The Influence of Diisocyanate Structure on Thermal Stability of Thermoplastic Polyurethane Elastomers Based on Diphenylmethane-Derivative Chain Extender with Sulfur Atoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
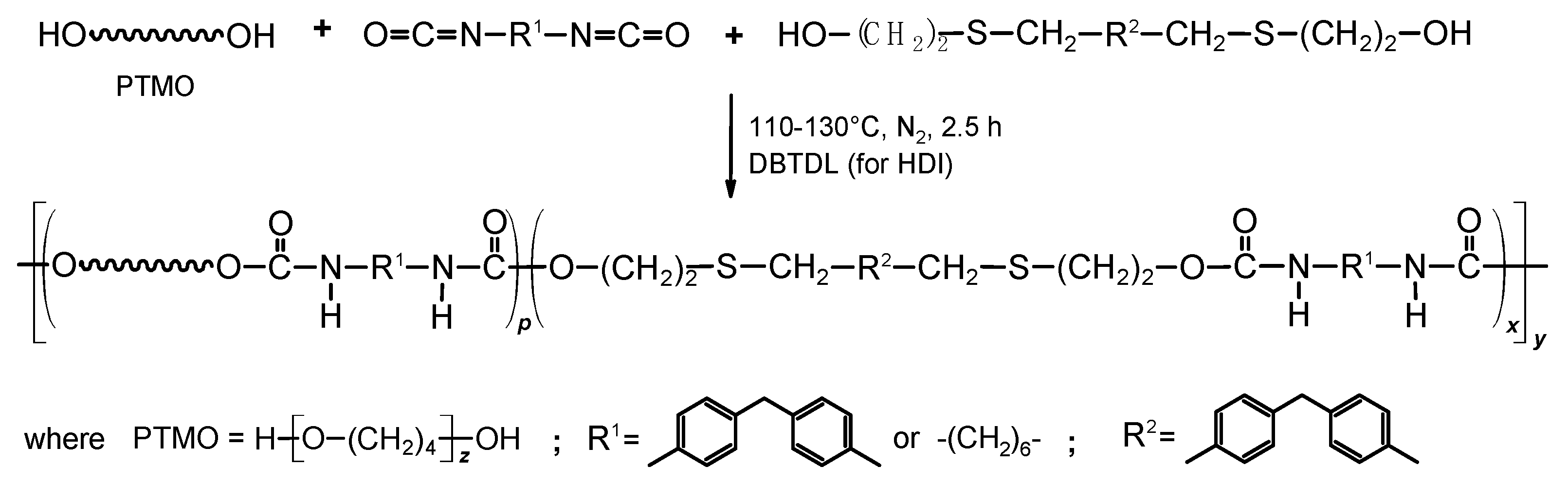
2.2. Synthesis of TPUs
2.3. Measurement Methods
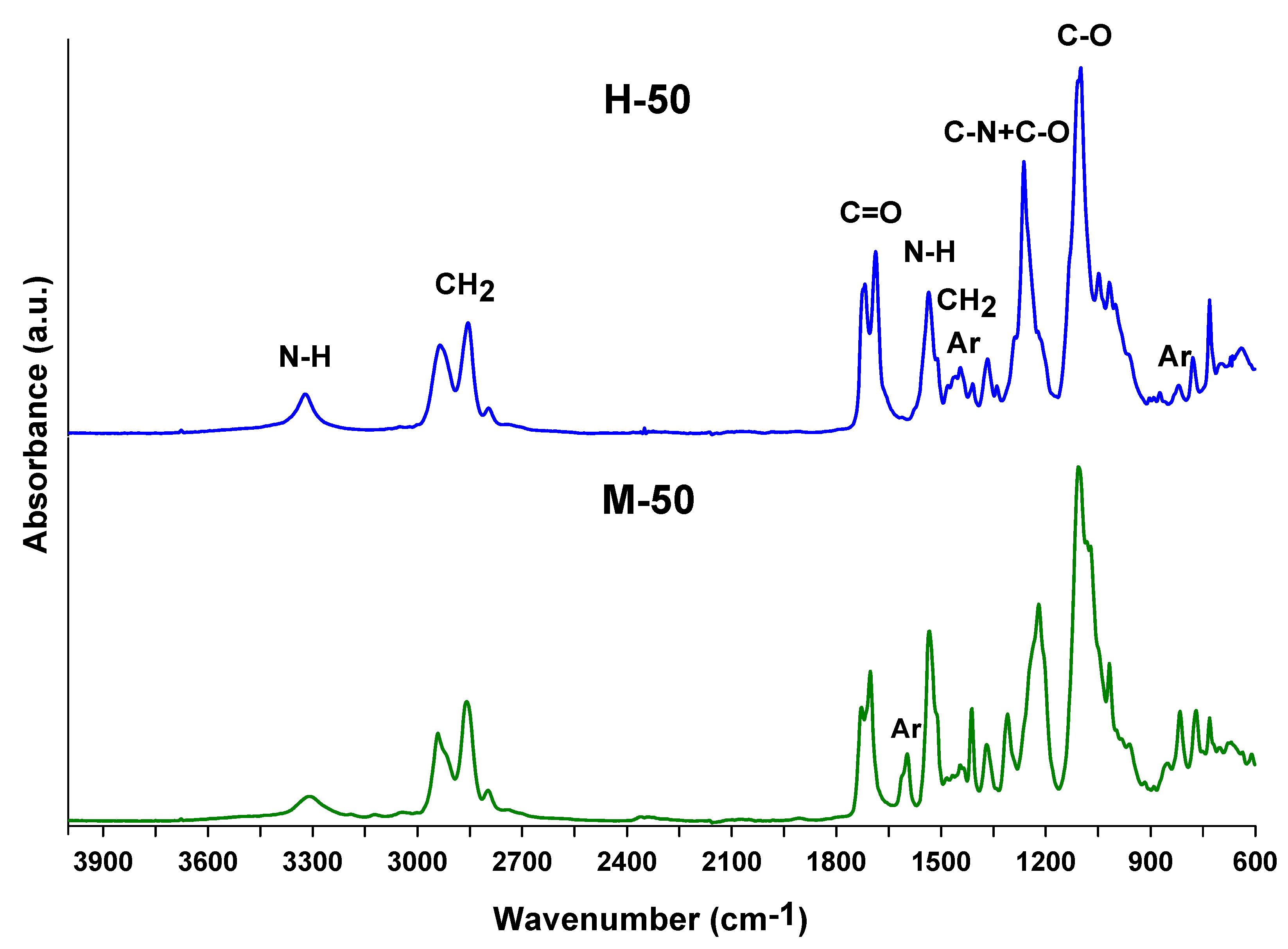
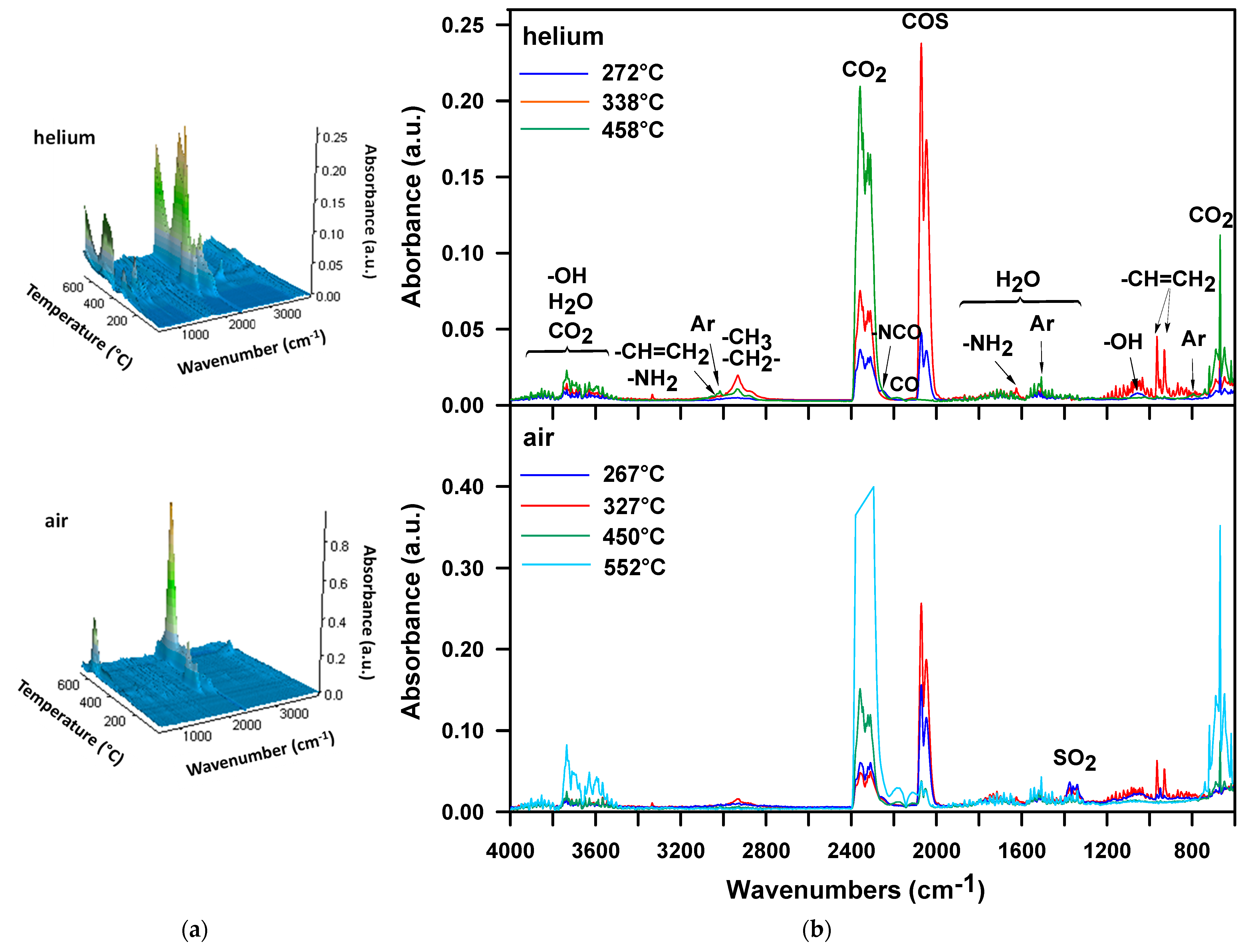
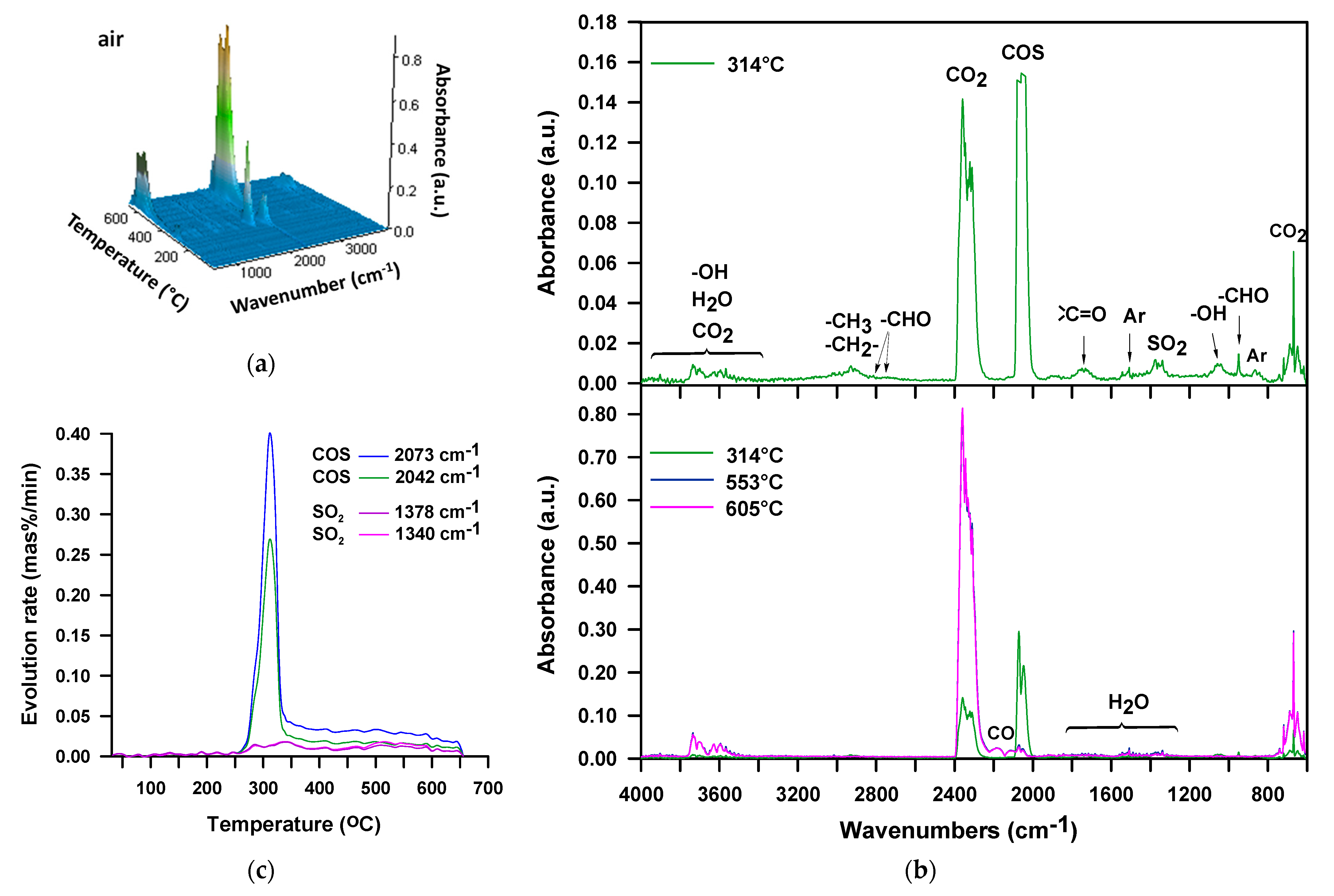
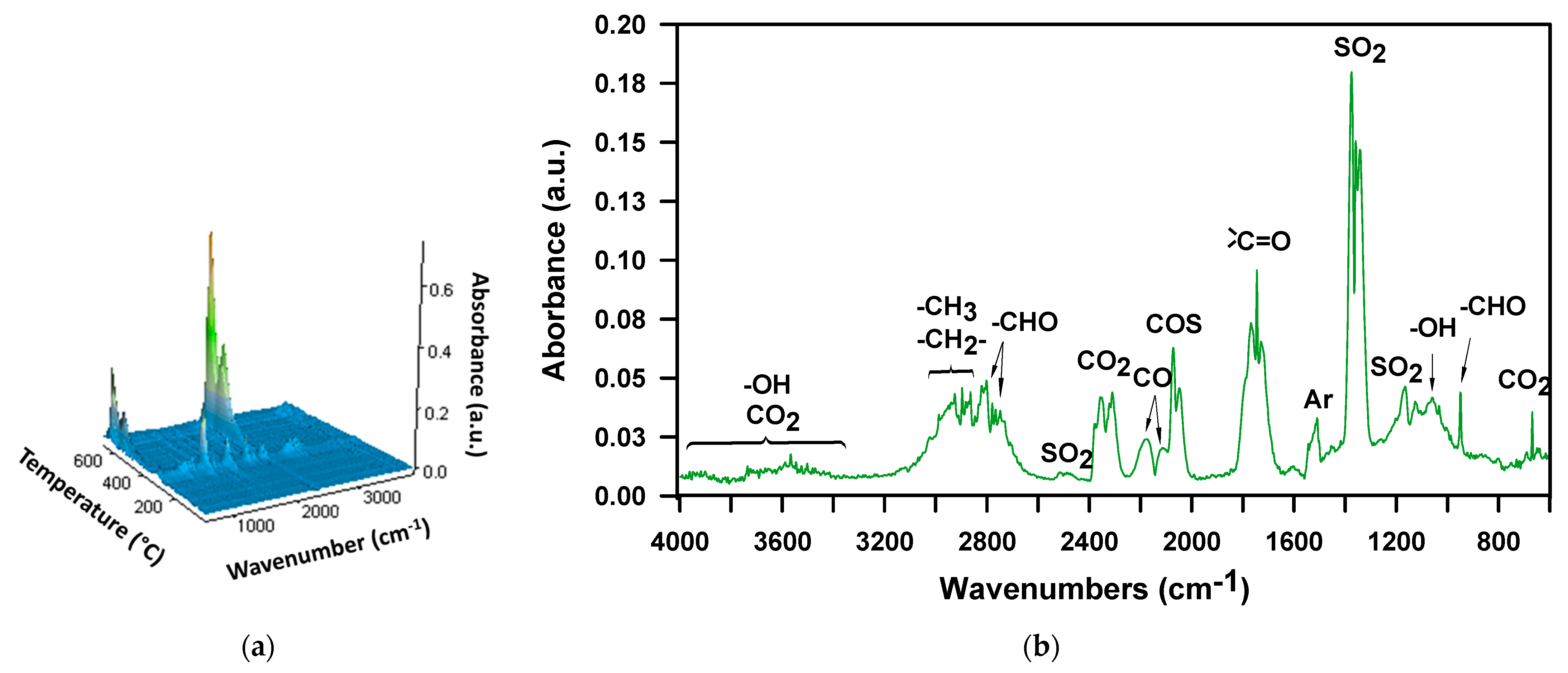
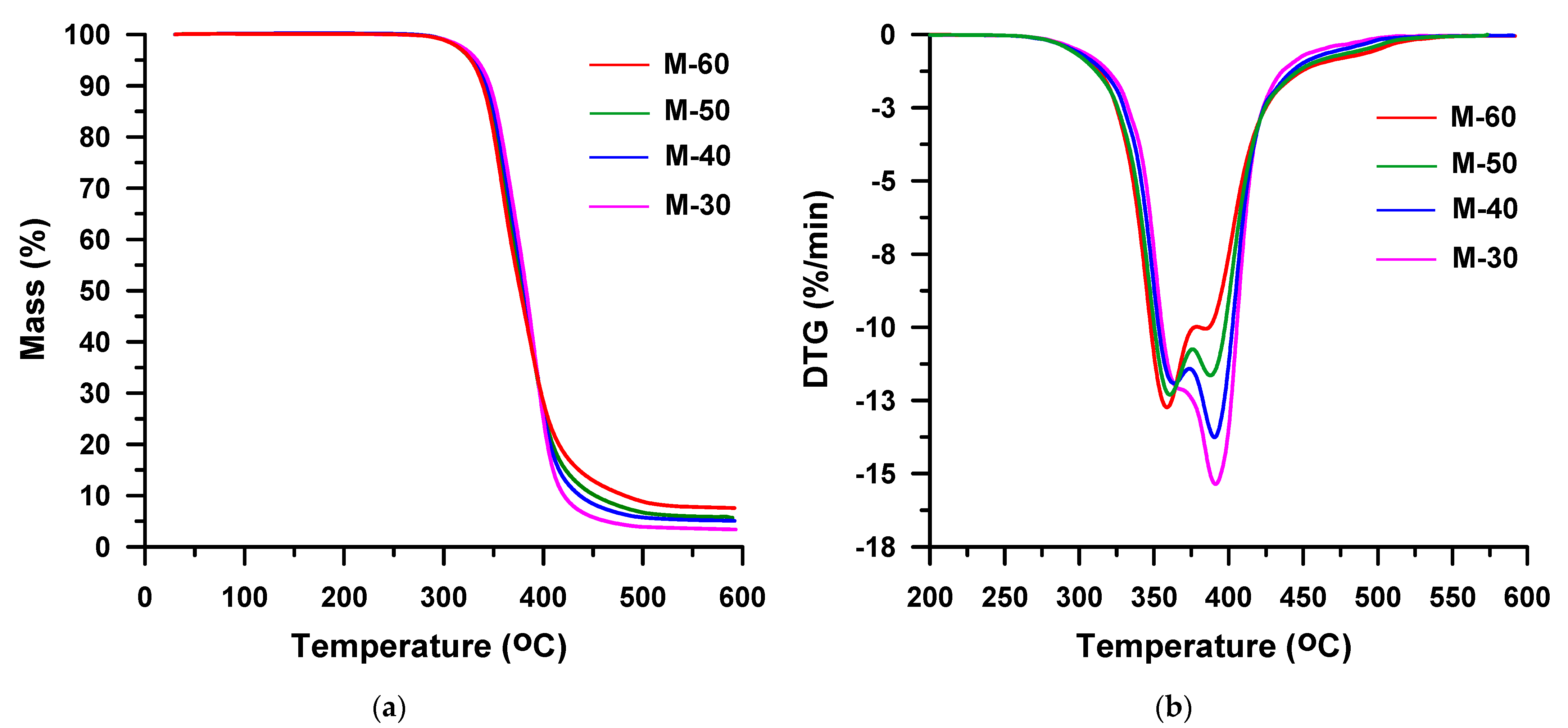
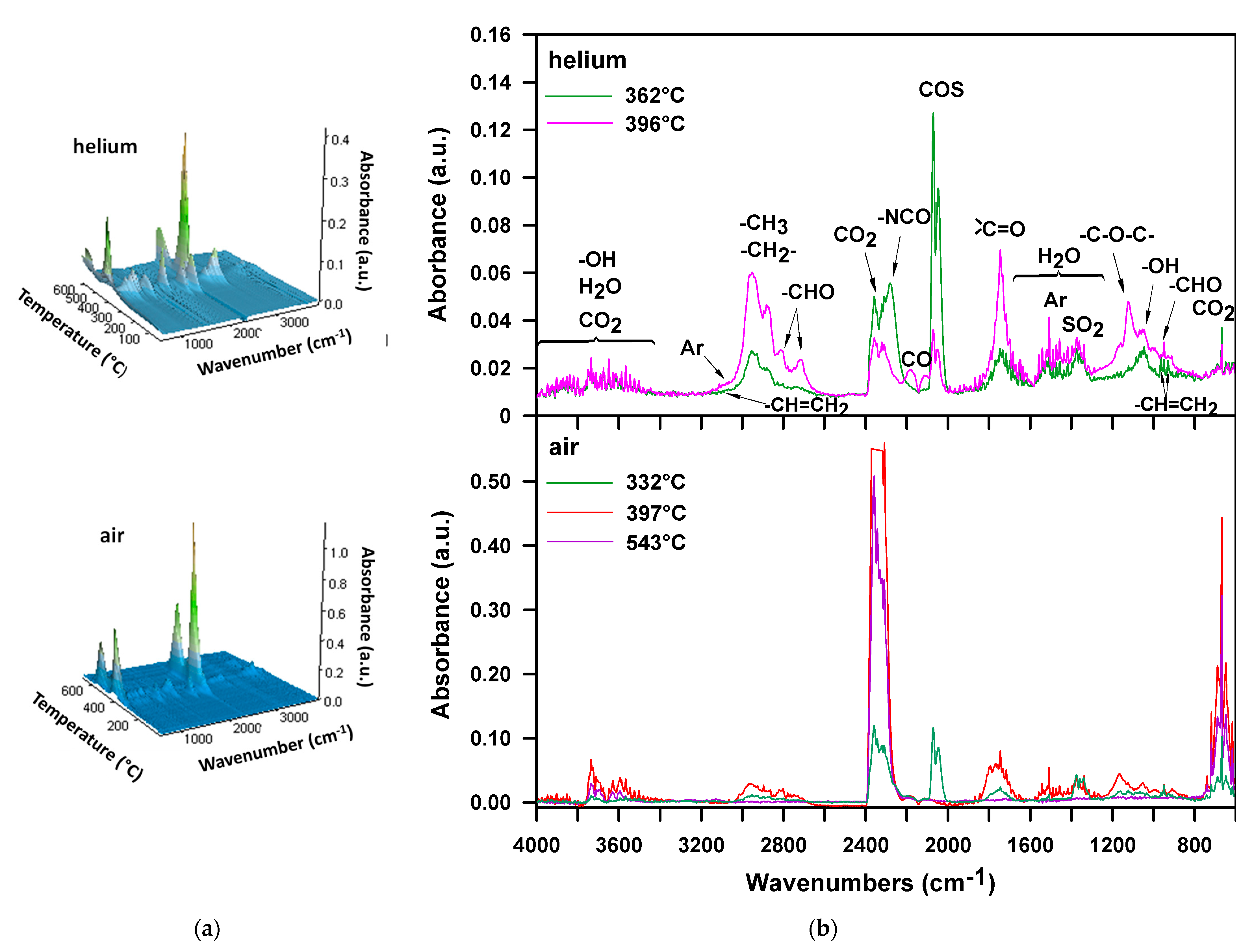
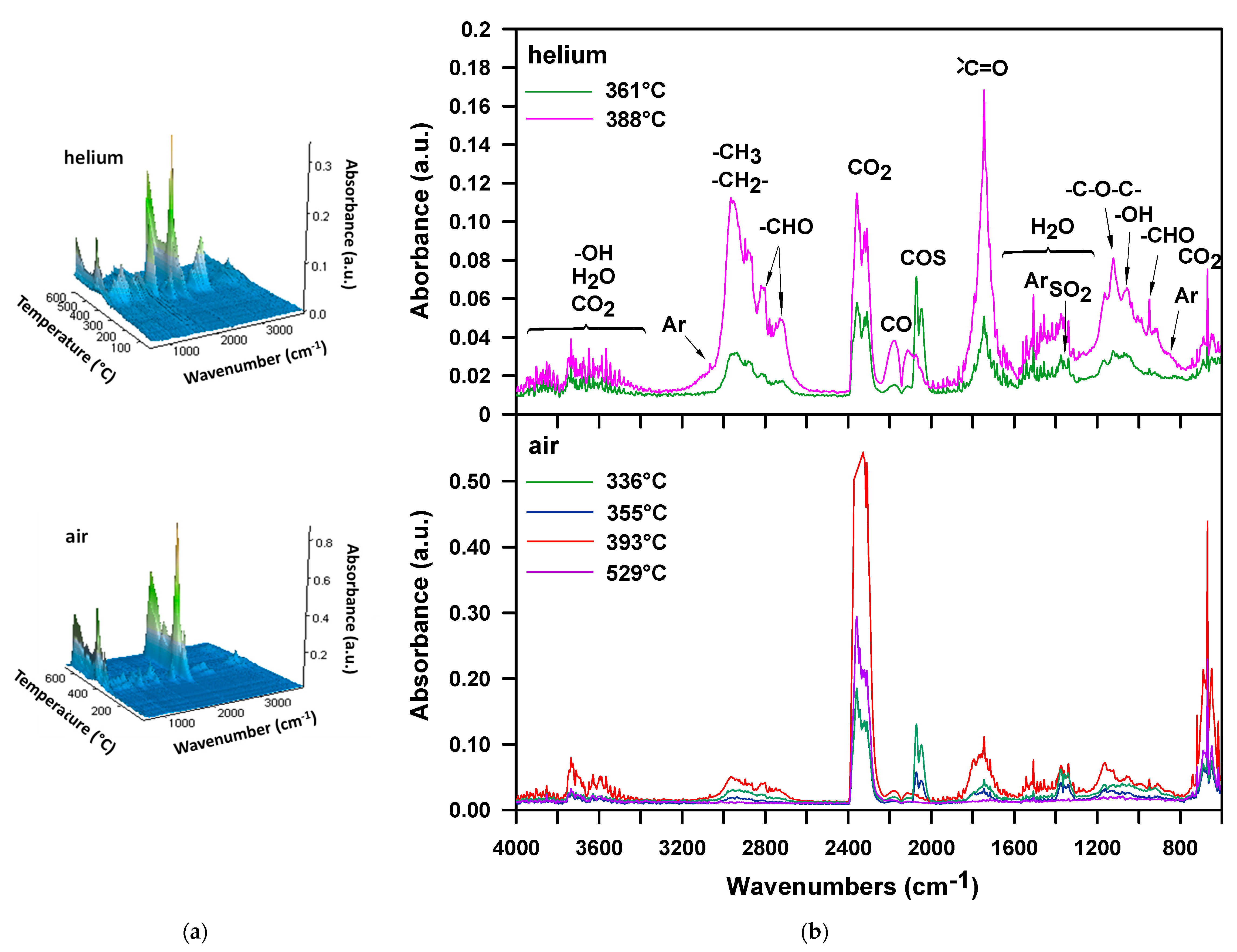
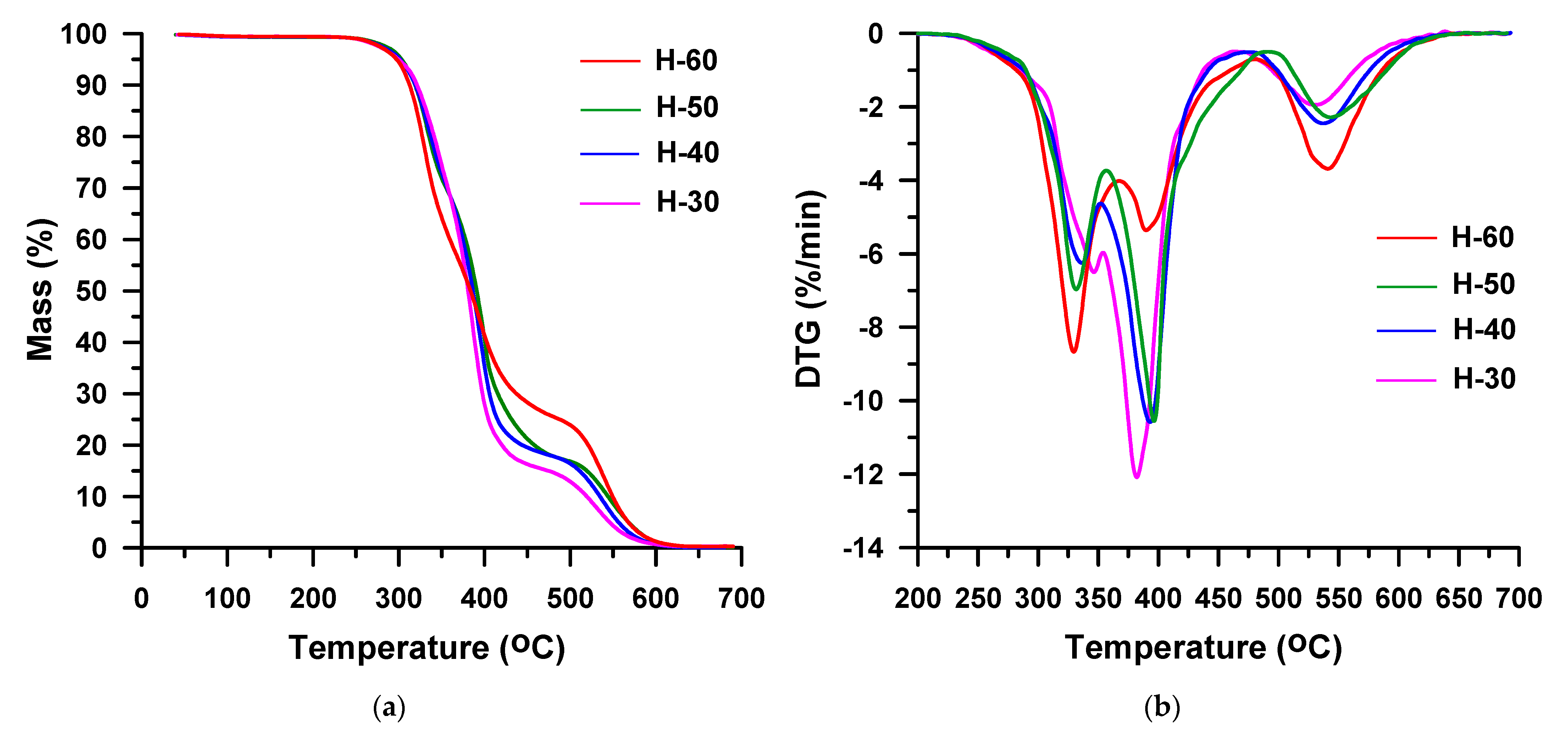
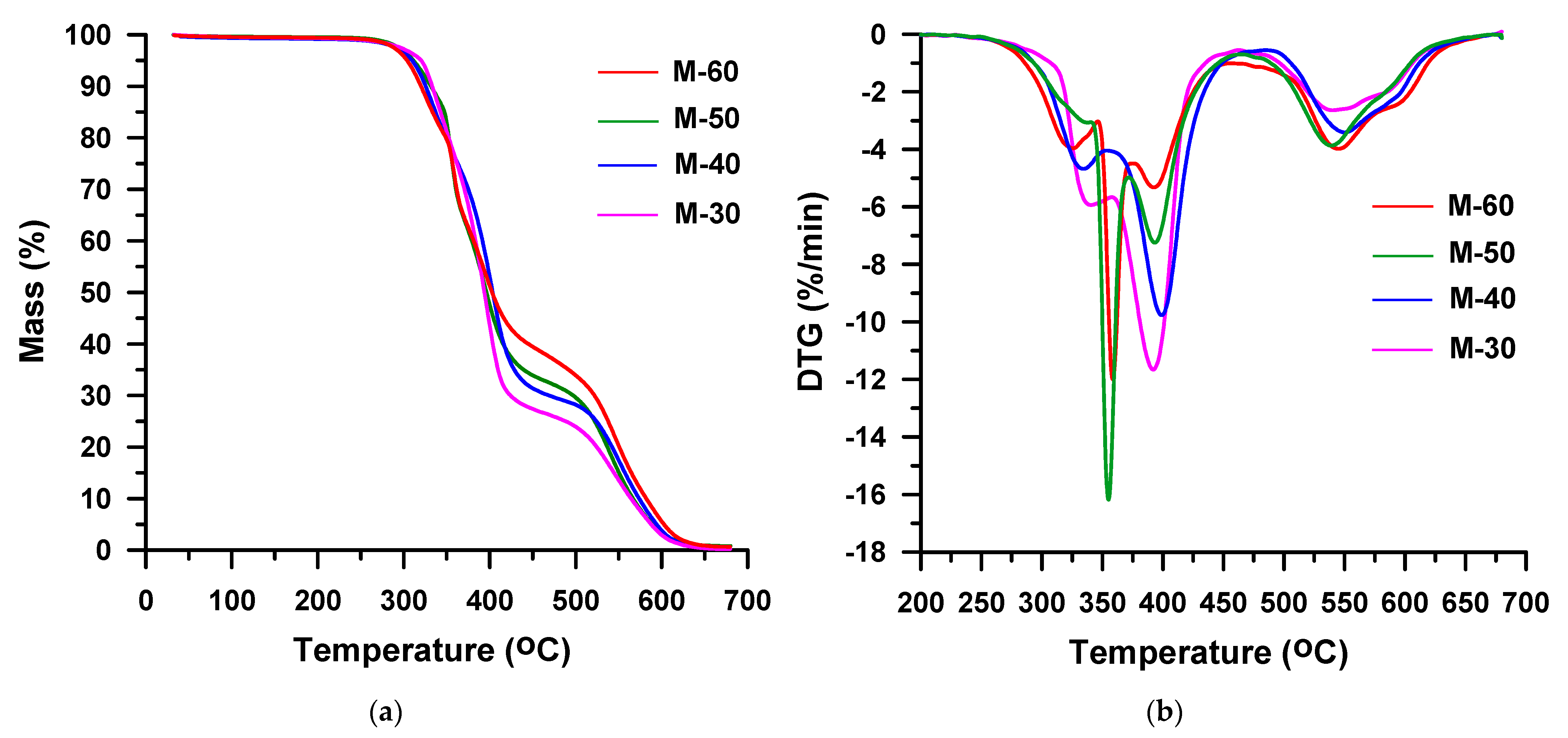
3. Results and Discussion
| TPU | Hard-Segment Content (Mas%) | Diisocyanate | T1% (°C) | T5% (°C) | T10% (°C) | T50% (°C) | Tmax (°C) | Mass Losses (%) |
|---|---|---|---|---|---|---|---|---|
| H-30 | 30 | HDI | 281 | 322 | 341 | 388 | 398 | 99 |
| H-40 | 40 | 280 | 316 | 335 | 383 | 370, 398 | 38, 61 | |
| H-50 | 50 | 282 | 315 | 334 | 380 | 362, 396 | 41, 58 | |
| H-60 | 60 | 282 | 313 | 328 | 370 | 357, 393 | 60, 39 | |
| H-100 | 100 | 203 | 254 | 282 | 353 | 272, 338, 458 | 10, 56, 17 | |
| M-30 | 30 | MDI | 301 | 333 | 346 | 383 | 370, 391 | 36, 62 |
| M-40 | 40 | 301 | 331 | 344 | 382 | 364, 391 | 44, 53 | |
| M-50 | 50 | 300 | 328 | 340 | 378 | 361, 388 | 47, 48 | |
| M-60 | 60 | 299 | 328 | 339 | 377 | 359, 385 | 53, 41 | |
| M-100 * | 100 | 259 | 315 | 344 | 380 | 373, 521 | - |
| TPU | T1% (°C) | T5% (°C) | T10% (°C) | T50% (°C) | Tmax (°C) | Mass Losses (%) |
|---|---|---|---|---|---|---|
| H-30 | 265 | 306 | 324 | 382 | 346, 382, 530 | 27, 57, 15 |
| H-40 | 264 | 306 | 322 | 387 | 336, 393, 537 | 28, 54, 17 |
| H-50 | 262 | 307 | 321 | 392 | 332, 397, 543 | 30, 52, 17 |
| H-60 | 252 | 302 | 315 | 386 | 330, 389, 541 | 42, 32, 25 |
| H-100 | 180 | 246 | 267 | 413 | 267, 327, 450, 552 | 14, 33, 12, 41 |
| M-30 | 272 | 320 | 333 | 352 | 340, 392, 540 | 23, 51, 20 |
| M-40 | 270 | 310 | 325 | 403 | 335, 399, 549 | 25, 49, 26 |
| M-50 | 268 | 311 | 331 | 395 | 336, 355, 393, 529 | 13, 25, 31, 31 |
| M-60 | 261 | 304 | 320 | 401 | 326, 359, 392, 545 | 19, 19, 24, 38 |
| M-100 | 237 | 283 | 297 | 529 | 314, 553, 605 | 38, 42, 20 |
