Recent Progress of Polymeric Corrosion Inhibitor: Structure and Application
Abstract
1. Introduction
2. Corrosion Inhibitor from Natural Polymer
3. Synthetic Polymeric Corrosion Inhibitor
3.1. Phosphorus-Containing Synthetic Polymeric Inhibitor
| Inhibitor | Metal | Corrosion Medium | Test Method | Highest IE (%) | Reference |
|---|---|---|---|---|---|
| P-1 | aluminum alloy 3003 | pH = 5 acetic acid | EIS | / | [44] |
| P-2 | copper | 200 × 10−3 g/L NaCl | weight loss, EIS | 92 | [46] |
| P-3 | mild steel | 3.5% NaCl | salt spray test | / | [47] |
| M-1/M-2 | low-carbon steel | 0.1 M NaCl | EIS | 85 | [49] |
| M-3 | mild steel | 3.5% NaCl | PDP, EIS | [54] | |
| P-4/P-5 | iron coin | 3% NaCl | PDP, EIS | 83.5 | [51] |
| P-6 | 316L stainless steel | trifluoroacetic acid | long term stability tests | / | [52] |
3.2. Sulfur-Containing Synthetic Polymeric Inhibitor
3.3. Nitrogen-Containing Synthetic Polymeric Inhibitor
3.3.1. Poly (Quaternary Ammonium)
3.3.2. Polyethyleneimine
3.3.3. Polyaniline
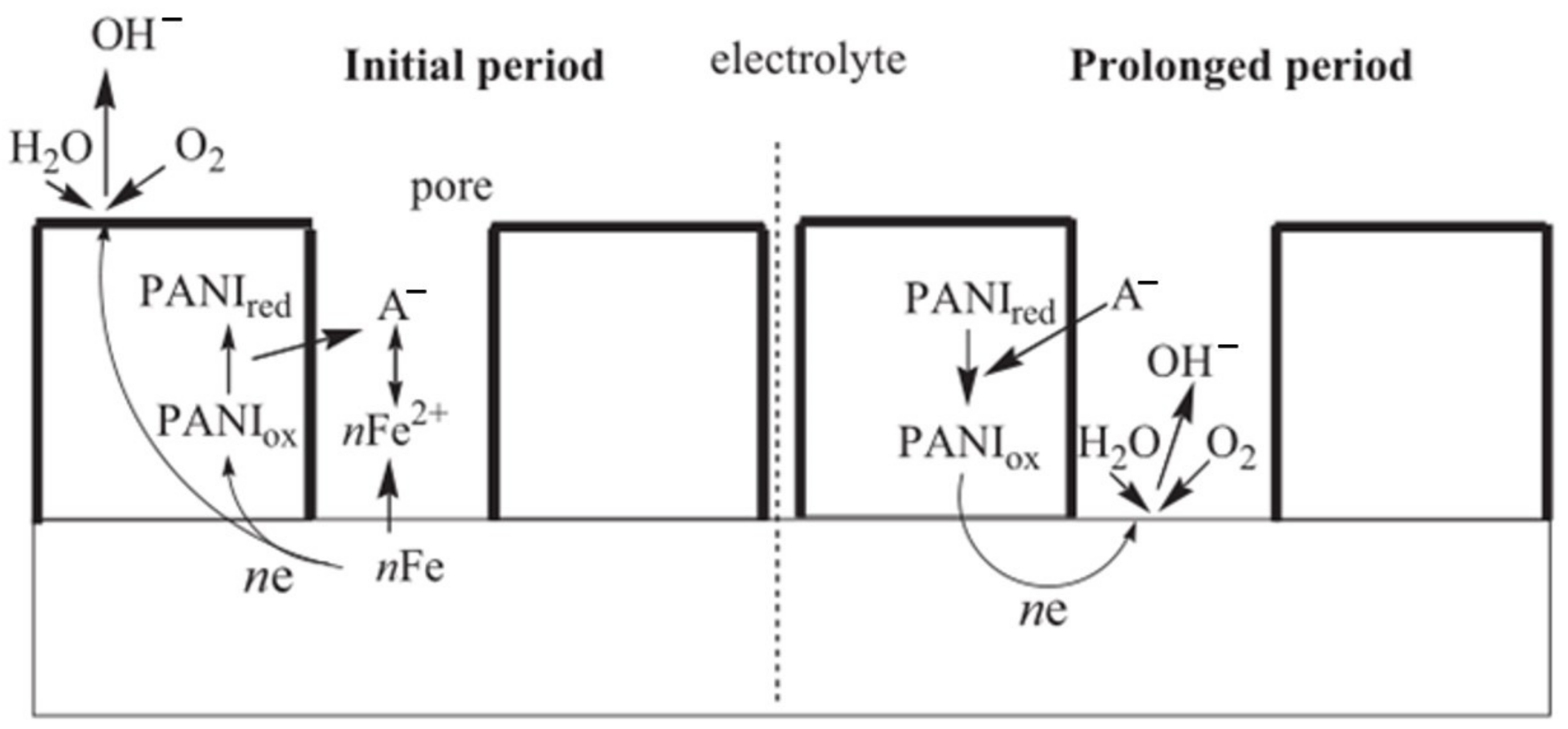
3.3.4. Inorganic Mineral-Doped Polyaniline-Based Inhibitor
3.3.5. Polyaniline-Based Inhibitors from Doping with Protonic Acids
3.3.6. Polyaniline-Based Inhibitor from Doping with Metals Oxides
3.4. Other Type of Polymeric Inhibitors
| Inhibitor | Metal | Corrosion Medium | Test Method | Highest IE (%) | Reference |
|---|---|---|---|---|---|
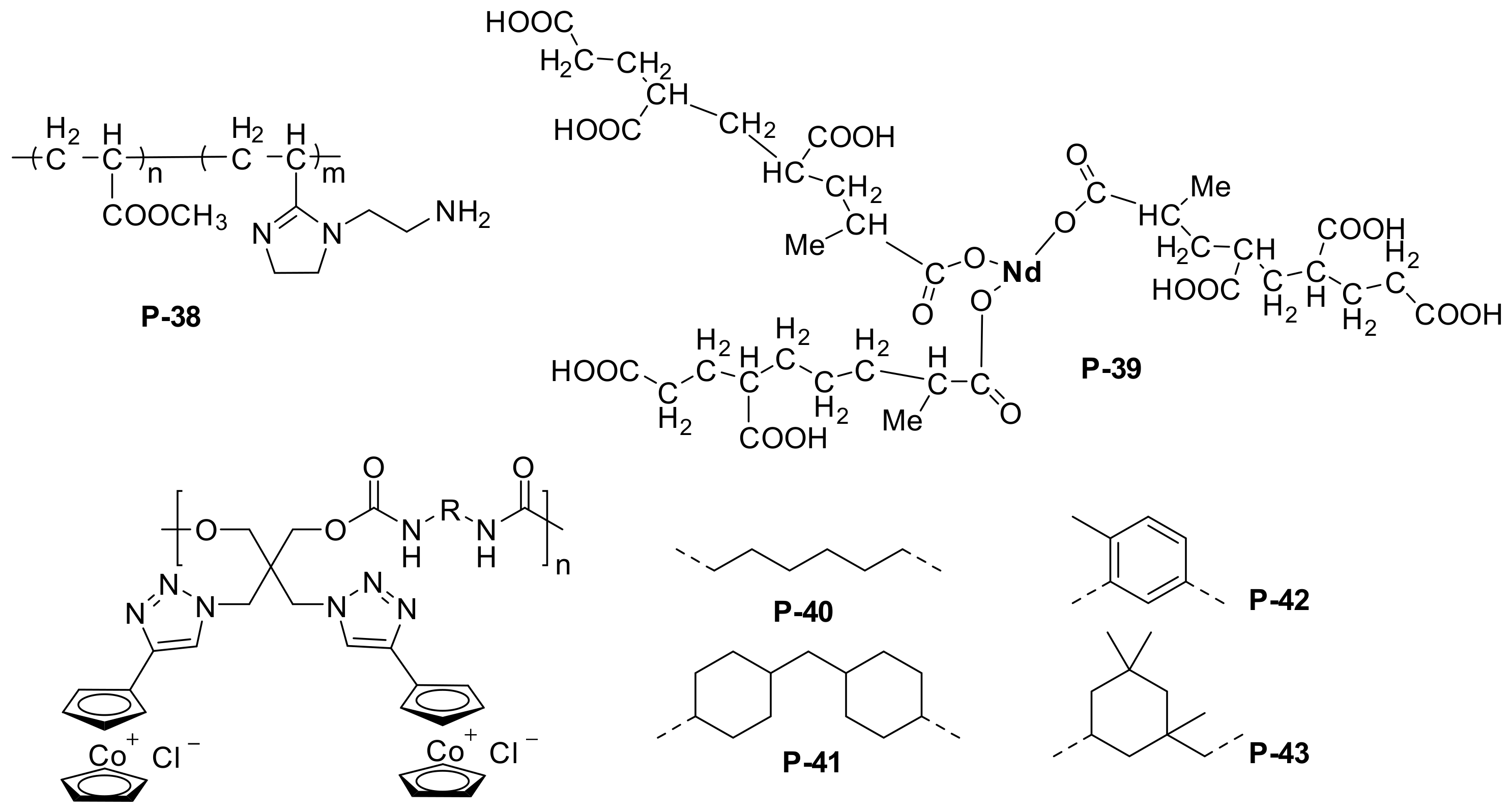
| P-38 | N80 steel sheet | 1 M H2SO4 | PDP, EIS | 90.2 | [117] |
| P-39 | ST-12 type steel sheets | 0.1 M NaCl | PDP, EIS | / | [118] |
| P-40/P-41/P-42/P-43 | Mild steel | 4 M HCl | weight loss, EIS, PDP | 98 | [120] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and nonferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An overview. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepkova, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Finsgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- El Ibrahimi, B.; Jmiai, A.; Bazzi, L.; El Issami, S. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Rhee, K.Y. Aqueous phase polymeric corrosion inhibitors: Recent advancements and future opportunities. J. Mol. Liq. 2022, 348, 118387. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: A review. J. Mol. Liq. 2018, 251, 100–118. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Molecular structural aspects of organic corrosion inhibitors: Influence of -CN and -NO2 substituents on designing of potential corrosion inhibitors for aqueous media. J. Mol. Liq. 2020, 316, 113874. [Google Scholar] [CrossRef]
- Maeztu, J.D.; Rivero, P.J.; Berlanga, C.; Bastidas, D.M.; Palacio, J.F.; Rodriguez, R. Effect of graphene oxide and fluorinated polymeric chains incorporated in a multilayered sol-gel nanocoating for the design of corrosion resistant and hydrophobic surfaces. Appl. Surf. Sci. 2017, 419, 138–149. [Google Scholar] [CrossRef]
- Chang, K.-C.; Hsu, M.-H.; Lu, H.-I.; Lai, M.-C.; Liu, P.-J.; Hsu, C.-H.; Ji, W.-F.; Chuang, T.-L.; Wei, Y.; Yeh, J.-M.; et al. Room-temperature cured hydrophobic epoxy/graphene composites as corrosion inhibitor for cold-rolled steel. Carbon 2014, 66, 144–153. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Rhee, K.Y.; Verma, C.; Quraishi, M.A.; Ebenso, E.E. Challenges and advantages of using plant extract as inhibitors in modern corrosion inhibition systems: Recent advancements. J. Mol. Liq. 2021, 321, 114666. [Google Scholar] [CrossRef]
