Recent Advances of Indium Oxide-Based Catalysts for CO2 Hydrogenation to Methanol: Experimental and Theoretical
Abstract
:1. Introductions
2. In2O3-Based Catalysts for CO2 Hydrogenation to Methanol
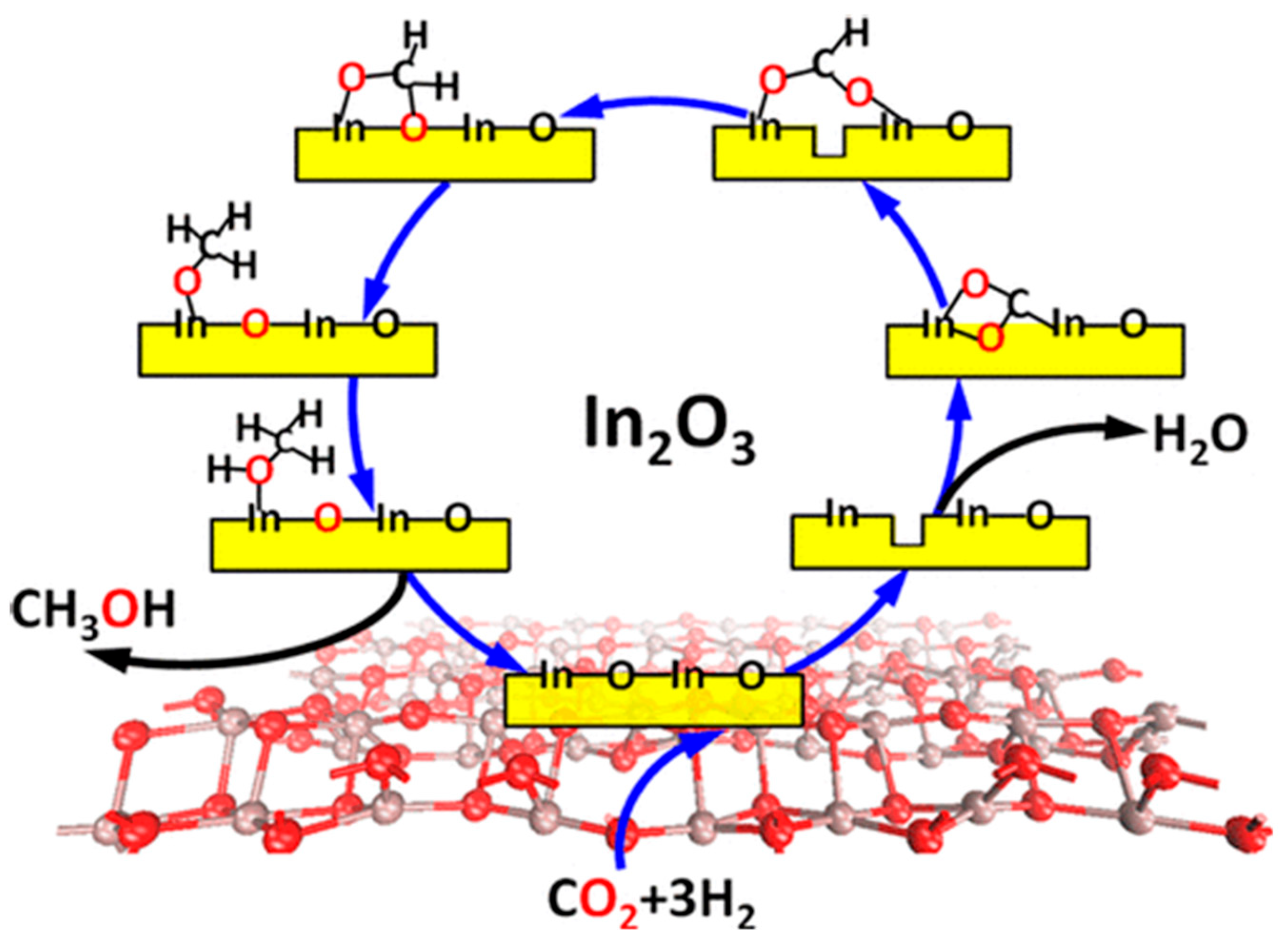
2.1. Pure In2O3 Catalyst
2.2. Metal/In2O3 Composite Catalysts
2.2.1. Noble Metal/In2O3 Catalysts
2.2.2. Base Metal/In2O3 Catalysts
2.3. In2O3/Metal Oxides Composite Catalysts
3. Conclusions and Further Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yue, Y.; Huang, Z.; Cai, D.; Ullah, S.; Ibrahim, A.-R.; Yang, X.; Huang, J.; Zhan, G. Fabrication of multi-layered Co3O4/ZnO nanocatalysts for spectroscopic visualization: Effect of spatial positions on CO2 hydrogenation performance. Fuel 2022, 321, 124042. [Google Scholar] [CrossRef]
- Tan, K.B.; Zhan, G.; Sun, D.; Huang, J.; Li, Q. The development of bifunctional catalysts for carbon dioxide hydrogenation to hydrocarbons via the methanol route: From single component to integrated components. J. Mater. Chem. A 2021, 9, 5197–5231. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Zhan, G.; Huang, J.; Li, Q. Hydrogenation of CO2 to Dimethyl Ether over Tandem Catalysts Based on Biotemplated Hierarchical ZSM-5 and Pd/ZnO. ACS Sustain. Chem. Eng. 2020, 8, 14058–14070. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Song, M.; Huang, Z.; Chen, B.; Liu, S.; Ullah, S.; Cai, D.; Zhan, G. Reduction treatment of nickel phyllosilicate supported Pt nanocatalysts determining product selectivity in CO2 hydrogenation. J. CO2 Util. 2021, 52, 101674. [Google Scholar] [CrossRef]
- Liu, S.; Song, M.; Cha, X.; Hu, S.; Cai, D.; Li, W.; Zhan, G. Nickel phyllosilicates functionalized with graphene oxide to boost CO selectivity in CO2 hydrogenation. Sep. Purif. Technol. 2022, 287, 120555. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Li, Y. Single-atom catalysis for carbon neutrality. Carbon Energy 2022, 4, 1021–1079. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; He, G.; Wang, H.; Zhang, X.; Lin, J.; Qi, Y.; Liang, X. Challenges and opportunities for carbon neutrality in China. Nat. Rev. Earth Environ. 2021, 3, 141–155. [Google Scholar] [CrossRef]
- Calzadiaz-Ramirez, L.; Meyer, A.S. Formate dehydrogenases for CO2 utilization. Curr. Opin. Biotechnol. 2022, 73, 95–100. [Google Scholar] [CrossRef]
- Kim, J.; Seong, A.; Yang, Y.; Joo, S.; Kim, C.; Jeon, D.H.; Dai, L.; Kim, G. Indirect surpassing CO2 utilization in membrane-free CO2 battery. Nano Energy 2021, 82, 105741. [Google Scholar] [CrossRef]
- Castro, S.; Albo, J.; Irabien, A. Photoelectrochemical Reactors for CO2 Utilization. ACS Sustain. Chem. Eng. 2018, 6, 15877–15894. [Google Scholar] [CrossRef] [Green Version]
- Mikulčić, H.; Ridjan Skov, I.; Dominković, D.F.; Wan Alwi, S.R.; Manan, Z.A.; Tan, R.; Duić, N.; Hidayah Mohamad, S.N.; Wang, X. Flexible Carbon Capture and Utilization technologies in future energy systems and the utilization pathways of captured CO2. Renew Sust. Energ. Rev. 2019, 114, 109338. [Google Scholar] [CrossRef]
- Reis Machado, A.S.; Nunes da Ponte, M. CO2 capture and electrochemical conversion. Curr. Opin. Green Sustain. Chem. 2018, 11, 86–90. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, Y.; Wang, X.; Qiu, H. Catalytic Hydrogenation of CO2 to Methanol: A Review. Catalysts 2022, 12, 403. [Google Scholar] [CrossRef]
- Murthy, P.S.; Liang, W.; Jiang, Y.; Huang, J. Cu-Based Nanocatalysts for CO2 Hydrogenation to Methanol. Energy Fuels 2021, 35, 8558–8584. [Google Scholar] [CrossRef]
