Plastic-Waste-Derived Char as an Additive for Epoxy Composite
Abstract
:1. Introduction
2. Char Production from Plastic Waste
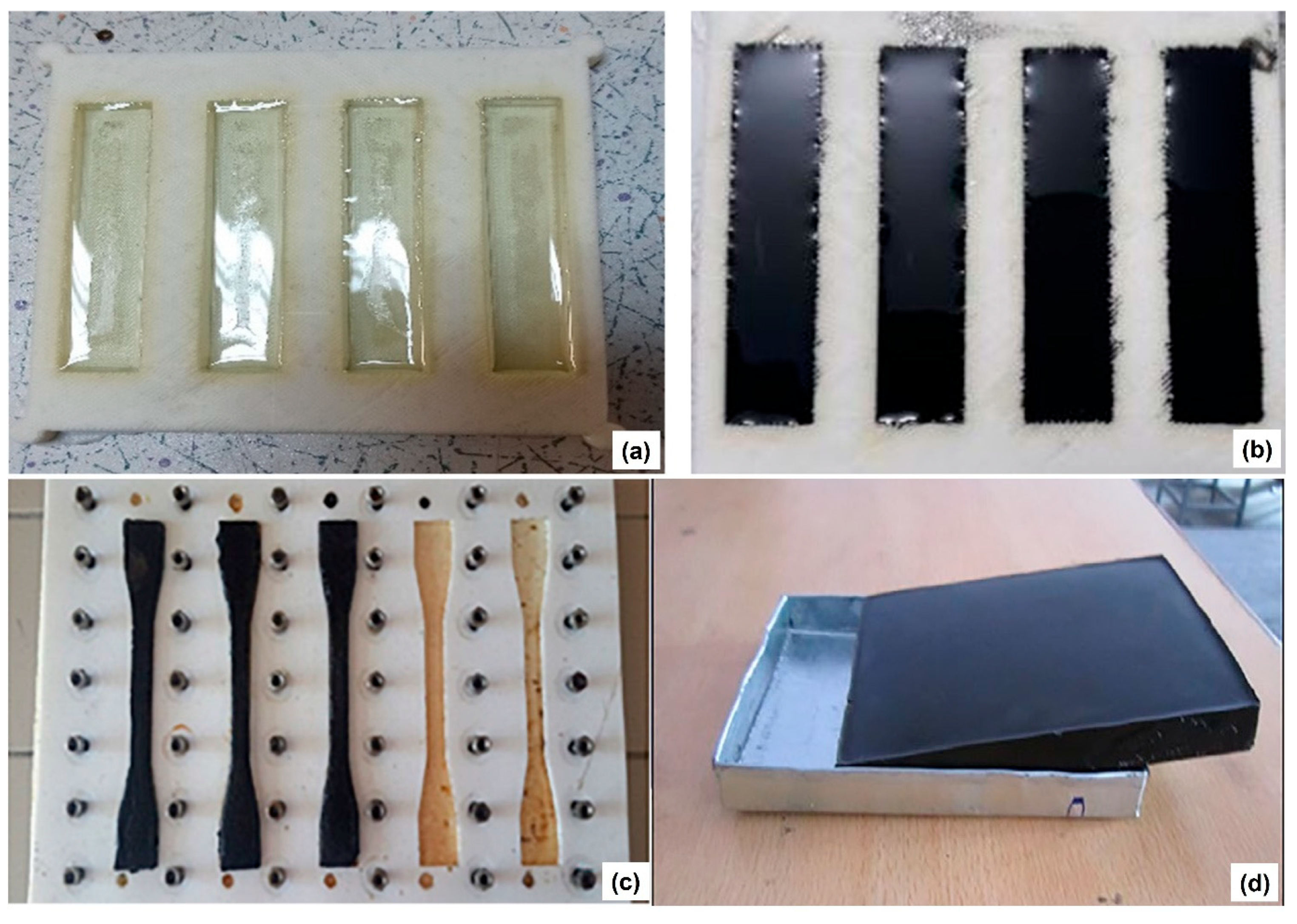
3. Application of Plastic-Waste-Derived Char as an Additive for Epoxy Composite
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gala, A.; Guerrero, M.; Serra, J.M. Characterization of post-consumer plastic film waste from mixed MSW in Spain: A key point for the successful implementation of sustainable plastic waste management strategies. Waste Manag. 2020, 111, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Mastellone, M.L. A feasibility assessment of an integrated plastic waste system adopting mechanical and thermochemical conversion processes. Resour. Conserv. Recycl. X 2019, 4, 100017. [Google Scholar] [CrossRef]
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a circular economy for plastic packaging wastes–the environmental potential of chemical recycling. Resour. Conserv. Recycl. 2020, 162, 105010. [Google Scholar] [CrossRef]
- De Weerdt, L.; Sasao, T.; Compernolle, T.; Van Passel, S.; De Jaeger, S. The effect of waste incineration taxation on industrial plastic waste generation: A panel analysis. Resour. Conserv. Recycl. 2020, 157, 104717. [Google Scholar] [CrossRef]
- Rajmohan, K.V.S.; Ramya, C.; Raja Viswanathan, M.; Varjani, S. Plastic pollutants: Effective waste management for pollution control and abatement. Curr. Opin. Environ. Sci. Health 2019, 12, 72–84. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, J.; Brown, R.J.C.; Kim, K.-H. Micro- and nano-plastic pollution: Behavior, microbial ecology, and remediation technologies. J. Clean. Prod. 2021, 291, 125240. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, J.; Brown, R.J.C.; Kim, K.-H. Environmental fate, ecotoxicity biomarkers, and potential health effects of micro- and nano-scale plastic contamination. J. Hazard. Mater. 2021, 403, 123910. [Google Scholar] [CrossRef]
- Mooij, D.; Muller, M.A. Decarbonisation Options for the Dutch Polycarbonate Industry. A Manufacturing Industry Decarbonisation Data Exchange Network (MIDDEN) report, PBL Netherlands Environmental Assessment Agency, PBL publication number: 4580. 2021. Available online: https://www.pbl.nl/en/publications/decarbonisation-options-for-the-dutch-polycarbonate-industry (accessed on 10 February 2023).
- Jiang, H.; Liu, W.; Zhang, X.; Qiao, J. Chemical recycling of plastics by microwave-assisted high-temperature pyrolysis. Glob. Chall. 2020, 4, 1900074. [Google Scholar] [CrossRef] [Green Version]
- Pohjakallio, M.; Vuorinen, T.; Oasmaa, A. Chemical routes for recycling—Dissolving, catalytic, and thermochemical technologies. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 359–384. [Google Scholar]
- Carniel, A.; Gomes, A.d.C.; Coelho, M.A.Z.; de Castro, A.M. Process strategies to improve biocatalytic depolymerization of post-consumer PET packages in bioreactors, and investigation on consumables cost reduction. Bioprocess Biosyst. Eng. 2021, 44, 507–516. [Google Scholar] [CrossRef]
