Optimization of Plating Process on Inner Wall of Metal Pipe and Research on Coating Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Device and Process Flow
2.2. Bath Composition and Process Conditions
2.3. Experimental Design
2.4. Test Instruments
- (1)
- The surface morphology of the coating was analyzed using a Quanta 200 scanning electron microscope (Quanta 200, FEI Company, Hillsboro, OR, USA) at a magnification of 2000 times.
- (2)
- The hardness of the deposited layer was characterized using a microhardness tester (VMH-002V, UHL, Asslar, Germany). A load of 25 gf was applied at 5 different positions for 15 s, and the average value of the obtained data were recorded.
- (3)
- The cross-section of the coating was observed using a Leica DMi8 M metallographic microscope at a magnification of 500 times (Leica DMi8 M, Leica, Wetzlar, Germany).
- (4)
- Electrochemical experiments were performed using an electrochemical workstation (CS2350, Wuhan Keist Instrument Co., Ltd., Wuhan, China) in a 3.5 wt% NaCl corrosion medium at room temperature. The three-electrode system had a saturated calomel electrode (SCE) as the reference electrode, a platinum electrode as the working electrode, and a potential range of 1500 mV to 1000 mV with a scan rate of 1 mV/s.
- (5)
- The deformation resistance of the coating was analyzed using a G200 nanoindenter (KLA, Hayward, CA, USA). A load of 100 mN was applied to 5 different points on the surface of the coating, with a Poisson’s ratio of 0.25 set based on the material properties.
3. Results and Discussion
3.1. Process Optimization
3.1.1. Optimization of Pretreatment Process and Bath Configuration
3.1.2. Trial Production of Pipe Inner Wall Coating after Process Optimization
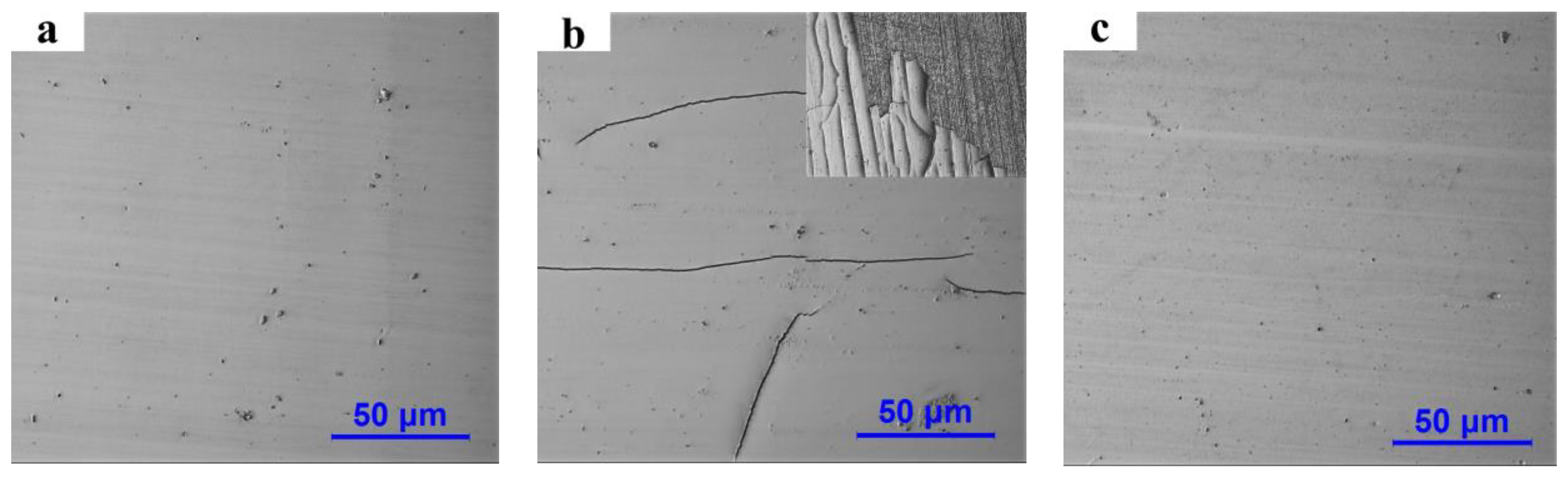
3.2. Surface Morphology of Pipe Inner Wall Coating under Different Voltage
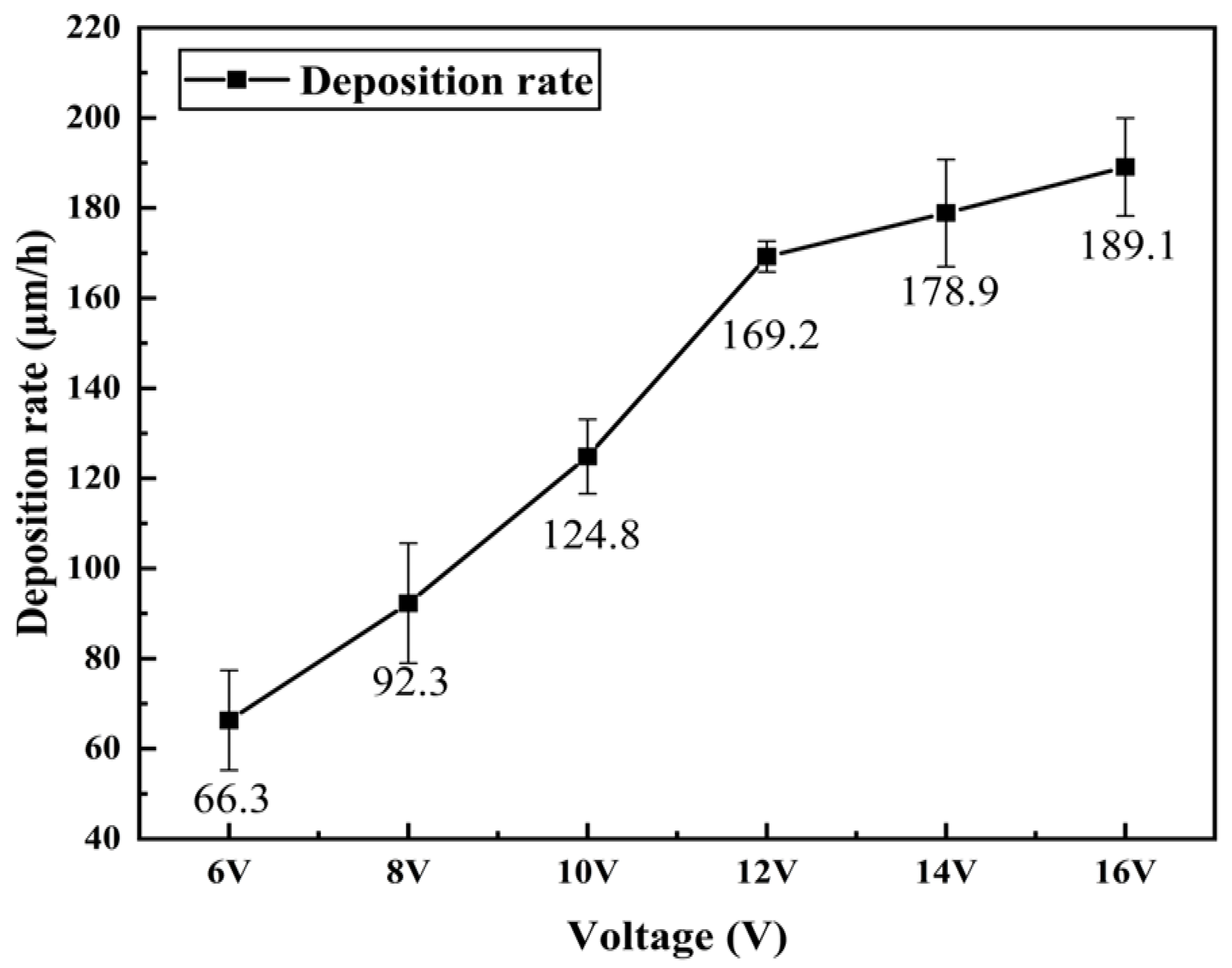
3.3. Cross-Sectional Morphology and Deposition Rate Analysis
