Progress and Perspective of Glass-Ceramic Solid-State Electrolytes for Lithium Batteries
Abstract
1. Introduction
- The non-flammable characteristics of SSEs make ASSLIBs have higher safety performance than LIBs [10].
- Compared to traditional LIBs, SSEs are able to replace the liquid electrolyte and separator to effectively reduce battery weight. Meanwhile, the energy density of the battery is increased by combining the application of a lithium-metal anode [11].
- Compared to conventional LIBs, ASSLIBs have greater structural design advantages because they can be connected in series internally to achieve higher voltages. Chen et al. [12] stacked one, two and three solid-state cells in a button battery to obtain open-circuit voltages of 3.08, 6.51 and 9.12 V, respectively.
2. Ionic Conduction Mechanism
- Cation vacancy diffusion, cation migration from the initial position to its adjacent vacancy lattice position.
- The cation occupying the interstitial migrates directly to the adjacent vacant interstitial.
- Interstitialcy mechanism, cation occupying a lattice interstitial migrates to an adjacent lattice node, migrating the cation occupying that lattice to the next site.

3. Synthesis and Characterization of Glass-Ceramic Solid-State Electrolytes
3.1. Oxide Glass-Ceramic SSE Systems
3.1.1. NASICON-Type Glass-Ceramic Systems
3.1.2. Other Oxide Glass-Ceramic Systems
3.2. Sulfide Glass-Ceramic SSE Systems
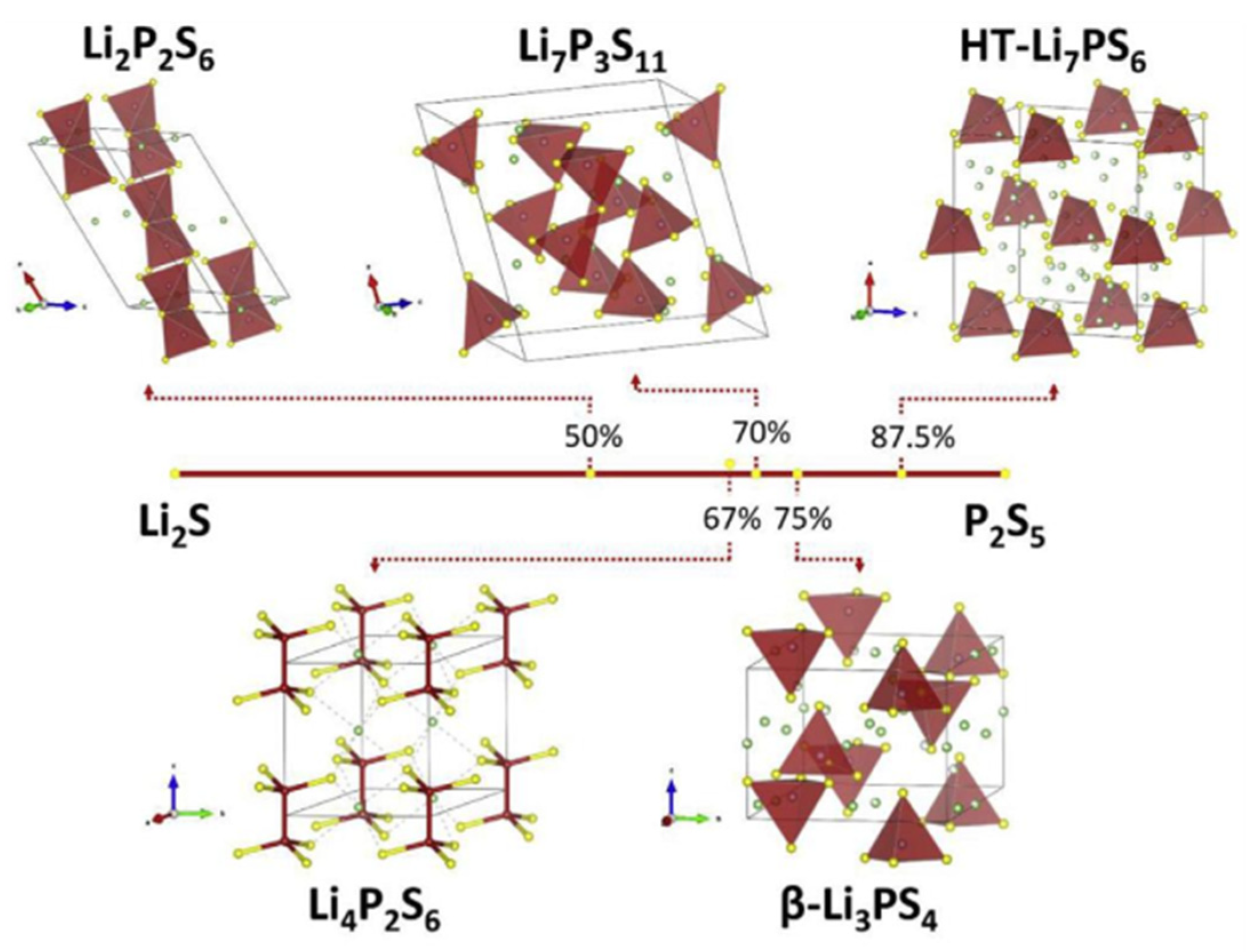
3.2.1. Li2S-P2S5 Binary System
3.2.2. Synthesis of LPS Glass-Ceramic SSEs
3.2.3. Enhancement of LPS Glass-Ceramic Performance
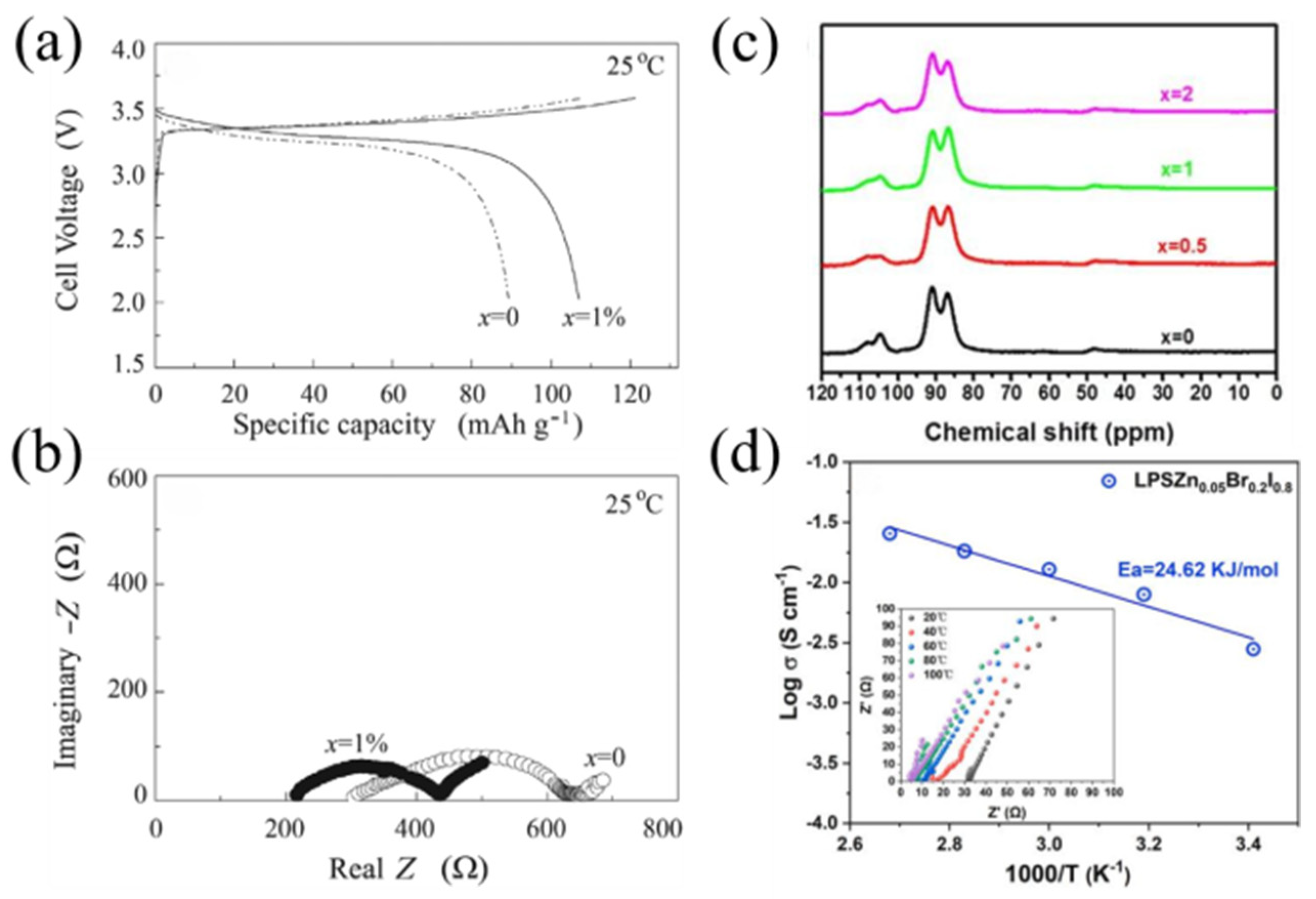
4. Interfacial Problems of Solid-State Electrolytes
4.1. Interface Problems and Optimization Methods
4.2. Enhancement of Interfacial Properties of Oxide Glass-Ceramic SSE Systems
4.3. Enhancement of Interfacial Properties of Sulfide Glass-Ceramic SSE Systems
5. Conclusions and Perspective
- Although the glass-ceramic SSE has a high ionic conductivity (10−4~10−2 S·cm−1), there is still a gap to its practical application. This is mainly because LPS electrolyte materials still have problems such as water sensitivity and a narrow electrochemical window. Optimization of preparation methods and structural modifications are important to improve the properties of glass-ceramic SSEs.
