Material Evidence of Sediments Recovered from Ancient Amphorae Found at the Potaissa Roman Fortress
Abstract
:1. Introduction
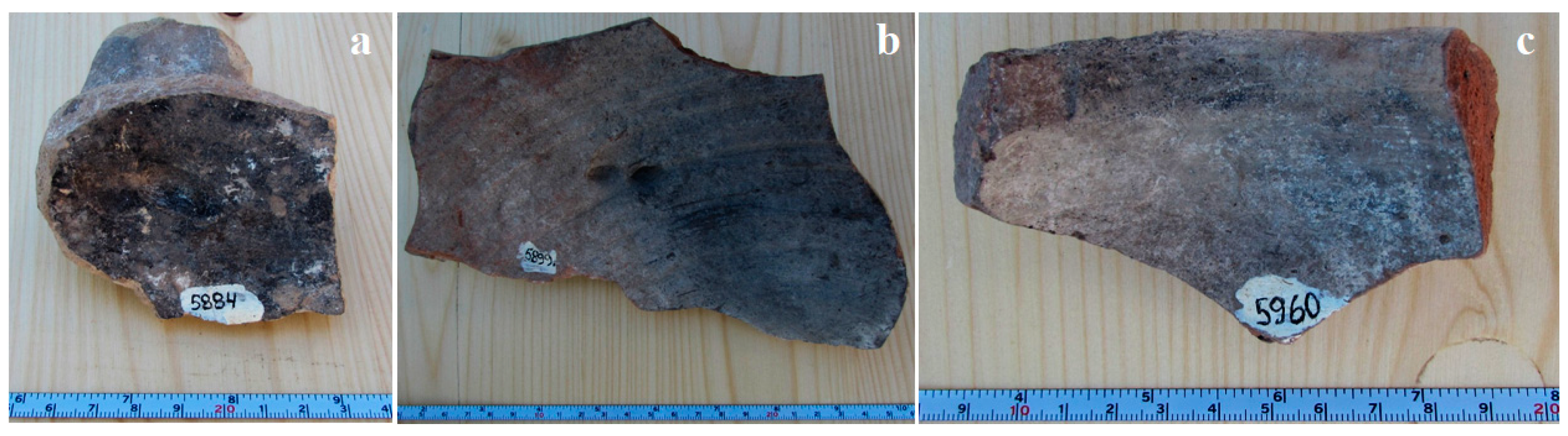
2. Materials and Methods
3. Results and Discussion
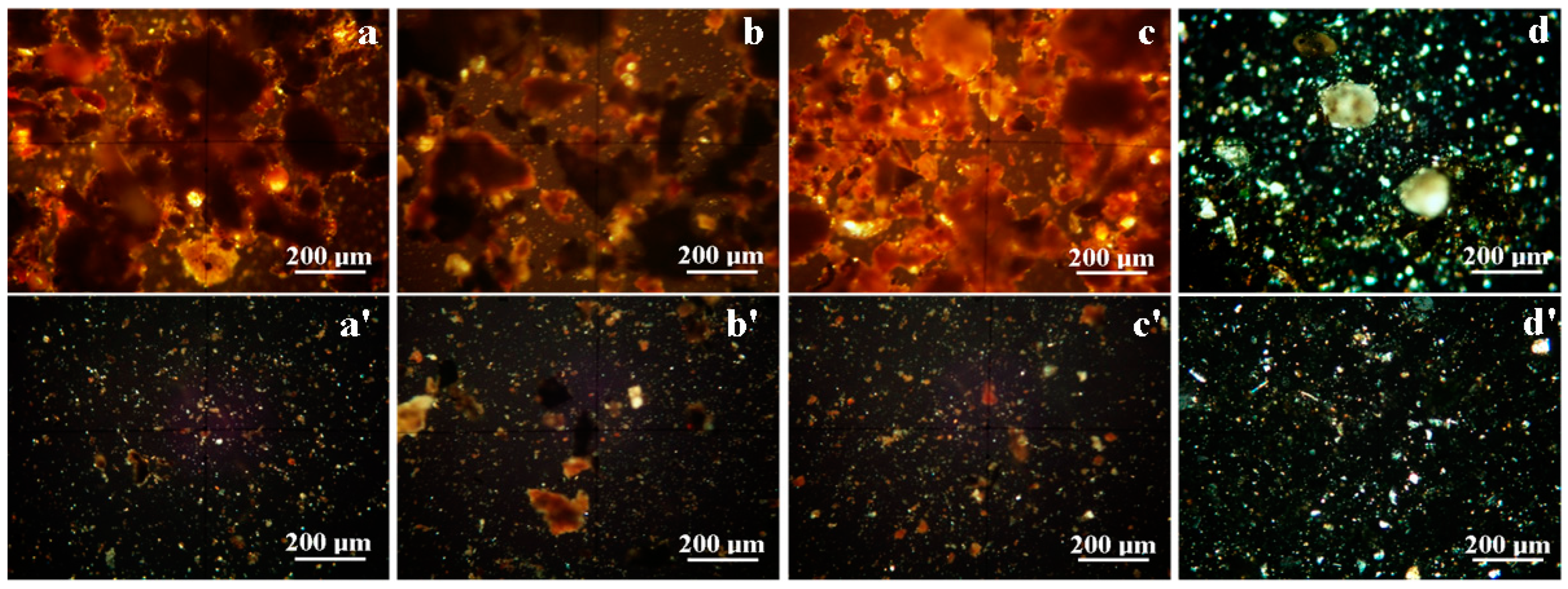
3.1. Optical Mineralogical Microscopy
3.2. X-Ray Diffraction (XRD)
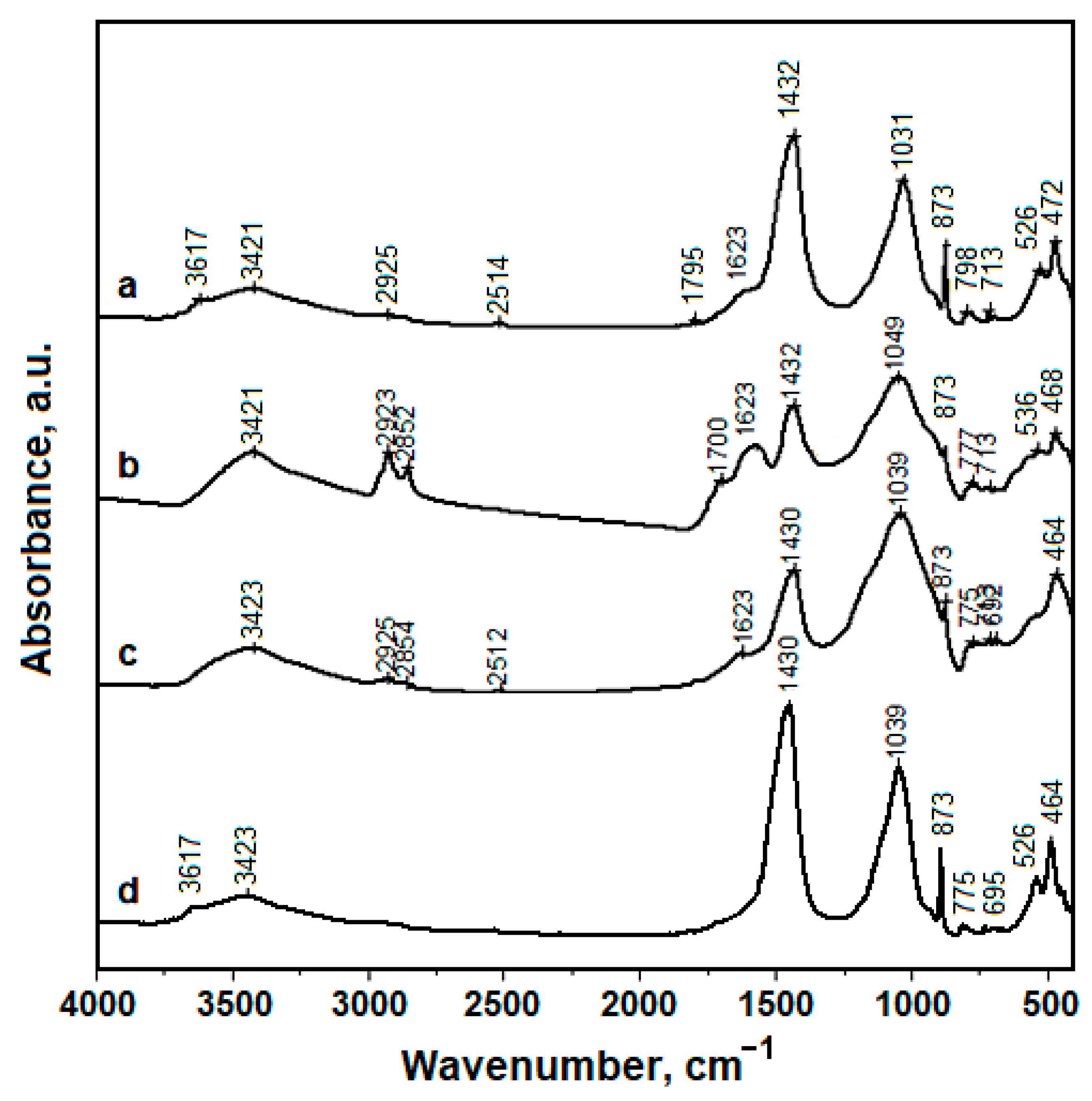
3.3. Fourier-Transformed Infrared Spectroscopy (FTIR)
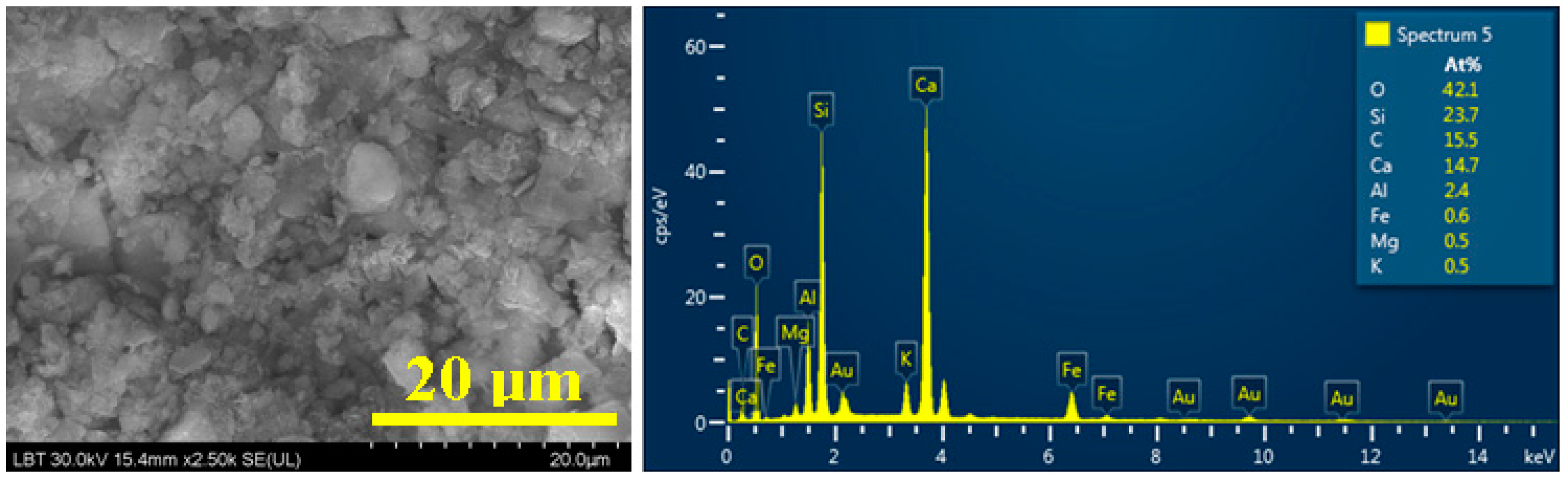
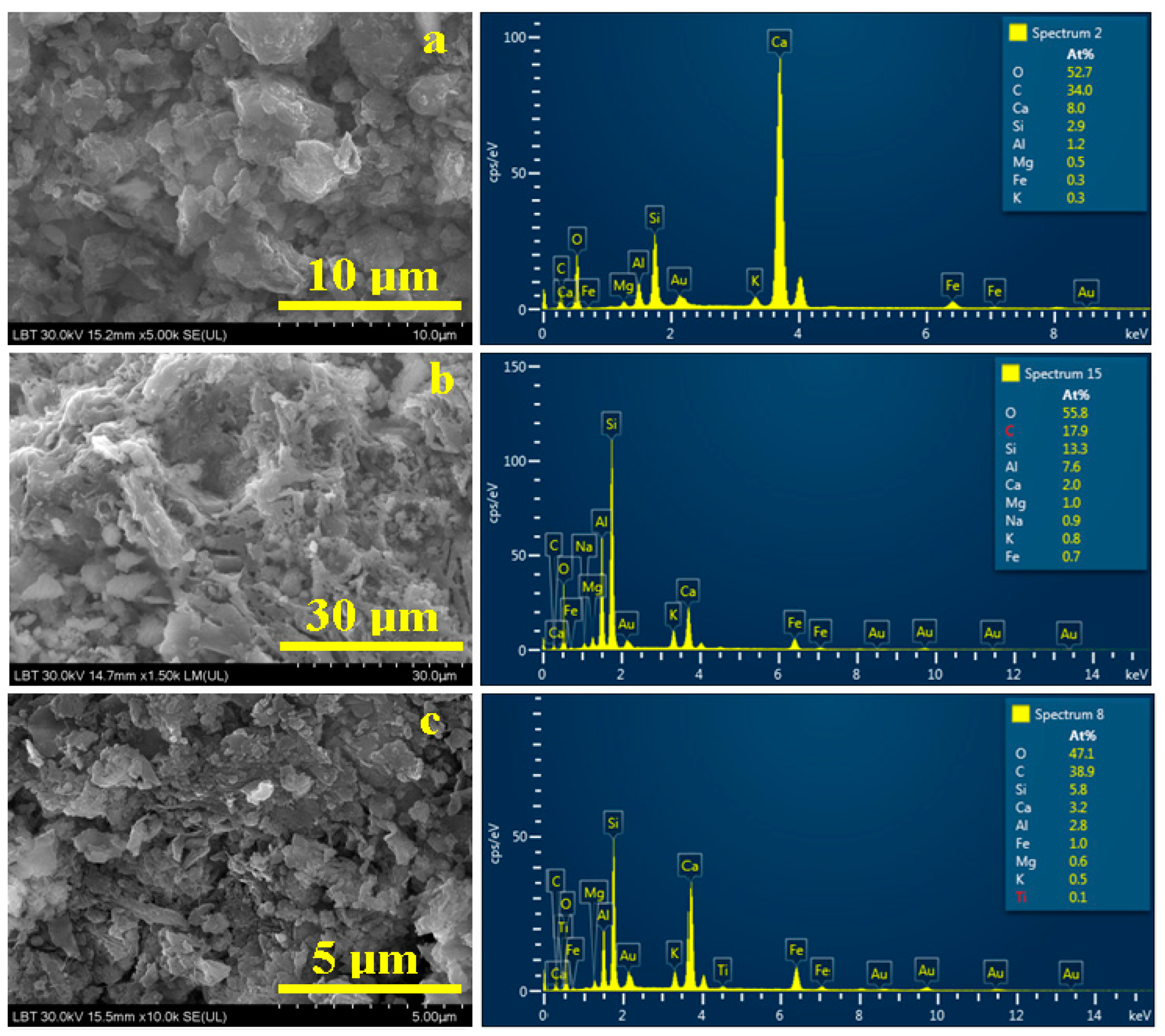
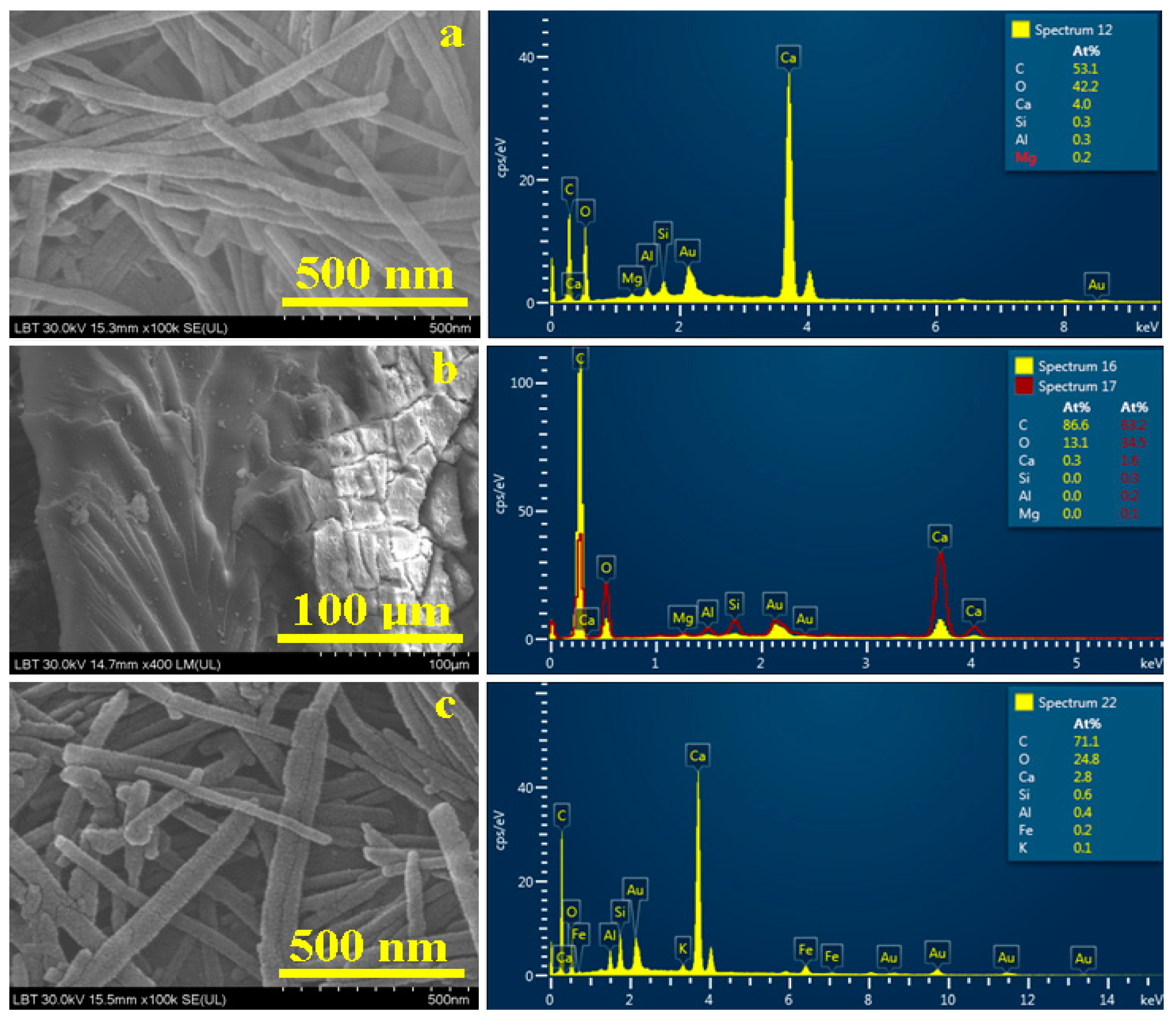
3.4. Scanning Electron Microscopy (SEM) and Elemental Analysis(EDS)
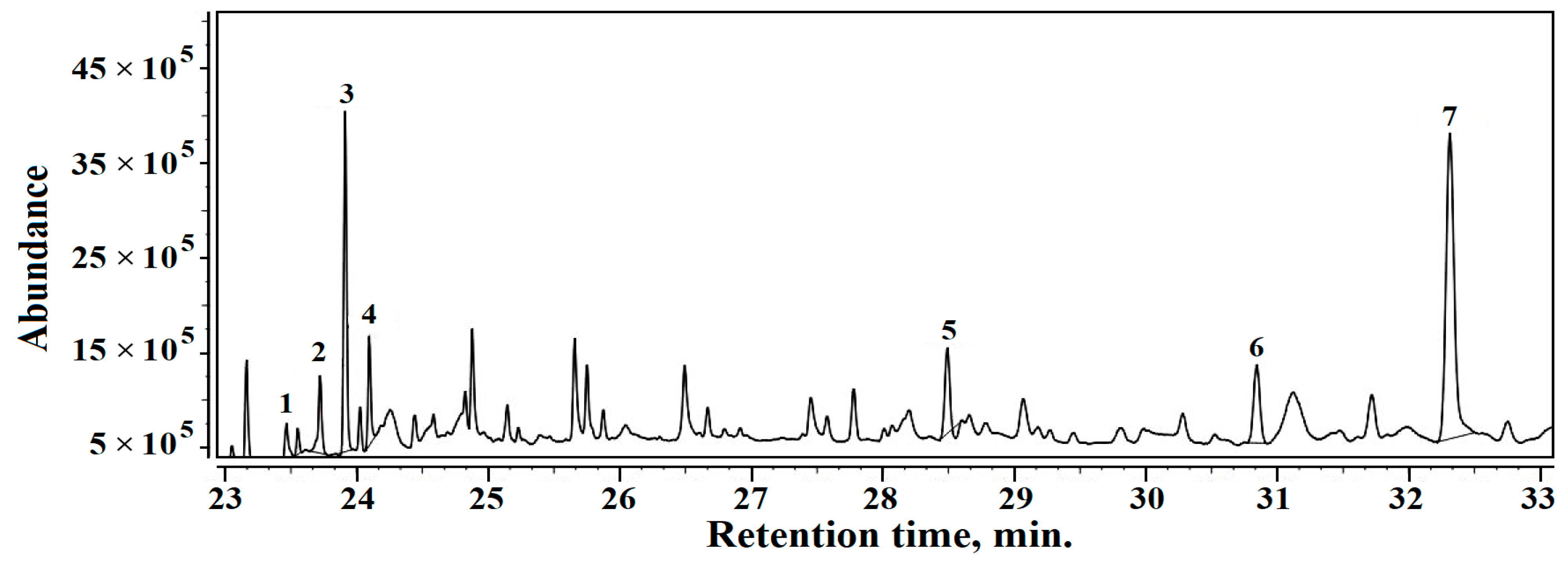
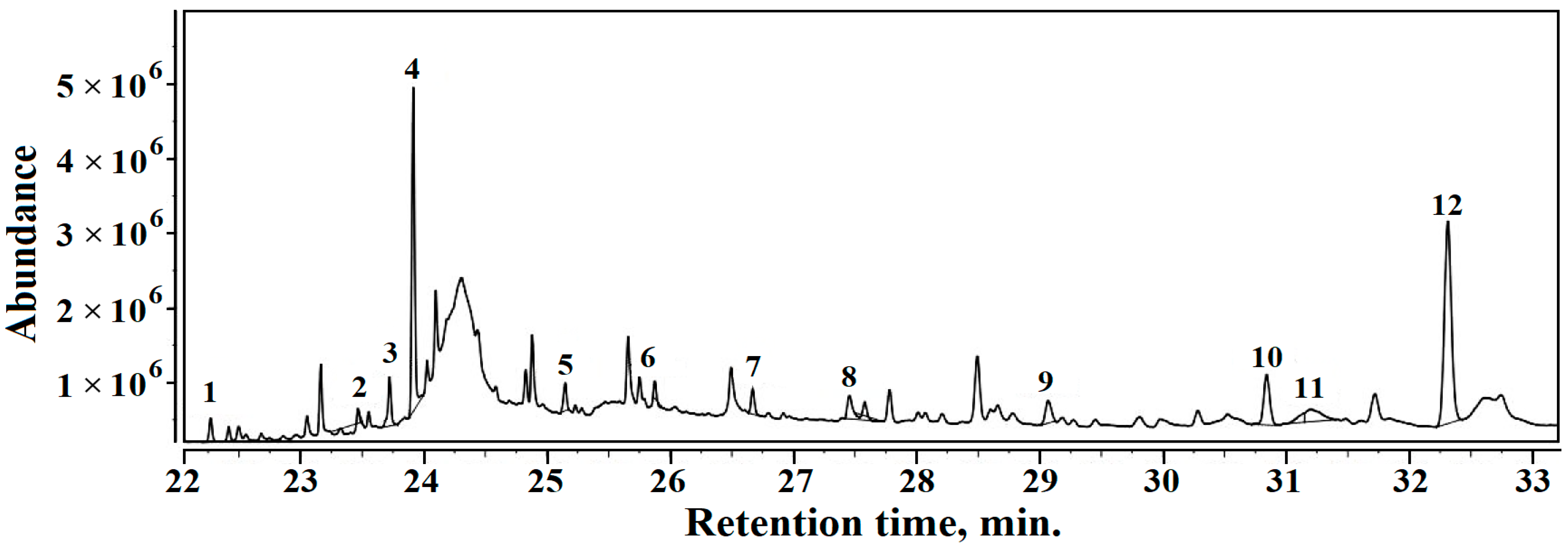
3.5. Gas Chromatography-Mass Spectrometry (GC–MS)
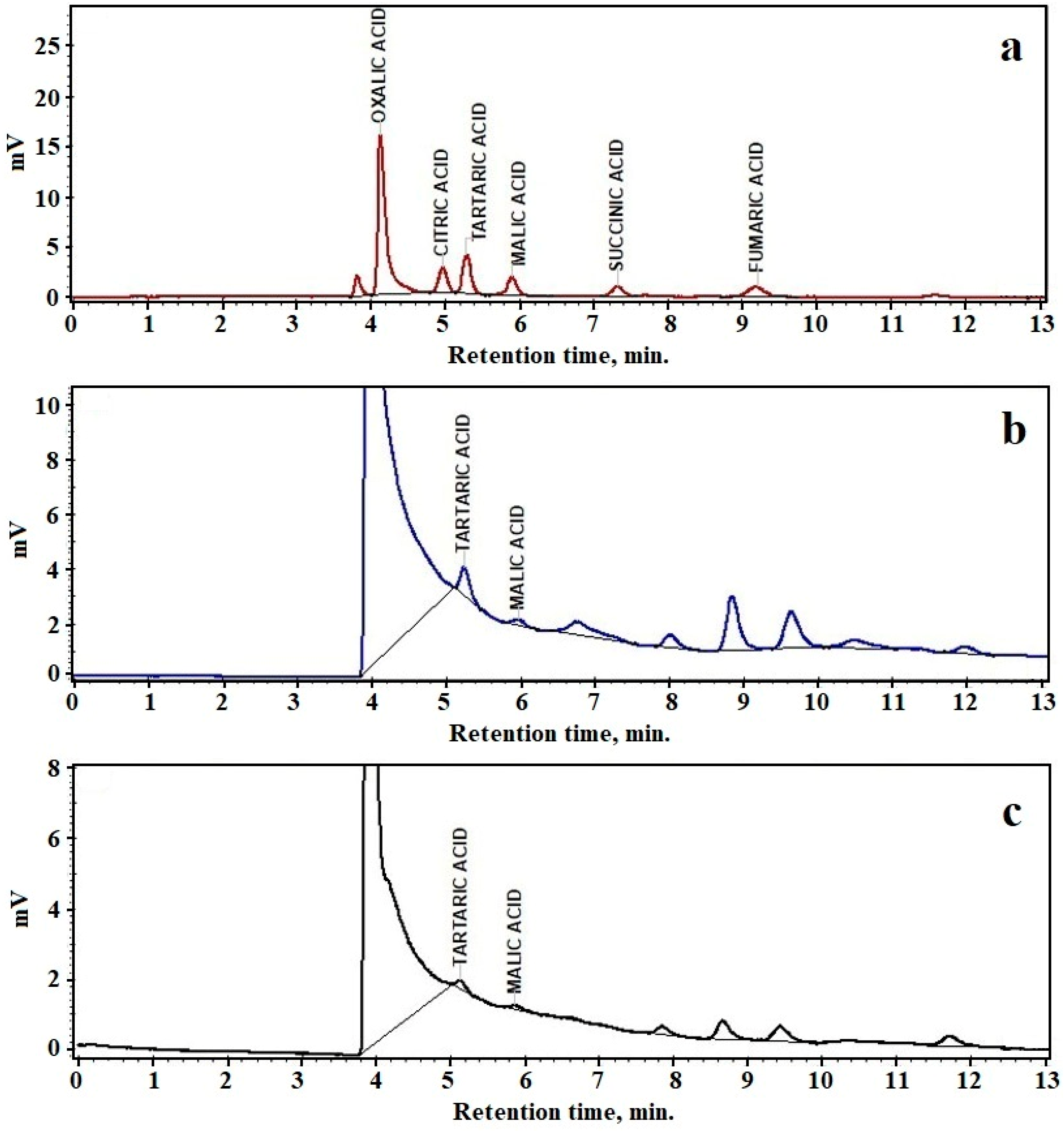
3.6. High-Performance Liquid Chromatography(HPLC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colomban, P.; Kremenović, A. Asbestos-Based Pottery from Corsica: The First Fiber-Reinforced Ceramic Matrix Composite. Materials 2020, 13, 3597. [Google Scholar] [CrossRef] [PubMed]
- Chiriu, D.; Pisu, F.A.; Ricci, P.C.; Carbonaro, C.M. Application of Raman Spectroscopy to Ancient Materials: Models and Results from Archaeometric Analyses. Materials 2020, 13, 2456. [Google Scholar] [CrossRef]
- Śliwka-Kaszyńska, M.; Ślebioda, M.; Brillowska-Dąbrowska, A.; Mroczyńska, M.; Karczewski, J.; Marzec, A.; Rybiński, P.; Drążkowska, A. Multi-Technique Investigation of Grave Robes from 17th and 18th Century Crypts Using Combined Spectroscopic, Spectrometric Techniques, and New-Generation Sequencing. Materials 2021, 14, 3535. [Google Scholar] [CrossRef]
- Šimić, K.; Soljačić, I.; Mudronja, D.; PetrovićLeš, T. Metal Content and Structure of Textiles in Textile Metal Threads in Croatia from 17th to 20th Century. Materials 2022, 15, 251. [Google Scholar] [CrossRef]
- Preusz, M.; Triska, J.; Vrchtova, N.; Vilimek, J.; Enei, F.; Preusz, K. Chemical profile of organic residues from ancient amphoras found in Pyrgi and Castrum Novum, Tyrrhenian Sea (Italy). J. Archaeol. Sci.: Rep. 2019, 24, 565–573. [Google Scholar] [CrossRef]