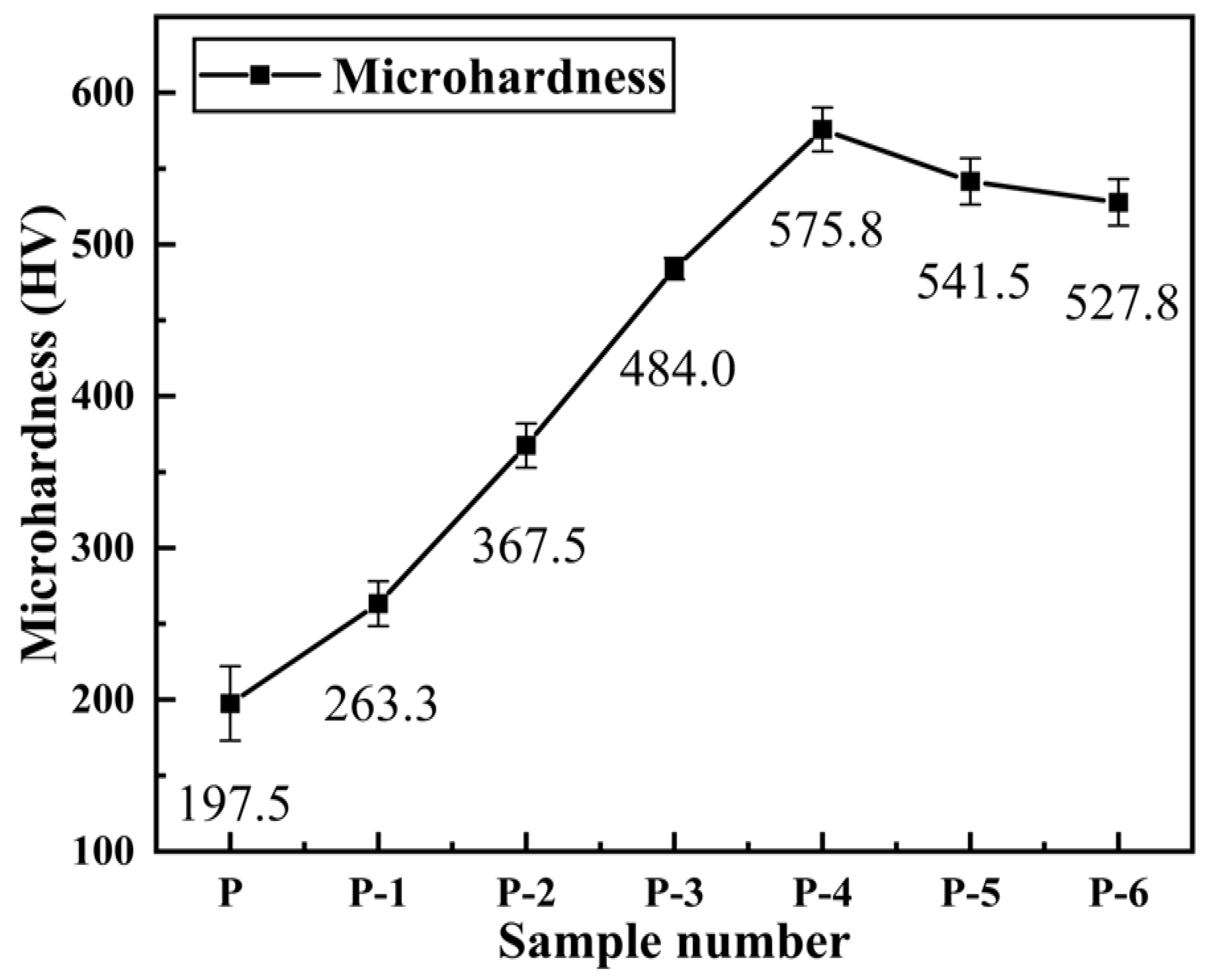
3.4. Hardness of Pipe Inner Wall Coating under Different Voltage
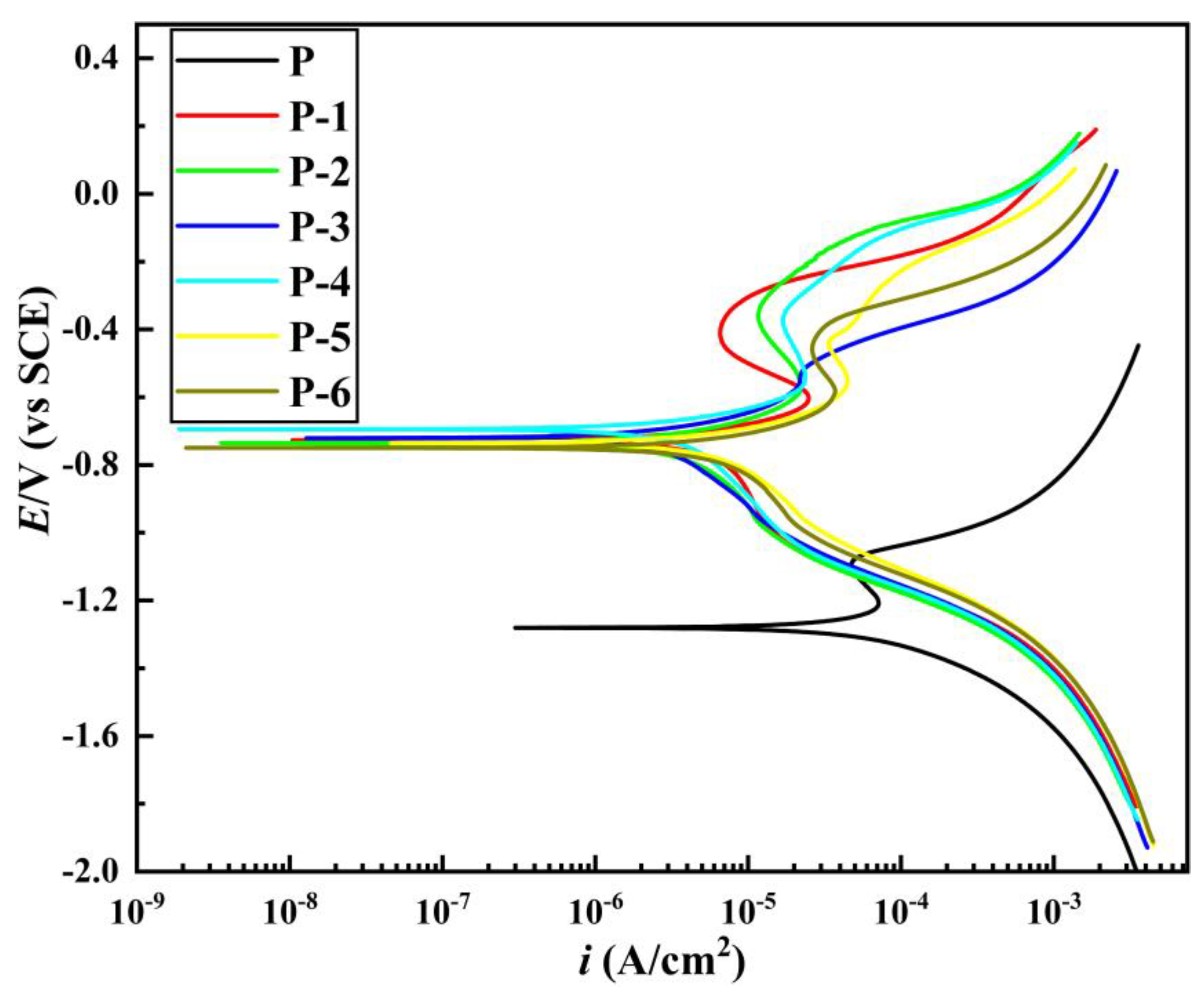
3.5. Corrosion Resistance of Pipe Inner Wall Coating under Different Voltage
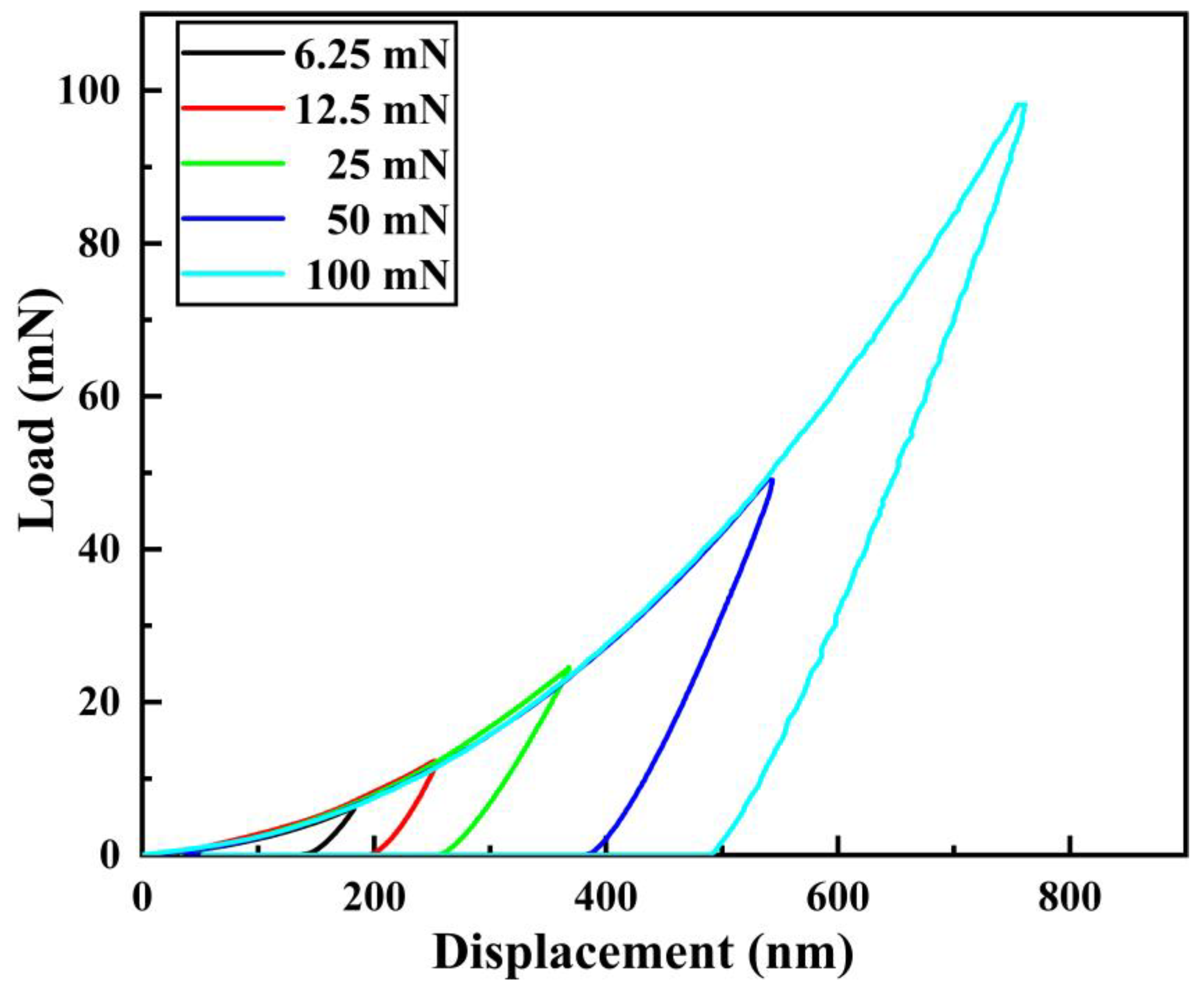
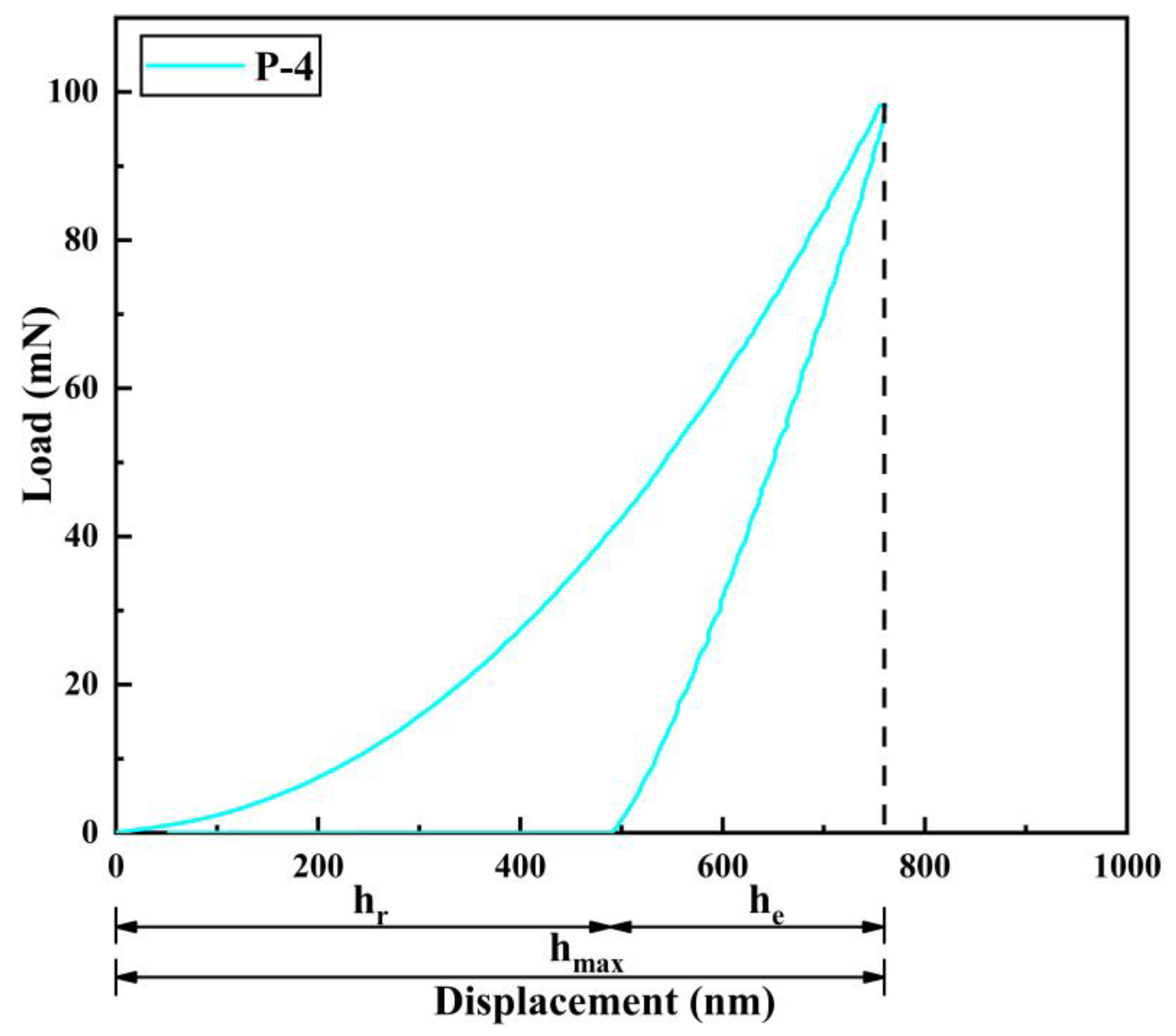
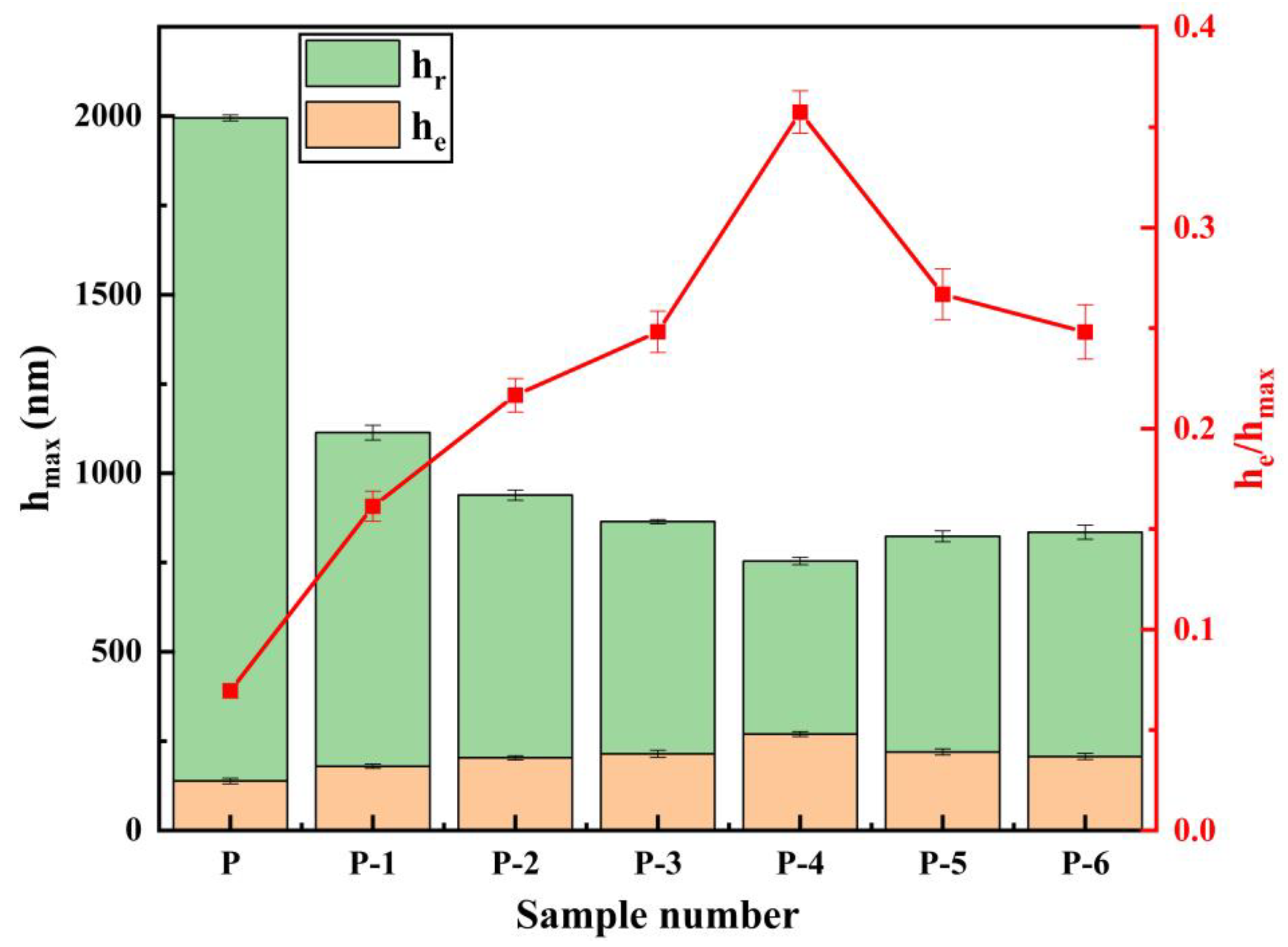
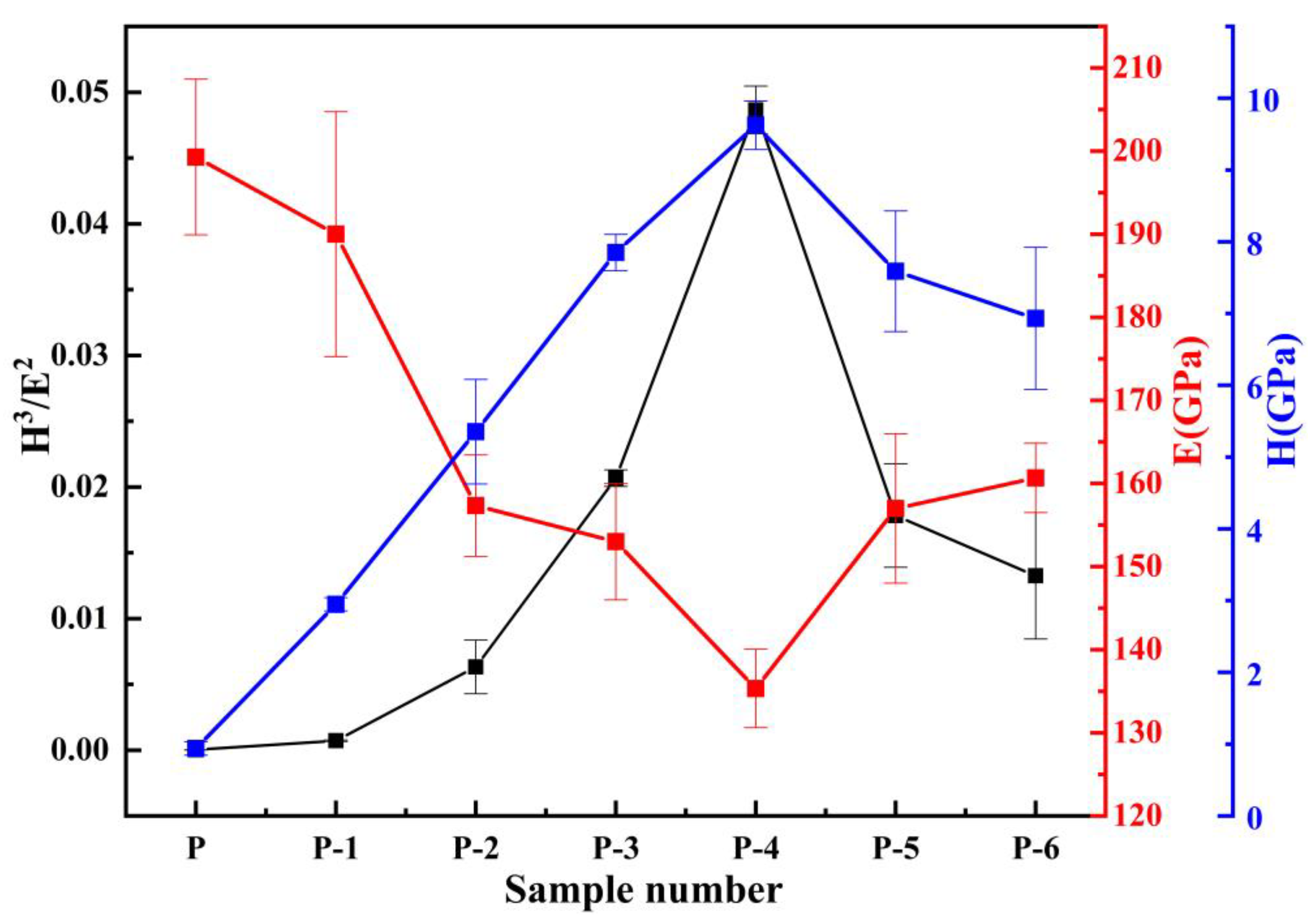
3.6. Elastic-Plastic Deformation Capacity of Pipe Inner Wall Coating under Different Voltage
4. Conclusions
- (1)
- Through the optimization of the pre-treatment process and plating solution formula, nine steps in the electro-brush plating process have been simplified to five steps. Moreover, Ni coatings with dense and uniform surface and good performance can be prepared conveniently and efficiently on the inner wall of metal pipes.
- (2)
- As the working voltage gradually increases, the surface and cross-sectional morphology of the pipe inner wall coating exhibit a trend of improvement followed by deterioration. The deposition rate shows a trend of acceleration followed by deceleration. By using an appropriate working voltage (12 V), a coating with superior surface and cross-sectional morphology, as well as good adhesion to the substrate, can be obtained while maintaining a high deposition rate (169.2 μm/h).
