The Effects of Curing Temperature on CH-Based Fly Ash Composites
Abstract
1. Introduction and Background
1.1. Research Background
1.2. Flotation Method
1.3. Research Purpose and Significance
2. Materials and Methods
2.1. Modification of Fly Ash
2.2. Materials
2.3. Curing Method and Sample Preparation
2.4. Measurement Method
3. Results and Discussion
3.1. Properties of Fly Ash
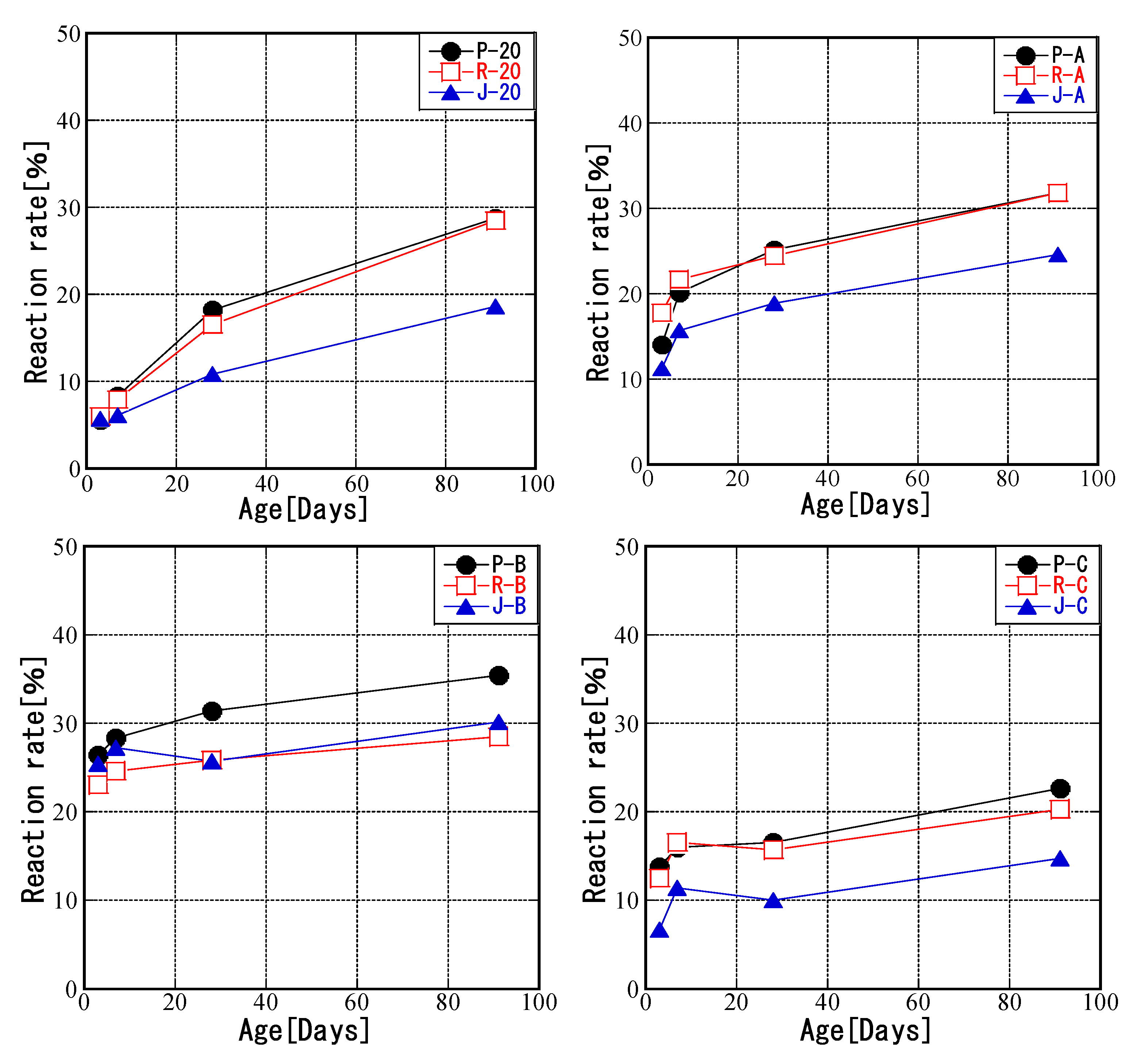
3.2. Relationship between Curing Temperature and FA Reaction Rate
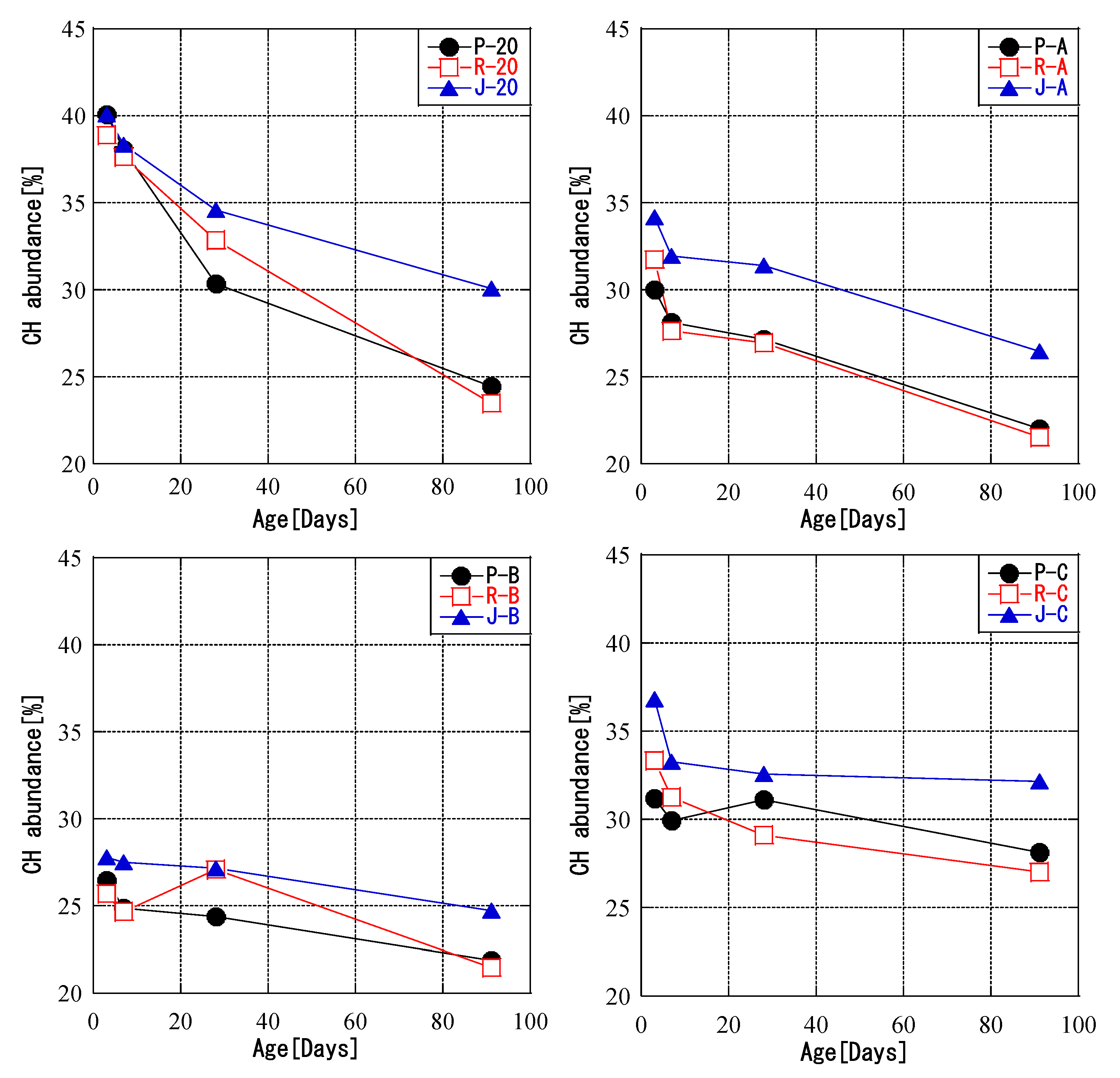
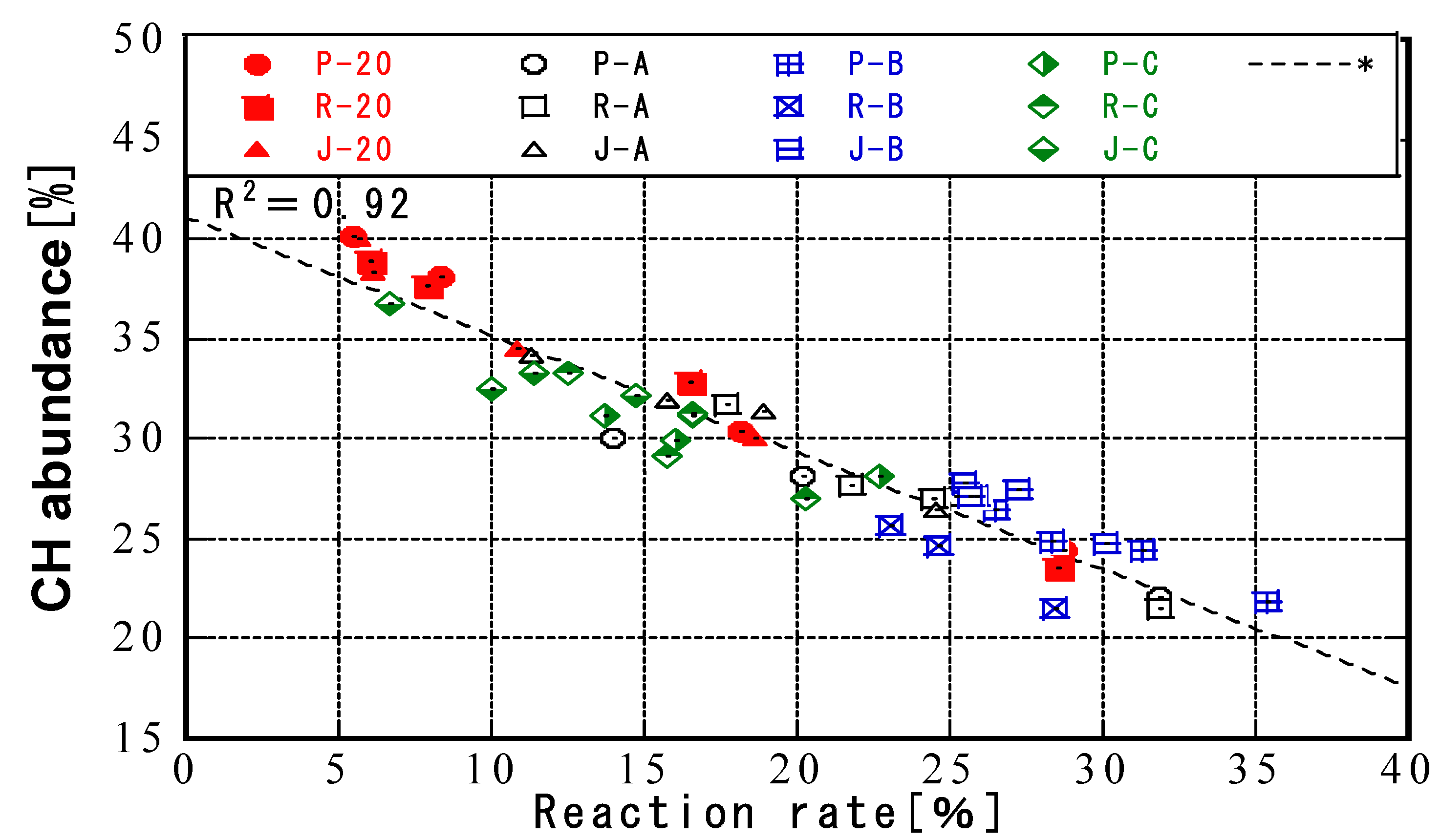
3.3. Relationship between Temperature History and CH Abundance
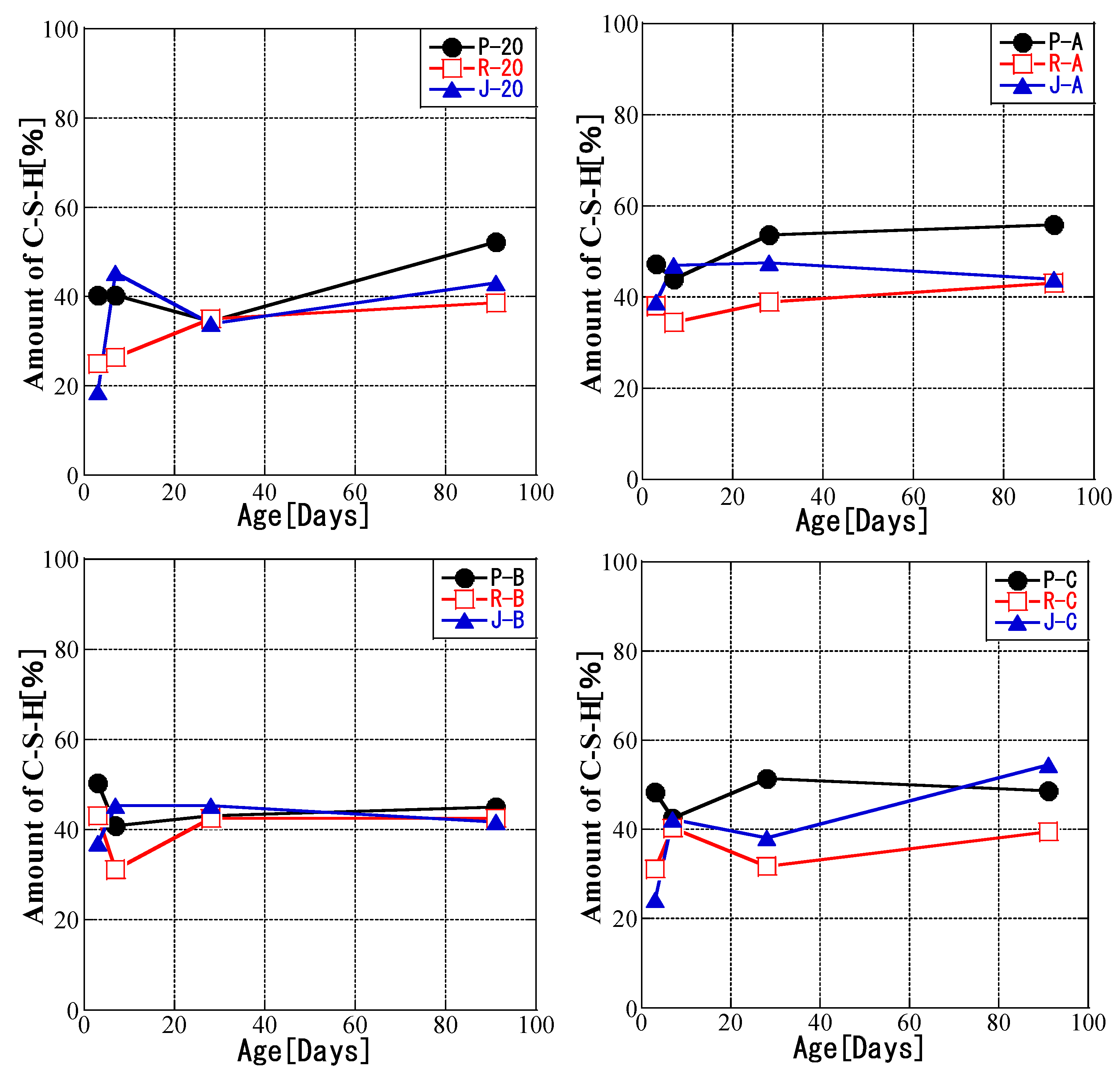
3.4. Relationship between Temperature History and C-S-H Production Amount
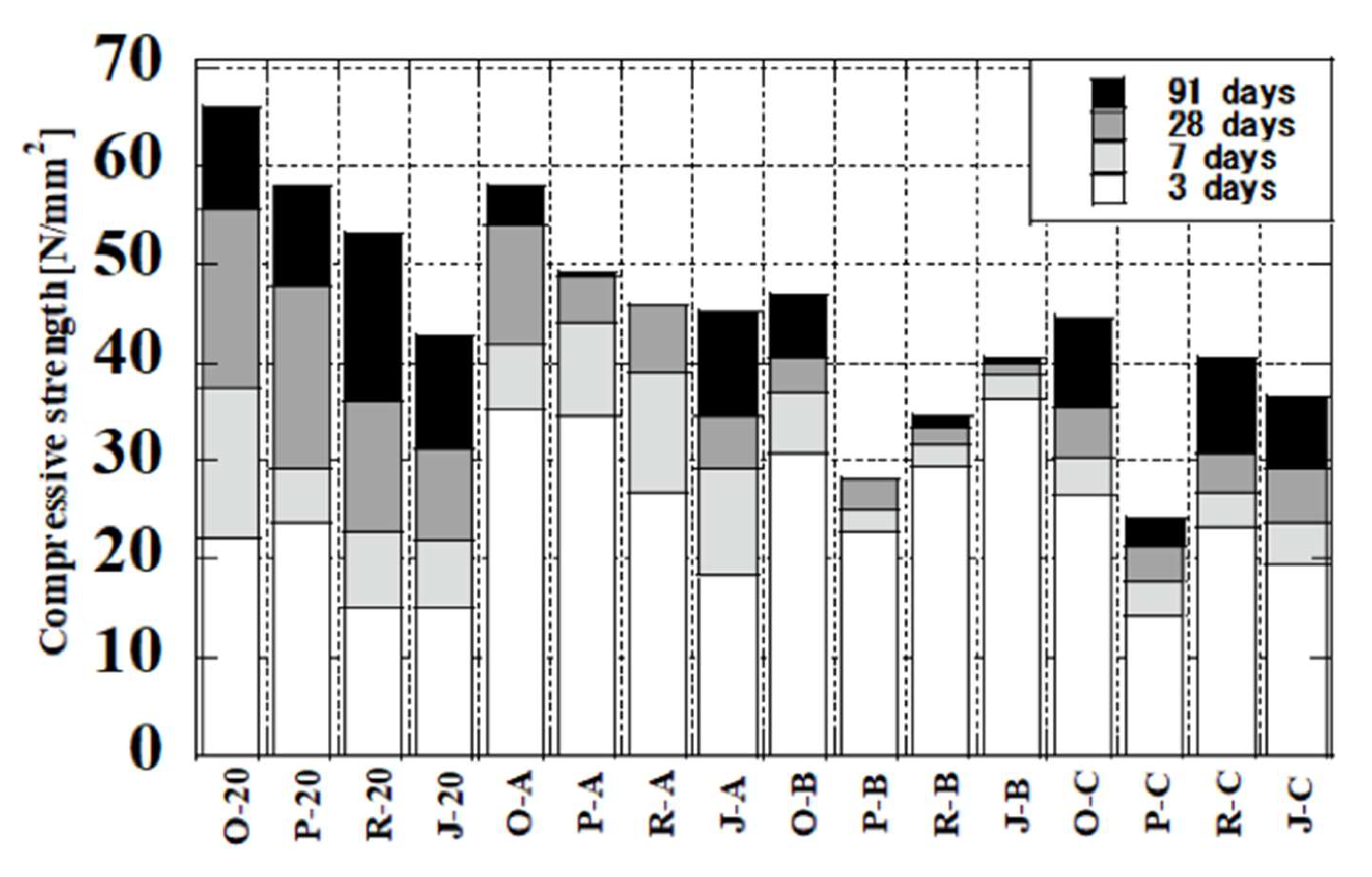
3.5. Relationship between Temperature History and Compressive Strength
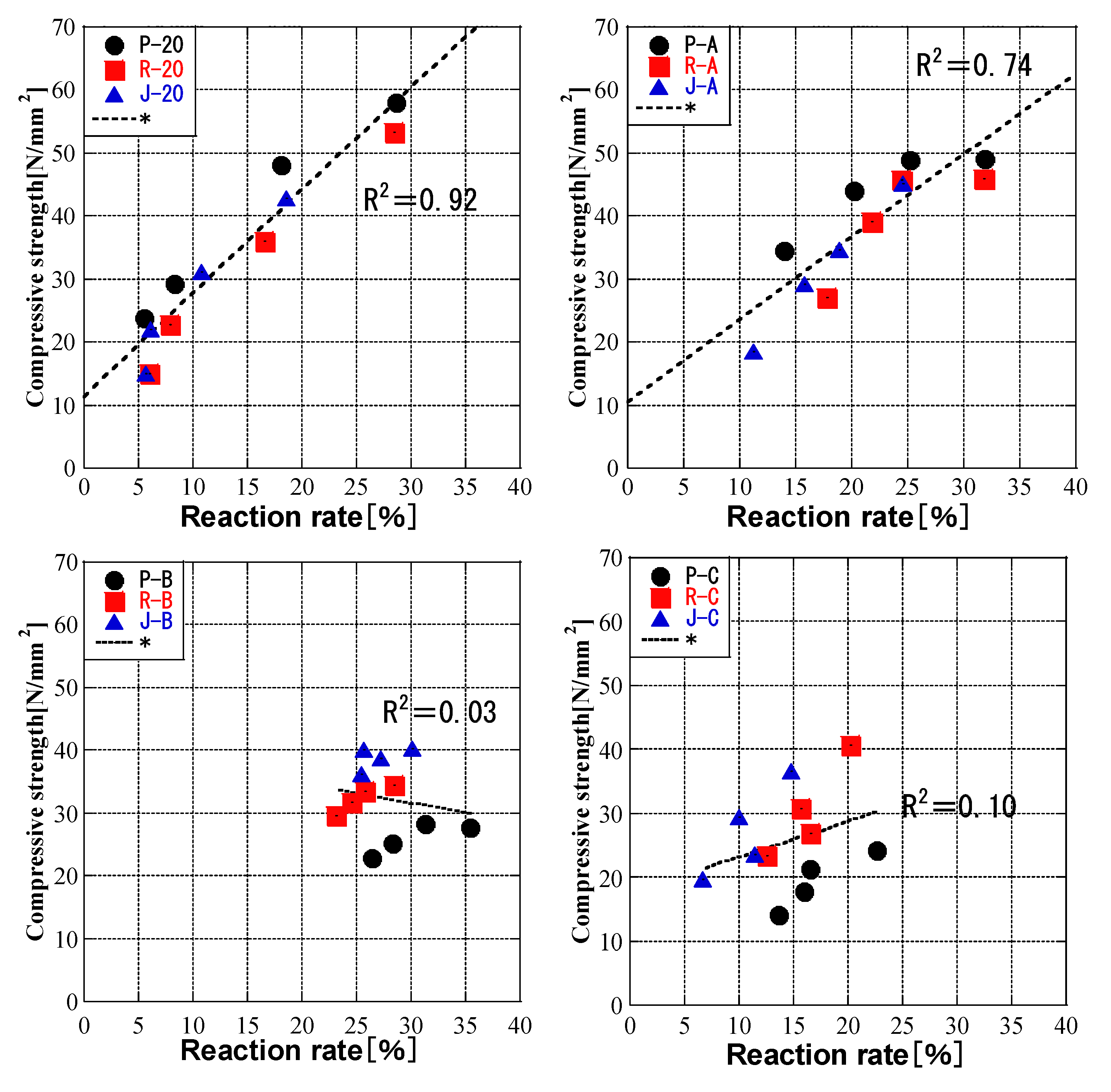