- Arobba, D.; Bulgarelli, F.; Camin, F.; Caramielo, R.; Larcher, R.; Martinelli, L. Palaeo botanical, chemical and physical investigation of the content of an ancient wine amphora from the northern Tyrrhenian sea in Italy. J. Archaeol. Sci. 2014, 45, 226–233. [Google Scholar] [CrossRef]
- Milanesi, C.; Bigliazzi, I.; Faleri, C.; Caterina, B.; Cresti, M. Microscope observations and DNA analysis of wine residues from Roman amphorae found in Ukraine and from bottles of recent Tuscan wines. J. Archaeol. Sci. 2011, 38, 3675–3680. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Barros, L.; Ferreira Machado, I.; Gonzalez, A.; Pereira, M.F.C.; Casimiro, T.M. Spectroscopic characterization of amphorae from the 8 th to the 7 th c. BCE found at the Almaraz settlement in Almada, Portugal. J. Archaeol. Sci. Rep. 2018, 21, 166–174. [Google Scholar] [CrossRef]
- Lettieri, M. Infrared spectroscopic characterization of residues on archaeological pottery through different spectra acquisition modes. Vib. Spectrosc. 2015, 76, 48–54. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2004; pp. 11–33. ISBN 978-0-470-09307-8. [Google Scholar] [CrossRef]
- Liu, X.; Colman, S.M.; Brown, E.T.; Minor, E.C.; Li, H. Estimation of carbonate, total organic carbon, and biogenic silica content by FTIR and XRF techniques in lacustrine sediments. J. Paleolimnol. 2013, 50, 387–398. [Google Scholar] [CrossRef]
- Barbulescu, M. The legionary fortress at Potaissa (Turda) 45 years of archeological research, (Castrul legionar de la Potaissa (Turda). 45 de ani de cercetări arheologice). Cercet. Arheol. 2020, 27, 421–439. [Google Scholar] [CrossRef]
- Howland Briggs, E. Ancient Shipwrecks and Archaeological Science: Characterising Cargo Items through Stable Isotope, Organic Residue, and DNA Analysis. PhD Thesis, University of Oxford, Oxford, UK, 2019; p. 279. [Google Scholar]