- (3)
- The microhardness, corrosion resistance, and elastic-plasticity of the Ni coating prepared on the inner wall of the pipe show a trend of initially increasing and then decreasing as the working voltage gradually increases. The coating exhibits the best surface quality, the fewest defects, and the most significant grain refinement effect when the working voltage is 12V. At this voltage, the coating has a hardness of 575.8 HV, a corrosion current density of 1.040 × 10−5 A·cm−2, a corrosion rate of 0.122 mm·a−1, and an elastic recovery ratio (he/hmax) of 0.36. The results indicate that the coating prepared at 12 V has the best microhardness, corrosion resistance, and deformation resistance.
- (4)
- Ni coating on the inner wall of a metal pipe via an optimized electro-brush plating deposition process. Compared with the coating on the inner wall of commercially available galvanized steel pipes, the resultant Ni coating exhibited significant enhancements in surface morphology, coating hardness, corrosion resistance, and elastoplasticity. Moreover, the proposed method features several advantages including simple and convenient equipment, uniform and dense surface morphology, high deposition rate, and simplified plating solution and process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pootakham, T.; Kumar, A. Bio-oil transport by pipeline: A techno-economic assessment. Bioresour. Technol. 2010, 101, 7137–7143. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Farhat, Z.N.; Ahmed, E.M.; Alfantazi, A. Erosion enhanced corrosion and corrosion enhanced erosion of API X-70 pipeline steel. Wear 2013, 302, 1592–1601. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Mohamed, A.M. Erosion-corrosion in oil and gas industry: A review. Int. J. Metall. Mater. Sci. Eng. 2014, 4, 7–28. [Google Scholar]
- Hu, X.; Neville, A. CO2 erosion–corrosion of pipeline steel (API X65) in oil and gas conditions—A systematic approach. Wear 2009, 267, 2027–2032. [Google Scholar] [CrossRef]
- EGIG. 9th Report of the European Gas Pipeline Incident Data Group. Available online: https://www.egig.eu/reports (accessed on 25 November 2022).
- Caleyo, F.; Alfonso, L.; Alcántara, J.; Hallen, J. On the estimation of failure rates of multiple pipeline systems. J. Press. Vessel Technol. 2008, 130, 021704. [Google Scholar] [CrossRef]
- Ryu, H.K.; Yoon, K.B. Leak failure at the TP316L welds of a water pipe caused by microbiologically influenced corrosion. Eng. Fail. Anal. 2021, 122, 105244. [Google Scholar]
- Tavanpour, N.; Noshadi, M.; Tavanpour, N. Scale formation and corrosion of drinking water pipes: A case study of drinking water distribution system of Shiraz city. Mod. Appl. Sci. 2016, 10, 166–177. [Google Scholar] [CrossRef]
- Alcantar-Martínez, L.M.; Ruiz-Trabolsi, P.A.; Tadeo-Rosas, R.; Miranda-Hernández, J.G.; Terán-Méndez, G.; Velázquez, J.C.; Hernández-Sánchez, E. Improving the Surface Properties of an API 5L Grade B Pipeline Steel by Applying the Boriding Process. Part I: Kinetics and Layer Characterization. Coatings 2023, 13, 298. [Google Scholar] [CrossRef]
- Ossai, C.I.; Boswell, B.; Davies, I.J. Pipeline failures in corrosive environments–A conceptual analysis of trends and effects. Eng. Fail. Anal. 2015, 53, 36–58. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Guo, Z.; Shu, H. Elastic buckling of cylindrical pipe linings with variable thickness encased in rigid host pipes. Thin Walled Struct. 2012, 51, 10–19. [Google Scholar] [CrossRef]
- Kharazmi, P. Experimental assessment of the state of the lining materials used in the rehabilitation of sewers in residential buildings. Case Stud. Constr. Mater. 2019, 11, e00291. [Google Scholar] [CrossRef]
- Kim, D.; Lee, Y.; Lee, S.; Kim, D. Investigating coating material and conditions for rehabilitation of water transmission pipe using a robotic system. J. Korean Soc. Water Wastewater 2016, 30, 725–736. [Google Scholar] [CrossRef]
- Li, X.H. Study on Erosion Wear Resistance of Internal Coating of Wear-Resistant FRP Compound Pipe. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2016; pp. 112–116. [Google Scholar]
- Kumar, K.; Kumar, S.; Singh, G.; Singh, J.P.; Singh, J. Erosion wear investigation of HVOF sprayed WC-10Co4Cr coating on slurry pipeline materials. Coatings 2017, 7, 54. [Google Scholar] [CrossRef]
- Wang, X.; Sui, X.; Zhang, S.; Yan, M.; Yang, J.; Hao, J.; Liu, W. Effect of deposition pressures on uniformity, mechanical and tribological properties of thick DLC coatings inside of a long pipe prepared by PECVD method. Surf. Coat. Technol. 2019, 375, 150–157. [Google Scholar] [CrossRef]