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ulrich, H. Polyurethanes. In Encyclopedia of Polymers Science and Technology; Mark, H.F., Ed.; Wiley: Hoboken, NJ, USA, 2003; Volume 4, pp. 26–72. [Google Scholar]
- Holden, G. Thermoplastic elastomers. In Kirk-Othmer Encyclopedia of Chemical Technology; Kroschwitz, J., Ed.; Wiley Interscience: New York, NY, USA, 2001; Volume 24, pp. 695–718. [Google Scholar]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Gogolewski, S. Selected topics in biomedical polyurethanes. A review. Colloid Polym. Sci. 1989, 267, 757–785. [Google Scholar] [CrossRef]
- Stokes, K.; McVenes, R.; Anderson, J.M. Polyurethane elastomer biostability. J. Biomater. Appl. 1995, 9, 321–354. [Google Scholar] [CrossRef] [PubMed]
- Zdrahala, R.J. Small Caliber Vascular Grafts. Part II: Polyurethanes Revisited. J. Biomater. Appl. 1996, 11, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Piegat, A.; El Fray, M. Thermoplastic Elastomers: Materials for Heart Assist Devices. Polim. Med. 2016, 46, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Sun, L.; Wei, W.; Taylor, D.K.; Su, S.; Yu, H. Structure and performance control of high-damping bio-based thermoplastic polyurethane. J. Appl. Polym. Sci. 2022, 139, e52059. [Google Scholar] [CrossRef]
- Yang, L.; Ou, Z.; Jiang, G. Research progress of elastomer materials and application of elastomers in drilling fluid. Polymers 2023, 15, 918. [Google Scholar] [CrossRef] [PubMed]
- Wirpsza, Z. Polyurethanes: Chemistry, Technology and Applications; Ellis Horwood: New York, NY, USA, 1993. [Google Scholar]
- Rosu, L.; Varganic, C.D.; Rosu, D.; Oprea, S. Effect of Thermal Aging on the Physico-Chemical and Optical Properties of Poly(ester urethane) Elastomers Designed for Passive Damping (Pads) of the Railway. Polymers 2021, 13, 192. [Google Scholar] [CrossRef]
- Hepburn, C. Polyurethane Elastomers; Elsevier Science Publishers Ltd.: London, UK, 1992. [Google Scholar]
- Jin, X.; Guo, N.; You, Z.; Tan, Y. Design and Performance of Polyurethane Elastomers Composed with Different Soft Segments. Materials 2020, 13, 4991. [Google Scholar] [CrossRef] [PubMed]