- Bowker, M. Methanol Synthesis from CO2 Hydrogenation. ChemCatChem 2019, 11, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Lee, Y.H.; Lee, D.-W.; Moon, D.-J.; Lee, K.-Y. Hydrogenation of CO2 to methanol over Pd–Cu/CeO2 catalysts. Mol. Catal. 2017, 434, 146–153. [Google Scholar] [CrossRef]
- Zhao, F.; Fan, L.; Xu, K.; Hua, D.; Zhan, G.; Zhou, S.-F. Hierarchical sheet-like Cu/Zn/Al nanocatalysts derived from LDH/MOF composites for CO2 hydrogenation to methanol. J. CO2 Util. 2019, 33, 222–232. [Google Scholar] [CrossRef]
- Li, Y.; Na, W.; Wang, H.; Gao, W. Hydrogenation of CO2 to methanol over Au–CuO/SBA-15 catalysts. J. Porous Mater. 2016, 24, 591–599. [Google Scholar] [CrossRef]
- García-Trenco, A.; Regoutz, A.; White, E.R.; Payne, D.J.; Shaffer, M.S.P.; Williams, C.K. PdIn intermetallic nanoparticles for the Hydrogenation of CO2 to Methanol. Appl. Catal. B 2018, 220, 9–18. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Zhu, J.; Zhang, X.; Ding, F.; Zhang, A.; Guo, X.; Song, C. CO2 Hydrogenation to Methanol over In2O3-Based Catalysts: From Mechanism to Catalyst Development. ACS Catal. 2021, 11, 1406–1423. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Wu, Y.; Wu, S.; Huang, S.; Gao, J. Solid-State Synthesis of Pd/In2O3 Catalysts for CO2 Hydrogenation to Methanol. Catal. Lett. 2022, 153, 903–910. [Google Scholar] [CrossRef]
- Choi, H.; Oh, S.; Trung Tran, S.B.; Park, J.Y. Size-controlled model Ni catalysts on Ga2O3 for CO2 hydrogenation to methanol. J. Catal. 2019, 376, 68–76. [Google Scholar] [CrossRef]
- Poerjoto, A.J.; Ashok, J.; Dewangan, N.; Kawi, S. The role of lattice oxygen in CO2 hydrogenation to methanol over La1−xSrxCuO catalysts. J. CO2 Util. 2021, 47, 101498. [Google Scholar] [CrossRef]
- Kothandaraman, J.; Dagle, R.A.; Dagle, V.L.; Davidson, S.D.; Walter, E.D.; Burton, S.D.; Hoyt, D.W.; Heldebrant, D.J. Condensed-phase low temperature heterogeneous hydrogenation of CO2 to methanol. Catal. Sci. Technol. 2018, 8, 5098–5103. [Google Scholar] [CrossRef]
- Frusteri, L.; Cannilla, C.; Todaro, S.; Frusteri, F.; Bonura, G. Tailoring of Hydrotalcite-Derived Cu-Based Catalysts for CO2 Hydrogenation to Methanol. Catalysts 2019, 9, 1058. [Google Scholar] [CrossRef] [Green Version]
- Din, I.U.; Shaharun, M.S.; Naeem, A.; Tasleem, S.; Johan, M.R. Carbon nanofiber-based copper/zirconia catalyst for hydrogenation of CO2 to methanol. J. CO2 Util. 2017, 21, 145–155. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.-X.; Zijlstra, B.; Filot, I.A.W.; Zhou, Z.; Sun, S.; Hensen, E.J.M. Optimum Cu nanoparticle catalysts for CO2 hydrogenation towards methanol. Nano Energy 2018, 43, 200–209. [Google Scholar] [CrossRef]
- Gutterod, E.S.; Lazzarini, A.; Fjermestad, T.; Kaur, G.; Manzoli, M.; Bordiga, S.; Svelle, S.; Lillerud, K.P.; Skulason, E.; Oien-Odegaard, S.; et al. Hydrogenation of CO2 to Methanol by Pt Nanoparticles Encapsulated in UiO-67: Deciphering the Role of the Metal-Organic Framework. J. Am. Chem. Soc. 2020, 142, 999–1009. [Google Scholar] [CrossRef]
- Toyao, T.; Kayamori, S.; Maeno, Z.; Siddiki, S.M.A.H.; Shimizu, K.-i. Heterogeneous Pt and MoOx Co-Loaded TiO2 Catalysts for Low-Temperature CO2 Hydrogenation To Form CH3OH. ACS Catal. 2019, 9, 8187–8196. [Google Scholar] [CrossRef]
- Ojelade, O.A.; Zaman, S.F.; Daous, M.A.; Al-Zahrani, A.A.; Malik, A.S.; Driss, H.; Shterk, G.; Gascon, J. Optimizing Pd:Zn molar ratio in PdZn/CeO2 for CO2 hydrogenation to methanol. Appl Catal. A-Gen. 2019, 584, 117185. [Google Scholar] [CrossRef]
