 0.75 | 29.10 ± 0.66 | 3.60 ± 0.30 | 260.20 ± 55.50 | 4.50 ± 0.30 |
| 6 | 0.24 ± 0.06 | - | 23.41 ± 1.98 | - | 22.32 ± 0.43 | - | 26.71 ± 0.73 | - | 210.25 ± 32.30 | - |
| 9 | 0.34 ± 0.07 | - | 18.20 ± 1.56 | - | 26.75 ± 1.22 | - | 20.88 ± 0.86 | - | 247.75 ± 31.30 | - |
| 12 | 0.17 ± 0.08 | - | 16.75 ± 2.04 | - | 20.11 ± 1.51 | - | 15.38 ± 0.93 | - | 30.15 ± 26.30 | - |
| 15 | 0.14 ± 0.09 | - | 18.61 ± 2.84 | - | 22.23 ± 2.01 | - | 16.46 ± 1.10 | - | 27.85 ± 5.30 | - |
| 18 | 0.19 ± 0.11 | - | * | - | * | - | 10.52 ± 1.20 | - | 7.90 ± 1.30 | - |
| 26 | 0.11 ± 0.12 | - | * | - | * | - | 9.01 ± 1.20 | - | 6.88 ± 1.20 | - |
| 34 | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strangis, G.; Rossi, D.; Cinelli, P.; Seggiani, M. Seawater Biodegradable Poly(butylene succinate-co-adipate)—Wheat Bran Biocomposites. Materials 2023, 16, 2593. https://doi.org/10.3390/ma16072593
Strangis G, Rossi D, Cinelli P, Seggiani M. Seawater Biodegradable Poly(butylene succinate-co-adipate)—Wheat Bran Biocomposites. Materials. 2023; 16(7):2593. https://doi.org/10.3390/ma16072593
Chicago/Turabian StyleStrangis, Giovanna, Damiano Rossi, Patrizia Cinelli, and Maurizia Seggiani. 2023. "Seawater Biodegradable Poly(butylene succinate-co-adipate)—Wheat Bran Biocomposites" Materials 16, no. 7: 2593. https://doi.org/10.3390/ma16072593
APA StyleStrangis, G., Rossi, D., Cinelli, P., & Seggiani, M. (2023). Seawater Biodegradable Poly(butylene succinate-co-adipate)—Wheat Bran Biocomposites. Materials, 16(7), 2593. https://doi.org/10.3390/ma16072593