- Salleh, S.Z.; Yusoff, A.H.; Zakaria, S.K.; Taib, M.A.A.; Abu Seman, A.; Masri, M.N.; Mohamad, M.; Mamat, S.; Sobri, S.A.; Ali, A.; et al. Plant extracts as green corrosion inhibitor for ferrous metal alloys: A review. J. Clean. Prod. 2021, 304, 127030. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M. Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 2016, 140, 314–341. [Google Scholar] [CrossRef] [PubMed]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Verma, C.; Verma, D.K.; Ebenso, E.E.; Quraishi, M.A. Sulfur and phosphorus heteroatom-containing compounds as corrosion inhibitors: An overview. Heteroat. Chem. 2018, 29, e21437. [Google Scholar] [CrossRef]
- Mobin, M.; Rizvi, M. Polysaccharide from Plantago as a green corrosion inhibitor for carbon steel in 1 M HCl solution. Carbohydr. Polym. 2017, 160, 172–183. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. Effect of starch on the corrosion behavior of Al-Mg-Si alloy processed by micro arc oxidation from an ecofriendly electrolyte system. Bioelectrochemistry 2019, 128, 133–139. [Google Scholar] [CrossRef]
- Thamer, A.N.; Kadham, L.H.; Alwaan, I.M. Preparation and Characteristics of Starch Nanocrystals as Corrosion Inhibitor: Experimental and Theoretical Studies. Asian J. Chem. 2022, 34, 2854–2864. [Google Scholar] [CrossRef]
- Sushmitha, Y.; Rao, P. Material conservation and surface coating enhancement with starch-pectin biopolymer blend: A way towards green. Surf. Interfaces 2019, 16, 67–75. [Google Scholar]
- Zhang, Z.; Ba, H.; Wu, Z. Sustainable corrosion inhibitor for steel in simulated concrete pore solution by maize gluten meal extract: Electrochemical and adsorption behavior studies. Constr. Build. Mater. 2019, 227, 117080. [Google Scholar] [CrossRef]
- Burrell, M.M. Starch: The need for improved quality or quantity—An overview. J. Exp. Bot. 2003, 54, 451–456. [Google Scholar] [CrossRef]
- Othman, N.K.; Salleh, E.M.; Dasuki, Z.; Lazim, A.M. Dimethyl Sulfoxide-Treated Starch of Dioescorea hispida as a Green Corrosion Inhibitor for Low Carbon Steel in Sodium Chloride Medium; IntechOpen: London, UK, 2018. [Google Scholar]
- Anyiam, C.K.; Ogbobe, O.; Oguzie, E.E.; Madufor, I.C. Synergistic Study of Modified Sweet Potato Starch and KI for Corrosion Protection of Mild Steel in Acidic Media. J. Bio- Tribo-Corros. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Anyiam, C.K.; Ogbobe, O.; Oguzie, E.E.; Madufor, I.C.; Nwanonenyi, S.C.; Onuegbu, G.C.; Obasi, H.C.; Chidiebere, M.A. Corrosion inhibition of galvanized steel in hydrochloric acid medium by a physically modified starch. SN Appl. Sci. 2020, 2, 520. [Google Scholar] [CrossRef]
- Deng, S.; Li, X.; Du, G. An efficient corrosion inhibitor of cassava starch graft copolymer for aluminum in phosphoric acid. Chin. J. Chem. Eng. 2021, 37, 222–231. [Google Scholar] [CrossRef]
- Hou, D.; Yang, W.; Dong, S. Corrosion inhibition of carbon steel in acid solution by starch-AA-CS terpolymer. Chem. Ind. Eng. 2022, 39, 100–107. [Google Scholar]
- Li, X.; Deng, S.; Lin, T.; Xie, X. Cassava starch graft copolymer as a novel inhibitor for the corrosion of aluminium in HNO3 solution. J. Mol. Liq. 2019, 282, 499–514. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Gao, P.; Liu, R.; Zhao, D.; Zhai, J.; Qu, G. Inhibition for Zn Corrosion by Starch Grafted Copolymer. J. Chin. Soc. Corros. Prot. 2021, 41, 131–138. [Google Scholar]
- Li, X.; Deng, S.; Lin, T.; Xie, X.; Du, G. Cassava starch ternary graft copolymer as a corrosion inhibitor for steel in HCl solution. J. Mater. Res. Technol. 2020, 9, 2196–2207. [Google Scholar] [CrossRef]
- Arukalam, I.O.; Madufor, I.C.; Ogbobe, O.; Oguzie, E.E. Experimental and Theoretical Studies of Hydroxyethyl Cellulose as Inhibitor for Acid Corrosion Inhibition of Mild Steel and Aluminium. Open Corros. J. 2014, 6, 1–10. [Google Scholar] [CrossRef]