- Mizera, K.; Sałasińska, K.; Ryszkowska, J.; Kurańska, M.; Kozera, R. Effect of the Addition of Biobased Polyols on the Thermal Stability and Flame Retardancy of Polyurethane and Poly(urea)urethane Elastomers. Materials 2021, 14, 1805. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Asensio, M.; Ferrer, J.F.; Nohales, A.; Culebras, M.; Gómez, C.M. The Role of Diisocyanate Structure to Modify Properties of Segmented Polyurethanes. Materials 2023, 16, 1633. [Google Scholar] [CrossRef] [PubMed]
- Prisacariu, C. Polyurethane Elastomers: From Morphology to Mechanical Aspects; Springer: Wien, Austria, 2011. [Google Scholar]
- Govorčin Bajsić, E.; Rek, V.; Agić, A. Thermal degradation of polyurethane elastomers: Determination of kinetic parameters. J. Elastom. Plast. 2003, 35, 311–323. [Google Scholar] [CrossRef]
- Gomez, C.M.; Gutierrez, D.; Asensio, M.; Costa, V.; Nohales, A. Transparent thermoplastic polyurethanes based on aliphatic diisocyanates and polycarbonate diol. J. Elastom. Plast. 2016, 49, 77–95. [Google Scholar] [CrossRef]
- Datta, J.; Kasprzyk, P. Thermoplastic polyurethanes derived from petrochemical or renewable resources: A comprehensive review. Polym. Eng. Sci. 2018, 58, E14–E35. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stab. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Patti, A.; Costa, F.; Perrotti, M.; Barbarino, D.; Acierno, D. Polyurethane Impregnation for Improving the Mechanical and the Water Resistance of Polypropylene-Based Textiles. Materials 2021, 14, 1951. [Google Scholar] [CrossRef]
- Karak, N. Vegetable Oil-Based Polymers. Properties, Processing and Applications; Woodhead Publishing Ltd.: Cambridge, UK, 2012. [Google Scholar]
- Oprea, S.; Joga, A.; Zorlescu, B.; Oprea, V. Effect of the hard segment structure on properties of resorcinol derivatives-based polyurethane elastomers. High Perform. Polym. 2014, 26, 859–866. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Li, J.; Cheng, J.; Zhang, J. Synthesis and properties of segmented polyurethanes with hydroquinone ether derivatives as chain extender. J. Polym. Res. 2015, 22, 149. [Google Scholar] [CrossRef]
- Su, S.K.; Gu, J.H.; Lee, H.T.; Yu, S.H.; Wu, C.L.; Suen, M.C. Effects of an Aromatic Fluoro-diol and Polycaprolactone on the Properties of the Resultant Polyurethanes. Adv. Polym. Technol. 2018, 37, 1142–1152. [Google Scholar] [CrossRef]