- Yang, R.; Xu, G.; Dong, B.; Guo, X.; Wang, Q. Selective, sequential, and “one-pot” depolymerization strategies for chemical recycling of commercial plastics and mixed plastics. ACS Sustain. Chem. Eng. 2022, 10, 9860–9871. [Google Scholar] [CrossRef]
- Miao, Y.; von Jouanne, A.; Yokochi, A. Current technologies in depolymerization process and the road ahead. Polymers 2021, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, S.A.; Raji, S.A.; Adeniyi, A.G. Development of particleboard from waste styrofoam and sawdust. Nigerian J. Technol. Develop. 2017, 14, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wan, K.; Zhang, Y.; Wang, Y. Waste to wealth: Chemical recycling and chemical upcycling of waste plastics for a great future. ChemSusChem 2021, 14, 4123–4136. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, E.E.; Lam, S.S.; Chen, W.-H.; Rinklebe, J.; Park, Y.-K. Chemical recycling of plastic waste via thermocatalytic routes. J. Clean. Prod. 2021, 321, 128989. [Google Scholar] [CrossRef]
- Remias, J.E.; Pavlosky, T.A.; Sen, A. Oxidative chemical recycling of polyethene. Comptes Rendus L’académie Sci.-Ser. IIC-Chem. 2000, 3, 627–629. [Google Scholar] [CrossRef]
- Szarka, G.; Domján, A.; Szakács, T.; Iván, B. Oil from poly(vinyl chloride): Unprecedented degradative chain scission under mild thermooxidative conditions. Polym. Degrad. Stab. 2012, 97, 1787–1793. [Google Scholar] [CrossRef]
- Szarka, G.; Iván, B. Thermal properties, degradation and stability of poly(vinyl chloride) predegraded thermooxidatively in the presence of dioctyl phthalate plasticizer. J. Macromol. Sci. A 2013, 50, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Vijeta, A.; Casadevall, C.; Gentleman, A.S.; Euser, T.; Reisner, E. Bridging plastic recycling and organic catalysis: Photocatalytic deconstruction of polystyrene via a C–H oxidation pathway. ACS Catal. 2022, 12, 8155–8163. [Google Scholar] [CrossRef]
- Pinsuwan, K.; Opaprakasit, P.; Petchsuk, A.; Dubas, L.; Opaprakasit, M. Chemical recycling of high-density polyethylene (HDPE) wastes by oxidative degradation to dicarboxylic acids and their use as value-added curing agents for acrylate-based materials. Polym. Degrad. Stab. 2023, 210, 110306. [Google Scholar] [CrossRef]
- Saeaung, K.; Phusunti, N.; Phetwarotai, W.; Assabumrungrat, S.; Cheirsilp, B. Catalytic pyrolysis of petroleum-based and biodegradable plastic waste to obtain high-value chemicals. Waste Manag. 2021, 127, 101–111. [Google Scholar] [CrossRef]
- Kosloski-Oh, S.C.; Wood, Z.A.; Manjarrez, Y.; de Los Rios, J.P.; Fieser, M.E. Catalytic methods for chemical recycling or upcycling of commercial polymers. Mater. Horizons 2021, 8, 1084–1129. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Lv, Y.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Chen, P.; Lei, H.; Ruan, R. Pyrolysis technology for plastic waste recycling: A state-of-the-art review. Prog. Energy Combust. Sci. 2022, 93, 101021. [Google Scholar] [CrossRef]