- Geng, F.; Bonita, Y.; Jain, V.; Magiera, M.; Rai, N.; Hicks, J.C. Bimetallic Ru–Mo Phosphide Catalysts for the Hydrogenation of CO2 to Methanol. Ind. Eng. Chem. Res. 2020, 59, 6931–6943. [Google Scholar] [CrossRef]
- Feng, W.-H.; Yu, M.-M.; Wang, L.-J.; Miao, Y.-T.; Shakouri, M.; Ran, J.; Hu, Y.; Li, Z.; Huang, R.; Lu, Y.-L.; et al. Insights into Bimetallic Oxide Synergy during Carbon Dioxide Hydrogenation to Methanol and Dimethyl Ether over GaZrOx Oxide Catalysts. ACS Catal. 2021, 11, 4704–4711. [Google Scholar] [CrossRef]
- Sha, F.; Tang, C.; Tang, S.; Wang, Q.; Han, Z.; Wang, J.; Li, C. The promoting role of Ga in ZnZrOx solid solution catalyst for CO2 hydrogenation to methanol. J. Catal. 2021, 404, 383–392. [Google Scholar] [CrossRef]
- Lee, K.; Anjum, U.; Araújo, T.P.; Mondelli, C.; He, Q.; Furukawa, S.; Pérez-Ramírez, J.; Kozlov, S.M.; Yan, N. Atomic Pd-promoted ZnZrO solid solution catalyst for CO2 hydrogenation to methanol. Appl. Catal. B 2022, 304, 120994. [Google Scholar] [CrossRef]
- Xu, D.; Hong, X.; Liu, G. Highly dispersed metal doping to ZnZr oxide catalyst for CO2 hydrogenation to methanol: Insight into hydrogen spillover. J. Catal. 2021, 393, 207–214. [Google Scholar] [CrossRef]
- Sun, K.; Fan, Z.; Ye, J.; Yan, J.; Ge, Q.; Li, Y.; He, W.; Yang, W.; Liu, C.-j. Hydrogenation of CO2 to methanol over In2O3 catalyst. J. CO2 Util. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Ghosh, S.; Sebastian, J.; Olsson, L.; Creaser, D. Experimental and kinetic modeling studies of methanol synthesis from CO2 hydrogenation using In2O3 catalyst. Chem. Eng. J. 2021, 416, 129120. [Google Scholar] [CrossRef]
- Baumgarten, R.; Naumann d’Alnoncourt, R.; Lohr, S.; Gioria, E.; Frei, E.; Fako, E.; De, S.; Boscagli, C.; Drieß, M.; Schunk, S.; et al. Quantification and Tuning of Surface Oxygen Vacancies for the Hydrogenation of CO2 on Indium Oxide Catalysts. Chem. Ing. Tech. 2022, 94, 1765–1775. [Google Scholar] [CrossRef]
- Medina, J.C.; Figueroa, M.; Manrique, R.; Rodríguez Pereira, J.; Srinivasan, P.D.; Bravo-Suárez, J.J.; Baldovino Medrano, V.G.; Jiménez, R.; Karelovic, A. Catalytic consequences of Ga promotion on Cu for CO2 hydrogenation to methanol. Catal. Sci. Technol. 2017, 7, 3375–3387. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Lobo, R.F. Direct conversion of CO2 into methanol over promoted indium oxide-based catalysts. Appl Catal. A-Gen. 2019, 583, 117144. [Google Scholar] [CrossRef]
- Fichtl, M.B.; Schlereth, D.; Jacobsen, N.; Kasatkin, I.; Schumann, J.; Behrens, M.; Schlögl, R.; Hinrichsen, O. Kinetics of deactivation on Cu/ZnO/Al2O3 methanol synthesis catalysts. Appl Catal. A-Gen. 2015, 502, 262–270. [Google Scholar] [CrossRef]
- Sun, J.T.; Metcalfe, I.S.; Sahibzada, M. Deactivation of Cu/ZnO/Al2O3 Methanol Synthesis Catalyst by Sintering. Ind. Eng. Chem. Res. 1999, 38, 3868–3872. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [Green Version]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Huang, Z.L.; Yuan, Y.J.; Song, M.M.; Hao, Z.M.; Xiao, J.R.; Cai, D.R.; Ibrahim, A.R.; Zhan, G.W. CO2 hydrogenation over mesoporous Ni-Pt/SiO2 nanorod catalysts: Determining CH4/CO selectivity by surface ratio of Ni/Pt. Chem. Eng. Sci. 2022, 247, 117106. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Huang, J.; Liu, X.; Fu, D.; Huang, J.; Li, Q.; Zhan, G. MxOy-ZrO2 (M = Zn, Co, Cu) Solid Solutions Derived from Schiff Base-Bridged UiO-66 Composites as High-Performance Catalysts for CO2 Hydrogenation. ACS Appl. Mater. Interfaces 2019, 11, 33263–33272. [Google Scholar] [CrossRef]