- Pecci, A.; Giorgi, G.; Salvini, L.; Ontiveros, M.A.C. Identifying wine markers in ceramics and plasters using gas chromatography–mass spectrometry. Experimental and archaeological materials. J. Archaeol. Sci. 2013, 40, 109–115. [Google Scholar] [CrossRef]
- Stern, B.; Heron, C.; Tellefsen, T.; Serpico, M. New investigation into the Uluburn resin cargo. J. Archaeol. Sci. 2008, 35, 2188–2203. [Google Scholar] [CrossRef]
- Hosu-Prack, A.G.; Petean, I.; Arghir, G.; Bobos, L.D.; Iurcut, I.; Tomoaia Cotisel, M. Marble nano erosion under acid rain evidenced by atomic force microscopy. Carpathian J. Earth Environ. Sci. 2013, 8, 75–82. [Google Scholar]
- Bălc, R.; Tămaș, T.; Popiță, G.; Vasile, G.; Bratu, M.C.; Gligor, D.M.; Moldovan, C. Assessment of Chemical Elements in Soil, Grapes and Wine from Two Representative Vineyards in Romania. Carpathian J. Earth Environ. Sci. 2018, 13, 435–446. [Google Scholar] [CrossRef]
- Nagy, R.; Zsófi, Z.; Papp, I.; Földvári, M.; Kerényi, A.; Szabó, S. Evaluation of the Relationship Between Soil Erosion and the Mineral Composition of the Soil: A Case Study from a Cool Climate Wine Region of Hungary. Carpathian J. Earth Environ. Sci. 2012, 7, 223–230. [Google Scholar]
- Negreanu, S.; Soare, B.; Avram, S. Clay Minerals Identified in Red Quaternary Deposits, from Valea Anilor Area (Mehedinţi County, Romania), Using XRD and IR Absorption Analysis. Carpathian J. Earth Environ. Sci. 2018, 13, 447–452. [Google Scholar] [CrossRef]
- Canevali, C.; Gentile, P.; Orlandi, M.; Modugno, F.; Lucejko, J.J.; Colombini, M.P.; Brambilla, L.; Goidanich, S.; Riedo, C.; Chiantore, O.; et al. A multi-analytical approach for the characterization of powders from the Pompeii archaeological site. Anal. Bioanal. Chem. 2011, 401, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Gao, C.; Zhong, D.; Liu, K.; Deng, Y.; Lin, Q.; Zhao, Q.; Zhang, X.; Zheng, Z. Characteristics and origin of the dolostones of the Ediacaran Dengying formation in the eastern Sichuan basin, south China. Carpathian J. Earth Environ. Sci. 2022, 17, 187–197. [Google Scholar] [CrossRef]