- Kulas, D.G.; Zolghadr, A.; Chaudhari, U.S.; Shonnard, D.R. Economic and environmental analysis of plastics pyrolysis after secondary sortation of mixed plastic waste. J. Clean. Prod. 2023, 384, 135542. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Lee, N.; Lin, K.-Y.A.; Lee, J. Carbon dioxide-mediated thermochemical conversion of banner waste using cobalt oxide catalyst as a strategy for plastic waste treatment. Environ. Res. 2022, 213, 113560. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Park, Y.-K. Simultaneous upcycling of biodegradable plastic and sea shell wastes through thermocatalytic monomer recovery. ACS Sustain. Chem. Eng 2022, 10, 13972–13979. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.; Lee, N.; Ahn, B.; Lee, J. Upcycling of abandoned banner via thermocatalytic process over a MnFeCoNiCu high-entropy alloy catalyst. J. Hazard. Mater. 2022, 440, 129825. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energ. Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Wijesekara, D.A.; Sargent, P.; Ennis, C.J.; Hughes, D. Prospects of using chars derived from mixed post waste plastic pyrolysis in civil engineering applications. J. Clean. Prod. 2021, 317, 128212. [Google Scholar] [CrossRef]
- Jamradloedluk, J.; Lertsatitthanakorn, C. Characterization and utilization of char derived from fast pyrolysis of plastic wastes. Procedia Eng. 2014, 69, 1437–1442. [Google Scholar] [CrossRef] [Green Version]
- Saptoadi, H.; Rohmat, T.A.; Sutoyo. Combustion of char from plastic wastes pyrolysis. AIP Conf. Proc. 2016, 1737, 030006. [Google Scholar]
- Kumar, A.; Singh, E.; Singh, L.; Kumar, S.; Kumar, R. Carbon material as a sustainable alternative towards boosting properties of urban soil and foster plant growth. Sci. Total Environ. 2021, 751, 141659. [Google Scholar] [CrossRef]
- Singh, E.; Kumar, A.; Khapre, A.; Saikia, P.; Shukla, S.K.; Kumar, S. Efficient removal of arsenic using plastic waste char: Prevailing mechanism and sorption performance. J. Water Process Eng. 2020, 33, 101095. [Google Scholar] [CrossRef]
- Chakraborty, I.; Das, S.; Dubey, B.K.; Ghangrekar, M.M. High-density polyethylene waste-derived carbon as a low-cost cathode catalyst in microbial fuel cell. Int. J. Environ. Res. 2021, 15, 1085–1096. [Google Scholar] [CrossRef]
- Gnanaraj, J.S.; Lee, R.J.; Levine, A.M.; Wistrom, J.L.; Wistrom, S.L.; Li, Y.; Li, J.; Akato, K.; Naskar, A.K.; Paranthaman, M.P. Sustainable waste tire derived carbon material as a potential anode for lithium-ion batteries. Sustainability 2018, 10, 2840. [Google Scholar] [CrossRef] [Green Version]