- Sha, F.; Tang, S.; Tang, C.; Feng, Z.; Wang, J.; Li, C. The role of surface hydroxyls on ZnZrOx solid solution catalyst in CO2 hydrogenation to methanol. Chin. J. Catal. 2023, 45, 162–173. [Google Scholar] [CrossRef]
- Pinheiro Araújo, T.; Morales-Vidal, J.; Zou, T.; Agrachev, M.; Verstraeten, S.; Willi, P.O.; Grass, R.N.; Jeschke, G.; Mitchell, S.; López, N.; et al. Design of Flame-Made ZnZrOx Catalysts for Sustainable Methanol Synthesis from CO2. Adv. Energy Mater. 2023, 2204122. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef] [Green Version]
- Sadeghinia, M.; Rezaei, M.; Kharat, A.N.; Jorabchi, M.N.; Nematollahi, B.; Zareiekordshouli, F. Effect of In2O3 on the structural properties and catalytic performance of the CuO/ZnO/Al2O3 catalyst in CO2 and CO hydrogenation to methanol. Mol. Catal. 2020, 484, 110776. [Google Scholar] [CrossRef]
- Ye, J.Y.; Liu, C.J.; Mei, D.H.; Ge, Q.F. Active Oxygen Vacancy Site for Methanol Synthesis from CO2 Hydrogenation on In2O3(110): A DFT Study. ACS Catal. 2013, 3, 1296–1306. [Google Scholar] [CrossRef]
- Qin, B.; Li, S. First principles investigation of dissociative adsorption of H2 during CO2 hydrogenation over cubic and hexagonal In2O3 catalysts. PCCP 2020, 22, 3390–3399. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Yan, T.; Hu, Z.; Jelle, A.A.; Meira, D.M.; Duchesne, P.N.; Loh, J.Y.Y.; Qiu, C.; Storey, E.E.; et al. Black indium oxide a photothermal CO2 hydrogenation catalyst. Nat. Commun. 2020, 11, 2432. [Google Scholar] [CrossRef]
- Lorenz, H.; Jochum, W.; Klötzer, B.; Stöger-Pollach, M.; Schwarz, S.; Pfaller, K.; Penner, S. Novel methanol steam reforming activity and selectivity of pure In2O3. Appl. Catal. A-Gen. 2008, 347, 34–42. [Google Scholar] [CrossRef]
- Bielz, T.; Lorenz, H.; Amann, P.; Klötzer, B.; Penner, S. Water–Gas Shift and Formaldehyde Reforming Activity Determined by Defect Chemistry of Polycrystalline In2O3. J. Phys. Chem. C 2011, 115, 6622–6628. [Google Scholar] [CrossRef]
- Martin, O.; Martin, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferre, D.; Perez-Ramirez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261–6265. [Google Scholar] [CrossRef]
- Frei, M.S.; Capdevila-Cortada, M.; Garcia-Muelas, R.; Mondelli, C.; Lopez, N.; Stewart, J.A.; Ferre, D.C.; Perez-Ramirez, J. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide. J. Catal. 2018, 361, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Tsoukalou, A.; Abdala, P.M.; Stoian, D.; Huang, X.; Willinger, M.G.; Fedorov, A.; Muller, C.R. Structural Evolution and Dynamics of an In2O3 Catalyst for CO2 Hydrogenation to Methanol: An Operando XAS-XRD and In Situ TEM Study. J. Am. Chem. Soc. 2019, 141, 13497–13505. [Google Scholar] [CrossRef]
- Sun, K.; Rui, N.; Zhang, Z.; Sun, Z.; Ge, Q.; Liu, C.-J. A highly active Pt-In2O3 catalyst for CO2 hydrogenation to methanol with enhanced stability. Green Chem. 2020, 22, 5059–5066. [Google Scholar] [CrossRef]
- Yang, B.; Li, L.; Jia, Z.; Liu, X.; Zhang, C.; Guo, L. Comparative study of CO2 hydrogenation to methanol on cubic bixbyite-type and rhombohedral corundum-type indium oxide. Chin. Chem. Lett. 2020, 31, 2627–2633. [Google Scholar] [CrossRef]