- Baricza, A.; Bajnoczi, B.; Szabo, M.; Toth, M.; Bendo, Z.; Szabo, C. Deterioration of glazed architectural ceramics due to environmental factors: A comparative study of two buildings in Budapest. Carpathian J. Earth Environ. Sci. 2016, 11, 449–462. [Google Scholar]
- Cutillas-Victoria, B.; Day, P.M. Pottery traditions, consumers’ choices and exchange networks at Late Bronze Age Cobatillas la Vieja (southeast Iberia). J. Archaeol. Sci. Rep. 2022, 45, 103560. [Google Scholar] [CrossRef]
- Koupadi, K.; Boyatzis, S.C.; Roumpou, M.; Kalogeropoulos, N.; Kotzamani, D. Organic remains in early Christian Egyptian metal vessels: Investigation with fourier transform infrared spectroscopy and gas chromatography–mass spectrometry. Heritage 2021, 4, 3611–3629. [Google Scholar] [CrossRef]
- Wu, H.; Lin, S.; Cheng, X.; Chen, J.; Ji, Y.; Xu, D.; Kang, D. Comparative study of strontium adsorption on muscovite, biotite and phlogopite. J. Env. Radioactiv. 2020, 225, 106446. [Google Scholar] [CrossRef]
- Selim, A.Q.; Mohamed, E.A.; Seliem, M.K.; Zayed, A.M. Synthesis of sole cancrinite phase from raw muscovite: Characterization and optimization. J. Alloy Comp. 2018, 762, 653–667. [Google Scholar] [CrossRef]
- Gopi, S.; Subramanian, V.K.; Palanisamy, K. Aragonite-calcite-vaterite: A temperature influenced sequential polymorphic transformation of CaCO3 in the presence of DTPA. Mater. Res. Bull. 2013, 48, 1906–1912. [Google Scholar] [CrossRef]
- Tarquini, G.; Nunziante Cesaro, S.; Campanella, L. Identification of oil residues in Roman amphorae (Monte Testaccio, Rome): A comparative FTIR spectroscopic study of archeological and artificially aged samples. Talanta 2014, 118, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Seetha, D.; Velraj, G. Spectroscopic and statistical approach of archaeological artifacts recently excavated from Tamilnadu, South India. Spectrochim. Acta -Part A Mol. Biomol. Spectrosc. 2015, 149, 59–68. [Google Scholar] [CrossRef]