- Lang, W.C.; Gao, B.; Nan, X.R. Process development of films deposited on inner wall of long tube by arc ion plating. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2012; pp. 1705–1710. [Google Scholar]
- Alcantar-Martínez, L.M.; Ruiz-Trabolsi, P.A.; Tadeo-Rosas, R.; Miranda-Hernández, J.G.; Cabrera-Sierra, R.; Velázquez, J.C.; Hernández-Sánchez, E. Improving the Surface Properties of an API 5L Grade B Pipeline Steel by Applying the Boriding Process—Part II: On the Changes in the Mechanical Properties. Coatings 2023, 13, 470. [Google Scholar]
- Manov, M.; Kalinov, T. Augmentation of ship’s operational availability through innovative reconditioning technologies. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; p. 012002. [Google Scholar]
- Vaishnu, Y.; Bindu Kumar, K.; Rani, S. Parameter optimization and wear characteristics of nano CuO/Ni composite coating by brush plating technique. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2020, 234, 292–300. [Google Scholar] [CrossRef]
- Qi, S.; Li, X.; Dong, H. Reduced friction and wear of electro-brush plated nickel composite coatings reinforced by graphene oxide. Wear 2019, 426, 228–238. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Ji, H. Fabrication of lotus-leaf-like superhydrophobic surfaces via Ni-based nano-composite electro-brush plating. Appl. Surf. Sci. 2014, 288, 341–348. [Google Scholar] [CrossRef]
- Jiang, X.; Xie, X.; Xu, Z. Double glow surface alloying of low carbon steel with electric brush plating Ni interlayer for improvement in corrosion resistance. Surf. Coat. Technol. 2003, 168, 156–160. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, X.; Jin, G.; Cai, Z.; Dong, M. Thermal stability of Ni-B/La2O3 coatings by electro-brush plating technique. Surf. Coat. Technol. 2018, 349, 1042–1047. [Google Scholar] [CrossRef]
- Du, L.; Xu, B.; Dong, S.; Yang, H.; Wu, Y. Preparation, microstructure and tribological properties of nano-Al2O3/Ni brush plated composite coatings. Surf. Coat. Technol. 2005, 192, 311–316. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Xiang, Y. Study on Repairing Technologies and Repairing Experiment by Electro-Brush Plating of Camshaft. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bangkok, Thailand, 24–26 February 2018; p. 022010. [Google Scholar]
- Hu, J.; Pei, Y.; Liu, Y.; Yang, X.; Li, H.; Xu, H.; Guo, N. Microstructure and Properties of Cr-Fe2B Composite Coatings Prepared by Pack-Preboronizing Combined with Electro Brush-Plating. Coatings 2020, 10, 519. [Google Scholar] [CrossRef]
- Zhong, Z.; Clouser, S.J. Nickel–tungsten alloy brush plating for engineering applications. Surf. Coat. Technol. 2014, 240, 380–386. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, Y.; Ma, H.; Zhao, R.; Wang, L.; Zhang, F. Microstructure and Mechanical Properties of Co-Deposited Ti-Ni Films Prepared by Magnetron Sputtering. Coatings 2023, 13, 524. [Google Scholar] [CrossRef]
- Zhu, Z.; Zou, L.; Li, H.; Zhang, H. Preparation and Characterization of Pulse Electrodeposited Ni/W-SiC Nanocomposite Coating on Mild Steel Substrate. Coatings 2023, 13, 484. [Google Scholar] [CrossRef]
- Gao, S.; Wu, C.; Yang, X.; Cheng, J.; Kang, R. Study on Adhesion Properties and Process Parameters of Electroless Deposited Ni-P Alloy for PEEK and Its Modified Materials. Coatings 2023, 13, 388. [Google Scholar] [CrossRef]
- Jiang, H.; Tong, J.; Zhan, Z.; Yao, Z.; Yu, S.; Min, F.; Wang, C.; Noudem, J.G.; Zhang, J. Comparative Study on the Densification, Microstructure and Properties of WC-10(Ni, Ni/Co) Cemented Carbides Using Electroless Plated and Coprecipitated Powders. Materials 2023, 16, 1977. [Google Scholar] [CrossRef]
- Li, B.; Su, J.; Li, J.; Wang, Y. Comparison of the Effect of Mechanical and Ultrasonic Agitation on the Properties of Electrodeposited Ni-Co/ZrO2 Composite Coatings. Metals 2023, 13, 478. [Google Scholar] [CrossRef]
- Yin, K.-M.; Wang, C. A study on the deposit uniformity of hard chromium plating on the interior of small-diameter tubes. Surf. Coat. Technol. 1999, 114, 213–223. [Google Scholar] [CrossRef]
- Balakrishna, P.; Murty, B.; Jha, S.; Krishnan, P.; Saibaba, N. Electroplating of copper on annular zirconium alloy billets. Plat. Surf. Finish. 2005, 92, 56–61. [Google Scholar]
- Asiabi, H.; Yamini, Y.; Seidi, S.; Esrafili, A.; Rezaei, F. Electroplating of nanostructured polyaniline–polypyrrole composite coating in a stainless-steel tube for on-line in-tube solid phase microextraction. J. Chromatogr. A 2015, 1397, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Venkatachalam, R. Brush plating-present state of art. Bull. Electrochem. 1998, 14, 472–475. [Google Scholar]