- Dang, S.; Qin, B.; Yang, Y.; Wang, H.; Cai, J.; Han, Y.; Li, S.; Gao, P.; Sun, Y. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity. Sci. Adv. 2020, 6, eaaz2060. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Tan, Q.; Wu, D. Mixed-Phase Indium Oxide as a Highly Active and Stable Catalyst for the Hydrogenation of CO2 to CH3OH. Ind. Eng. Chem. Res. 2021, 60, 3532–3542. [Google Scholar] [CrossRef]
- Cao, A.; Wang, Z.; Li, H.; Nørskov, J.K. Relations between Surface Oxygen Vacancies and Activity of Methanol Formation from CO2 Hydrogenation over In2O3 Surfaces. ACS Catal. 2021, 11, 1780–1786. [Google Scholar] [CrossRef]
- Qin, B.; Zhou, Z.; Li, S.; Gao, P. Understanding the structure-performance relationship of cubic In2O3 catalysts for CO2 hydrogenation. J. CO2 Util. 2021, 49, 101543. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Zhang, M. Facet effect of In2O3 for methanol synthesis by CO2 hydrogenation: A mechanistic and kinetic study. Surf. Interfaces 2021, 25, 101244. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.-j. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Cai, Z.; Dai, J.; Li, W.; Tan, K.B.; Huang, Z.; Zhan, G.; Huang, J.; Li, Q. Pd Supported on MIL-68(In)-Derived In2O3 Nanotubes as Superior Catalysts to Boost CO2 Hydrogenation to Methanol. ACS Catal. 2020, 10, 13275–13289. [Google Scholar] [CrossRef]
- Guanfeng, T.; Youqing, W.; Shiyong, W.; Sheng, H.; Jinsheng, G. CO2 hydrogenation to methanol over Pd/MnO/In2O3 catalyst. J. Environ. Chem. Eng. 2021, 10, 106965. [Google Scholar] [CrossRef]
- Han, Z.; Tang, C.; Wang, J.; Li, L.; Li, C. Atomically dispersed Ptn+ species as highly active sites in Pt/In2O3 catalysts for methanol synthesis from CO2 hydrogenation. J. Catal. 2021, 394, 236–244. [Google Scholar] [CrossRef]
- Wang, J.; Sun, K.; Jia, X.; Liu, C.-j. CO2 hydrogenation to methanol over Rh/In2O3 catalyst. Catal. Today 2021, 365, 341–347. [Google Scholar] [CrossRef]
- Dostagir, N.H.M.D.; Thompson, C.; Kobayashi, H.; Karim, A.M.; Fukuoka, A.; Shrotri, A. Rh promoted In2O3 as a highly active catalyst for CO2 hydrogenation to methanol. Catal. Sci. Technol. 2020, 10, 8196–8202. [Google Scholar] [CrossRef]
- Qinglei, W.; Chenyang, S.; Ning, R.; Kaihang, S.; Chang-jun, L. Experimental and theoretical studies of CO2 hydrogenation to methanol on Ru/In2O3. J. CO2 Util. 2021, 53, 101720. [Google Scholar] [CrossRef]
- Rui, N.; Zhang, F.; Sun, K.; Liu, Z.; Xu, W.; Stavitski, E.; Senanayake, S.D.; Rodriguez, J.A.; Liu, C.-J. Hydrogenation of CO2 to Methanol on a Auδ+–In2O3–x Catalyst. ACS Catal. 2020, 10, 11307–11317. [Google Scholar] [CrossRef]
- Shen, C.; Sun, K.; Zhang, Z.; Rui, N.; Jia, X.; Mei, D.; Liu, C.-j. Highly Active Ir/In2O3 Catalysts for Selective Hydrogenation of CO2 to Methanol: Experimental and Theoretical Studies. ACS Catal. 2021, 11, 4036–4046. [Google Scholar] [CrossRef]
- Jia, X.; Sun, K.; Wang, J.; Shen, C.; Liu, C.-j. Selective hydrogenation of CO2 to methanol over Ni/In2O3 catalyst. J. Energy Chem. 2020, 50, 409–415. [Google Scholar] [CrossRef]
- Zhu, J.; Cannizzaro, F.; Liu, L.; Zhang, H.; Kosinov, N.; Filot, I.A.W.; Rabeah, J.; Bruckner, A.; Hensen, E.J.M. Ni-In Synergy in CO2 Hydrogenation to Methanol. ACS Catal. 2021, 11, 11371–11384. [Google Scholar] [CrossRef]
- Li, L.; Yang, B.; Gao, B.; Wang, Y.; Zhang, L.; Ishihara, T.; Qi, W.; Guo, L. CO2 hydrogenation selectivity shift over In-Co binary oxides catalysts: Catalytic mechanism and structure-property relationship. Chin. J. Catal. 2022, 43, 862–876. [Google Scholar] [CrossRef]