- In addition to the properties of the materials themselves, the industrial production of the materials is another factor that hinders their practical application. Traditional solid-state reactions, mechanical ball milling and melt quenching require much time and effort. All these ways are difficult to apply to the practical production of glass-ceramic SSEs. The liquid-phase synthesis method seems to be a potential method for industrial production. However, for the present studies, the liquid-phase synthesis method is also not ready for practical production. Therefore, more research on industrial production methods for glass-ceramic SSEs is still necessary in the future.
- The small interfacial contact area caused by interfacial problems leads to poor contact, insufficient interfacial reactions and high interfacial resistance, which is still the most difficult obstacle to break through to further the practical application of ASSLIBs. The design of a good electrode/electrolyte contact interface through structural modification, interface engineering and optimization of preparation methods is the main way to improve the interfacial properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- La Monaca, A.; Paolella, A.; Guerfi, A.; Rosei, F.; Zaghib, K. Electrospun ceramic nanofibers as 1D solid electrolytes for lithium batteries. Electrochem. Commun. 2019, 104, 106483. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A review of composite polymer-ceramtowardic electrolytes for lithium batteries. Energy Storage Mater. 2021, 34, 282–300. [Google Scholar] [CrossRef]
- Li, W.D.; Song, B.H.; Manthiram, A. High-voltage positive electrode materials for lithium-ion batteries. Chem. Soc. Rev. 2017, 46, 3006–3059. [Google Scholar] [CrossRef]
- Li, L.; Deng, Y.; Chen, G. Status and prospect of garnet/polymer solid composite electrolytes for all-solid-state lithium batteries. J. Energy Chem. 2020, 50, 154–177. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO based polymer-ceramic hybrid solid electrolytes: A review. Nano Converg. 2021, 8, 2. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Shen, L.; Liu, Q.; Ma, J.; Lv, W.; He, Y.; Yang, Q. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef] [PubMed]
- Funke, K. Solid State Ionics: From Michael Faraday to green energy-the European dimension. Sci. Technol. Adv. Mater. 2013, 14, 043502. [Google Scholar] [CrossRef]
- Kundu, S.; Kraytsberg, A.; Ein-Eli, Y. Recent development in the field of ceramics solid-state electrolytes: I-oxide ceramic solid-state electrolytes. J. Solid State Electrochem. 2022, 26, 1809–1838. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tang, W.; Zhang, X.; Deng, L.; Luo, J. High energy density solid state lithium metal batteries enabled by sub-5 µm solid polymer electrolytes. Adv. Mater. 2021, 33, 2105329. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, W.; Ding, L.X.; Wang, S.; Wang, H. Enhancing interfacial contact in all solid state batteries with a cathode-supported solid electrolyte membrane framework. Energy Environ. Sci. 2019, 12, 938–944. [Google Scholar] [CrossRef]
- Sakamoto, A.; Yamamoto, S. Glass-Ceramics: Engineering Principles and Applications. Int. J. Appl. Glass Sci. 2010, 1, 237–247. [Google Scholar] [CrossRef]
- Gandi, S.; Vaddadi, V.S.C.S.; Panda, S.S.S.; Goona, N.K.; Parne, S.R.; Lakavat, M.; Bhaumik, A. Recent progress in the development of glass and glass-ceramic cathode/ solid electrolyte materials for next-generation high capacity all-solid-state sodium-ion batteries: A review. J. Power Sources 2022, 521, 230930. [Google Scholar] [CrossRef]
- Pietrzak, T.K.; Wasiucionek, M.; Garbarczyk, J.E. Towards Higher Electric Conductivity and Wider Phase Stability Range via Nanostructured Glass-Ceramics Processing. Nanomaterials 2021, 11, 1321. [Google Scholar] [CrossRef]
- Dias, J.A.; Santagneli, S.H.; Messaddeq, Y. Methods for Lithium Ion NASICON Preparation: From Solid-State Synthesis to Highly Conductive Glass-Ceramics. J. Phys. Chem. C 2020, 124, 26518–26539. [Google Scholar] [CrossRef]
- Jian, Z.; Hu, Y.; Ji, X.; Chen, W. NASICON-Structured Materials for Energy Storage. Adv. Mater. 2017, 29, 1601925. [Google Scholar] [CrossRef]
- Kudu, Ö.U.; Famprikis, T.; Fleutot, B.; Braida, M.-D.; Le Mercier, T.; Islam, M.S.; Masquelier, C. A review of structural properties and synthesis methods of solid electrolyte materials in the Li2S−P2S5 binary system. J. Power Sources 2018, 407, 31–43. [Google Scholar] [CrossRef]
- Lau, J.; DeBlock, R.H.; Butts, D.M.; Ashby, D.S.; Choi, C.S.; Dunn, B.S. Sulfide Solid Electrolytes for Lithium Battery Applications. Adv. Energy Mater. 2018, 8, 1800933. [Google Scholar] [CrossRef]
- Hayashi, A.; Tatsumisago, M. Invited Paper: Recent Development of Bulk-Type Solid-State Rechargeable Lithium Batteries with Sulfide Glass-ceramic Electrolytes. Electron. Mater. Lett. 2012, 8, 199–207. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, W.; Feng, Z.; Guerfi, A.; Vijh, A.; Zaghib, K. Recent progress in sulfide-based solid electrolytes for Li-ion batteries. Mater. Sci. Eng. B 2016, 213, 169–176. [Google Scholar] [CrossRef]
- Hou, M.; Liang, F.; Chen, K.; Dai, Y.; Xue, D. Challenges and perspectives of NASICON-type solid electrolytes for all solid-state lithium batteries. Nanotechnology 2020, 31, 132003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, 1901131. [Google Scholar] [CrossRef]