- Eid, S.; Abdallah, M.; Kamar, E.M.; El-Etre, A. Corrosion Inhibition of Aluminum and Aluminum Silicon Alloys in Sodium Hydroxide Solutions by Methyl Cellulose. J. Mater. Environ. Sci. 2015, 6, 892–901. [Google Scholar]
- Sobhi, M.; Eid, S. Chemical, Electrochemical and Morphology Studies on Methyl Hydroxyethyl Cellulose as Green Inhibitor for Corrosion of Copper in Hydrochloric acid Solutions. Prot. Met. Phys. Chem. Surf. 2018, 54, 893–898. [Google Scholar] [CrossRef]
- Nwanonenyi, S.C.; Obasi, H.C.; Eze, I.O. Hydroxypropyl Cellulose as an Efficient Corrosion Inhibitor for Aluminium in Acidic Environments: Experimental and Theoretical Approach. Chem. Afr. -A J. Tunis. Chem. Soc. 2019, 2, 471–482. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Y.; Jiang, Z.N.; Zhang, Q.H.; Li, Y.Y.; Liu, H.F.; Zhang, G.A. Developing two thiocarbohydrazide modified glucose derivatives as high-efficiency green corrosion inhibitors for carbon steel. Ind. Crops Prod. 2022, 188, 115680. [Google Scholar] [CrossRef]
- Yang, S.F.; Wen, Y.; Yi, P.; Xiao, K.; Dong, C.F. Effects of chitosan inhibitor on the electrochemical corrosion behavior of 2205 duplex stainless steel. Int. J. Miner. Metall. Mater. 2017, 24, 1260–1266. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, C.; Yan, X.; Xiao, R.; Wang, T.; Li, M. β-Cyclodextrin Modified Natural Chitosan as a Green Inhibitor for Carbon Steel in Acid Solutions. Ind. Eng. Chem. Res. 2015, 54, 5664–5672. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Hou, B.S.; Li, Y.Y.; Zhu, G.Y.; Liu, H.F.; Zhang, G.A. Two novel chitosan derivatives as high efficient eco-friendly inhibitors for the corrosion of mild steel in acidic solution. Corros. Sci. 2020, 164, 108346. [Google Scholar] [CrossRef]
- Fares, M.M.; Maayta, A.K.; Al-Mustafa, J.A. Corrosion inhibition of iota-carrageenan natural polymer on aluminum in presence of zwitterion mediator in HCl media. Corros. Sci. 2012, 65, 223–230. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Halambek, J.; Cindric, I.; Grassino, A.N. Evaluation of pectin isolated from tomato peel waste as natural tin corrosion inhibitor in sodium chloride/acetic acid solution. Carbohydr. Polym. 2020, 234, 115940. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Cui, J.; Li, Y.; Liang, Y.; Cao, G. Molecular dynamics simulation and DFT calculation of "green" scale and corrosion inhibitor. Comput. Mater. Sci. 2021, 188, 110229. [Google Scholar] [CrossRef]
- He, C.; Li, X.-Q.; Feng, G.-L.; Long, W.-J. A universal strategy for green and in situ synthesis of carbon dot-based pickling solution. Green Chem. 2022, 24, 5842–5855. [Google Scholar] [CrossRef]
- Coquery, C.; Negrell, C.; Causse, N.; Pebere, N.; David, G. Synthesis of new high molecular weight phosphorylated chitosans for improving corrosion protection. Pure Appl. Chem. 2019, 91, 509–521. [Google Scholar] [CrossRef]
- Solimando, X.; Kennedy, E.; David, G.; Champagne, P.; Cunningham, M.F. Phosphorus-containing polymers synthesised via nitroxide-mediated polymerisation and their grafting on chitosan by grafting to and grafting from approaches. Polym. Chem. 2020, 11, 4133–4142. [Google Scholar] [CrossRef]
- Coquery, C.; Carosio, F.; Negrell, C.; Causse, N.; Pebere, N.; David, G. New bio-based phosphorylated chitosan/alginate protective coatings on aluminum alloy obtained by the LbL technique. Surf. Interfaces 2019, 16, 59–66. [Google Scholar] [CrossRef]
- Ouarga, A.; Noukrati, H.; Iraola-Arregui, I.; Elaissari, A.; Barroug, A.; Ben Youcef, H. Development of anti-corrosion coating based on phosphorylated ethyl cellulose microcapsules. Prog. Org. Coat. 2020, 148, 70. [Google Scholar] [CrossRef]