- Pustovarenko, A.; Dikhtiarenko, A.; Bavykina, A.; Gevers, L.; Ramírez, A.; Russkikh, A.; Telalovic, S.; Aguilar, A.; Hazemann, J.-L.; Ould-Chikh, S.; et al. Metal–Organic Framework-Derived Synthesis of Cobalt Indium Catalysts for the Hydrogenation of CO2 to Methanol. ACS Catal. 2020, 10, 5064–5076. [Google Scholar] [CrossRef]
- Shi, Z.; Pan, M.; Wei, X.; Wu, D. Cu-In intermetallic compounds as highly active catalysts for CH3OH formation from CO2 hydrogenation. Int. J. Energy Res. 2021, 46, 1285–1298. [Google Scholar] [CrossRef]
- Yang, C.; Pei, C.; Luo, R.; Liu, S.; Wang, Y.; Wang, Z.; Zhao, Z.J.; Gong, J. Strong Electronic Oxide-Support Interaction over In2O3/ZrO2 for Highly Selective CO2 Hydrogenation to Methanol. J. Am. Chem. Soc. 2020, 142, 19523–19531. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-y.; Cao, C.; Chen, T.-b.; Ding, X.; Huang, H.; Shen, L.; Cao, X.; Zhu, M.; Xu, J.; Gao, J.; et al. Unraveling Highly Tunable Selectivity in CO2 Hydrogenation over Bimetallic In-Zr Oxide Catalysts. ACS Catal. 2019, 9, 8785–8797. [Google Scholar] [CrossRef]
- Akkharaphatthawon, N.; Chanlek, N.; Cheng, C.K.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Tuning adsorption properties of GaxIn2-xO3 catalysts for enhancement of methanol synthesis activity from CO2 hydrogenation at high reaction temperature. Appl. Surf. Sci. 2019, 489, 278–286. [Google Scholar] [CrossRef]
- Regalado Vera, C.Y.; Manavi, N.; Zhou, Z.; Wang, L.-C.; Diao, W.; Karakalos, S.; Liu, B.; Stowers, K.J.; Zhou, M.; Luo, H.; et al. Mechanistic understanding of support effect on the activity and selectivity of indium oxide catalysts for CO2 hydrogenation. Chem. Eng. J. 2021, 426, 131767. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; Cesarini, A.; Krumeich, F.; Hauert, R.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Role of Zirconia in Indium Oxide-Catalyzed CO2 Hydrogenation to Methanol. ACS Catal. 2019, 10, 1133–1145. [Google Scholar] [CrossRef]
- Ye, J.; Liu, C.-j.; Mei, D.; Ge, Q. Methanol synthesis from CO2 hydrogenation over a Pd4/In2O3 model catalyst: A combined DFT and kinetic study. J. Catal. 2014, 317, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Huang, M.; Dai, J.; Zhan, G.; Sun, F.-l.; Zhuang, G.-L.; Wang, Y.; Tian, P.; Chen, B.; Ullah, S.; et al. Fabrication of Pd/In2O3 Nanocatalysts Derived from MIL-68(In) Loaded with Molecular Metalloporphyrin (TCPP(Pd)) Toward CO2 Hydrogenation to Methanol. ACS Catal. 2022, 12, 709–723. [Google Scholar] [CrossRef]
- Pan, T.; Zhongjie, C.; Guowu, Z.; Jiale, H.; Qingbiao, L. Preparation of supported In2O3/Pd nanocatalysts using natural pollen as bio-templates for CO2 hydrogenation to methanol: Effect of acid-etching on template. Mol. Catal. 2021, 516, 111945. [Google Scholar] [CrossRef]
- Bing Tan, K.; Tian, P.; Zhang, X.; Tian, J.; Zhan, G.; Huang, J.; Li, Q. Green synthesis of microspherical-confined nano-Pd/In2O3 integrated with H-ZSM-5 as bifunctional catalyst for CO2 hydrogenation into dimethyl ether: A carbonized alginate templating strategy. Sep. Purif. Technol. 2022, 297, 121559. [Google Scholar] [CrossRef]
- Frei, M.S.; Mondelli, C.; García-Muelas, R.; Kley, K.S.; Puértolas, B.; López, N.; Safonova, O.V.; Stewart, J.A.; Curulla Ferré, D.; Pérez-Ramírez, J. Atomic-scale engineering of indium oxide promotion by palladium for methanol production via CO2 hydrogenation. Nat. Commun. 2019, 10, 3377. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Lin, J.; Wu, X.; Wang, W.; Chen, Y.; Zhang, M. Efficient hydrogenation of CO2 to methanol over Pd/In2O3/SBA-15 catalysts. J. CO2 Util. 2020, 36, 33–39. [Google Scholar] [CrossRef]