- Hussin, F.; Aroua, M.K.; Kassim, M.A.; Ali, U.F.M. Transforming plastic waste into porous carbon for capturing carbon dioxide: A review. Energies 2021, 14, 8421. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Behaviour of waste polypropylene pyrolysis char-based epoxy composite materials. Environ. Sci. Pollut. Res. 2020, 27, 3871–3884. [Google Scholar] [CrossRef]
- Verma, A.; Baurai, K.; Sanjay, M.R.; Siengchin, S. Mechanical, microstructural, and thermal characterization insights of pyrolyzed carbon black from waste tires reinforced epoxy nanocomposites for coating application. Polym. Compos. 2020, 41, 338–349. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011, 173, 62–71. [Google Scholar] [CrossRef]
- Kwapinski, W. Char production technology. In Char and Carbon Materials Derived from Biomass; Jeguirim, M., Limousy, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Park, C.; Lee, N.; Cho, I.S.; Ahn, B.; Yu, H.K.; Lee, J. Effects of cobalt oxide catalyst on pyrolysis of polyester fiber. Korean J. Chem. Eng. 2022, 39, 3343–3349. [Google Scholar] [CrossRef]
- Juma, M.; Koreňová, Z.; Markoš, J.; Annus, J.; Jelemenský, Ľ. Pyrolysis and combustion of scrap tire. Petroleum Coal 2006, 48, 15–26. [Google Scholar]
- Burra, K.G.; Gupta, A.K. Synergistic effects in steam gasification of combined biomass and plastic waste mixtures. Appl. Energy 2018, 211, 230–236. [Google Scholar] [CrossRef]
- Xu, D.; Xiong, Y.; Ye, J.; Su, Y.; Dong, Q.; Zhang, S. Performances of syngas production and deposited coke regulation during co-gasification of biomass and plastic wastes over Ni/γ-Al2O3 catalyst: Role of biomass to plastic ratio in feedstock. Chem. Eng. J. 2020, 392, 123728. [Google Scholar] [CrossRef]
- Ahmetli, G.; Kocaman, S.; Ozaytekin, I.; Bozkurt, P. Epoxy composites based on inexpensive char filler obtained from plastic waste and natural resources. Polym. Compos. 2013, 34, 500–509. [Google Scholar] [CrossRef]
- Sogancioglu, M.; Yel, E.; Ahmetli, G. Pyrolysis of waste high density polyethylene (HDPE) and low density polyethylene (LDPE) plastics and production of epoxy composites with their pyrolysis chars. J. Clean. Prod. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Yucel, A.; Sogancioglu, M.; Yel, E.; Ahmetli, G. Evaluation of poly (ethylene terephtalate) waste char in epoxy based composites. In Proceedings of the 2nd International Conference on Sustainable Agriculture and Environment, Konya, Turkey, 30 September–3 October 2015; pp. 425–429. [Google Scholar]
- Sogancioglu, M.; Yucel, A.; Yel, E.; Ahmetli, G. Production of epoxy composite from the pyrolysis char of washed PET wastes. Energy Procedia 2017, 118, 216–220. [Google Scholar] [CrossRef]
- Öner, G.A. Flexural strength and thermal properties of carbon black nanoparticle reinforced epoxy composites obtained from waste tires. Open Chem. 2022, 20, 863–872. [Google Scholar] [CrossRef]