- Rios-Reyes, C.A.; Nunez-Alarcon, E.D.; Puentes-Arguello, L.S.; BarriosLopez, J.C.; Moreno-Gonzalez, L.; Martinez, J.A.H. Mineralogical characterization of an ancient pottery from the la Candelaria archaeological site, Santa Helena del Opón, Santander (Colombia). Mater. Sci. Eng. Int. J. 2019, 3, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Nyquist, R.A. Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra, 1st ed.; Academic Press Elsevier: Amsterdam, The Netherlands, 2001; Volume 1, pp. 172–203. ISBN 978-0-12-523475-7. [Google Scholar]
- Filopoulou, V.; Vlachou, S.; Boyatzis, S.C. Fatty Acids and Their Metal Salts: A Review of Their Infrared Spectra in Light of Their Presence in Cultural Heritage. Molecules 2021, 26, 6005. [Google Scholar] [CrossRef]
- Lim, V.; Peh, K.K.; Sahudin, S. Synthesis, Characterisation, and Evaluation of a Cross-Linked Disulphide Amide-Anhydride-Containing Polymer Based on Cysteine for Colonic Drug Delivery. Int. J. Mol. Sci. 2013, 14, 24670–24691. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and Algal-Based Beads for the Sorption of Metal Cations: Cu(II) and Pb(II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, R.E.; Stojanovic, S.; Besch, L.; Alkildani, S.; Schröder, R.; Jung, O.; Bogram, C.; Görke, O.; Najman, S.; Tremel, W.; et al. In Vivo Biocompatibility Investigation of an Injectable Calcium Carbonate (Vaterite) as a Bone Substitute including Compositional Analysis via SEM-EDX Technology. Int. J. Mol. Sci. 2022, 23, 1196. [Google Scholar] [CrossRef]
- Teodorescu, L.; Ben Amara, A.; Cantin, N.; Chapoulie, R.; Ducu, C.; Ciucă, S.; Tulugea, C.; Terteci, C.; Abrudeanu, M. Characterization of Archaeological Artefacts Using Methods Specific to Materials Science: The Case Study of Dacian Ceramics from 2nd c. BC to 1st c. AD. Materials 2021, 14, 3908. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, S.; Zhang, S. Geochemical and Mineralogical Characteristics of the Middle Jurassic Coals from the Tongjialiang Mine in the Northern Datong Coalfield, Shanxi Province, China. Minerals 2019, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Lander, R.H.; Bonell, L.M. A model for fibrous illite nucleation and growth in sandstones. AAPG Bull. 2010, 94, 1161–1187. [Google Scholar] [CrossRef]
- Wilson, M.J.; Wilson, L.; Patey, I. The influence of individual clay minerals on formation damage of reservoir sandstones: A critical review with some new insights. Clay Miner. 2014, 49, 147–164. [Google Scholar] [CrossRef]
- Naziri, E.; Mantzouridou, F.; Tsimidou, M.Z. Recovery of squalene from wine lees using ultrasound assisted extraction-a feasibility study. J. Agric. Food Chem. 2012, 60, 9195–9201. [Google Scholar] [CrossRef]
- Chassouant, L. Organic Residue Analysis in Archaeological Amphorae. PhD Thesis, Université d’Avignon, Università degli studi La Sapienza, Rome, Italy, 2021. [Google Scholar]
- González-Rámila, S.; Mateos, R.; García-Cordero, J.; Seguido, M.A.; Bravo-Clemente, L.; Sarriá, B. Olive Pomace Oil versus High Oleic Sunflower Oil and Sunflower Oil: A Comparative Study in Healthy and Cardiovascular Risk Humans. Foods 2022, 11, 2186. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Stanzione, V.; Mariotti, R.; Mastio, V.; Azariadis, A.; Passeri, V.; Valeri, M.C.; Baldoni, L.; Bufacchi, M. Bioactive Compound Profiling of Olive Fruit: The Contribution of Genotype. Antioxidants 2022, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Mastralexi, A.; Tsimidou, M.Z. On the Squalene Content of CV Chondrolia Chalkidikis and Chalkidiki (Greece) Virgin Olive Oil. Molecules 2021, 26, 6007. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kourkoutas, Y. High-Temperature Semi-Dry and Sweet Low Alcohol Wine-Making Using Immobilized Kefir Culture. Fermentation 2021, 7, 45. [Google Scholar] [CrossRef]
- Lukić, I.; Carlin, S.; Vrhovsek, U. Utility of Comprehensive GC×GC Gas Chromatography in Finding Varietal Markers among Volatile Compounds in Non-Aromatic Red Wines. Agronomy 2022, 12, 2512. [Google Scholar] [CrossRef]