- Men, Y.-L.; Liu, Y.; Wang, Q.; Luo, Z.-H.; Shao, S.; Li, Y.-B.; Pan, Y.-X. Highly dispersed Pt-based catalysts for selective CO2 hydrogenation to methanol at atmospheric pressure. Chem. Eng. Sci. 2019, 200, 167–175. [Google Scholar] [CrossRef]
- Pinheiro Araújo, T.; Morales-Vidal, J.; Zou, T.; García-Muelas, R.; Willi, P.O.; Engel, K.M.; Safonova, O.V.; Faust Akl, D.; Krumeich, F.; Grass, R.N.; et al. Flame Spray Pyrolysis as a Synthesis Platform to Assess Metal Promotion in In2O3-Catalyzed CO2 Hydrogenation. Adv. Energy Mater. 2022, 12, 2103707. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, Z.; Shen, C.; Rui, N.; Liu, C.-j. The feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol. Green Energy Environ. 2022, 7, 807–817. [Google Scholar] [CrossRef]
- Shen, C.; Bao, Q.; Xue, W.; Sun, K.; Zhang, Z.; Jia, X.; Mei, D.; Liu, C.-j. Synergistic effect of the metal-support interaction and interfacial oxygen vacancy for CO2 hydrogenation to methanol over Ni/In2O3 catalyst: A theoretical study. J. Energy Chem. 2022, 65, 623–629. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, C.; Sun, K.; Liu, C.-J. Improvement in the activity of Ni/In2O3 with the addition of ZrO2 for CO2 hydrogenation to methanol. Catal. Commun. 2022, 162, 106386. [Google Scholar] [CrossRef]
- Fang, T.; Liu, B.; Lian, Y.; Zhang, Z. Selective Methanol Synthesis from CO2 Hydrogenation over an In2O3/Co/C-N Catalyst. Ind. Eng. Chem. Res. 2020, 59, 19162–19167. [Google Scholar] [CrossRef]
- Stangeland, K.; Navarro, H.H.; Huynh, H.L.; Tucho, W.M.; Yu, Z. Tuning the interfacial sites between copper and metal oxides (Zn, Zr, In) for CO2 hydrogenation to methanol. Chem. Eng. Sci. 2021, 238, 116603. [Google Scholar] [CrossRef]
- Zhang, X.; Kirilin, A.V.; Rozeveld, S.; Kang, J.H.; Pollefeyt, G.; Yancey, D.F.; Chojecki, A.; Vanchura, B.; Blum, M. Support Effect and Surface Reconstruction in In2O3/m-ZrO2 Catalyzed CO2 Hydrogenation. ACS Catal. 2022, 12, 3868–3880. [Google Scholar] [CrossRef]
- Numpilai, T.; Kidkhunthod, P.; Cheng, C.K.; Wattanakit, C.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. CO2 hydrogenation to methanol at high reaction temperatures over In2O3/ZrO2 catalysts: Influence of calcination temperatures of ZrO2 support. Catal. Today 2021, 375, 298–306. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Abdala, P.M.; Armutlulu, A.; Willinger, E.; Fedorov, A.; Müller, C.R. Operando X-ray Absorption Spectroscopy Identifies a Monoclinic ZrO2:In Solid Solution as the Active Phase for the Hydrogenation of CO2 to Methanol. ACS Catal. 2020, 10, 10060–10067. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Serykh, A.I.; Willinger, E.; Kierzkowska, A.; Abdala, P.M.; Fedorov, A.; Müller, C.R. Hydrogen dissociation sites on indium-based ZrO2-supported catalysts for hydrogenation of CO2 to methanol. Catal. Today 2022, 387, 38–46. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, F.; Ma, J.; Yang, C.; Wang, X.; Cao, J. Catalytic roles of In2O3 in ZrO2-based binary oxides for CO2 hydrogenation to methanol. Mol. Catal. 2022, 525, 112354. [Google Scholar] [CrossRef]
- Salomone, F.; Sartoretti, E.; Ballauri, S.; Castellino, M.; Novara, C.; Giorgis, F.; Pirone, R.; Bensaid, S. CO2 hydrogenation to methanol over Zr- and Ce-doped indium oxide. Catal. Today 2023, accepted. [Google Scholar] [CrossRef]