- Macarie, L.; Ilia, G. Poly(vinylphosphonic acid) and its derivatives. Prog. Polym. Sci. 2010, 35, 1078–1092. [Google Scholar] [CrossRef]
- Posner, R.; Sundell, P.E.; Bergman, T.; Roose, P.; Heylen, M.; Grundmeier, G.; Keil, P. UV-Curable Polyester Acrylate Coatings: Barrier Properties and Ion Transport Kinetics Along Polymer/Metal Interfaces. J. Electrochem. Soc. 2011, 158, C185–C193. [Google Scholar] [CrossRef]
- Millet, F.; Auvergne, R.; Caillol, S.; David, G.; Manseri, A.; Pebere, N. Improvement of corrosion protection of steel by incorporation of a new phosphonated fatty acid in a phosphorus-containing polymer coating obtained by UV curing. Prog. Org. Coat. 2014, 77, 285–291. [Google Scholar] [CrossRef]
- Macarie, L.; Pekar, M.; Simulescu, V.; Plesu, N.; Iliescu, S.; Ilia, G.; Tara-Lunga-Mihali, M. Properties in Aqueous Solution of Homo- and Copolymers of Vinylphosphonic Acid Derivatives Obtained by UV-Curing. Macromol. Res. 2017, 25, 214–221. [Google Scholar] [CrossRef]
- Kousar, F.; Moratti, S.C. Synthesis of fluorinated phosphorus-containing copolymers and their immobilization and properties on stainless steel. RSC Adv. 2021, 11, 38189–38201. [Google Scholar] [CrossRef]
- Sheffer, M.; Groysman, A.; Starosvetsky, D.; Savchenko, N.; Mandler, D. Anion embedded sol-gel films on Al for corrosion protection. Corros. Sci. 2004, 46, 2975–2985. [Google Scholar] [CrossRef]
- Kannan, A.G.; Choudhury, N.R.; Dutta, N.K. Electrochemical performance of sol-gel derived phospho-silicate-methacrylate hybrid coatings. J. Electroanal. Chem. 2010, 641, 28–34. [Google Scholar] [CrossRef]
- Kannan, A.G.; Choudhury, N.R.; Dutta, N.K. Synthesis and characterization of methacrylate phospho-silicate hybrid for thin film applications. Polymer 2007, 48, 7078–7086. [Google Scholar] [CrossRef]
- Tuken, T.; Yazici, B.; Erbil, M. The use of polythiophene for mild steel protection. Prog. Org. Coat. 2004, 51, 205–212. [Google Scholar] [CrossRef]
- Zhi, D.; Smyrl, W.H. Application of Electroactive Films in Corrosion Protection.2.Metal Hwxacyanometalate Films on TiO2/Ti Surfaces. J. Electrochem. Soc. 1991, 138, 1911–1918. [Google Scholar]
- Wang, W.L.; Lai, Y.H. Synthesis and characterization of a novel 1,4-naphthalene-based thiophene copolymers. Thin Solid Film. 2002, 417, 211–214. [Google Scholar] [CrossRef]
- Medrano-Vaca, M.G.; Gonzalez-Rodriguez, J.G.; Nicho, M.E.; Casales, M.; Salinas-Bravo, V.M. Corrosion protection of carbon steel by thin films of poly (3-alkyl thiophenes) in 0.5 M H2SO4. Electrochim. Acta 2008, 53, 3500–3507. [Google Scholar] [CrossRef]
- Branzoi, F.; Mihai, M.A.; Petrescu, S. Corrosion Protection Efficacy of the Electrodeposit of Poly (N-Methyl Pyrrole-Tween20/3-Methylthiophene) Coatings on Carbon Steel in Acid Medium. Coatings 2022, 12, 1062. [Google Scholar] [CrossRef]
- Emira, H.S.A.; Abu-Ayana, Y.M.; El-Sawy, S.M. Modified amino resins for corrosion prevention in organic coatings. Pigment Resin Technol. 2013, 42, 298–308. [Google Scholar] [CrossRef]
- McAndrew, T.P. Corrosion prevention with electrically conductive polymers. Trends Polym. Sci. 1997, 5, 7–12. [Google Scholar]
- Leon-Silva, U.; Nicho, M.E. Poly(3-octylthiophene) and polystyrene blends thermally treated as coatings for corrosion protection of stainless steel 304. J. Solid State Electrochem. 2010, 14, 1487–1497. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Yang, C.-R.; Tsai, J.-H.; Yu, Y.-H.; Huang, P.-T. Enhanced Corrosion Protection of Iron by Poly(3-hexylthiophene)/Poly (styrene-co-hydroxystyrene) Blends. Coatings 2018, 8, 383. [Google Scholar] [CrossRef]
- Al-Muallem, H.A.; Mazumder, M.A.J.; Estaitie, M.K.; Ali, S.A. A novel cyclopolymer containing residues of essential amino acid methionine: Synthesis and application. Iran. Polym. J. 2015, 24, 541–547. [Google Scholar] [CrossRef]