- Padmavathy, T.; Srinivasan, K.S.V. Liquid crystalline polyurethanes—A review. J. Macromol. Sci. Polym. Rev. 2003, C43, 45–85. [Google Scholar] [CrossRef]
- Liaw, D.J. The relative physical and thermal properties of polyurethane elastomers: Effect of chain extenders of bisphenols, diisocyanate, and polyol structures. J. Appl. Polym. Sci. 1997, 66, 1251–1265. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A. Aliphatic polycarbonate-based thermoplastic polyurethane elastomers containing diphenyl sulfide units. J. Therm. Anal. Calorim. 2016, 126, 225–243. [Google Scholar] [CrossRef]
- Puszka, A.; Kultys, A. The influence of soft segments on some properties of new transparent segmented polyurethanes. Polym. Adv. Technol. 2017, 28, 1937–1944. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Puszka, A. New thermoplastic poly(carbonate-urethane)s based on chain extenders with sulfur atoms. Chem. Pap. 2017, 71, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Rogulska, M. Transparent sulfur-containing thermoplastic polyurethanes with polyether and polycarbonate soft segments. Polym. Bull. 2018, 75, 1211–1235. [Google Scholar] [CrossRef]
- Rogulska, M. Polycarbonate-based thermoplastic polyurethane elastomers modified by DMPA. Polym. Bull. 2019, 76, 4719–4733. [Google Scholar] [CrossRef]
- Puszka, A. Thermal and Mechanical Behavior of New Transparent Thermoplastic Polyurethane Elastomers Derived from Cycloaliphatic Diisocyanate. Polymers 2018, 10, 537. [Google Scholar] [CrossRef]
- Rogulska, M. New thermoplastic poly(carbonate-urethane)s based on diphenylethane-derivative chain extenders—The effect of chain extender structure on thermal and mechanical properties. J. Therm. Anal. Calorim. 2020, 139, 3107–3121. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Olszewska, E. New thermoplastic poly(thiourethane-urethane) elastomers based on hexane-1,6-diyl diisocyanate (HDI). J. Therm. Anal. Calorim. 2013, 114, 903–916. [Google Scholar] [CrossRef]
- Kultys, A.; Puszka, A. Transparent poly(thiourethane-urethane)s based on dithiol chain extender. J. Therm. Anal. Calorim. 2014, 117, 1427–1439. [Google Scholar] [CrossRef]