- Yang, H.; Wu, N. Ionic conductivity and ion transport mechanisms of solid-state lithium-ion battery electrolytes: A review. Energy Sci. Eng. 2022, 10, 1643–1671. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Mo, Y. Origin of fast ion diffusion in superionic conductors. Nat. Commun. 2017, 8, 15893. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, R.; Yang, L.; Zheng, J.; Zhang, K.; Mo, S.; Lin, Z.; Pan, F. Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Mater. 2018, 10, 139–159. [Google Scholar] [CrossRef]
- Reddy, M.V.; Julien, C.M.; Mauger, A.; Zaghib, K. Sulfide and Oxide Inorganic Solid Electrolytes for All-Solid-State Li Batteries: A Review. Nanomaterials 2020, 10, 1606. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Chandra, A.; Bhatt, A.; Chandra, A. Ion Conduction in Superionic Glassy Electrolytes: An Overview. J. Mater. Sci. Technol. 2013, 29, 193–208. [Google Scholar] [CrossRef]
- Funke, K.; Banhatti, R.D. Ionic motion in materials with disordered structures. Solid State Ion. 2006, 177, 1551–1557. [Google Scholar] [CrossRef]
- Miura, A.; Rosero-Navarro, N.C.; Sakuda, A.; Tadanaga, K.; Phuc, N.H.H.; Matsuda, A.; Machida, N.; Hayashi, A.; Tatsumisago, M. Liquid-phase syntheses of sulfide electrolytes for all-solid-state lithium battery. Nat. Rev. Chem. 2019, 3, 189–198. [Google Scholar] [CrossRef]
- Xu, R.; Xia, X.; Yao, Z.; Wang, X.; Gu, C.; Tu, J. Preparation of Li7P3S11 glass-ceramic electrolyte by dissolution-evaporation method for all-solid-state lithium ion batteries. Electrochim. Acta 2016, 219, 235–240. [Google Scholar] [CrossRef]
- Calpa, M.; Rosero-Navarro, N.C.; Miura, A.; Tadanaga, K. Instantaneous preparation of high lithium-ion conducting sulfide solid electrolyte Li7P3S11 by a liquid phase process. RSC Adv. 2017, 7, 46499–46504. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.; Park, J.; Nichols, W.T.; Shin, D. Facile synthesis of Li2S-P2S5 glass-ceramics electrolyte with micron range particles for all-solid-state batteries via a low-temperature solution technique (LTST). Appl. Surf. Sci. 2018, 444, 10–14. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Recent progress in solid oxide and lithium ion conducting electrolytes research. Ionics 2006, 12, 81–92. [Google Scholar] [CrossRef]
- Mariappan, C.R.; Yada, C.; Rosciano, F.; Roling, B. Correlation between micro-structural properties and ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 ceramics. J. Power Sources 2011, 196, 6456–6464. [Google Scholar] [CrossRef]
- Mariappan, C.R.; Galven, C.; Crosnier-Lopez, M.P.; Le Berre, F.; Bohnke, O. Synthesis of nanostructured LiTi2(PO4)3 powder by a pechini-type polymerizable complex method. J. Solid State Chem. 2006, 179, 450–456. [Google Scholar] [CrossRef]
- Rossbach, A.; Tietz, F.; Grieshammer, S. Structural and transport properties of lithium-conducting NASICON materials. J. Power Sources 2018, 391, 1–9. [Google Scholar] [CrossRef]
- Francisco, B.E.; Stoldt, C.R. Lithium-Ion Trapping from Local Structural Distortions in Sodium Super Ionic Conductor (NASICON) Electrolytes. Chem. Mater. 2014, 26, 4741–4749. [Google Scholar] [CrossRef]
- Giarola, M.; Sanson, A.; Tietz, F.; Pristat, S.; Dashjav, E.; Rettenwander, D.; Redhammer, G.J.; Mariotto, G. Structure and vibrational dynamics of NASICON-type LiTi2(PO4)3. J. Phys. Chem. C 2017, 121, 3697–3706. [Google Scholar] [CrossRef]
- Narváez-Semanate, J.L.; Martins Rodrigues, A.C.; Muñoz-Meneses, R.A.; Muñoz-Hoyos, J.R.; Villamarín-Muñoz, J.A. Obtention and Characterization of Lithium Superionic Conductors Using the Glass-Ceramic Method. Dyna 2018, 85, 148–156. [Google Scholar] [CrossRef]
- Pershina, S.V.; Antonov, B.D.; Farlenkov, A.S.; Vovkotrub, E.G. Glass-ceramics in Li1+xAlxGe2−x(PO4)3 system: The effect of Al2O3 addition on microstructure, structure and electrical properties. J. Alloys Compd. 2020, 835, 155281. [Google Scholar] [CrossRef]
- Breuer, S.; Prutsch, D.; Ma, Q.; Epp, V.; Preishuber-Pflügl, F.; Tietzb, F.; Wilkening, M. Separating Bulk from Grain Boundary Li Ion Conductivity in the Sol-Gel Prepared Solid Electrolyte Li1.5Al0.5Ti1.5(PO4)3. J. Mater. Chem. A 2015, 3, 21343–21350. [Google Scholar] [CrossRef]
- Hartmann, P.; Leichtweiss, T.; Busche, M.R.; Schneider, M.; Reich, M.; Sann, J.; Adelhelm, P.; Janek, J. Degradation of NASICON-Type Materials in Contact with Lithium Metal: Formation of Mixed Conducting Interphases (MCI) on Solid Electrolytes. J. Phys. Chem. C 2013, 117, 21064–21074. [Google Scholar] [CrossRef]
- Das, A.; Goswami, M.; Krishnan, M. Crystallization kinetics of Li2O-Al2O3-GeO2-P2O5 glass-ceramics system. J. Therm. Anal. Calorim. 2018, 131, 2421–2431. [Google Scholar] [CrossRef]
- Feng, J.K.; Yan, B.G.; Liu, J.C.; Lai, M.O.; Li, L. All solid state lithium ion rechargeable batteries using NASICON structured electrolyte. Mater. Technol. 2013, 28, 276–279. [Google Scholar] [CrossRef]
- He, K.; Zu, C.; Wang, Y.; Han, B.; Yin, X.; Zhao, H.; Liu, Y.; Chen, J. Stability of lithium ion conductor NASICON structure glass ceramic in acid and alkaline aqueous solution. Solid State Ion. 2014, 254, 78–81. [Google Scholar] [CrossRef]
- Pershina, S.V.; Pankratov, A.A.; Vovkotrub, E.G.; Antonov, B.D. Promising high-conductivity Li1.5Al0.5Ge1.5(PO4)3 solid electrolytes: The effect of crystallization temperature on the microstructure and transport properties. Ionics 2019, 25, 4713–4725. [Google Scholar] [CrossRef]