- Ali, S.A.; Saeed, M.T. Synthesis and corrosion inhibition study of some 1,6-hexanediamine-based N,N-diallyl quaternary ammonium salts and their polymers. Polymer 2001, 42, 2785–2794. [Google Scholar] [CrossRef]
- Ali, S.A.; Goni, L.; Mazumder, M.A.J. Butler’s cyclopolymerizaton protocol in the synthesis of diallylamine salts/sulfur dioxide alternate polymers containing amino acid residues. J. Polym. Res. 2017, 24, 1022–9760. [Google Scholar] [CrossRef]
- Haladu, S.A.; Umoren, S.A.; Ali, S.A.; Solomon, M.M.; Mohammed, A.R.I. Synthesis, characterization and electrochemical evaluation of anticorrosion property of a tetrapolymer for carbon steel in strong acid media. Chin. J. Chem. Eng. 2019, 27, 965–978. [Google Scholar] [CrossRef]
- Liu, S.; Yan, J.; Shi, J.Q.; Li, X.Z.; Zhang, J.P.; Wang, X.Y.; Cai, N.J.; Fang, Q.H.; Zhang, Q.Y.; Yan, Y. Rational design of cobaltocenium-containing polythioether type metallo-polyelectrolytes as HCl corrosion inhibitors for mild steel. Polym. Chem. 2023, 14, 330–342. [Google Scholar] [CrossRef]
- Braun, R.D.; Lopez, E.E.; Vollmer, D.P. Low-Molecular-Weight Straight-Chain Amines as Corrosion-Inhibitors. Corros. Sci. 1993, 34, 1251–1257. [Google Scholar] [CrossRef]
- Finsgar, M.; Fassbender, S.; Nicolini, F.; Milosev, I. Polyethyleneimine as a corrosion inhibitor for ASTM 420 stainless steel in near-neutral saline media. Corros. Sci. 2009, 51, 525–533. [Google Scholar] [CrossRef]
- Finsgar, M.; Fassbender, S.; Hirth, S.; Milosev, I. Electrochemical and XPS study of polyethyleneimines of different molecular sizes as corrosion inhibitors for AISI 430 stainless steel in near-neutral chloride media. Mater. Chem. Phys. 2009, 116, 198–206. [Google Scholar] [CrossRef]
- Ansari, K.; Chauhan, D.S.; Quraishi, M.A.; Adesina, A.Y.; Saleh, T.A. The synergistic influence of polyethyleneimine-grafted graphene oxide and iodide for the protection of steel in acidizing conditions. RSC Adv. 2020, 10, 17739–17751. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, X.; Sheng, Y. Studies on preparing and corrosion inhibition behaviour of quaternized polyethyleneimine for low carbon steel in sulfuric acid. Mater. Chem. Phys. 2008, 108, 375–381. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Gao, B. Studies on Cationic Property of Quaternary Polyethyleneimine. Mater. Sci. Forum 2011, 689, 432–439. [Google Scholar] [CrossRef]
- Chen, T.; Chen, M.; Chen, Z.; Fu, C. Comprehensive investigation of modified polyethyleneimine as an efficient polymeric corrosion inhibitor in neutral medium: Synthesis, experimental and theoretical assessments. J. Mol. Liq. 2021, 339, 116803. [Google Scholar] [CrossRef]
- Chen, T.; Chen, M.; Fu, C. Effect of molecular weight on inhibition performance of modified polyethyleneimine as polymer corrosion inhibitor for carbon steel in neutral medium. J. Appl. Polym. Sci. 2022, 139, e51922. [Google Scholar] [CrossRef]
- Mcandrew, T.P.; Miller, S.A.; Gilicinski, A.G.; Robeson, L.M. Polyaniline in Corrision-Resistant Coatings. ACS Symp. Ser. 1998, 689, 396–408. [Google Scholar]
- Wessling, B. Passivation of Metals by Coating with Polyaniline-Corrosion Potential Shift and Morphological-Changes. Adv. Mater. 1994, 6, 226–228. [Google Scholar] [CrossRef]
- Deberry, D.W. Modification Of The Electrochemical And Corrosion Behavior Of Stainless-Steels With An Electroactive Coating. J. Electrochem. Soc. 1985, 132, 1022–1026. [Google Scholar] [CrossRef]
- Mirmohseni, A.; Oladegaragoze, A. Anti-corrosive properties of polyaniline coating on iron. Synth. Met. 2000, 114, 105–108. [Google Scholar] [CrossRef]