- Puszka, A.; Sikora, J.W. Synthesis and Characterization of New Polycarbonate-Based Poly(thiourethane-urethane)s. Polymers 2022, 14, 2933. [Google Scholar] [CrossRef] [PubMed]
- Puszka, A.; Sikora, J.W. New Segmented Poly(Thiourethane-Urethane)s Based on Poly(ε-Caprolactone)Diol Soft Segment: Synthesis and Characterization. Materials 2022, 15, 4940. [Google Scholar] [CrossRef] [PubMed]
- Duda, A.; Penczek, S. Sulfur-containing polymers. In Encyclopedia of Polymers Science and Technology; Mark, H.F., Ed.; Wiley: Hoboken, NJ, USA, 1989; Volume 16, pp. 246–368. [Google Scholar]
- Kultys, A. Sulfur-containing polymers. In Encyclopedia of Polymers Science and Technology; Mark, H.F., Ed.; Wiley: Hoboken, NJ, USA, 2003; Volume 4, pp. 336–393. [Google Scholar]
- Kausar, A.; Zulfiqar, S.; Sarwar, M.I. Recent Developments in Sulfur-Containing Polymers. Polym. Rev. 2014, 54, 185–267. [Google Scholar] [CrossRef]
- Hirano, H.; Kadota, J.; Agari, Y.; Harada, T.; Tanaka, M.; Hasegawa, K. Linear polymers with sulfur in the main chain. IV. Synthesis of thermotropic liquid-crystalline polythioesters based on 4,4′-biphenyldithiol with excellent adhesive properties. Polym. Eng. Sci. 2007, 47, 262–269. [Google Scholar] [CrossRef]
- Rahmawati, R.; Nozaki, S.; Kojio, K.; Takahara, A.; Shinohara, N.; Yamasaki, S. Microphase-separated structure and mechanical properties of cycloaliphatic diisocyanate-based thiourethane elastomers. Polym. J. 2009, 51, 265–273. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, H.; Wicks, D.A.; Hoyle, C.E.; Magers, D.H.; McAlexander, H.R. Comparison of Small Molecule and Polymeric Urethanes, Thiourethanes, and Dithiourethanes: Hydrogen Bonding and Thermal, Physical, and Mechanical Properties. Macromolecules 2009, 42, 1824–1833. [Google Scholar] [CrossRef]
- Shin, J.; Matsushima, H.; Chan, J.W.; Hoyle, C.E. Segmented polythiourethane elastomers through sequential thiol-ene and thiol-isocyanate reactions. Macromolecules 2009, 42, 3294–3301. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, D. A Novel Self-Healing Polyurethane Based on Disulfide Bonds. Macromol. Chem. Phys. 2016, 217, 1191–1196. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Yang, B.; Wang, L.; Sun, H. A colorless, transparent and self-healing polyurethane elastomer modulated by dynamic disulfide and hydrogen bonds. New J. Chem. 2020, 44, 5746. [Google Scholar] [CrossRef]