- Mohekar, P.; Osborne, J.; Tomasino, E. Effects of Fining Agents, Reverse Osmosis and Wine Age on Brown Marmorated Stink Bug (Halyomorphahalys) Taint in Wine. Beverages 2018, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Verde, M.; De Bonis, A.; D’Uva, F.; Guarino, V.; Izzo, F.; Rispoli, C.; Borriello, G.; Giglio, M.; Iavarone, S.; Morra, V. Minero-petrographic investigation on Roman pottery found in a dump in the workshop area of Cumae (southern Italy). J. Archaeol. Sci. Rep. 2022, 42, 103376. [Google Scholar] [CrossRef]
- Dias, M.I.; Prudêncio, M.I.; Gouveia, M.A.; Trindade, M.J.; Marques, R.; Franco, D.; Raposo, J.; Fabião, C.S.; Guerra, A. Chemical tracers of Lusitanian amphorae kilns from the Tagus estuary (Portugal). J. Archaeol. Sci. 2010, 37, 784–798. [Google Scholar] [CrossRef]
- Filip, M.; Moldovan, M.; Vlassa, M.; Sarosi, C.; Cojocaru, I. HPLC Determination of the Main Organic Acids in Teeth Bleaching Gels Prepared with the Natural Fruit Juices. Rev. Chim. 2016, 67, 2440–2445. [Google Scholar]
- Wohlfahrt, Y.; Patz, C.-D.; Schmidt, D.; Rauhut, D.; Honermeier, B.; Stoll, M. Responses on Must and Wine Composition of Vitis vinifera L. cvs. Riesling and Cabernet Sauvignon under a Free Air CO2 Enrichment (FACE). Foods 2021, 10, 145. [Google Scholar] [CrossRef]
- Gajek, M.; Pawlaczyk, A.; Szynkowska-Jozwik, M.I. Multi-Elemental Analysis of Wine Samples in Relation to Their Type, Origin, and Grape Variety. Molecules 2021, 26, 214. [Google Scholar] [CrossRef]
- Acevedo, W.; Cañón, P.; Gómez-Alvear, F.; Huerta, J.; Aguayo, D.; Agosin, E. l-Malate (−2) Protonation State is Required for Efficient Decarboxylation to l-Lactate by the Malolactic Enzyme of Oenococcusoeni. Molecules 2020, 25, 3431. [Google Scholar] [CrossRef]

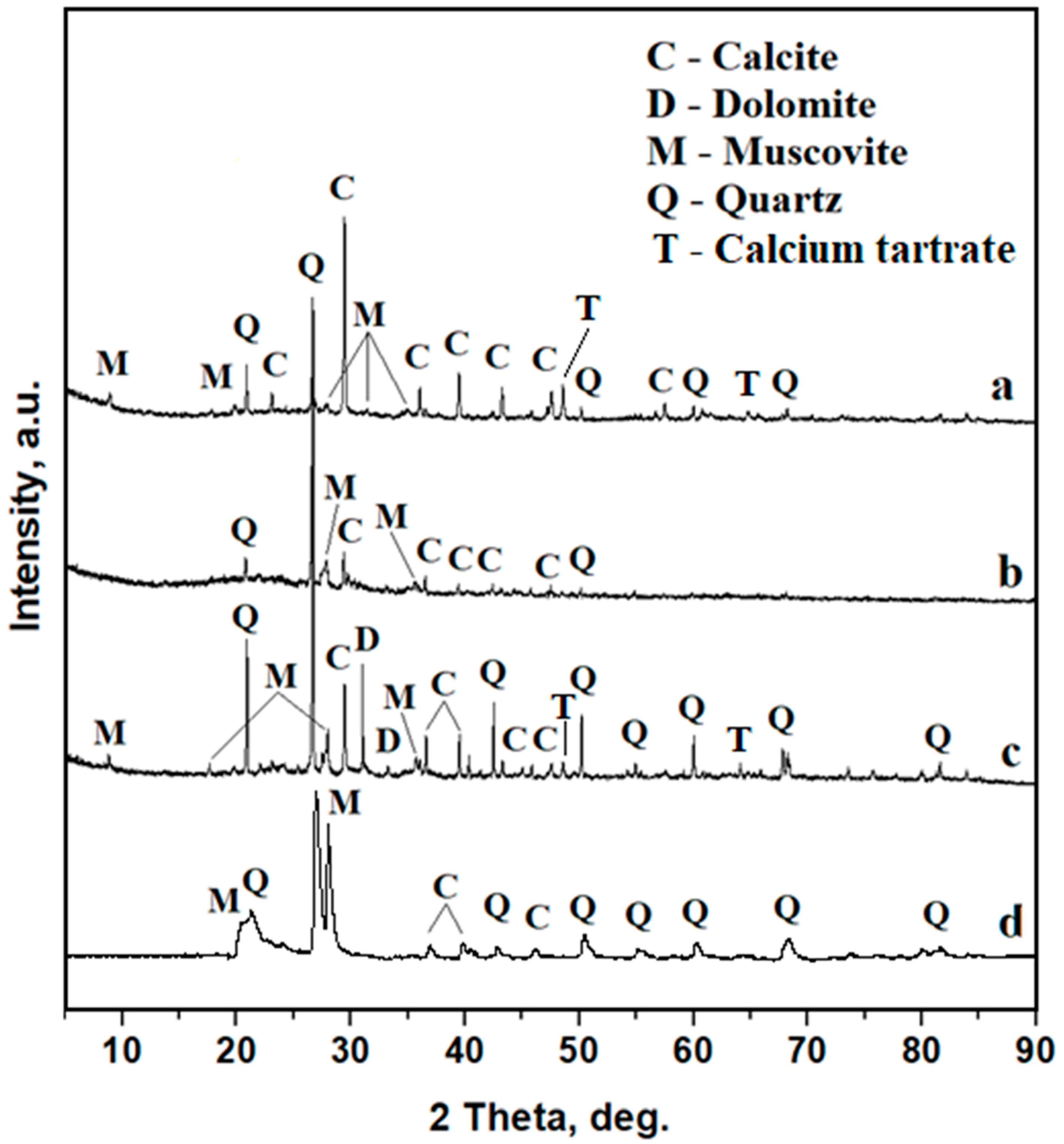

| Sample | β Quartz Wt.% | α Quartz Wt.% | Calcite Wt.% | Muscovite Wt.% | Dolomite Wt.% | Ca Tartrate Wt.% |