- Tian, G.; Wu, Y.; Wu, S.; Huang, S.; Gao, J. Influence of Mn and Mg oxides on the performance of In2O3 catalysts for CO2 hydrogenation to methanol. Chem. Phys. Lett. 2022, 786, 139173. [Google Scholar] [CrossRef]










| Strategies | Catalysts | P (MPa) | T (°C) | H2/CO2 Molar Ratio | CO2 Conversion (%) | Methanol Selectivity (%) | STY (gMeOH h−1 gcat−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Intrinsic activity | In2O3 | 4 | 330 | 3:1 | 7.1 | 39.7 | ~0.12 | [38] |
| In2O3 | 5 | 300 | 4:1 | a | 100 | ~0.18 | [58] | |
| In2O3 | 5 | 300 | 4:1 | 9.4 | ~62.5 | ~0.34 | [61] | |
| c-In2O3 | 4 | 340 | 4:1 | ~12.0 | ~19.0 | ~0.09 | [62] | |
| rh-In2O3 | 4 | 340 | 4:1 | ~5.0 | ~30.0 | ~0.05 | [62] | |
| c-In2O3 | 3 | 300 | 3:1 | ~4.0 | ~70.5 | 0.06 | [64] | |
| h-In2O3 | 3 | 300 | 3:1 | ~4.7 | ~71.0 | 0.07 | [64] | |
| c/h-In2O3-1 | 3 | 300 | 3:1 | ~5.7 | ~72.3 | 0.09 | [64] | |
| c/h-In2O3-2 | 3 | 300 | 3:1 | ~6.2 | ~73.0 | 0.10 | [64] | |
| c/h-In2O3-3 | 3 | 300 | 3:1 | ~5.0 | ~72.1 | 0.08 | [64] | |
| Introducing other metal elements into In2O3 | Pd-P/In2O3 | 5 | 300 | 4:1 | ~20.0 | ~70.0 | 0.89 | [68] |
| h-In2O3/Pd | 3 | 300 | 3:1 | ~10.5 | 72.4 | 0.53 | [69] | |
| Pd/MnO/In2O3 | 3 | 280 | 3:1 | 4.5 | 71.3 | 0.24 | [70] | |
| Pt/In2O3 | 5 | 300 | 4:1 | 17.3 | ~54.0 | 0.54 | [61] | |
| Pt/In2O3 | 4 | 300 | 3:1 | 5.7 | ~71.5 | ~0.75 | [71] | |
| Rh/In2O3 | 5 | 300 | 4:1 | 17.1 | 56.1 | 0.54 | [72] | |
| Rh-5-In2O3 | 5 | 270 | 4:1 | 10.0 | 71.0 | 0.52 | [73] | |
| Ru/In2O3 | 5 | 300 | 4:1 | 14.3 | 69.7 | 0.57 | [74] | |
| Au/In2O3 | 5 | 300 | 4:1 | 11.7 | 67.8 | 0.47 | [75] | |
| Ir/In2O3 | 5 | 300 | 4:1 | 17.7 | ~70.0 | ~0.77 | [76] | |
| Ni/In2O3 | 5 | 300 | 4:1 | 18.4 | ~54.0 | 0.55 | [77] | |
| Ni/In2O3 | 3 | 250 | 3:1 | 3.0 | ~52.0 | ~0.25 | [78] | |
| Co/In2O3 | 4 | 300 | 3:1 | ~9.0 | ~40.0 | ~0.31 | [79] | |
| In2O3@Co3O4 | 5 | 250 | 4:1 | 8.3 | ~87.0 | 0.65 | [80] | |
| Cu11In9-In2O3 | 3 | 260 | 3:1 | 10.3 | 86.2 | ~0.19 | [81] | |
| Combining In2O3 with other metal oxides | In2O3/ZrO2 | 5 | 300 | 4:1 | a | ~100 | ~0.31 | [58] |
| In2O3/m-ZrO2 | 3 | 280 | 3:1 | 12.1 | 84.6 | a | [82] | |
| In2.5/ZrO2 | 5 | 280 | 4:1 | a | 60.0 | ~0.07 | [83] | |
| Ga0.4In1.6O3 | 3 | 320 | 3:1 | ~12.0 | ~28.0 | a | [84] | |
| InCe oxides | 0.1 | 290 | 3:1 | a | ~10.0 | ~0.12 b | [85] | |
| In2O3/Al2O3 | 5 | 280 | 4:1 | a | a | ~0.04 | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, D.; Cai, Y.; Tan, K.B.; Zhan, G. Recent Advances of Indium Oxide-Based Catalysts for CO2 Hydrogenation to Methanol: Experimental and Theoretical. Materials 2023, 16, 2803. https://doi.org/10.3390/ma16072803
Cai D, Cai Y, Tan KB, Zhan G. Recent Advances of Indium Oxide-Based Catalysts for CO2 Hydrogenation to Methanol: Experimental and Theoretical. Materials. 2023; 16(7):2803. https://doi.org/10.3390/ma16072803
Chicago/Turabian StyleCai, Dongren, Yanmei Cai, Kok Bing Tan, and Guowu Zhan. 2023. "Recent Advances of Indium Oxide-Based Catalysts for CO2 Hydrogenation to Methanol: Experimental and Theoretical" Materials 16, no. 7: 2803. https://doi.org/10.3390/ma16072803
APA StyleCai, D., Cai, Y., Tan, K. B., & Zhan, G. (2023). Recent Advances of Indium Oxide-Based Catalysts for CO2 Hydrogenation to Methanol: Experimental and Theoretical. Materials, 16(7), 2803. https://doi.org/10.3390/ma16072803