- Kalendova, A.; Vesely, D.; Stejskal, J. Organic coatings containing polyaniline and inorganic pigments as corrosion inhibitors. Prog. Org. Coat. 2008, 62, 105–116. [Google Scholar] [CrossRef]
- Duran, B.; Turhan, M.C.; Bereket, G.; Sarac, A.S. Electropolymerization, characterization and corrosion performance of poly(N-ethylaniline) on copper. Electrochim. Acta 2009, 55, 104–112. [Google Scholar] [CrossRef]
- Manivel, P.; Sathiyanarayanan, S.; Venkatachari, G. Synthesis of Poly(p-phenylene diamine) and Its Corrosion Inhibition Effect on Iron in 1M HC1. J. Appl. Polym. Sci. 2008, 110, 2807–2814. [Google Scholar] [CrossRef]
- Jeyaprabha, C.; Sathiyanarayanan, S.; Phani, K.L.N.; Venkatachari, G. Investigation of the inhibitive effect of poly(diphenylamine) on corrosion of iron in 0.5 M H2SO4 solutions. J. Electroanal. Chem. 2005, 585, 250–255. [Google Scholar] [CrossRef]
- Shi, F.; Wang, X.; Yu, J.; Hou, B. Corrosion inhibition by polyaniline copolymer of mild steel in hydrochloric acid solution. Anti-Corros. Methods Mater. 2011, 58, 111–115. [Google Scholar]
- Shukla, S.K.; Quraishi, M.A. Effect of some substituted anilines-formaldehyde polymers on mild steel corrosion in hydrochloric acid medium. J. Appl. Polym. Sci. 2012, 124, 5130–5137. [Google Scholar] [CrossRef]
- Seegmiller, J.C.; da Silva, J.E.P.; Buttry, D.A.; de Torresi, S.I.C.; Torresi, R.M. Mechanism of action of corrosion protection coating for AA2024-T3 based on poly(aniline)-poly(methylmethacrylate) blend. J. Electrochem. Soc. 2005, 152, B45–B53. [Google Scholar] [CrossRef]
- Torresi, R.M.; de Souza, S.; da Silvaa, J.E.P.; de Torresi, S.I.C. Galvanic coupling between metal substrate and polyaniline acrylic blends: Corrosion protection mechanism. Electrochim. Acta 2005, 50, 2213–2218. [Google Scholar] [CrossRef]
- Lan, T.; Kaviratna, P.D.; Pinnavaia, T.J. On The Nature Of Polyimide Clay Hybrid Composites. Chem. Mater. 1994, 6, 573–575. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Shao, Y.W.; Liu, X.L.; Shi, C.; Wang, Y.Q.; Meng, G.Z.; Zeng, X.G.; Yang, Y. A study on corrosion protection of different polyaniline coatings for mild steel. Prog. Org. Coat. 2017, 111, 240–247. [Google Scholar] [CrossRef]
- Yeh, J.M.; Chen, C.L.; Chen, Y.C.; Ma, C.Y.; Lee, K.R.; Wei, Y.; Li, S.X. Enhancement of corrosion protection effect of poly(o-ethoxyaniline) via the formation of poly (o-ethoxyaniline)-clay nanocomposite materials. Polymer 2002, 43, 2729–2736. [Google Scholar] [CrossRef]
- Olad, A.; Rashidzadeh, A. Preparation and anticorrosive properties of PANI/Na-MMT and PANI/O-MMT nanocomposites. Prog. Org. Coat. 2008, 62, 293–298. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Katsuma, S.; Fujimoto, S.; Hiromoto, S. Electrochemical study of Type 304 and 316L stainless steels in simulated body fluids and cell cultures. Acta Biomater. 2006, 2, 709–715. [Google Scholar] [CrossRef]
- Ganash, A.A.; Al-Nowaiser, F.M.; Al-Thabaiti, S.A.; Hermas, A.A. Comparison study for passivation of stainless steel by coating with polyaniline from two different acids. Prog. Org. Coat. 2011, 72, 480–485. [Google Scholar] [CrossRef]
- Shabani-Nooshabadi, M.; Ghoreishi, S.M.; Jafari, Y.; Kashanizadeh, N. Electrodeposition of polyaniline-montmorrilonite nanocomposite coatings on 316L stainless steel for corrosion prevention. J. Polym. Res. 2014, 21, 416. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Shao, Y.W.; Zhang, T.; Meng, G.Z.; Wang, F.H. High corrosion protection of a polyaniline/organophilic montmorillonite coating for magnesium alloys. Prog. Org. Coat. 2013, 76, 804–811. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.H.; Yu, Z.Z.; Koratkar, N. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, Y.D.; Choi, H.J. Fabrication of semiconducting graphene oxide/polyaniline composite particles and their electrorheological response under an applied electric field. Carbon 2012, 50, 290–296. [Google Scholar] [CrossRef]
- Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Rogers, B.R.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating (vol 6, pg 1102, 2012). ACS Nano 2012, 6, 4540. [Google Scholar]
- Chang, C.H.; Huang, T.C.; Peng, C.W.; Yeh, T.C.; Lu, H.I.; Hung, W.I.; Weng, C.J.; Yang, T.I.; Yeh, J.M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Rui, M.; Jiang, Y.L.; Zhu, A.P. Sub-micron calcium carbonate as a template for the preparation of dendrite-like PANI/CNT nanocomposites and its corrosion protection properties. Chem. Eng. J. 2020, 385, 123396. [Google Scholar] [CrossRef]
- Hou, X.M.; Wang, Y.N.; Hou, J.Z.; Sun, G.E.; Zhang, C.L. Effect of polyaniline-modified glass fibers on the anticorrosion performance of epoxy coatings. J. Coat. Technol. Res. 2017, 14, 407–415. [Google Scholar] [CrossRef]
- Chen, X.; Shen, K.; Zhang, J. Preparation and anticorrosion properties of polyaniline-SiO2-containing coating on Mg-Li alloy. Pigment Resin Technol. 2010, 39, 322–326. [Google Scholar] [CrossRef]
- Kohl, M.; Kalendova, A. Effect of polyaniline salts on the mechanical and corrosion properties of organic protective coatings. Prog. Org. Coat. 2015, 86, 96–107. [Google Scholar] [CrossRef]
- Bagherzadeh, M.R.; Mahdavi, F.; Ghasemi, M.; Shariatpanahi, H.; Faridi, H.R. Using nanoemeraldine salt-polyaniline for preparation of a new anticorrosive water-based epoxy coating. Prog. Org. Coat. 2010, 68, 319–322. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Dehghanian, C.; Kosari, A. In situ synthesis of polyaniline-camphorsulfonate particles in an epoxy matrix for corrosion protection of mild steel in NaCl solution. Corros. Sci. 2014, 85, 204–214. [Google Scholar] [CrossRef]
- Olad, A.; Ramazani, Z. Preparation, Characterization, and Anticorrosive Properties of Polyaniline Nanotubes. Int. J. Polym. Mater. 2012, 61, 949–962. [Google Scholar] [CrossRef]
- Elkais, A.R.; Gvozdenovic, M.M.; Jugovic, B.Z.; Grgur, B.N. The influence of thin benzoate-doped polyaniline coatings on corrosion protection of mild steel in different environments. Prog. Org. Coat. 2013, 76, 670–676. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. Preparation of polyaniline-Fe2O3 composite and its anticorrosion performance. Synth. Met. 2007, 157, 751–757. [Google Scholar] [CrossRef]
- Lee, S.Y.; Regnault, W.F.; Antonucci, J.M.; Skrtic, D. Effect of particle size of an amorphous calcium phosphate filler on the mechanical strength and ion release of polymeric composites. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2007, 80, 11–17. [Google Scholar] [CrossRef]
- Siviour, C.R.; Gifford, M.J.; Walley, S.M.; Proud, W.G.; Field, J.E. Particle size effects on the mechanical properties of a polymer bonded explosive. J. Mater. Sci. 2004, 39, 1255–1258. [Google Scholar] [CrossRef]
- Olad, A.; Barati, M.; Shirmohammadi, H. Conductivity and anticorrosion performance of polyaniline/zinc composites: Investigation of zinc particle size and distribution effect. Prog. Org. Coat. 2011, 72, 599–604. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Siju, C.R.; Mahanta, D.; Patil, S.; Madras, G. Conducting polyaniline-nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta 2009, 54, 1249–1254. [Google Scholar] [CrossRef]