- Liu, D.; Fan, C.-J.; Xiao, Y.; Yang, K.-K.; Wang, Y.-Z. High strength, self-healing polyurethane elastomer based on synergistic multiple dynamic interactions in multiphase. Polymer 2022, 263, 125513. [Google Scholar] [CrossRef]
- Rogulska, M.; Puszka, A.; Malm, A.; Łoś, R. New thermoplastic polyurethane elastomers with polyether soft segment (in Polish). In Science and Industry-Spectroscopic Methods in Practice, New Challenges and Possibilities; Hubicki, Z., Ed.; UMCS: Lublin, Poland, 2018; pp. 130–133. [Google Scholar]
- Kultys, A.; Pikus, S. Polyurethanes containing sulfur. III. New thermoplastic HDI-based segmented polyurethanes with diphenylmethane unit in their structure. J. Polym. Sci. A Polym. Chem. 2001, 39, 1733–1742. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M.; Pikus, S. New thermoplastic segmented polyurethanes with hard segments derived from 4,4’-diphenylmethane diisocyanate and methylenebis(1,4-phenylenemethylenethio)dialcanols. J. Appl. Polym. Sci. 2012, 123, 331–346. [Google Scholar] [CrossRef]
- Pielichowski, K.; Słotwińska, D.; Dziwiński, E. Segmented MDI/HMDI-Based Polyurethanes with Lowered Flammability. J. Appl. Polym. Sci. 2004, 91, 3214–3224. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Rogulska, M.; Maciejewska, M.; Olszewska, E. New thermoplastic poly(carbonate-urethane)s based on diphenylethane derivative chain extender: The effect of diisocyanate structure on some properties. J. Therm. Anal. Calorim. 2020, 139, 1049–1068. [Google Scholar] [CrossRef]
- Simon, J.; Barla, F.; Kelemen-Haller, A.; Farkas, F.; Kraxner, M. Thermal stability of polyurethanes. Chromatographia. 1988, 25, 99–106. [Google Scholar] [CrossRef]
- Shufen, L.; Zhi, J.; Kaijun, Y.; Shuqin, Y.; Chow, W.K. Studies on the Thermal Behavior of Polyurethanes. Polym. Plast. Technol. Eng. 2006, 45, 95–108. [Google Scholar] [CrossRef]
- Govorčin Bajsić, E.; Rek, V. Thermal stability of polyurethane elastomers before and after UV irradiation. J. Appl. Polym. Sci. 2001, 79, 864–873. [Google Scholar] [CrossRef]