- Karabork, F. Investigation of the mechanical, tribological and corrosive properties of epoxy composite coatings reinforced with recycled waste tire products. Express Polym. Lett. 2022, 16, 1114–1127. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Subadra, S.P.; Striūgas, N. Functionalization of char derived from pyrolysis of metallised food packaging plastics waste and its application as a filler in fiberglass/epoxy composites. Process Saf. Environ. Prot. 2021, 147, 723–733. [Google Scholar] [CrossRef]
- de Paula, F.G.F.; de Castro, M.C.M.; Ortega, P.F.R.; Blanco, C.; Lavall, R.L.; Santamaría, R. High value activated carbons from waste polystyrene foams. Microporous Mesoporous Mater. 2018, 267, 181–184. [Google Scholar] [CrossRef]
- Samal, B.; Vanapalli, K.R.; Dubey, B.K.; Bhattacharya, J.; Chandra, S.; Medha, I. Influence of process parameters on thermal characteristics of char from co-pyrolysis of eucalyptus biomass and polystyrene: Its prospects as a solid fuel. Energy 2021, 232, 121050. [Google Scholar] [CrossRef]
- Özaytekin, İ.; Kar, Y. Synthesis and properties of composites of oligoazomethine with char. J. Appl. Polym. Sci. 2012, 123, 815–823. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, N.; Yang, H.; Wu, C. Application of carbon nanotubes from waste plastics as filler to epoxy resin composite. ACS Sustain. Chem. Eng. 2022, 10, 2204–2213. [Google Scholar] [CrossRef] [PubMed]

| No. | Plastic Waste | Pyrolysis Conditions | Pyrolysate Yield (%) | Char Properties | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | Heating Rate (°C min−1) | Gas | Oil | Char | Surface Area (m2 g−1) | Particle Size (µm) | Elemental Composition (wt%) | |||
| 1 | Polypropylene (PP) waste | 300–700 | 5 | 17.7–22.8 | 75.1–79.6 | 2.2–2.7 | 13.5–22.0 | - | - | [41] |
| 2 | Polyethylene terephthalate (PET) waste | 450 | - | - | - | - | - | <63 | C = 74.7, O = 21.8, K = 2.4, Mg = 0.3, Ca = 0.8 | [49] |
| 3 | High-density polyethylene (HDPE) waste | 300–700 | 5 | 9.1–14 | 83.8–88.5 | 2.1–2.3 | - | <63 | - | [50] |
| 4 | Low-density polyethylene (LDPE) waste | 300–700 | 5 | 11.5–21.4 | 72.9–78.4 | 6.4–10.1 | - | <63 | - | [50] |
| 5 | PET waste | 300–700 | - | - | - | - | - | <63 | - | [51] |
| 6 | PET waste | 300–700 | - | - | - | - | - | ~63 | - | [52] |
| 7 | Tire waste | - | - | - | - | - | - | - | - | [53] |
| 8 | Tire waste | ~525 | - | - | - | - | 30.4 | <45 | C = 79.2, S = 1.5 | [54] |
| 9 | Tire waste | ~315 | - | - | - | - | - | 50–70 nm (8-h milling at >2500 rpm) | C = 86.0, O = 5.4, S = 2.3, Zn = 5.1, Al = 0.4, Si = 0.7 | [42] |
| 10 | Food packaging plastic waste | 600 | 25 | - | - | 18.6 | - | 10–15 | - | [55] |
| 11 | Expanded polystyrene (PS) foam waste | 530 a | 10 | - | - | - | 2712 | - | C = 94.4, O = 3.8, H = 0.2, N = 0.2 | [56] |
| 12 | PS waste + Eucalyptus biomass b | 300–550 | 10 | - | - | 18–38 | - | - | Fixed C = 4.5–34.2 | [57] |