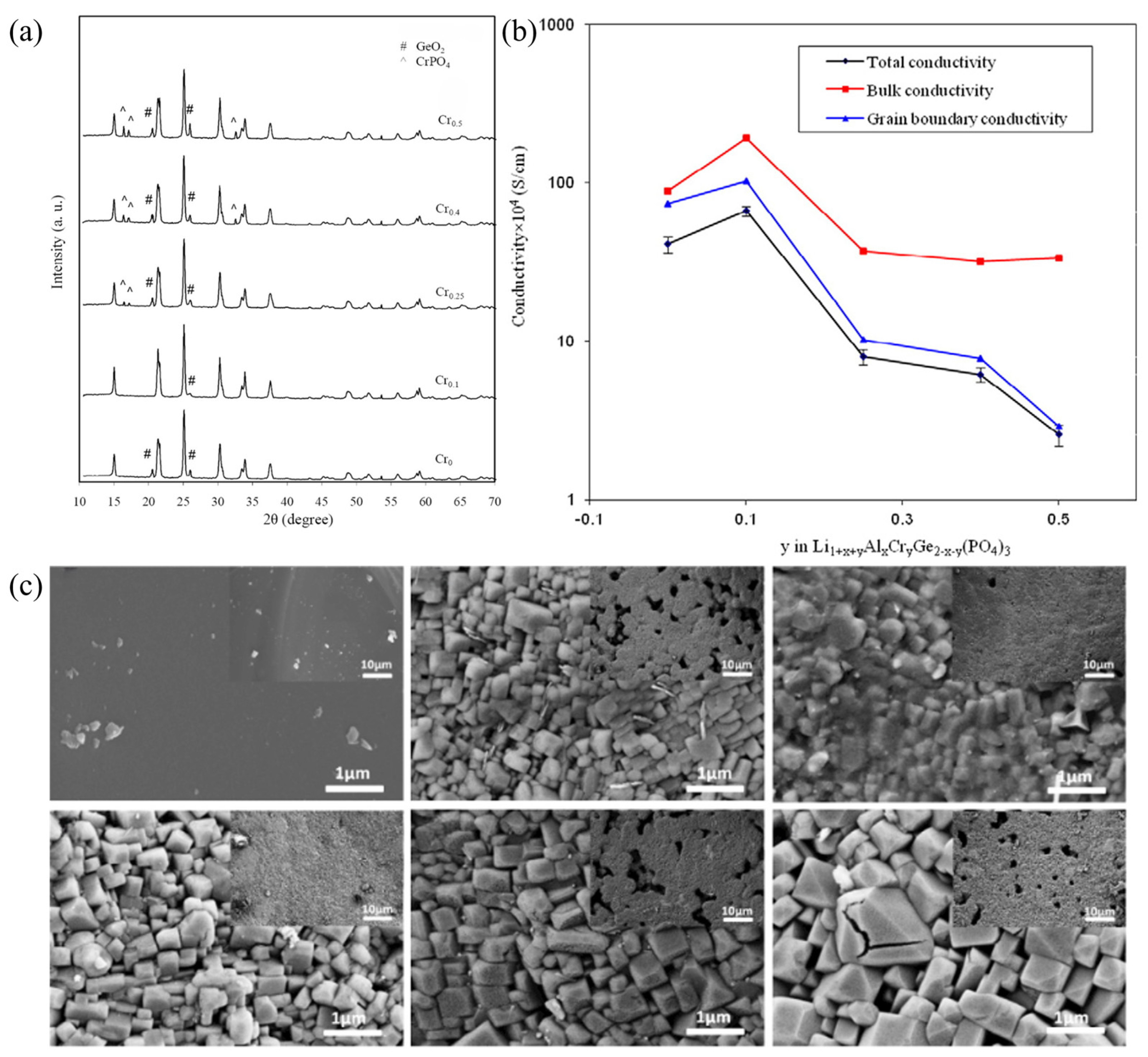
- Illbeigi, M.; Fazlali, A.; Kazazi, M.; Mohammadi, A.M. Effect of simultaneous addition of aluminum and chromium on the lithium ionic conductivity of LiGe2(PO4)3 NASICON-type glass-ceramics. Solid State Ion. 2016, 289, 180–187. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Lu, L. Influence of crystallization temperature on ionic conductivity of lithium aluminum germanium phosphate glass-ceramic. J. Power Sources 2015, 290, 123–129. [Google Scholar] [CrossRef]
- Nikodimos, Y.; Tsai, M.C.; Abrha, L.H.; Weldeyohannis, H.H.; Chiu, S.F.; Bezabh, H.K.; Shitaw, K.N.; Fenta, F.W.; Wu, S.H.; Su, W.N.; et al. Al-Sc Dual Doped LiGe2(PO4)3—A NASICON-Type Solid Electrolyte with Improved Ionic Conductivity. J. Mater. Chem. A 2020, 8, 11302–11313. [Google Scholar] [CrossRef]
- Yi, E.; Wang, W.; Mohanty, S.; Kieffer, J.; Tamaki, R.; Laine, R.M. Materials that can replace liquid electrolytes in Li batteries: Superionic conductivities in Li1.7Al0.3Ti1.7Si0.4P2.6O12. Processing combustion synthesized nanopowders to free standing thin films. J. Power Sources 2014, 269, 577–588. [Google Scholar] [CrossRef]
- Yan, B.; Kang, L.; Kotobuki, M.; Wang, F.; Huang, X.; Song, X.; Jiang, K. NASICON-structured solid-state electrolyte Li1.5Al0.5−xGaxGe1.5(PO4)3 prepared by microwave sintering. Mater. Technol. 2019, 34, 356–360. [Google Scholar] [CrossRef]
- Wang, H.; Okubo, K.; Inada, M.; Hasegawa, G.; Enomoto, N.; Hayashi, K. Low temperature-densified NASICON-based ceramics promoted by Na2O-Nb2O5-P2O5 glass additive and spark plasma sintering. Solid State Ion. 2018, 322, 54–60. [Google Scholar] [CrossRef]
- Kobayashi, E.; Plashnitsa, L.S.; Doi, T.; Okada, S.; Yamaki, J.-I. Electrochemical properties of Li symmetric solid-state cell with NASICON-type solid electrolyte and electrodes. Electrochem. Commun. 2010, 12, 894–896. [Google Scholar] [CrossRef]
- Thokchom, J.S.; Kumar, B. Microstructural Effects on the Superionic Conductivity of a Lithiated Glass-Ceramic. Solid State Ion. 2006, 177, 727–732. [Google Scholar] [CrossRef]
- Zhong, Y.; Luo, J.; Shang, F.; Chen, G. Preparation, microstructure and ionic conductivity of Li1.3Al0.3Ti1.7(PO4)3/50Li2O-50P2O5 glass ceramic electrolytes. J. Mater. Sci. Mater. Electron. 2022, 33, 7869–7882. [Google Scholar] [CrossRef]
- Leo, C.J.; Chowdari, B.V.R.; Rao, G.V.S.; Souquet, J.L. Lithium Conducting Glass Ceramic with Nasicon Structure. Mater. Res. Bull. 2002, 37, 1419–1430. [Google Scholar] [CrossRef]
- Santagneli, S.H.; Baldacim, H.V.A.; Ribeiro, S.J.L.; Kundu, S.; Rodrigues, A.C.M.; Doerenkamp, C.; Eckert, H. Preparation, Structural Characterization, and Electrical Conductivity of Highly Ion-Conducting Glasses and Glass Ceramics in the System Li1+xAlxSnyGe2−(x+y)(PO4)3. J. Phys. Chem. C 2016, 120, 14556–14567. [Google Scholar] [CrossRef]
- Xu, X.; Wen, Z.; Wu, X.; Yang, X.; Gu, Z. Lithium Ion Conducting Glass-Ceramics of Li1.5Al0.5Ge1.5(PO4)3−xLi2O (x = 0.0–0.20) with Good Electrical and Electrochemical Properties. J. Am. Ceram. Soc. 2007, 90, 2802–2806. [Google Scholar] [CrossRef]
- Pershina, S.V.; Vovkotrub, E.G.; Antonov, B.D. Effects of B2O3 on crystallization kinetics, microstructure and properties of Li1.5Al0.5Ge1.5(PO4)3-based glass-ceramics. Solid State Ion. 2022, 383, 115990. [Google Scholar] [CrossRef]
- Nuernberg, R.B.; Rodrigues, A.C.M. A New NASICON Lithium Ion- Conducting Glass-Ceramic of the Li1+xCrx(GeyTi1−y)2−x(PO4)3 System. Solid State Ion. 2017, 301, 1–9. [Google Scholar] [CrossRef]
- Nuernberg, R.B.; Pradel, A.; Rodrigues, A.C.M. A systematic study of glass stability, crystal structure and electrical properties of lithium ion-conducting glass-ceramics of the Li1+xCrx(GeyTi1−y)2−x(PO4)3 system. J. Power Sources 2017, 371, 167–177. [Google Scholar] [CrossRef]
- Nagao, K.; Hayashi, A.; Tatsumisago, M. Mechanochemical synthesis and crystallization of Li3BO3-Li2CO3 glass electrolytes. J. Ceram. Soc. Jpn. 2016, 124, 915–919. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Takano, R.; Nose, M.; Nagao, K.; Kato, A.; Sakuda, A.; Tadanaga, K.; Hayashi, A. Electrical and mechanical properties of glass and glass-ceramic electrolytes in the system Li3BO3-Li2SO4. J. Ceram. Soc. Jpn. 2017, 125, 433–437. [Google Scholar] [CrossRef]
- Yoneda, Y.; Shigeno, M.; Kimura, T.; Nagao, K.; Hotehama, C.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Preparation and characterization of hexagonal Li4GeO4-based glass-ceramic electrolytes. Solid State Ion. 2021, 363, 115605. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Takano, R.; Tadanaga, K.; Hayashi, A. Preparation of Li3BO3-Li2SO4 glass-ceramic electrolytes for all-oxide lithium batteries. J. Power Sources 2014, 270, 603–607. [Google Scholar] [CrossRef]
- Yoneda, Y.; Hotehama, C.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Glassy oxide electrolytes in the system Li4SiO4-Li2SO4 with excellent formability. J. Ceram. Soc. Jpn. 2021, 129, 458–463. [Google Scholar] [CrossRef]
- Widanarto, W.; Ramdhan, A.M.; Ghoshal, S.K.; Effendi, M.; Cahyanto, W.T. Improved ionic conductivity of lithium-zinc-tellurite glass-ceramic electrolytes. Results Phys. 2017, 7, 2277–2280. [Google Scholar] [CrossRef]
- Tezuka, N.; Okawa, Y.; Kajihara, K.; Kanamura, K. Synthesis and characterization of lithium-ion-conductive glass-ceramics of lithium chloroboracite Li4+xB7O12+x/2Cl (x = 0–1). J. Ceram. Soc. Jpn. 2017, 125, 348–352. [Google Scholar] [CrossRef]