3.6. Relationship between Compressive Strength and FA Reaction Rate
4. Conclusions
- In this work, we found that flotation modification had little effect on the chemical composition of fly ash. The factor that affected the pozzolanic reaction was the lower glass content in fly ash before modification and the fact that the quality of the fly ash is affected by the use of flotation to modify the fly ash. RMFA has a particle size distribution in the range of 0.1–1 µm, which is about 4 times larger than that of the original ash, with a larger increase in small particle size than WMFA. WMFA is less active than RMFA in the first, middle, and late stages, but it can reach 96.53% at 91 days and can meet the JIS Class II ash specification.
- The reaction rate of fly ash did not depend on the amount of glass, and the reaction rates of the modified fly ashes for all periods were higher than that of JIS Class II fly ash. The highest early reaction rate was observed at an initial temperature of 80 °C, but the reaction rate increased slowly after 28 days. The reaction rate increases slowly after 7 days when the temperature is lower than 20 °C, and when the curing temperature is lower than 10 °C at 14 days, the average increase in response rate in the middle and late stages of the reaction is 5.55%. When the curing temperature is raised from the age of the feed and the temperature is continuously maintained at a high level, the increase in the reaction rate slows down after 28 days of feeding. In addition, curing at temperatures below 20 °C also slowed the increase in reaction rate.
- For all fly ashes, the amount of CH decreased with increasing curing temperature. Furthermore, the fly ash reaction rate was correlated with the amount of CH for all fly ash types and curing conditions. The amount of CH consumed by the pozzolanic reaction was related to the fly ash reaction rate during curing with a given temperature history. Initial high-temperature curing slowed down the production of C-S-H. The fly ash could be divided into types that were and were not affected by temperature history.
- The cement paste without fly ash developed high compressive strength under all curing conditions, and its compressive strength can reach up to 65.83 N/mm2. The compressive strength at three days increased with the initial curing temperature. After 7 days, the increase in strength was largest for curing at 20 °C. The modified fly ash samples exhibited higher strength development than JIS Class II fly ash cured at 20 °C and at initial temperatures of 50 and 80 °C. The strengths of modified fly ash samples were lower for curing at an ultra-high temperature.