- Ferm, R.J. The chemistry of carbonyl sulfide. Chem. Rev. 1957, 57, 621–640. [Google Scholar] [CrossRef]
- Kultys, A.; Rogulska, M.; Gluchowska, H. The effect of soft-segment structure on the properties of novel thermoplastic polyurethane elastomers based on an unconventional chain extender. Polym. Int. 2011, 60, 652–659. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Lubczak, J. New thermoplastic polyurethane elastomers based on aliphatic-aromatic chain extenders with different content of sulfur atoms. J. Therm. Anal. Calorim. 2015, 121, 397–410. [Google Scholar] [CrossRef]
- Kumagai, S.; Motokucho, S.; Yabuki, R.; Anzai, A.; Kameda, T.; Watanabe, A.; Nakatani, H.; Yoshioka, T. Effects of hard- and soft-segment composition on pyrolysis characteristics of MDI, BD, and PTMG-based polyurethane elastomers. J. Anal. Appl. Pyrolysis 2017, 126, 337–345. [Google Scholar] [CrossRef]
- Król, P.; Urama, Ł.; Król, B.; Pielichowska, K.; Walczak, M. Study of chemical, physico-mechanical and biological properties of 4,4′-methylenebis(cyclohexyl isocyanate)-based polyurethane films. Mater. Sci. Eng. C. 2018, 93, 483–494. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Błażek, K.; Parcheta, P.; Datta, J. Green thermoplastic poly(ether-urethane)s—Synthesis, chemical structure and selected properties investigation. Polimery 2020, 65, 672–680. [Google Scholar] [CrossRef]
- Kasprzyk, P.; Głowińska, E.; Parcheta-Szwindowska, P.; Rohde, K.; Datta, J. Green TPUs from Prepolymer Mixtures Designed by Controlling the Chemical Structure of Flexible Segments. Int. J. Mol. Sci. 2021, 22, 7438. [Google Scholar] [CrossRef]
- Jung, Y.S.; Woo, J.; Lee, E.; Lee, S.; Shin, E.J. Synthesis and properties of bio-based thermoplastic poly(ether urethane) for soft actuators. J. Polym. Res. 2022, 29, 521. [Google Scholar] [CrossRef]
- Herrera, M.; Matuschek, G.; Kettrup, A. Thermal degradation of thermoplastic polyurethane elastomers (TPU) based on MDI. Polym. Degrad. Stab. 2002, 78, 323–331. [Google Scholar] [CrossRef]
- Otto, K.; Bombicz, P.; Madarász, J.; Acik, I.O.; Krunks, M.; Pokol, G. Structure and evolved gas analyses (TG/DTA-MS and TG-FTIR) of mer-trichlorotris(thiourea)-indium(III), a precursor for indium sulfide thin films. J. Therm. Anal. Calorim. 2011, 105, 83–91. [Google Scholar] [CrossRef]