- Nagao, K.; Nose, M.; Kato, A.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Preparation and characterization of glass solid electrolytes in the pseudoternary system Li3BO3-Li2SO4-Li2CO3. Solid State Ion. 2017, 308, 68–76. [Google Scholar] [CrossRef]
- Okumura, T.; Takeuchi, T.; Kobayashi, H. Enhancement of lithium-ion conductivity for Li2.2C0.8B0.2O3 by spark plasma sintering. J. Ceram. Soc. Jpn. 2017, 125, 276–280. [Google Scholar] [CrossRef]
- Shin, R.-H.; Son, S.I.; Han, Y.S.; Kim, Y.D.; Kim, H.-T.; Ryu, S.-S.; Pan, W. Sintering behavior of garnet-type Li7La3Zr2O12-Li3BO3 composite solid electrolytes for all-solid-state lithium batteries. Solid State Ion. 2017, 301, 10–14. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Hayashi, A. Superionic glasses and glass-ceramics in the Li2S-P2S5 system for all-solid-state lithium secondary batteries. Solid State Ion. 2012, 225, 342–345. [Google Scholar] [CrossRef]
- Mi, C.; Hall, S.R. Preparation and degradation of high air stability sulfide solid electrolyte 75Li2S-25P2S5 glass-ceramic. Solid State Ion. 2023, 389, 116106. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Liu, T.; Shen, Y.; Lin, Y.; Nan, C.W. High Capacity, Superior Cyclic Performances in All-Solid-State Lithium-Ion Batteries Based on 78Li2S-22P2S5 Glass-Ceramic Electrolytes Prepared via Simple Heat Treatment. ACS Appl. Mater. Interfaces 2017, 9, 28542–28548. [Google Scholar] [CrossRef]
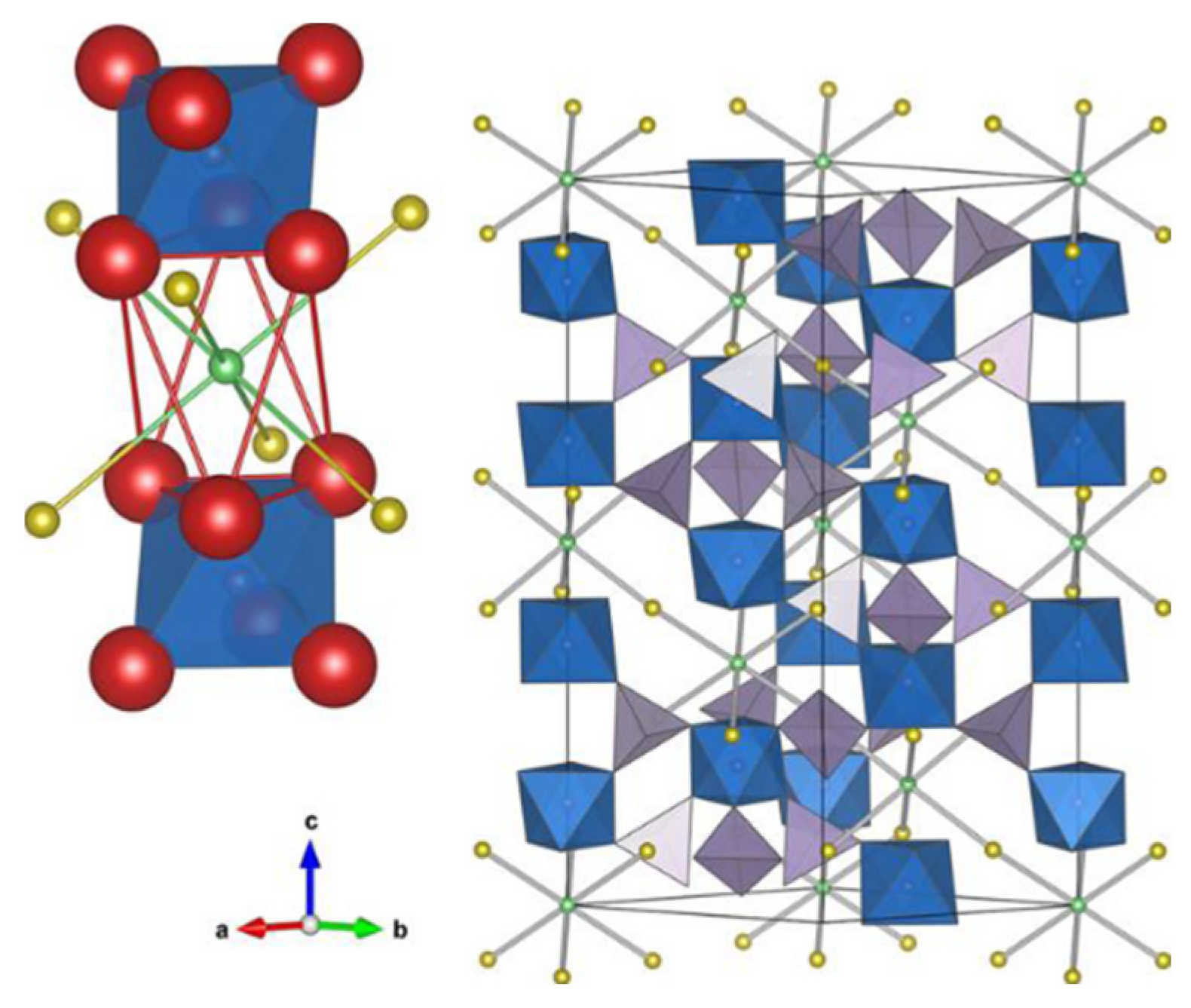
- Yamane, H.; Shibata, M.; Shimane, Y.; Junke, T.; Seino, Y.; Adams, S.; Minami, K.; Hayashi, A.; Tatsumisago, M. Crystal Structure of a Superionic Conductor, Li7P3S11. Solid State Ion. 2007, 178, 1163–1167. [Google Scholar] [CrossRef]
- Wang, Y.; Richards, W.D.; Ong, S.P.; Miara, L.J.; Kim, J.C.; Mo, Y.; Ceder, G. Design Principles for Solid-State Lithium Superionic Conductors. Nat. Mater. 2015, 14, 1026–1031. [Google Scholar] [CrossRef]
- Ohtomo, T.; Hayashi, A.; Tatsumisago, M.; Tsuchida, Y.; Hama, S.; Kawamoto, K. All-solid-state lithium secondary batteries using the 75Li2S-25P2S5 glass and the 70Li2S-30P2S5 glass-ceramic as solid electrolytes. J. Power Sources 2013, 233, 231–235. [Google Scholar] [CrossRef]
- Homma, K.; Yonemura, M.; Kobayashi, T.; Nagao, M.; Hirayama, M.; Kanno, R. Crystal structure and phase transitions of the lithium ionic conductor Li3PS4. Solid State Ion. 2011, 182, 53–58. [Google Scholar] [CrossRef]
- Chen, S.; Xie, D.; Liu, G.; Mwizerwa, J.P.; Zhang, Q.; Zhao, Y.; Xu, X.; Yao, X. Sulfide Solid Electrolytes for All-Solid-State Lithium Batteries: Structure, Conductivity, Stability and Application. Energy Storage Mater. 2018, 14, 58–74. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, Y.; Lee, J.; Shin, D. Formation of the high lithium ion conducting phase from mechanically milled amorphous Li2S-P2S5 system. J. Power Sources 2011, 196, 6920–6923. [Google Scholar] [CrossRef]
- Lu, S.; Kosaka, F.; Shiotani, S.; Tsukasaki, H.; Mori, S.; Otomo, J. Optimization of lithium ion conductivity of Li2S-P2S5 glass ceramics by microstructural control of crystallization kinetics. Solid State Ion. 2021, 362, 115583. [Google Scholar] [CrossRef]
- Yu, C.; Ganapathy, S.; van Eck, E.R.; van Eijck, L.; de Klerk, N.; Kelder, E.M.; Wagemaker, M. Investigation of Li-ion transport in Li7P3S11 and solid-state lithium batteries. J. Energy Chem. 2019, 38, 1–7. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Z.; Yu, C.; Wei, C.; Peng, L.; Wang, L.; Cheng, S.; Xie, J. Low temperature ensures FeS2 cathode superior cycling stability in Li7P3S11-based all-solid-state lithium batteries. Front. Energy Res. 2023, 10, 1108789. [Google Scholar] [CrossRef]
- Trevey, J.E.; Jung, Y.S.; Lee, S. High lithium ion conducting Li2S-GeS2-P2S5 glass-ceramic solid electrolyte with sulfur additive for all solid-state lithium secondary batteries. Electrochim. Acta 2011, 56, 4243–4247. [Google Scholar] [CrossRef]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2014, 7, 627–631. [Google Scholar] [CrossRef]
- Preefer, M.B.; Grebenkemper, J.H.; Schroeder, F.; Bocarsly, J.D.; Pilar, K.; Cooley, J.A.; Zhang, W.; Hu, J.; Misra, S.; Seeler, F.; et al. Rapid and Tunable Assisted-Microwave Preparation of Glass and Glass-Ceramic Thiophosphate “Li7P3S11” Li-Ion Conductors. ACS Appl. Mater. Interfaces 2019, 11, 42280–42287. [Google Scholar] [CrossRef]
- Muramatsu, H.; Hayashi, A.; Ohtomo, T.; Hama, S.; Tatsumisago, M. Structural change of Li2S-P2S5 sulfide solid electrolytes in the atmosphere. Solid State Ion. 2011, 182, 116–119. [Google Scholar] [CrossRef]
- Trevey, J.E.; Jung, Y.S.; Lee, S. Preparation of Li2S-GeSe2-P2S5 electrolytes by a single step ball milling for all-solid-state lithium secondary batteries. J. Power Sources 2010, 195, 4984–4989. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, Y.; Eom, M.; Shin, D. Characterization of amorphous and crystalline Li2S-P2S5-P2Se5 solid electrolytes for all-solid-state lithium ion batteries. Solid State Ion. 2012, 225, 626–630. [Google Scholar] [CrossRef]
- Lu, P.; Ding, F.; Xu, Z.; Liu, J.; Liu, X.; Xu, Q. Study on (100 − x)(70Li2S-30P2S5)-xLi2ZrO3 glass-ceramic electrolyte for all-solid-state lithium-ion batteries. J. Power Sources 2017, 356, 163–171. [Google Scholar] [CrossRef]