- Wu, B.; Xu, B.-S.; Zhang, B.; Dong, S.-Y. The effects of parameters on the mechanical properties of Ni-based coatings prepared by automatic brush plating technology. Surf. Coat. Technol. 2007, 201, 5758–5765. [Google Scholar] [CrossRef]
- Wu, B.; Xu, B.-S.; Zhang, B.; Lü, Y.-H. Preparation and properties of Ni/nano-Al2O3 composite coatings by automatic brush plating. Surf. Coat. Technol. 2007, 201, 6933–6939. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Shi, Z. Mechanisms and influences of electro-brush plating micro-force on coatings performances. J. Mater. Res. 2016, 31, 2337–2346. [Google Scholar] [CrossRef]
- Rajasekaran, N.; Mohan, S. Structure, microstructure and corrosion properties of brush-plated Cu–Ni alloy. J. Appl. Electrochem. 2009, 39, 1911–1916. [Google Scholar] [CrossRef]
- Bazarnik, P.; Huang, Y.; Lewandowska, M.; Langdon, T.G. Structural impact on the Hall–Petch relationship in an Al–5Mg alloy processed by high-pressure torsion. Mater. Sci. Eng. A 2015, 626, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Shen, X.; Xin, G. Electro-brush plating Ni-Graphene oxide composite coating enhanced corrosion resistance of fluoride ion. Mater. Res. Express 2021, 8, 026406. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Zhang, K.; Qu, Z. Effect of duty cycle on properties of Ni-P-(sol) Al2O3 nanocomposite coatings prepared by pulse-assisted sol-gel method. Kov. Mater 2023, 61, 23–33. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Han, H.; Liu, M.; Zhang, M.; Wang, Z.; Wu, T. Effects of duty ratio on properties of Ni–P–(sol) Al2O3 coating prepared by pulse-assisted chemical deposition. J. Mater. Res. Technol. 2021, 15, 924–935. [Google Scholar] [CrossRef]
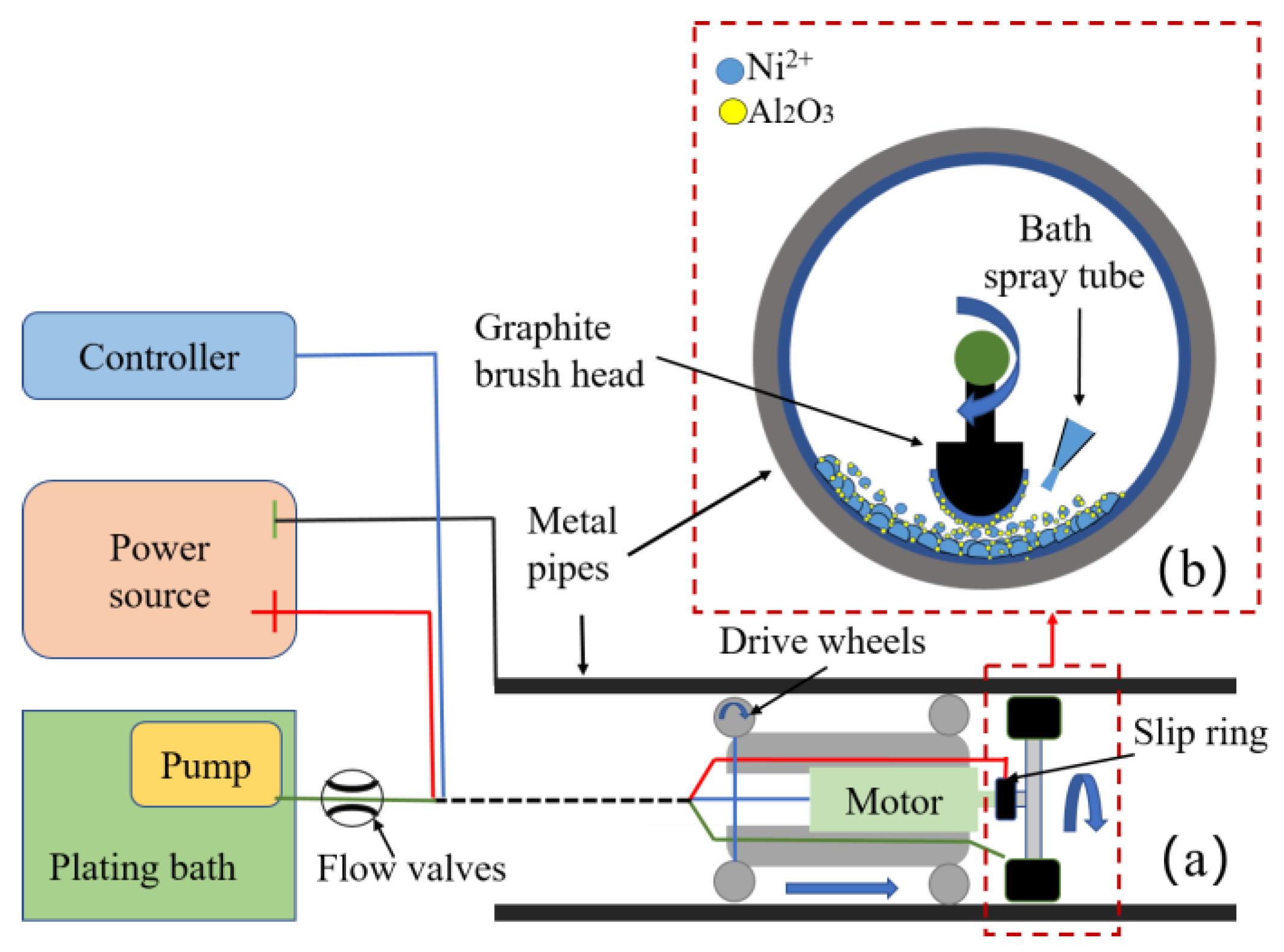




| Bath Compositions | Concentration | Depositing Parameters | Values |
|---|---|---|---|
| NiSO4·6H2O | 250 (g·L−1) | Temperature | 25 (°C) |
| C2H7NO2 | 45 (g·L−1) | pH | 6.5–7.5 |
| (NH4)3C6H5O7 | 35 (g·L−1) | Brush head speed | 10 (m/min) |
| C2H8N2O4·H2O | 1 (g·L−1) | Time | 5 (min) |
| NH3·H2O | 100 (mL/L) | working voltage | 8 V |
| Bath supply speed | 16 (mL/min) |
| Bath Compositions | Concentration | Depositing Parameters | Values |
|---|---|---|---|
| NiSO4·6H2O | 250 (g·L−1) | Temperature | 25 (°C) |
| C2H7NO2 | 45 (g·L−1) | pH | 6.5–7.5 |
| (NH4)3C6H5O7 | 35 (g·L−1) | Brush head speed | 100,180,260 (r/min) |
| C2H8N2O4·H2O | 1 (g·L−1) | Time | 5 (min) |
| NH3·H2O | 100(mL/L) | working voltage | 6 V, 8 V, 10 V, 12 V, 14 V, 16 V |
| Surfactant | 0.1 (g·L−1) | Bath supply speed | 16 (mL/min) |
| First Pre-Treatment | Second Pre-Treatment | Third Pre-Treatment | |
|---|---|---|---|
| Step | Operation | Operation | Operation |
| 1 | Electroclean | Lye degreasing | Lye degreasing |
| 2 | Rinse | Rinse | Rinse |
| 3 | Activate 1 | Mixed acid activation (15%HNO3 + 15% H3PO4) | Impurity removal and activate (5%HCl) |
| 4 | Rinse | Rinse | Rinse |
| 5 | Activate 2 | Pure Ni plating | Pure Ni plating |
| 6 | Rinse | ||
| 7 | Pre-nickel plating | ||
| 8 | Rinse | ||
| 9 | Pure Ni plating |
| Number | Corrosion Potential/V | Corrosion Current Density/A·cm−2 | Corrosion Rate/mm·a−1 |
|---|---|---|---|
| P | –1.281 | 7.651 × 10−5 | 0.898 |
| P-1 | –0.727 | 3.615 × 10−5 | 0.424 |
| P-2 | –0.735 | 1.749 × 10−5 | 0.205 |
| P-3 | –0.721 | 1.262 × 10−5 | 0.148 |
| P-4 | –0.695 | 1.040 × 10−5 | 0.122 |
| P-5 | –0.735 | 2.589 × 10−5 | 0.304 |
| P-6 | −0.749 | 3.107 × 10−5 | 0.364 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, Y.; Xu, X.; Zhang, M.; Leng, H.; Sun, B. Optimization of Plating Process on Inner Wall of Metal Pipe and Research on Coating Performance. Materials 2023, 16, 2800. https://doi.org/10.3390/ma16072800
Zhang C, Li Y, Xu X, Zhang M, Leng H, Sun B. Optimization of Plating Process on Inner Wall of Metal Pipe and Research on Coating Performance. Materials. 2023; 16(7):2800. https://doi.org/10.3390/ma16072800
Chicago/Turabian StyleZhang, Chenming, Yongfeng Li, Xiaochang Xu, Mingming Zhang, Haoyuan Leng, and Bin Sun. 2023. "Optimization of Plating Process on Inner Wall of Metal Pipe and Research on Coating Performance" Materials 16, no. 7: 2800. https://doi.org/10.3390/ma16072800
APA StyleZhang, C., Li, Y., Xu, X., Zhang, M., Leng, H., & Sun, B. (2023). Optimization of Plating Process on Inner Wall of Metal Pipe and Research on Coating Performance. Materials, 16(7), 2800. https://doi.org/10.3390/ma16072800