- The relationship between the compressive strength of the cement paste and the fly ash reaction rate of the CH paste showed that the compressive strength of the cement paste was determined by the fly ash reaction rate, not by the fly ash properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcés, P.; Andión, L.G.; Zornoza, E.; Bonilla, M.; Payá, J. The effect of processed fly ashes on the durability and the corrosion of steel rebars embedded in cement–modified fly ash mortars. Cem. Concr. Compos. 2010, 32, 204–210. [Google Scholar] [CrossRef]
- Katja, O.; Janne, P.; Juho, Y.; Mirja, I. Utilization of Fly Ashes from Fluidized Bed Combustion: A Review. Sustainability 2020, 12, 2988. [Google Scholar]
- Wang, Q.; Wang, D.; Chen, H. The role of fly ash microsphere in the microstructure and macroscopic properties of high-strength concrete. Cem. Concr. Compos. 2017, 83, 25–137. [Google Scholar] [CrossRef]
- Fasihihour, N.; Abad, J.M.N.; Karimipour, A.; Mohebbi, M.R. Experimental and numerical model for mechanical properties of concrete containing fly ash: Systematic review. Measurement 2022, 188, 110547. [Google Scholar] [CrossRef]
- Stratoura, M.C.; Lazari, G.-E.D.; Badogiannis, E.G.; Papadakis, V.G. Perlite and Rice Husk Ash Re-Use As Fine Aggregates in Lightweight Aggregate Structural Concrete—Durability Assessment. Sustainability 2023, 15, 4217. [Google Scholar] [CrossRef]
- Lu, D.; Huo, Y.; Jiang, Z.; Zhong, J. Carbon nanotube polymer nanocomposites coated aggregate enabled highly conductive concrete for structural health monitoring. Carbon 2023, 206, 340–350. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Yang, X.; Zhang, A.; Yu, M. Monte Carlo simulations of deformation behaviour of unbound granular materials based on a real aggregate library. Int. J. Pavement Eng. 2023, 24, 2165650. [Google Scholar] [CrossRef]
- Xiao, R.; Jiang, X.; Zhang, M.; Polaczyk, P.; Huang, B. Analytical investigation of phase assemblages of alkali-activated materials in CaO-SiO2-Al2O3 systems: The management of reaction products and designing of precursors. Mater. Des. 2020, 194, 108975. [Google Scholar] [CrossRef]
- Narmluk, M.; Nawa, T. Effect of fly ash on the kinetics of Portland cement hydration at different curing temperatures. Cem. Concr. Res. 2011, 41, 579–589. [Google Scholar] [CrossRef]
- Narmluk, M.; Nawa, T. Effect of curing temperature on pozzolanic reaction of fly ash in blended cement paste. Int. J. Chem. Eng. Appl. 2014, 5, 31. [Google Scholar] [CrossRef]
- Wang, X.; Lee, H.S. Modeling the hydration of concrete incorporating fly ash or slag. Cem. Concr. Res. 2010, 40, 984–996. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Sun, Y.H. Hydration of high-volume fly ash cement pastes. Cem. Concr. Compos. 2000, 22, 445–452. [Google Scholar] [CrossRef]
- Termkhajornkit, P.; Nawa, T.; Kurumisawa, K. Effect of water curing conditions on the hydration degree and compressive strengths of fly ash–cement paste. Cem. Concr. Compos. 2006, 28, 781–789. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Z.; Li, D.; Yan, X.; Zhang, H. Effects of particle size on the flotation behavior of coal fly ash. Waste Manag. 2019, 85, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, F.; Tao, Y. Removal of unburned carbon from fly ash using enhanced gravity separation and the comparison with froth flotation. Fuel 2020, 259, 116282. [Google Scholar] [CrossRef]
- Soong, Y.; Schoffstall, M.R.; Gray, M.L. Dry beneficiation of high loss-on-ignition fly ash. Sep. Purif. Technol. 2002, 26, 177–184. [Google Scholar] [CrossRef]
- He, X.; Sun, H.; Chen, X.; Zhao, B.; Zhang, X.; Komarneni, S. Charging mechanism analysis of macerals during triboelectrostatic enrichment process: Insights from relative dielectric constant, specific resistivity and X-ray diffraction. Fuel 2018, 225, 533–541. [Google Scholar] [CrossRef]
- Choo, H.; Yeboah, N.N.N.; Burns, S.E. Impact of unburned carbon particles on the electrical conductivity of fly ash slurry. J. Geotech. Geoenviron. Eng. 2014, 140, 195–205. [Google Scholar] [CrossRef]
- Iuga, A.; Calin, L.; Neamtu, V.; Mihalcioiu, A.; Dascalescu, L. Tribocharging of plastics granulates in a fluidized bed device. J. Electrost. 2005, 63, 937–942. [Google Scholar] [CrossRef]
- Dodbiba, G.; Sadaki, J.; Okaya, K.; Shibayama, A.; Fujita, T. The use of air tabling and triboelectric separation for separating a mixture of three plastics. Miner. Eng. 2005, 18, 1350–1360. [Google Scholar] [CrossRef]
- Baltrus, J.P.; Diehl, J.R.; Soong, Y. Triboelectrostatic separation of fly ash and charge reversal. Fuel 2002, 81, 757–762. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, Y.J.; Yang, L. Research on flow field and kinematic characteristics of fly ash particles in rotary triboelectrostatic separator. Powder Technol. 2018, 336, 168–179. [Google Scholar] [CrossRef]
- Tao, D.; Fan, M.M.; Jiang, X.K. Dry coal fly ash cleaning using rotary triboelectrostatic separator. Min. Sci. Technol. 2009, 19, 642–647. [Google Scholar] [CrossRef]
- Cangialosi, F.; Notarnicola, M.; Liberti, L.; Stencel, J. The role of weathering on fly ash charge distribution during triboelectrostatic beneficiation. J. Hazard. Mater. 2009, 164, 683–688. [Google Scholar] [CrossRef]
- Cao, Y.J.; Li, G.S.; Liu, J.T.; Zhang, H.J.; Zhai, X. Removal of unburned carbon from fly ash using a cyclonic-static microbubble flotation column. J. S. Afr. Inst. Min. Metall. 2012, 112, 891–896. [Google Scholar]
- Drzymala, J.; Gorke, J.T.; Wheelock, T.D. A flotation collector for the separation of unburned carbon from fly ash. Coal Prep. 2005, 25, 67–80. [Google Scholar] [CrossRef]
- Zhou, Q.; Duan, Y.F.; Zhu, C.; Zhang, J.; She, M.; Wei, H.Q.; Hong, Y.G. Adsorption equilibrium, kinetics and mechanism studies of mercury on coal-fired fly ash Korean. J. Chem. Eng. 2015, 32, 1405–1413. [Google Scholar]
- Laskowski, J. Oil assisted fine particle processing. In Colloid Chemistry in Mineral Processing; Elsevier: Amsterdam, The Netherlands, 1992; pp. 361–394. [Google Scholar]
- Zhou, F.; Yan, C.; Wang, H. The result of surfactants on froth flotation of unburned carbon from coal fly ash. Fuel 2017, 90, 182–188. [Google Scholar] [CrossRef]
- Cheng, G.; Cao, Y.J.; Zhang, C.X. Application of novel flotation systems to fine coal cleaning. Int. J. Coal. Prep. Util. 2018, 38, 1–13. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Taulbee, D.N.; Hower, J.C. Characterization of differing forms of unburned carbon present in fly ash separated by density gradient centrifugation. Fuel 2001, 80, 795–800. [Google Scholar] [CrossRef]
- Zhou, M.; Kökkılıç, O.; Langlois, R. Size-by-size analysis of dry gravity separation using a 3-in. Knelson Concentrator. Miner. Eng 2016, 91, 42–54. [Google Scholar] [CrossRef]
- Lin, H.; Takasu, K.; Koyamada, H.; Suyama, H. Development of Flotation Device for Removing Unburnt Carbon in Fly Ash for Use in Hardened Cementitious Materials. Materials 2021, 14, 14216517. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ishida, T.; Gu, R. Experimental investigation of pozzolanic reaction and curing temperature-dependence of low-calcium fly ash in cement system and Ca-Si-Al element distribution of fly ash-blended cement paste. Constr. Build. Mater. 2021, 267, 121012. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and pozzolanic reactivity of glass powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Zheng, K. Pozzolanic reaction of glass powder and its role in controlling alkali–silica reaction. Cem. Concr. Compos. 2016, 67, 30–38. [Google Scholar] [CrossRef]
- Tangpagasit, J.; Cheerarot, R.; Jaturapitakkul, C.; Kiattikomol, K. Packing effect and pozzolanic reaction of fly ash in mortar. Cem. Concr. Res. 2005, 35, 1145–1151. [Google Scholar] [CrossRef]
- Durdziński, P.T.; Dunant, C.F.; Haha, M.B. A new quantification method based on SEM-EDS to assess fly ash composition and study the reaction of its individual components in hydrating cement paste. Cem. Concr. Res. 2015, 73, 111–122. [Google Scholar] [CrossRef]
- JIS A 6201:2015; Fly Ash for Use in Concrete. Japanese Industrial Standard Committee: Tokyo, Japan, 2015. Available online: www.jisc.go.jp (accessed on 15 July 2021).