- Costa, L.; Luda, M.P.; Cameron, G.G.; Qureshi, M.Y. The thermal and thermo-oxidative degradation of poly(tetrahydrofuran) and its complexes with LiBr and LiI. Polym. Degrad. Stabil. 2000, 67, 527–533. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Espinosa, J.I.; Cauich-Rodrıguez, J.V.; Avila-Ortega, A.; Vazquez-Torres, H.; Marcos-Fernandez, A.; San Roman, J. TGA/FTIR studies of segmented aliphatic polyurethanes and their nanocomposites prepared with commercial montmorillonites. Polym. Degrad. Stabil. 2009, 94, 1666–1677. [Google Scholar] [CrossRef]
- Lei, W.; Fang, C.; Zhou, X.; Cheng, Y.; Yang, R.; Liu, D. Morphology and thermal properties of polyurethane elastomer based on representative structural chain extenders. Thermochim. Acta 2017, 653, 116–125. [Google Scholar] [CrossRef]
- Rogulska, M.; Kultys, A.; Pikus, S. Studies on thermoplastic polyurethanes based on new diphenylethane-derivative diols. III. The effect of molecular weight and structure of soft segment on some properties of segmented polyurethanes. J. Appl. Polym. Sci. 2008, 110, 1677–1689. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogulska, M. The Influence of Diisocyanate Structure on Thermal Stability of Thermoplastic Polyurethane Elastomers Based on Diphenylmethane-Derivative Chain Extender with Sulfur Atoms. Materials 2023, 16, 2618. https://doi.org/10.3390/ma16072618
Rogulska M. The Influence of Diisocyanate Structure on Thermal Stability of Thermoplastic Polyurethane Elastomers Based on Diphenylmethane-Derivative Chain Extender with Sulfur Atoms. Materials. 2023; 16(7):2618. https://doi.org/10.3390/ma16072618
Chicago/Turabian StyleRogulska, Magdalena. 2023. "The Influence of Diisocyanate Structure on Thermal Stability of Thermoplastic Polyurethane Elastomers Based on Diphenylmethane-Derivative Chain Extender with Sulfur Atoms" Materials 16, no. 7: 2618. https://doi.org/10.3390/ma16072618
APA StyleRogulska, M. (2023). The Influence of Diisocyanate Structure on Thermal Stability of Thermoplastic Polyurethane Elastomers Based on Diphenylmethane-Derivative Chain Extender with Sulfur Atoms. Materials, 16(7), 2618. https://doi.org/10.3390/ma16072618