- Choi, S.; Eom, M.; Park, C.; Son, S.; Lee, G.; Shin, D. Effect of Li2SO4 on the properties of Li2S-P2S5 glass-ceramic solid electrolytes. Ceram. Int. 2016, 42, 6738–6742. [Google Scholar] [CrossRef]
- Trevey, J.E.; Gilsdorf, J.R.; Miller, S.W.; Lee, S. Li2S-Li2O-P2S5 solid electrolyte for all-solid-state lithium batteries. Solid State Ion. 2012, 214, 25–30. [Google Scholar] [CrossRef]
- Liu, G.; Xie, D.; Wang, X.; Yao, X.; Chen, S.; Xiao, R.; Li, H.; Xu, X. High air-stability and superior lithium ion conduction of Li3+3xP1−xZnxS4−xOx by aliovalent substitution of ZnO for all-solid-state lithium batteries. Energy Storage Mater. 2019, 17, 266–274. [Google Scholar] [CrossRef]
- Ahmad, N.; Sun, S.; Yu, P.; Yang, W. Design Unique Air-Stable and Li-Metal Compatible Sulfide Electrolyte via Exploration of Anion Functional Units for All-Solid-State Lithium-Metal Batteries. Adv. Funct. Mater. 2022, 32, 2201528. [Google Scholar] [CrossRef]
- Jiang, Z.; Liang, T.; Liu, Y.; Zhang, S.; Li, Z.; Wang, D.; Wang, X.; Xia, X.; Gu, C.; Tu, J. Improved Ionic Conductivity and Li Dendrite Suppression Capability toward Li7P3S11-Based Solid Electrolytes Triggered by Nb and O Cosubstitution. ACS Appl. Mater. Interfaces 2020, 12, 54662–54670. [Google Scholar] [CrossRef]
- Huang, B.; Yao, X.; Huang, Z.; Guan, Y.; Jin, Y.; Xu, X. Li3PO4-doped Li7P3S11 glass-ceramic electrolytes with enhanced lithium ion conductivities and application in all-solid-state batteries. J. Power Sources 2019, 284, 206–211. [Google Scholar] [CrossRef]
- Tsukasaki, H.; Morimoto, H.; Mori, S. Thermal behavior and microstructure of the Li3PS4-ZnO composite electrolyte. J. Power Sources 2019, 436, 226865. [Google Scholar] [CrossRef]
- Minami, K.; Hayashi, A.; Tatsumisago, M. Preparation and Characterization of Lithium Ion Conducting Li2S-P2S5-GeS2 Glasses and Glass -Ceramics. J. Non-Cryst. Solids 2010, 356, 2666–2669. [Google Scholar] [CrossRef]
- Hayashi, A.; Minami, K.; Ujiie, S.; Tatsumisago, M. Preparation and Ionic Conductivity of Li7P3S11-z Glass-Ceramic Electrolytes. J. Non-Cryst. Solids 2010, 356, 2670–2673. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.; Kim, M.; Min, S.; Kim, G.; Park, S.; Shin, D. Li metal stability enhancement of Sn-doped Li2S-P2S5 glass-ceramics electrolyte. Electrochim. Acta 2021, 390, 138808. [Google Scholar] [CrossRef]
- Park, M.; Jung, H.-G.; Jung, W.D.; Cho, S.Y.; Yun, B.-N.; Lee, Y.S.; Choi, S.; Ahn, J.; Lim, J.; Sung, J.Y.; et al. Chemically Evolved Composite Lithium-Ion Conductors with Lithium Thiophosphates and Nickel Sulfides. ACS Energy Lett. 2017, 2, 1740–1745. [Google Scholar] [CrossRef]
- Dong, P.; Jiao, Q.; Zhang, Z.; Jiang, M.; Lin, C.; Zhang, X.; Ma, H.; Ma, B.; Dai, S.; Xu, T. Controllable Li3PS4-Li4SnS4 solid electrolytes with affordable conductor and high conductivity for solid-state battery. J. Am. Ceram. Soc. 2022, 105, 3252–3260. [Google Scholar] [CrossRef]
- Zhou, L.; Tufail, M.K.; Yang, L.; Ahmad, N.; Chen, R.; Yang, W. Cathode-doped sulfide electrolyte strategy for boosting all-solid-state lithium batteries. Chem. Eng. J. 2020, 391, 123529. [Google Scholar] [CrossRef]
- Otoyama, M.; Kuratani, K.; Kobayashi, H. A systematic study on structure, ionic conductivity, and air-stability of xLi4SnS4·(1 x)Li3PS4 solid electrolytes. Ceram. Int. 2021, 47, 28377–28383. [Google Scholar] [CrossRef]
- Han, F.; Yue, J.; Zhu, X.; Wang, C. Suppressing Li Dendrite Formation in Li2S-P2S5 Solid Electrolyte by LiI Incorporation. Adv. Energy Mater. 2018, 8, 1703644. [Google Scholar] [CrossRef]
- Bui, A.D.; Choi, S.H.; Choi, H.; Lee, Y.J.; Doh, C.H.; Park, J.W.; Kim, B.G.; Lee, W.J.; Lee, S.M.; Ha, Y.C. Origin of the Outstanding Performance of Dual Halide Doped Li7P2S8X (X = I, Br) Solid Electrolytes for All-Solid-State Lithium Batteries. ACS Appl. Energy Mater. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- El Kharbachi, A.; Hu, Y.; Yoshida, K.; Vajeeston, P.; Kim, S.; Sørby, M.H.; Orimo, S.-I.; Fjellvåg, H.; Hauback, B.C. Lithium ionic conduction in composites of Li(BH4)0.75I0.25 and amorphous 0.75Li2S-0.25P2S5 for battery applications. Electrochim. Acta 2018, 278, 332–339. [Google Scholar] [CrossRef]
- Ujiie, S.; Inagaki, T.; Hayashi, A.; Tatsumisago, M. Conductivity of 70Li2S-30P2S5 glasses and glass-ceramics added with lithium halides. Solid State Ion. 2014, 263, 57–61. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Wang, Z.; Ma, W.; Shi, Y.; Jiang, Y.; Jiang, J.; Liu, X.; Xu, Y.; Zhang, J. Incorporation of lithium halogen in Li7P3S11 glass-ceramic and the interface improvement mechanism. Electrochim. Acta 2021, 390, 138849. [Google Scholar] [CrossRef]
- Zhang, N.; Ding, F.; Yu, S.; Zhu, K.; Li, H.; Zhang, W.; Liu, X.; Xu, Q. Novel Research Approach Combined with Dielectric Spectrum Testing for Dual-Doped Li7P3S11 Glass-Ceramic Electrolytes. ACS Appl. Mater. Interfaces 2019, 11, 27897–27905. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liang, B.; Lin, C.; Gao, C.; Shen, X.; Liu, Y.; Jiao, Q. Design of cation doped Li7P2S8Br(1−x)Ix sulfide electrolyte with improved conductivity and stable interfacial properties for all-solid-state lithium batteries. Appl. Mater. Today 2022, 29, 101692. [Google Scholar] [CrossRef]
- Jung, W.D.; Yun, B.N.; Jung, H.G.; Choi, S.; Son, J.W.; Lee, J.H.; Lee, J.H.; Kim, H. Configuring PSx tetrahedral clusters in Li-excess Li7P3S11 solid electrolyte. APL Mater. 2018, 6, 047902. [Google Scholar] [CrossRef]