- Aasaga, K.; Ohaswa, S.; Uwanishi, G.; Ohta, K.; Daimon, M. Determination of Uncombined Quartz in Hydrothermal Reaction of Quartz and Portland Cement. Kiln Ind. Assoc. J. 1982, 90, 397–400. [Google Scholar] [CrossRef]
- Kobayakawa, M.; Ozu, H.; Habara, S. Effect of mixing and curing temperature on pozzolanic reaction rate of fly ash. J. Res. Taiheiyo Cem. Corp. 2000, 139, 14–27. [Google Scholar]
- Hoshino, S.; Yamada, K.; Hirao, S.; Yamashita, H. A Study on the Hydration Analysis by X-Ray Diffraction/Rietveld Method and the Mechanism of Strength Development of Cement Including Limestone Powder. Cem. Sci. Concr. Technol. 2007, 60, 47–54. [Google Scholar]
- Ai, G.; Yang, X.; Li, X. Flotation characteristics and flotation kinetics of fine wolframite. Powder Technol. 2017, 305, 377–381. [Google Scholar] [CrossRef]
- Uçurum, M. Influences of Jameson flotation operation variables on the kinetics and recovery of unburned carbon. Powder Technol. 2009, 191, 240–246. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The limits of fine particle flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, J.T.; Ma, L.Q.; Cao, Y.J.; Li, J.H.; Huang, G. Study on energy consumption in fine coal flotation. Int. J. Coal Prep. Util. 2014, 34, 38–48. [Google Scholar] [CrossRef]
- Jones, M.R.; McCarthy, A.; Booth, A. Characteristics of the ultrafine component of fly ash. Fuel 2006, 85, 2250–2259. [Google Scholar] [CrossRef]
- Li, D.; Sun, R.; Wang, D.; Ren, C.; Fang, K. Study on the pozzolanic activity of ultrafine circulating fluidized-bed fly ash prepared by jet mill. Fuel 2021, 29, 120220. [Google Scholar] [CrossRef]
- Sun, H.; Hohl, B.; Cao, Y.; Handerker, C.; Rushing, T.S.; Cummins, T.K.; Weiss, J. Jet mill grinding of Portland cement, limestone, and fly ash: Impact on particle size, hydration rate, and strength. Cem. Concr. Compos. 2013, 44, 41–49. [Google Scholar] [CrossRef]
- Wang, X.Y.; Lee, H.S. Simulation of a temperature rise in concrete incorporating fly ash or slag. Mater. Struct. 2010, 43, 737–754. [Google Scholar] [CrossRef]
- Otsuka, T.; Mori, S.; Ishikawa, M.; Sakai, E. Relation between mineral compositions of fly ash and its pozzolanic reaction. Cem. Sci. Concr. Technol. 2009, 63, 16–21. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Malhotra, V.M. Effect of curing on the compressive strength, resistance to chloride-ion penetration and porosity of concretes incorporating slag, fly ash or silica fume. Cem. Concr. Compos. 1995, 17, 125–133. [Google Scholar] [CrossRef]
- Hanehara, S.; Tomosawa, F.; Kobayakawa, M.; Hwang, K. Effects of water/powder ratio, mixing ratio of fly ash, and curing temperature on pozzolanic reaction of fly ash in cement paste. Cem. Concr. Res. 2001, 31, 31–39. [Google Scholar] [CrossRef]
- Sato, M.; Koizumi, K.; Umemura, Y. Effect of the temperature dependence of the Pozzolanic reaction in fly ash and calucium Hydroxido. Cem. Sci. Concr. Technol. 2016, 70, 69–76. [Google Scholar] [CrossRef]