| No. (Same as No. in Table 1) | Epoxy Resin | Char Feedstock | Additive Dosage (%) | Condition for Epoxy Composite Synthesis | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Char | Other Supplement (Dosage) | Preparation | Degassing | Curing | ||||
| 1 | Not specified | PP waste | 10–50 |
|
|
|
| [41] |
| 2 | NPEL-128 | PET waste | 5–30 |
|
|
|
| [49] |
| 3 | NPEK-114 | HDPE waste | 10–50 |
|
|
|
| [50] |
| 4 | NPEK-114 | LDPE waste | 10–50 |
|
|
|
| [50] |
| 5 | NPEK-114 | PET waste | 10–50 |
|
|
|
| [51] |
| 6 | NPEK-114 | PET waste | 10–50 |
|
|
|
| [52] |
| 7 | DTE-1200 | Tire waste | - |
|
| - | - | [53] |
| 8 | Polires-188 | Tire waste | 3 |
|
|
|
| [54] |
| 9 | CY-230 | Tire waste | 5–15 |
|
|
|
| [42] |
| 10 | MGS RIMR-135 | Food packaging plastic waste | 0.25–1 |
|
|
|
| [55] |
| No. (Same as No. in Table 1) | Epoxy Composite | Elongation at Break (%) | Tensile Strength (MPa) | Young’s Modulus (GPa) | Hardness (Shore D, Otherwise Mentioned) | Electrical Conductivity (S cm−1) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neat Epoxy | Composite | Neat Epoxy | Composite | Neat Epoxy | Composite | Neat Epoxy | Composite | Neat Epoxy | Composite | |||
| 1 | PP waste char/epoxy resin a | 0.71 | 0.62 | 85 | 99 | 6.2 | 7.7 | 80 | 83 | 10−14 | 4.2 × 10−7 | [41] |
| 2 | PET waste char/NPEL-128 b | 0.53 | 0.52 | 0.47 | 0.59 | 82 | 110.7 | 83 | 87.6 | 10−14 | 2.0 × 10−5 | [49] |
| 3 | HDPE waste char/NPEK-114 a | 0.52 | 0.55 | 62 | 72 | - | - | 80 | 85 | 8.4 × 10−13 | 4.7 × 10−5 | [50] |
| 4 | LDPE waste char/NPEK-114 a | 0.52 | 0.25 | 62 | 42 | - | - | 80 | 73 | 8.4 × 10−13 | 4.3 × 10−8 | [50] |
| 5 | PET waste char/NPEK-114 a | 0.72 | 0.69 | 86 | 97 | 6.2 | 9.4 | - | - | - | - | [51] |
| 6 | PET waste char/NPEK-114 a | - | - | 62 | 98 | - | - | 80 | 85 | - | 7.98 × 10−5 | [52] |
| 7 | Tire waste char/DTE-1200 c | - | - | - | - | - | - | - | - | - | - | [53] |
| 8 | Tire waste char/Polires-188 | - | - | - | - | 6.7 | 3.0 | 415.9 MPa | 165.7 MPa | - | - | [54] |
| 9 | Tire waste char/CY-230 d | 7.1 | 7.6 | 33.8 | 34.6 | 0.63 | 0.74 | 130 HRL | 140.7 HRL | 1.96 × 10−3 | 2.4 × 10−3 | [42] |
| 10 | Food packaging plastic waste char/MGS RIMR 135 e | 2.3 | 1.8 | 188.2 | 176.4 | 6.58 | 7.79 | - | - | - | - | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kim, Y.T.; Lin, K.-Y.A.; Lee, J. Plastic-Waste-Derived Char as an Additive for Epoxy Composite. Materials 2023, 16, 2602. https://doi.org/10.3390/ma16072602
Lee S, Kim YT, Lin K-YA, Lee J. Plastic-Waste-Derived Char as an Additive for Epoxy Composite. Materials. 2023; 16(7):2602. https://doi.org/10.3390/ma16072602
Chicago/Turabian StyleLee, Seonho, Yong Tae Kim, Kun-Yi Andrew Lin, and Jechan Lee. 2023. "Plastic-Waste-Derived Char as an Additive for Epoxy Composite" Materials 16, no. 7: 2602. https://doi.org/10.3390/ma16072602
APA StyleLee, S., Kim, Y. T., Lin, K.-Y. A., & Lee, J. (2023). Plastic-Waste-Derived Char as an Additive for Epoxy Composite. Materials, 16(7), 2602. https://doi.org/10.3390/ma16072602




_Lin.png)