- Wei, J.; Kim, H.; Lee, D.-C.; Hu, R.; Wu, F.; Zhao, H.; Alamgir, F.M.; Yushin, G. Influence of annealing on ionic transfer and storage stability of Li2S-P2S5 solid electrolyte. J. Power Sources 2015, 294, 494–500. [Google Scholar] [CrossRef]
- Raj, V.; Aetukuri, N.P.B.; Nanda, J. Solid state lithium metal batteries-Issues and challenges at the lithium-solid electrolyte interface. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100999. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; GongGong, Y. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef]
- Agostini, M.; Aihara, Y.; Yamada, T.; Scrosati, B.; Hassoun, J. A lithium-sulfur battery using a solid, glass-type P2S5-Li2S electrolyte. Solid State Ion. 2013, 244, 48–51. [Google Scholar] [CrossRef]
- Fu, K.; Gong, Y.; Xu, S.; Zhu, Y.; Li, Y.; Dai, J.; Wang, C.; Liu, B.; Pastel, G.; Xie, H.; et al. Stabilizing the garnet solid-electrolyte/polysulfide interface in Li-S batteries. Chem. Mater. 2017, 29, 8037–8041. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Zheng, B.; Yang, Y. Recent Progress in All-Solid-State Lithium-Sulfur Batteries Using High Li-Ion Conductive Solid Electrolytes. Electrochem. Energy Rev. 2019, 2, 199–230. [Google Scholar] [CrossRef]
- Tufail, M.K.; Ahmad, N.; Zhou, L.; Faheem, M.; Yang, L.; Chen, R.; Yang, W. Insight on air-induced degradation mechanism of Li7P3S11 to design a chemical-stable solid electrolyte with high Li2S utilization in all-solid-state Li/S batteries. Chem. Eng. J. 2021, 425, 130535. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Zhang, Q.; Yan, L.; Wang, T.-B.; Hou, P.-Y. A review of interfaces within solid-state electrolytes: Fundamentals, issues and advancements. Chem. Eng. J. 2022, 437, 135179. [Google Scholar] [CrossRef]
- Yu, S.; Siegel, D.J. Grain boundary contributions to Li-ion transport in the solid electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 2017, 29, 9639–9647. [Google Scholar] [CrossRef]
- Shin, B.R.; Nam, Y.J.; Oh, D.Y.; Kim, D.H.; Kim, J.W.; Jung, Y.S. Comparative Study of TiS2/Li-In All-Solid-State Lithium Batteries Using Glass-Ceramic Li3PS4 and Li10GeP2S12 Solid Electrolytes. Electrochim. Acta 2014, 146, 395–402. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Liu, X.; Zhong, H.; Xu, H.; Xu, Z.; Shao, H.; Ding, F. Suppression of Lithium Dendrite Formation by Using LAGP-PEO (LiTFSI) Composite Solid Electrolyte and Lithium Metal Anode Modified by PEO (LiTFSI) in All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 13694–13702. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Shen, M.; Fang, Z.; Kong, T.; Feng, W.; Xie, Y.; Wang, F.; Hu, B.; Wang, Y. A Highly Stable Li-Organic All-Solid-State Battery Based on Sulfide Electrolytes. Adv. Energy Mater. 2022, 12, 2103932. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Sumita, M.; Tanaka, Y.; Ikeda, M.; Ohno, T. Charged and Discharged States of Cathode/Sulfide Electrolyte Interfaces in All-Solid-State Lithium-Ion Batteries. J. Phys. Chem. C 2016, 120, 13332–13339. [Google Scholar] [CrossRef]
- Kato, A.; Hayashi, A.; Tatsumisago, M. Enhancing utilization of lithium metal electrodes in all-solid-state batteries by interface modification with gold thin films. J. Power Sources 2016, 309, 27–32. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Zeng, X.-X.; Zhang, X.-D.; Wang, P.-F.; Ma, J.-Y.; Yin, Y.-X.; Wu, X.-W.; Guo, Y.-G.; Wan, L.-J. Mitigating Interfacial Potential Drop of Cathode-Solid Electrolyte via Ionic Conductor Layer To Enhance Interface Dynamics for Solid Batteries. J. Am. Chem. Soc. 2018, 140, 6767–6770. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, H.S.; Cho, M.-S.; Kalubarme, R.S.; Lee, J.-S.; Jung, K.-N.; Shin, K.-H.; Park, C.-J. Influence of B2O3 addition on the ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 glass ceramics. J. Power Sources 2013, 241, 502–508. [Google Scholar] [CrossRef]
- Saffirio, S.; Falco, M.; Appetecchi, G.B.; Smeacetto, F.; Gerbaldi, C. Li1.4Al0.4Ge0.4Ti1.4(PO4)3 promising NASICON-structured glass-ceramic electrolyte for all-solid-state Li-based batteries: Unravelling the effect of diboron trioxide. J. Eur. Ceram. Soc. 2022, 42, 1023–1032. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yoshida, R.; Sato, T.; Matsumoto, H.; Kurobe, H.; Hamanaka, T.; Kato, T.; Iriyama, Y.; Hirayama, T. Nano-scale simultaneous observation of Li-concentration profile and Ti-, O electronic structure changes in an all-solid-state Li-ion battery by spatially-resolved electron energy-loss spectroscopy. J. Power Sources 2014, 266, 414–421. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Li, B.; Song, H.; Cheng, Z.; Chen, M.; He, P.; Zhou, H. Germanium Thin Film Protected Lithium Aluminum Germanium Phosphate for Solid-State Li Batteries. Adv. Energy Mater. 2018, 8, 1702374. [Google Scholar] [CrossRef]
- Hu, F.; Li, Y.; Wei, Y.; Yang, J.; Hu, P.; Rao, Z.; Chen, X.; Yuan, L.; Li, Z. Construct an Ultrathin Bismuth Buffer for Stable Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2020, 12, 12793–12800. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Iriyama, Y.; Muto, S. STEM-EELS analysis of the interface structures of composite ASSLIB electrodes fabricated via aerosol deposition. J. Am. Ceram. Soc. 2020, 103, 1454–1462. [Google Scholar] [CrossRef]