|---|---|---|---|---|---|---|
| 1 | 31.2 ± 2.5 | - | 46.3 ± 3.7 | 20.2 ± 2.3 | traces | 2.3 ± 0.3 |
| 2 | 80.0 ± 5.5 | 8.8 ± 0.6 | 11.2± 0.9 | traces | traces | - |
| 3 | 63.8 ± 4.7 | - | 11.8 ± 1.0 | 4.6 ± 0.3 | 18.2 ± 1.2 | 1.7 ± 0.2 |
| 4 | 42.9 ± 3.2 | - | 25.5 ± 1.8 | 31.2 ± 2.2 | - | - |
| Samples | Elemental Composition, at.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | C | Ca | Si | Al | K | Mg | Fe | Ti | |
| Sediment 1 | 52.7 | 34.0 | 8.0 | 2.9 | 1.2 | 0.3 | 0.5 | 0.3 | - |
| Sediment 2 | 55.8 | 17.9 | 2.0 | 13.3 | 7.6 | 0.8 | 1.0 | 0.7 | - |
| Sediment 3 | 47.1 | 38.9 | 3.2 | 5.8 | 2.8 | 0.5 | 0.6 | 1.0 | 0.1 |
| Samples | Elemental Composition, at.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | C | Ca | Si | Al | K | Mg | Fe | Ti | |
| Sediment 1 | 42.2 | 53.1 | 4.0 | 0.3 | 0.3 | - | 0.2 | - | - |
| Sediment 2 | 13.1 | 86.6 | 0.3 | - | - | - | - | - | - |
| Sediment 3 | 24.8 | 71.1 | 2.8 | 0.6 | 0.4 | 0.1 | - | 0.2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farcas, I.A.; Dippong, T.; Petean, I.; Moldovan, M.; Filip, M.R.; Ciotlaus, I.; Tudoran, L.B.; Borodi, G.; Paltinean, G.A.; Pripon, E.; et al. Material Evidence of Sediments Recovered from Ancient Amphorae Found at the Potaissa Roman Fortress. Materials 2023, 16, 2628. https://doi.org/10.3390/ma16072628
Farcas IA, Dippong T, Petean I, Moldovan M, Filip MR, Ciotlaus I, Tudoran LB, Borodi G, Paltinean GA, Pripon E, et al. Material Evidence of Sediments Recovered from Ancient Amphorae Found at the Potaissa Roman Fortress. Materials. 2023; 16(7):2628. https://doi.org/10.3390/ma16072628
Chicago/Turabian StyleFarcas, Iulia Alexandra, Thomas Dippong, Ioan Petean, Marioara Moldovan, Miuta Rafila Filip, Irina Ciotlaus, Lucian Barbu Tudoran, Gheorghe Borodi, Gertrud Alexandra Paltinean, Emanoil Pripon, and et al. 2023. "Material Evidence of Sediments Recovered from Ancient Amphorae Found at the Potaissa Roman Fortress" Materials 16, no. 7: 2628. https://doi.org/10.3390/ma16072628