- Deschner, F.; Lothenbach, B.; Winnefeld, F.; Neubauer, J. Effect of temperature on the hydration of Portland cement blended with siliceous fly ash. Cem. Concr. Res. 2013, 52, 169–181. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Detwiler, R.J.; Gjørv, O.E. Development of microstructures in plain cement pastes hydrated at different temperatures. Cem. Concr. Res. 1991, 21, 179–189. [Google Scholar] [CrossRef]
- Saengsoy, W.; Nawa, T.; Termkhajornkit, P. Influence of relative humidity on compressive strength of fly ash cement paste. J. Struct. Constr. Eng. (Trans. AIJ) 2008, 73, 1433–1441. [Google Scholar] [CrossRef]
- Wang, T.; Ishida, T.; Gu, R. A study of the influence of crystal component on the reactivity of low-calcium fly ash in alkaline conditions based on SEM-EDS. Constr. Build. Mater. 2020, 243, 118227. [Google Scholar] [CrossRef]
- Makoto, K. Pozzolanic Reaction Analysis Method for Fly Ash and its Application to Existing Structures. Ph.D. Thesis, Tokyo Institute of Technology, Tokyo, Japan, 2009. [Google Scholar]
- Girao, A.V.; Richardson, I.G.; Taylor, R.; Brydson, R.M.D. Composition, morphology and nanostructure of C–S–H in 70% white Portland cement–30% fly ash blends hydrated at 55 °C. Cem. Concr. Res. 2010, 40, 1350–1359. [Google Scholar] [CrossRef]
- Chen, J.J.; Thomas, J.J.; Taylor, H.F.W.; Jennings, H.M. Solubility and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 1499–1519. [Google Scholar] [CrossRef]
- Sato, S.; Hattori, K.; Ogata, H.; Takada, R. The Influence of Initial Curing Temperature to the Strength Development and Compressive Strength of Normal Concret. Trans. Jpn. Soc. Irrig. Drain. Reclam. Eng. 2002, 220, 69–76. [Google Scholar]
- Naito, D.; Igarashi, S. Quantitative evaluation of changes in capillary pore structure as a function of curing temperature. Proc. Jpn. Concr. Inst. 2008, 30, 561–566. [Google Scholar]
- Konopacka-Łyskawa, D. Synthesis methods and favorable conditions for spherical vaterite precipitation: A review. Crystals 2019, 9, 223. [Google Scholar] [CrossRef]
- Nebel, H.; Neumann, M.; Mayer, C.; Epple, M. On the structure of amorphous calcium carbonate A detailed study by solid-state NMR spectroscopy. Inorg. Chem. 2008, 47, 7874–7879. [Google Scholar] [CrossRef]
- Yamazaki, H. Basic Research on the Effect of Mineral Powder on the Strength of Concrete. Jpn. Soc. Civ. Eng. 1962, 85, 15–46. [Google Scholar]
- Wu, F.R.; Masuda, Y.; Sugiyama, H. Pozzolanic Reaction and Strength Development of Cement Paste and Mortar Using Fly Ash, Transactions of AIJ. J. Struct. Constr. Eng. 2005, 590, 17–23. [Google Scholar] [CrossRef]
- Neville, A.M. Neville on Concrete: An Examination of Issues in Concrete Practice; ACI: Farmington Hills, MI, USA, 2003; pp. 51–57. [Google Scholar]

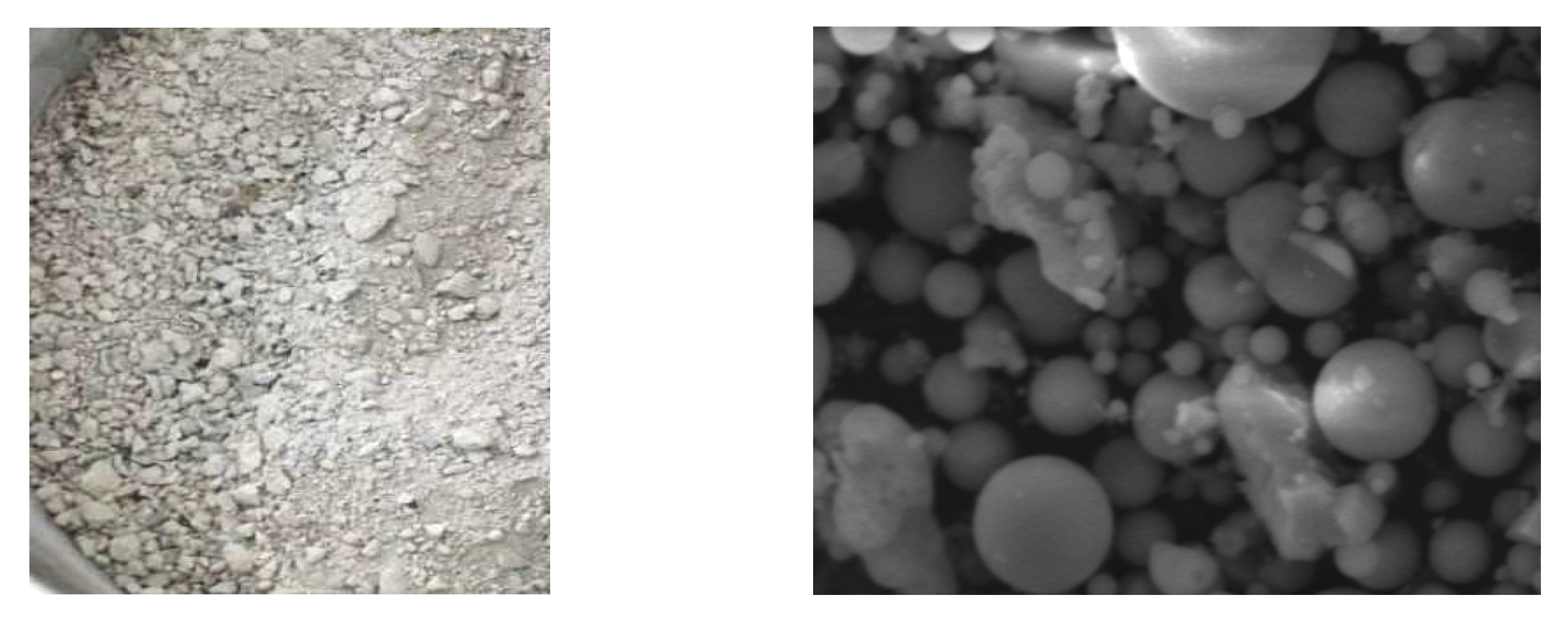



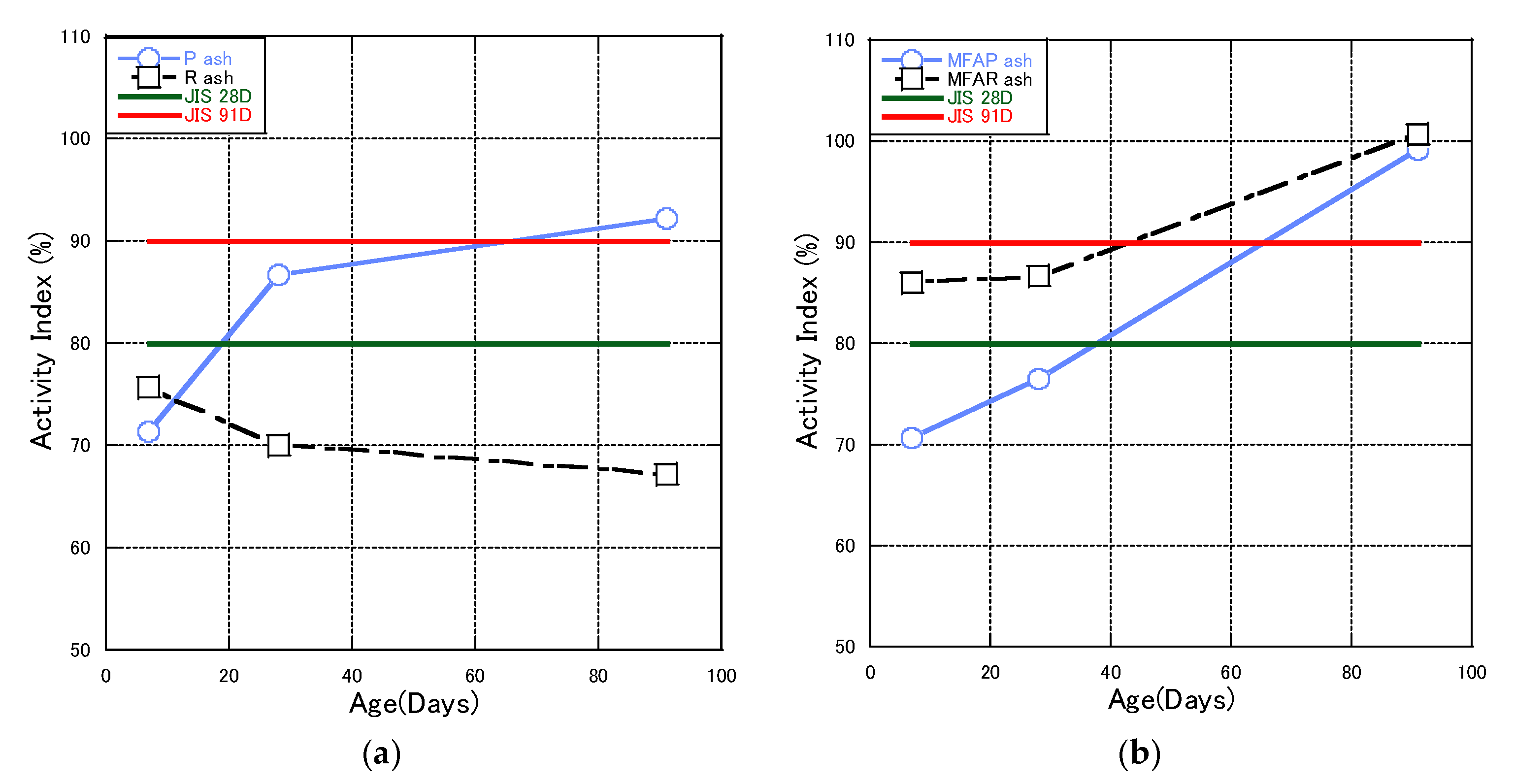
| Type | JIS Specification Year 1958 | JIS Specification Year 1974 | JIS Specification Year 1966 | |
|---|---|---|---|---|
| loss on ignition (%) | ≤5 | ≤5 | ≤5.0 | |
| Power | 45 m sieve residue (Tuna sieving method: %) | ≤25 | __ | ≤40 |
| Specific surface area (cm2/g) Blaine method | ≥2700 | ≥2400 | ≥2400 | |
| Unit water ratio (%) | ≤100 | ≤102 | ||
| Flow ratio (%) | ≥92 | |||
| Compressive strength ratio (%) | 28D | ≥63 | ≥60 | |
| Activity index(%) | 28D | ≥80 | ||