- Shubha, N.; Prasanth, R.; Hng, H.H.; Srinivasan, M. Study on effect of poly (ethylene oxide) addition and in-situ porosity generation on poly (vinylidene fluoride)-glass ceramic composite membranes for lithium polymer batteries. J. Power Sources 2014, 267, 48–57. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Z.; Yoshida, A.; An, X.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Novel SeS2 doped Li2S-P2S5 solid electrolyte with high ionic conductivity for all-solid-state lithium sulfur batteries. Chem. Eng. J. 2020, 380, 122419. [Google Scholar]
- Feng, X.; Chien, P.-H.; Patel, S.; Zheng, J.; Immediato-Scuotto, M.; Xin, Y.; Hung, I.; Gan, Z.; Hu, Y.-Y. Synthesis and characterizations of highly conductive and stable electrolyte Li10P3S12I. Energy Storage Mater. 2019, 22, 397–401. [Google Scholar] [CrossRef]
- Xu, R.; Han, F.; Ji, X.; Fan, X.; Tu, J.; Wang, C. Interface engineering of sulfide electrolytes for all-solid-state lithium batteries. Nano Energy 2018, 53, 958–966. [Google Scholar] [CrossRef]

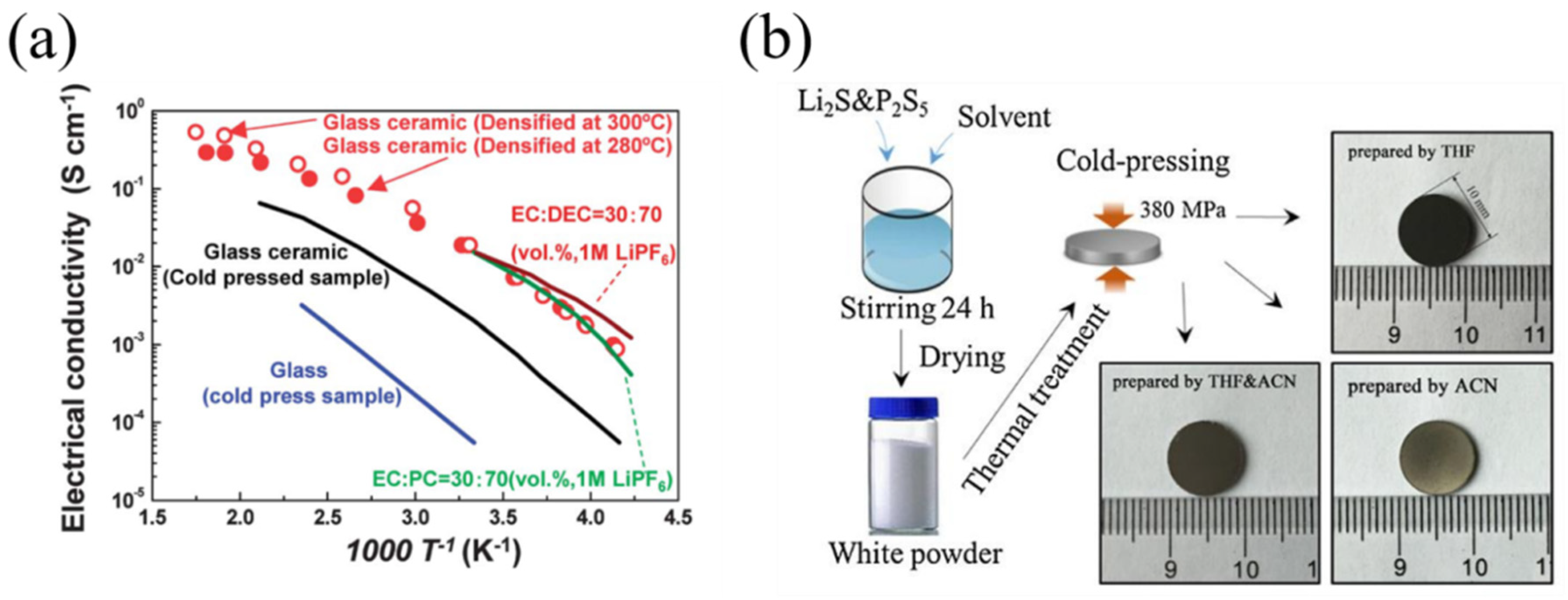
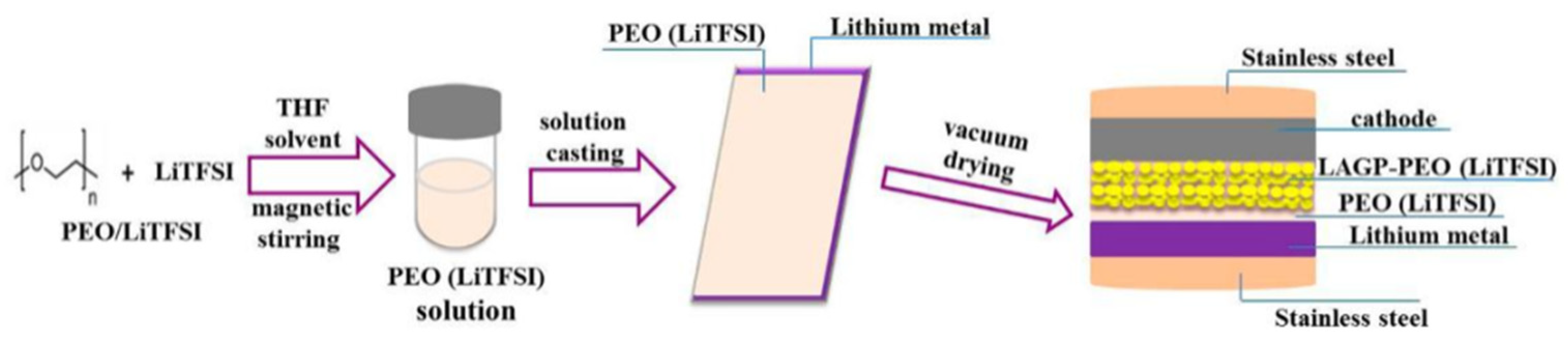
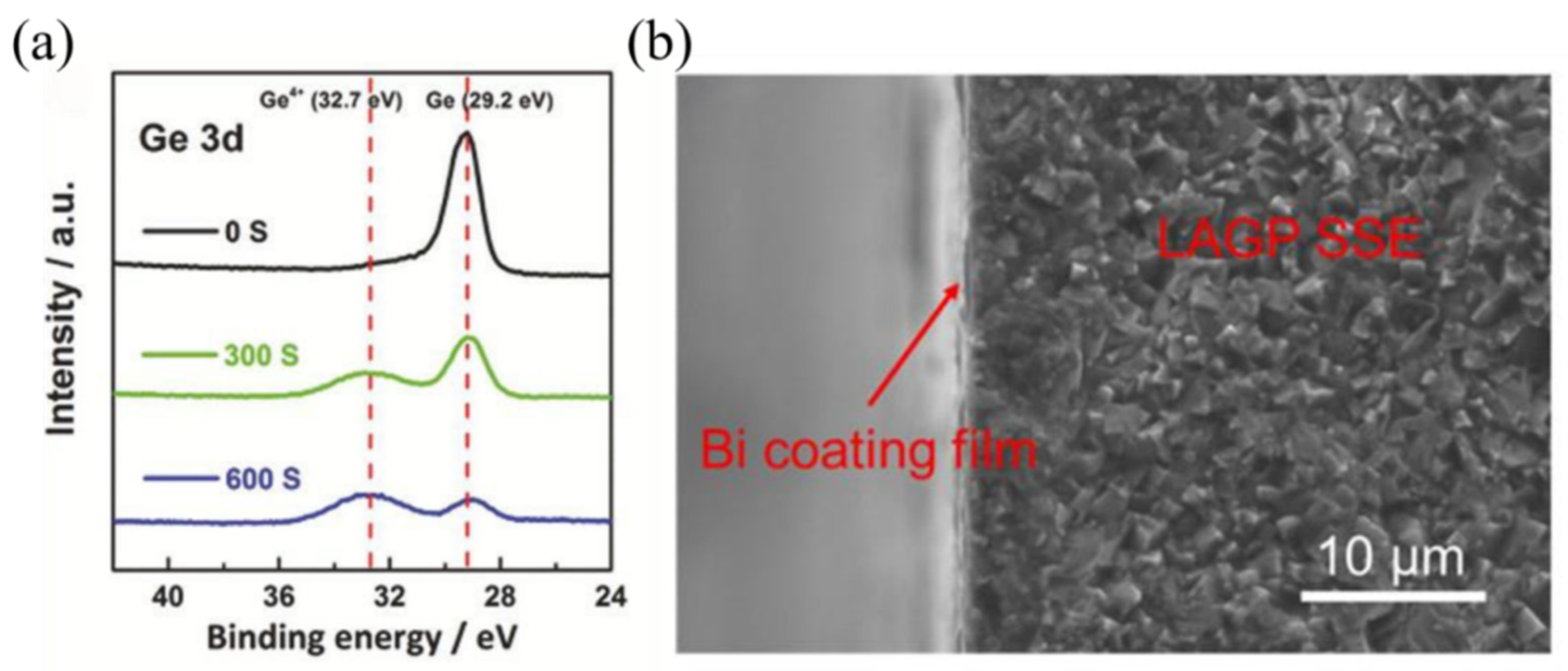
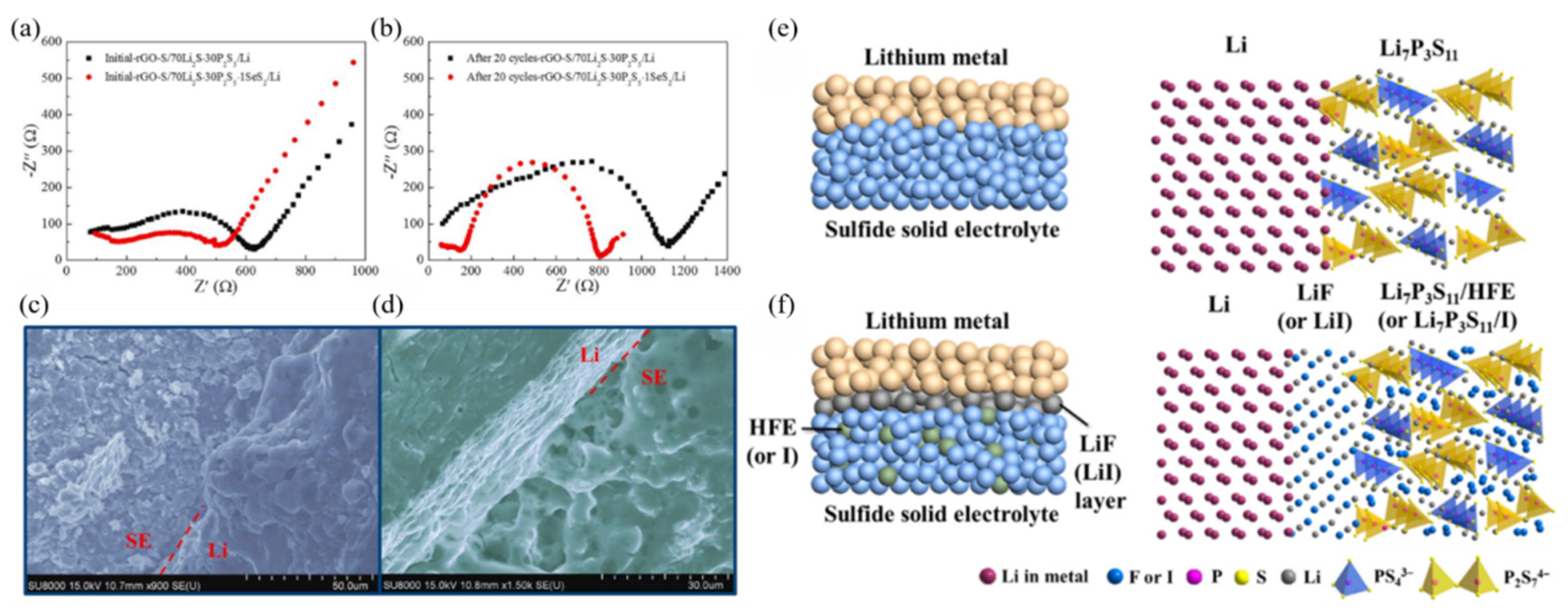
| Composition | Tg (°C) | Tc (°C) | Crystallization | σ (S·cm−1) | Ea (eV) | Reference |
|---|---|---|---|---|---|---|
| Li1.3Al0.3Ti1.7(PO4)3 | 624 | 660 | 1000 °C/0.33 h | 1.3 × 10−3 | 0.27 | [43] |
| Li1.3Al0.3Ti1.7(PO4)3 | 640 | 670 | 950 °C/70 h | 1.23 × 10−4 | 0.37 | [58] |
| Li1.3Al0.3Ti1.7(PO4)3-50P2O5 | 632 | 750 | 850 °C/10 h | 8.5 × 10−4 | 0.26 | [59] |
| Li1.4Al0.4Ge1.6(PO4)3 | 534 | 614 | 650 °C/96 h | 3.8 × 10−5 | 0.52 | [60] |
| Li1.5Al0.5Ge1.5(PO4)3 | 508.4 | 598.4 | 820 °C/2 h | 5.03 × 10−4 | 0.36 | [44] |
| Li1.5Al0.5Ge1.5(PO4)3 | 524 | 589 | 800 °C/8 h | 2.9 × 10−3 | 0.29 | [52] |
| Li1.25Al0.25Sn0.25Ge1.75(PO4)3 | 518 | 622 | 628 °C/1 h | 3.9 × 10−5 | 0.36 | [61] |
| Li1.5Al0.33Sc0.17Ge1.5(PO4)3 | 800 °C/8 h | 5.8 × 10−3 | 0.28 | [53] | ||
| Li1.5Al0.5Ge1.5(PO4)3 + 0.05Li2O | 532 | 629 | 829 °C/6 h | 7.3 × 10−4 | 0.38 | [62] |
| Li1.5Al0.5Ge1.5(PO4)3-0.05B2O3 | 526.0 | 636.4 | 820 °C/2 h | 5.5 × 10−4 | [63] | |
| Li1.4Cr0.4Ge0.64Ti0.96(PO4)3 | 623 | 692 | 900 °C/12 h | 6.6 × 10−5 | 0.40 | [64] |
| Li1.6Cr0.6Ge0.28Ti1.12(PO4)3 | 682.5 | 725.8 | 900 °C/2 h | 2.9 × 10−4 | 0.26 | [65] |
| Composition | σ (S·cm−1) | Structure of the Battery | Initial Energy Density | Electrochemical Window | Ref |
| 70Li2S∙30P2S5 | 1.7 × 10−2 | −0.1~5 V vs. Li/Li+ | [89] | ||
| Li7P3S11 | 6.3 × 10−4 | Li2S/Li7P3S11/Li-In | 1139.5 mAh/g at 0.064 mA/cm2 | [86] | |
| Li7P3S11 | 1.27 × 10−3 | FeS2/Li7P3S11/Li-In | 620.8 mAh/g at 0.1C | [87] | |
| Li7P3S11 | 9.7 × 10−4 | −0.5~5 V vs. Li/Li+ | [33] | ||
| Li7P3S11 | 1.0 × 10−3 | −0.5~5 V vs. Li/Li+ | [34] | ||
| Li7.25P3S11 | 2.5 × 10−3 | LiNi0.8Co0.15Al0.05O2/ Li7.25P3S11/In | 106.2 mAh/g at 0.1C | 2.0~3.6 V vs. Li-In | [116] |
| 99(70Li2S∙30P2S5)-1Li2ZrO3 | 2.85 × 10−3 | LiCoO2/99(70Li2S∙30P2S5)- 1Li2ZrO3/Li-In | 134.5 mAh/g at 0.1C | [94] | |
| Li7P2.88Nb0.12S10.7O0.3 | 3.59 × 10−3 | Li2S/Li7P2.88Nb0.12S10.7O0.3/Li | 642.1 mAh/g at 0.1C | [99] | |
| 70Li2S∙29P2S5-1Li3PO4 | 1.87 × 10−3 | LiCoO2/ 70Li2S∙29P2S5-1Li3PO4/Li-In | 108 mAh/g at 0.1C | [100] | |
| 99.5(70Li2S∙30P2S5)-0.5FeS2 | 2.22 × 10−3 | FeS2 composite/ 99.5(70Li2S-30P2S5)-0.5FeS2/Li–Ln | 543 mAh/g at 0.03 mA/cm2 | −0.5~5 V vs. Li/Li+ | [107] |
| 80Li7P3S11-20LiBr | 3.39 × 10−3 | LiCoO2/80Li7P3S11-20LiBr/Li | 120 mAh/g at 0.1 mA/cm2 | [113] | |
| 90(0.7Li2S-0.29P2S5-0.01WS2)-10LiBr | LiCoO2/90(0.7Li2S-0.29P2S5-0.01WS2)-10LiBr/Li-In | 129.6 mAh/g at 0.1C | [114] | ||
| 75Li2S∙25P2S5 | 3.1 × 10−4 | LiCoO2/75Li2S∙25P2S5/electrical conductive carbon | 115 mAh/g at 0.05C | −1~5 V vs. Li/Li+ | [35] |
| Li3.06P0.98Zn0.02S3.98O0.02 | 1.12 × 10−3 | LiCoO2/LGPS/Li3.06P0.98Zn0.02S3.98O0.02/Li | 139.1 mAh/g at 0.1C | −0.5~6 V vs. Li/Li+ | [97] |
| Li2.96P0.98S3.92O0.06-Li3N | 1.58 × 10−3 | LiNbO3@NCA/ Li2.96P0.98S3.92O0.06-Li3N/Li | 107.89 mAh/g at 0.064 mA/cm2 | −0.5~5 V vs. Li/Li+ | [98] |
| (Li2S)9-(P2S5)3-(Ni3S2)1 (LPN 9:3:1) | 2.0 × 10−3 | LPN(9:3:1)-NCM/ LPN(9:3:1)/In | 117 mAh/g at 0.1C | −0.5~10 V vs. Li/Li+ | [105] |
| 2.5Li3PS4-0.5Li4SnS4 | 2.1 × 10−3 | LiCoO2/2.5Li3PS4-0.5Li4SnS4/Li | 93 mAh/g at 0.1C | −0.1~5 V vs. Li/Li+ | [106] |
| Li(BH4)0.75I0.25- (Li2S)0.75∙(P2S5)0.25 | 1 × 10−3 | TiS2/Li(BH4)0.75I0.25- (Li2S)0.75∙(P2S5)0.25/Li | 239 mAh/g at 0.05C | −0.5~5 V vs. Li/Li+ | [111] |
| 78.3Li2S·21.7P2S5 | 6.3 × 10−4 | −0.3~5 V vs. Li/Li+ | [84] | ||
| Li7.05Zn0.05P1.95S8Br0.2I0.8 | 3.98 × 10−3 | −0.5~5 V vs. Li/Li+ | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Guo, W.; Li, M.; Qing, J.; Cai, C.; Yi, P.; Deng, Q.; Chen, W. Progress and Perspective of Glass-Ceramic Solid-State Electrolytes for Lithium Batteries. Materials 2023, 16, 2655. https://doi.org/10.3390/ma16072655
Lin L, Guo W, Li M, Qing J, Cai C, Yi P, Deng Q, Chen W. Progress and Perspective of Glass-Ceramic Solid-State Electrolytes for Lithium Batteries. Materials. 2023; 16(7):2655. https://doi.org/10.3390/ma16072655
Chicago/Turabian StyleLin, Liyang, Wei Guo, Mengjun Li, Juan Qing, Chuang Cai, Ping Yi, Qibo Deng, and Wei Chen. 2023. "Progress and Perspective of Glass-Ceramic Solid-State Electrolytes for Lithium Batteries" Materials 16, no. 7: 2655. https://doi.org/10.3390/ma16072655
APA StyleLin, L., Guo, W., Li, M., Qing, J., Cai, C., Yi, P., Deng, Q., & Chen, W. (2023). Progress and Perspective of Glass-Ceramic Solid-State Electrolytes for Lithium Batteries. Materials, 16(7), 2655. https://doi.org/10.3390/ma16072655