| 91D | ≥90 | |||
| Density (g/cm3) | ≥1.95 | ≥1.95 | ≥1.95 | |
| Humidity (%) | ≤1 | ≤1 | ≤1.0 | |
| Symbol | Type | Physical Characteristics | |
|---|---|---|---|
| Cement | C | Portland cement | Density: 3.16 g/cm3 |
| Admixture | FA | Raw ash fly ash (P ash) | Density: 2.30 g/cm3, |
| Specific surface area: 5070 g/cm3 | |||
| Loss on ignition: 12.9%, | |||
| Flow value ratio: 96.7%, | |||
| 28 days, 91 days Activity index: 86.7%, 92.2% | |||
| Modified fly ash equivalent to JIS type IV (PMFA ash) | Density: 2.41 g/cm3, | ||
| Specific surface area: 5520 g/cm3 | |||
| Loss on ignition: 1.7%, | |||
| Flow value ratio: 111.1%, | |||
| 28 days, 91 days Activity index: 76.5%, 99.2% | |||
| Raw ash fly ash (R ash) | Density: 2.19 g/cm3, | ||
| Specific surface area: 4200 g/cm3 | |||
| Loss on ignition: 8.5%, | |||
| Flow value ratio: 66.6%, | |||
| 28 days, 91 days Activity index: 70.0%, 67.2% | |||
| Modified fly ash equivalent to JIS type II (RMFA ash) | Density: 2.41 g/cm3, | ||
| Specific surface area: 5480 g/cm3 | |||
| Loss on ignition: 1.2%, | |||
| Flow value ratio: 91.2%, | |||
| 28 days, 91 days Activity index: 86.8%, 100.7% | |||
| Fly ash (J ash) JIS type II—certified product | Density: 2.30 g/cm3, | ||
| Specific surface area: 4000 g/cm3 | |||
| Loss on ignition: 1.3%, | |||
| Flow value ratio: 111%, | |||
| 28 days, 91 days Activity index: 90.0%, 102.0% | |||
| Water | W | Tap water | —— |
| FA | SiO2 | Fe2O3 | Al2O3 | CaO | K2O | TiO2 | SO3 | MgO | ZrO2 |
|---|---|---|---|---|---|---|---|---|---|
| P FA | 44.70 | 22.80 | 16.00 | 5.33 | 3.72 | 2.13 | 2.12 | 1.84 | 0.71 |
| P MFA | 46.70 | 18.90 | 16.90 | 3.69 | 3.37 | 1.84 | 0.22 | 1.96 | 0.70 |
| R FA | 59.70 | 9.53 | 16.90 | 2.82 | 3.16 | 4.02 | 1.63 | 1.16 | 0.66 |
| R MFA | 62.40 | 8.70 | 17.60 | 2.30 | 3.06 | 3.49 | 0.14 | 1.14 | 0.73 |
| J | 54.80 | 10.20 | 13.00 | 13.70 | 1.83 | 2.97 | 1.03 | 1.20 | 0.77 |
| FA | Mullite (%) | Quartz (%) | Magnetite (%) | Glass (%) |
|---|---|---|---|---|
| P FA | 13.26 | 9.68 | 2.70 | 54.20 |
| P MFA | 14.13 | 12.87 | 1.33 | 42.58 |
| R FA | 18.48 | 17.81 | 1.33 | 45.58 |
| R MFA | 20.32 | 21.29 | 0.77 | 37.74 |
| J | 9.86 | 8.26 | 0.97 | 59.13 |
| Name | W/C (%) | Unit Weight (kg/m3) | ||
|---|---|---|---|---|
| W | C | FA | ||
| FA0 | 50.0 | 180 | 360 | 0 |
| FA30 | 50.0 | 180 | 252 | 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Takasu, K.; Suyama, H.; Koyamada, H. The Effects of Curing Temperature on CH-Based Fly Ash Composites. Materials 2023, 16, 2645. https://doi.org/10.3390/ma16072645
Ji X, Takasu K, Suyama H, Koyamada H. The Effects of Curing Temperature on CH-Based Fly Ash Composites. Materials. 2023; 16(7):2645. https://doi.org/10.3390/ma16072645
Chicago/Turabian StyleJi, Xiangnan, Koji Takasu, Hiroki Suyama, and Hidehiro Koyamada. 2023. "The Effects of Curing Temperature on CH-Based Fly Ash Composites" Materials 16, no. 7: 2645. https://doi.org/10.3390/ma16072645
APA StyleJi, X., Takasu, K., Suyama, H., & Koyamada, H. (2023). The Effects of Curing Temperature on CH-Based Fly Ash Composites. Materials, 16(7), 2645. https://doi.org/10.3390/ma16072645