- Al-Masoud, M.A.; Khalaf, M.M.; Gouda, M.; Dao, V.D.; Mohamed, I.M.A.; Shalabi, K.; Abd El-Lateef, H.M. Synthesis and Characterization of the Mixed Metal Oxide of ZnO-TiO2 Decorated by Polyaniline as a Protective Film for Acidic Steel Corrosion: Experimental, and Computational Inspections. Materials 2022, 15, 7589. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhong, Y.; Zeng, T. Preparation and Performance Evaluation of Poly Methyl Acrylate-acrylic Acid Imidazoline with Corrosion Inhibition and Viscosity Reduction Effect. Oilfield Chem. 2018, 35, 144–149. [Google Scholar]
- Majd, M.T.; Shahrabi, T.; Ramezanzadeh, B.; Bahlakeh, G. Development of a high-performance corrosion protective functional nano-film based on poly acrylic acid-neodymium nitrate on mild steel surface. J. Taiwan Inst. Chem. Eng. 2019, 96, 610–626. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lin, Y.-Y.; Lin, C.-H.; Chan, C.-C.; Huang, Y.-C. High-performance polystyrene/graphene-based nanocomposites with excellent anti-corrosion properties. Polym. Chem. 2014, 5, 535–550. [Google Scholar] [CrossRef]
- Yan, J.; Li, X.; Zhang, X.; Liu, S.; Zhong, F.; Zhang, J.; Zhang, Q.; Yan, Y. Metallo-Polyelectrolyte-Based Waterborne Polyurethanes as Robust HCl Corrosion Inhibitor Mediated by Inter/intramolecular Hydrogen Bond. ACS Appl. Polym. Mater. 2022, 4, 3844–3854. [Google Scholar] [CrossRef]

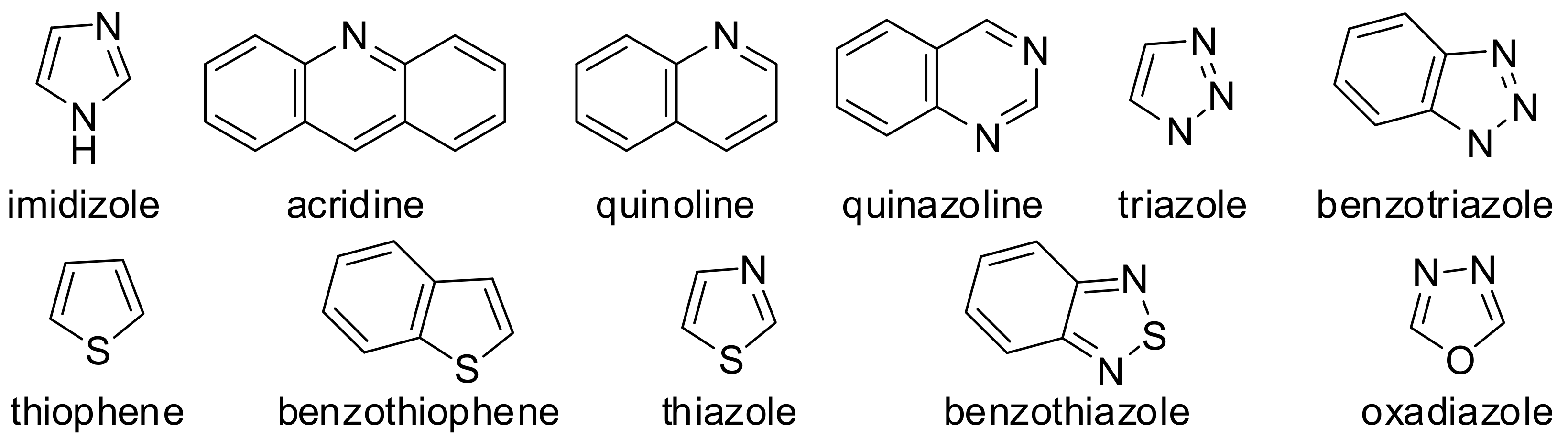


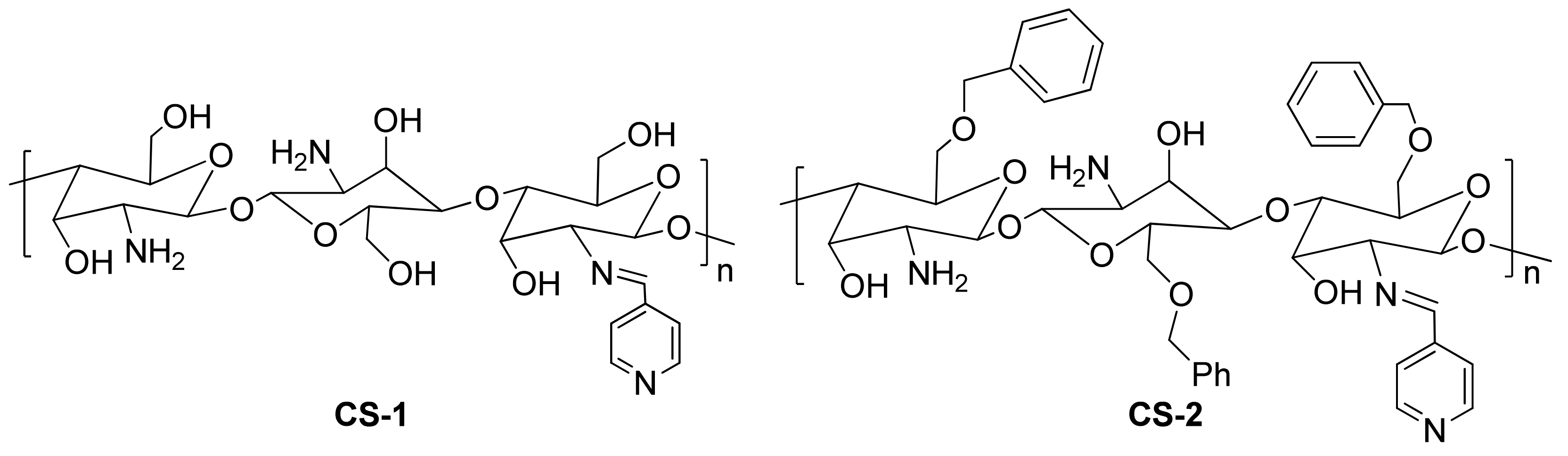
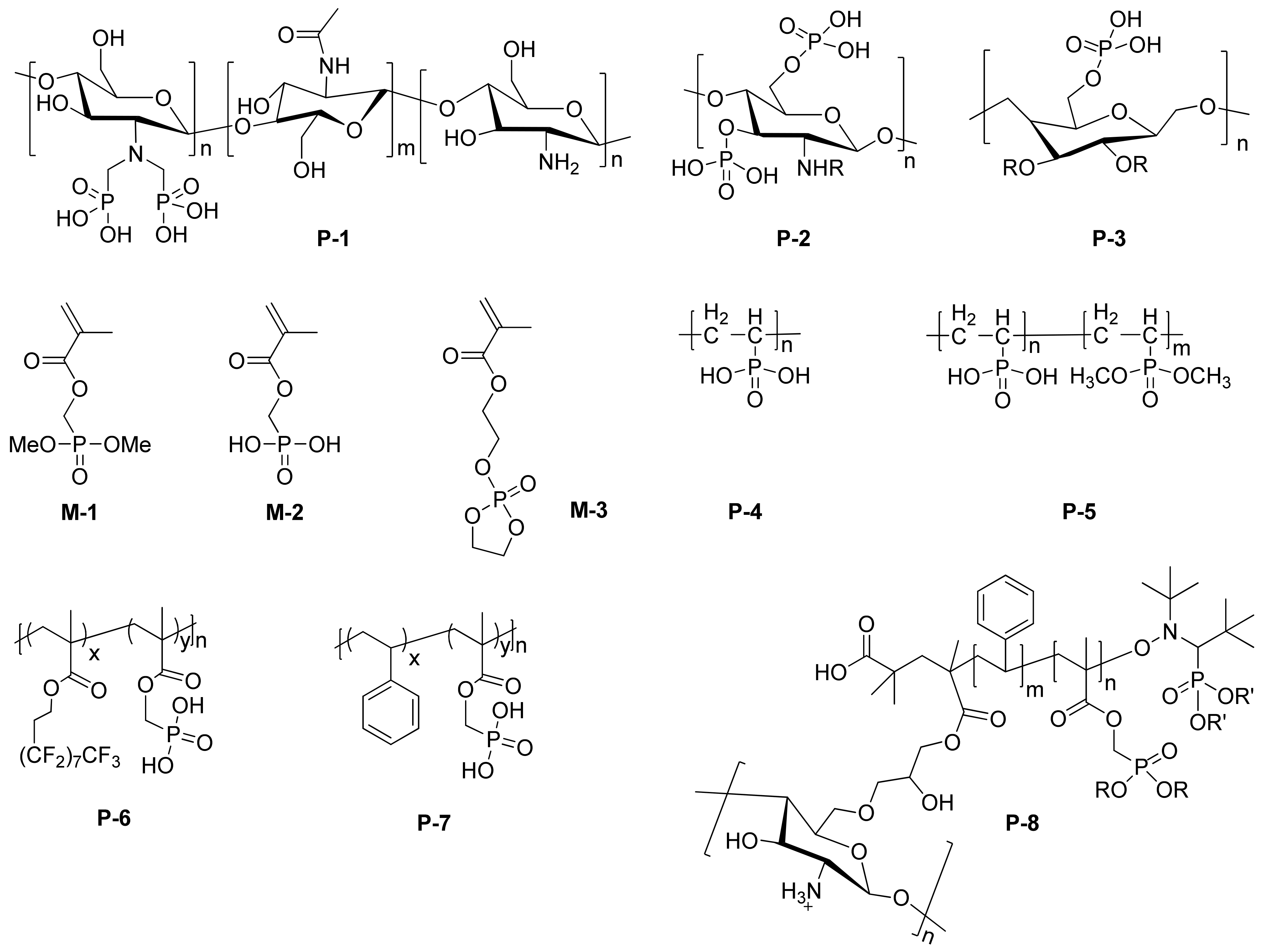

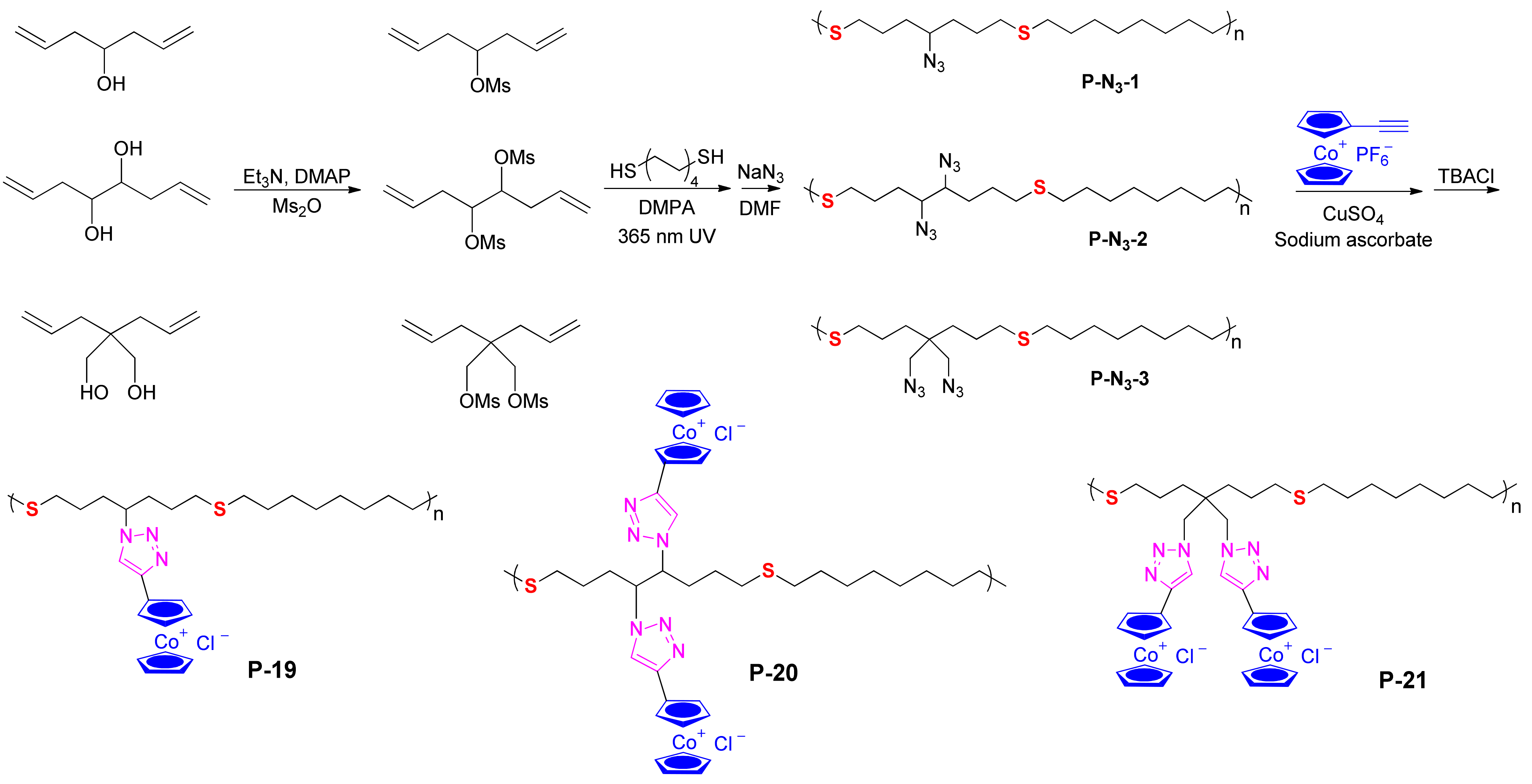


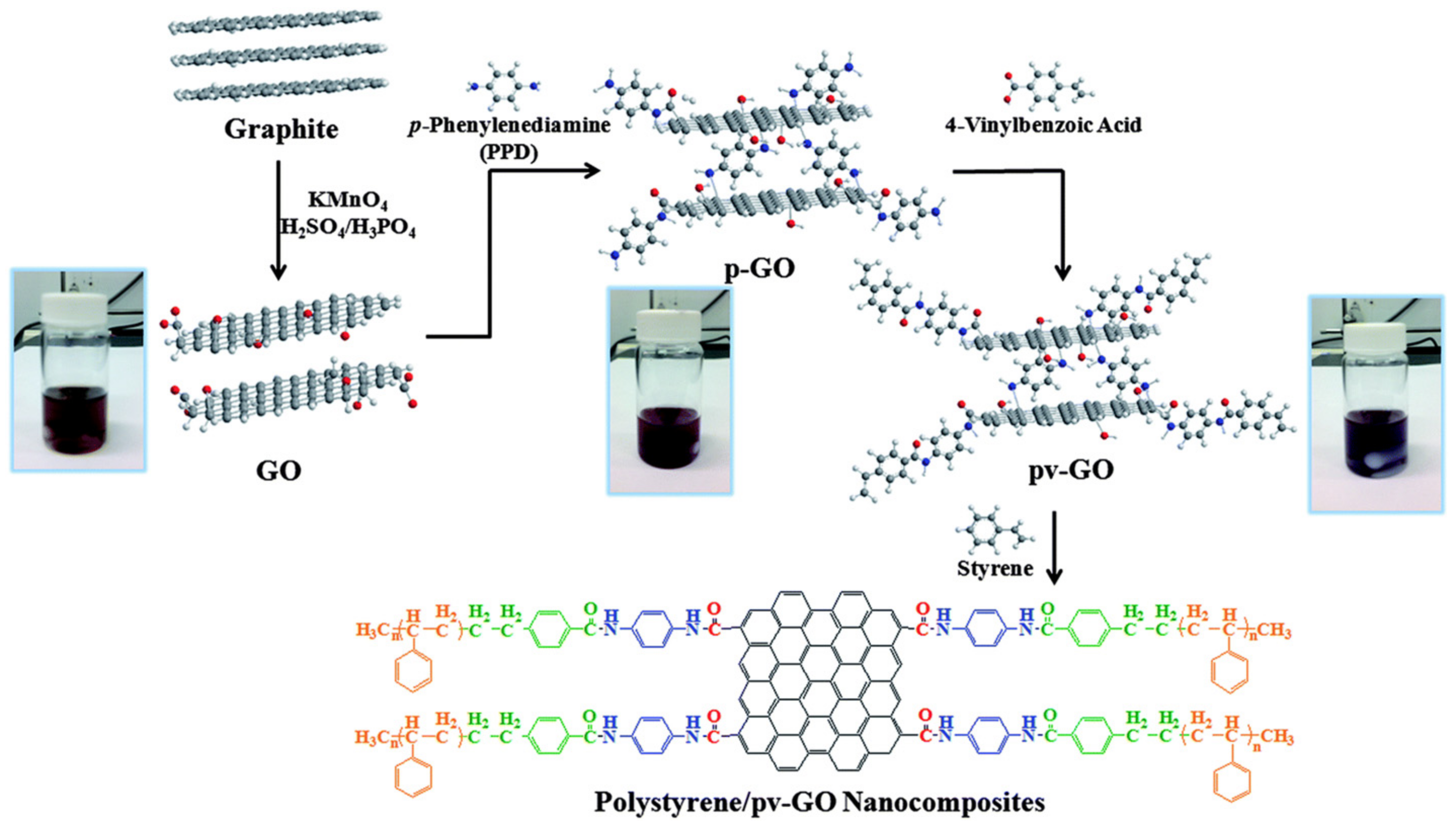
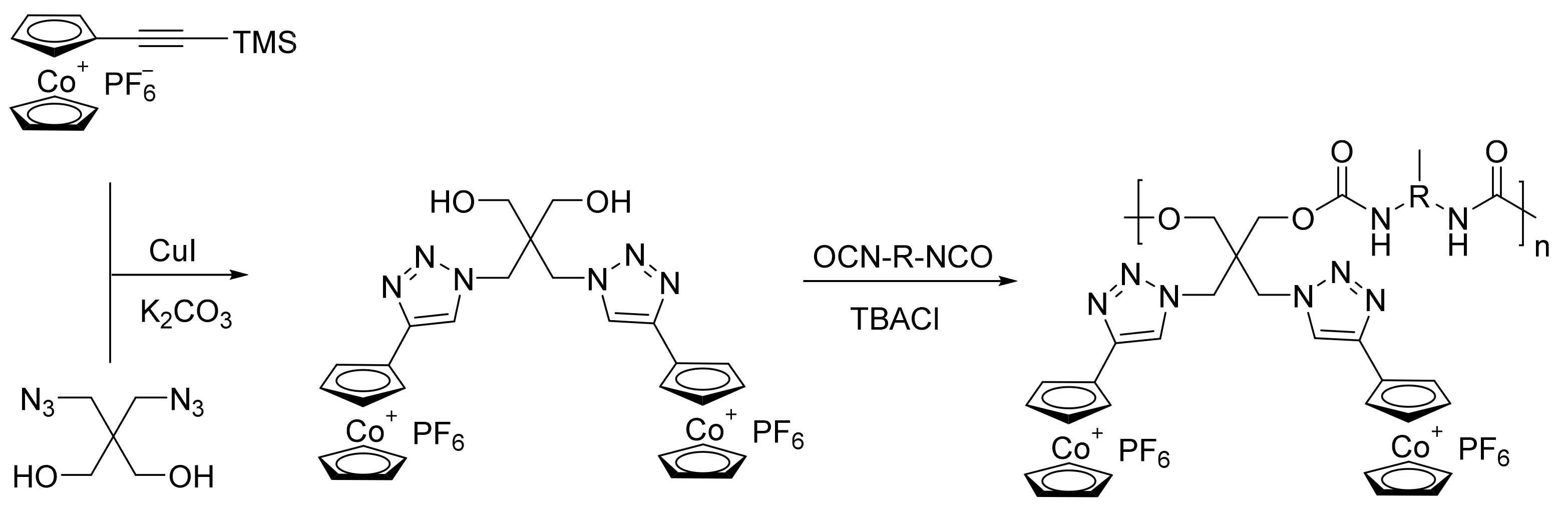
| Inhibitor | Metal | Corrosion Medium | TEST METHOD | Highest IE (%) | Reference |
|---|---|---|---|---|---|
| starch | Al-Mg-Si alloy | 3.5% NaCl | PDP, EIS | / | [18] |
| starch nanocrystals | mild steel (ST37-2) | 1 M HCl | weight loss, PDP, EIS | 67.0 | [19] |
| starch-pectin blend | mild steel | 1 M HCl | PDP, EIS | 88.9 | [20] |
| maize gluten meal extract | steel | simulated concrete pore solution with 3.0% NaCl | PDP, EIS | 88.1 | [21] |
| alkaline modified starch | mild steel | 0.25 M H2SO4 | PDP, weight loss | 84.2 | [24] |
| AM-grafted cassava starch | aluminum | 1 M H3PO4 | weight loss, PDP, EIS | 91.9 | [26] |
| starch-AA-CS copolymer | Q235 carbon steel | 1 M HCl | weight loss, EIS, PDP | 90.1 | [27] |
| AM grafted cassava starch | aluminum | 1 M HNO3 | weight loss, EIS, PDP | 97.8 | [28] |
| AM grafted starch | Zn | 1 M HCl | weight loss, EIS, PDP | 92.2 | [29] |
| AM grafted starch | Zn | 1 M HCl | weight loss, EIS, PDP | 97.2 | [30] |
| Inhibitor | Metal | Corrosion Medium | Test Method | Highest IE (%) | Reference |
|---|---|---|---|---|---|
| P-9 | 1018 carbon steel | 0.5 M H2SO4 | EIS | / | [59] |
| P-10 | iron | 3.5% NaCl | EIS, PDP | 96 | [64] |
| P-11/P-14 | mild steel | 1 M HCl | EIS | 99 | [66] |
| P-12/P-13 | mild steel | 1 M HCl | Weight loss | P-12: 94 P-13: 87 | [67] |
| P-15 | St37 carbon steel | 15% HCl/15% H2SO4 | EIS, PDP, linear polarization resistance, electrochemical frequency modulation | 79.5/61.1 | [62] |
| P-16/P-17/P-18 | mild steel | 1 M HCl | Weight loss | P-16: 92.3 P-18: 95.7 | [68] |
| P-19/P-20/P-21 | mild steel | 5% HCl | weight loss, EIS, PDP | 95 | [69] |
| P-22/P-23 | cold-rolled mild steel | 3.5% NaCl | weight loss, | / | [61] |
| Inhibitor | Metal | Corrosion Medium | Test Method | Highest IE (%) | Reference |
|---|---|---|---|---|---|
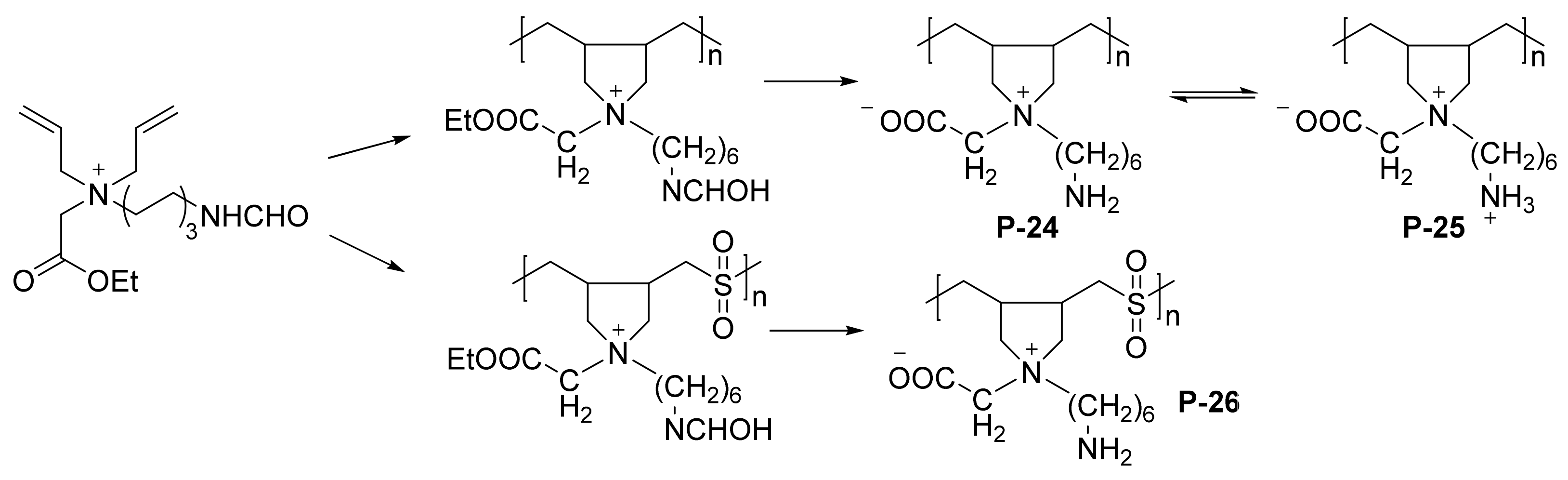
| P-24/P-25/P-26 | mild steel | 1 M HCl | weight loss | P-25: 82 P-26: 82 | [66] |
| P-27 | ASTM 420 stainless steel | 3% NaCl | linear polarization, cyclic polarization | 81.9 | [71] |
| P-28 | mild steel (A3 steel) | 0.5 M H2SO4 | weight loss, PDP | 92 | [74] |
| P-29 | mild steel | simulated neutral medium | weight loss tests, PDP, EIS | 88 | [76] |
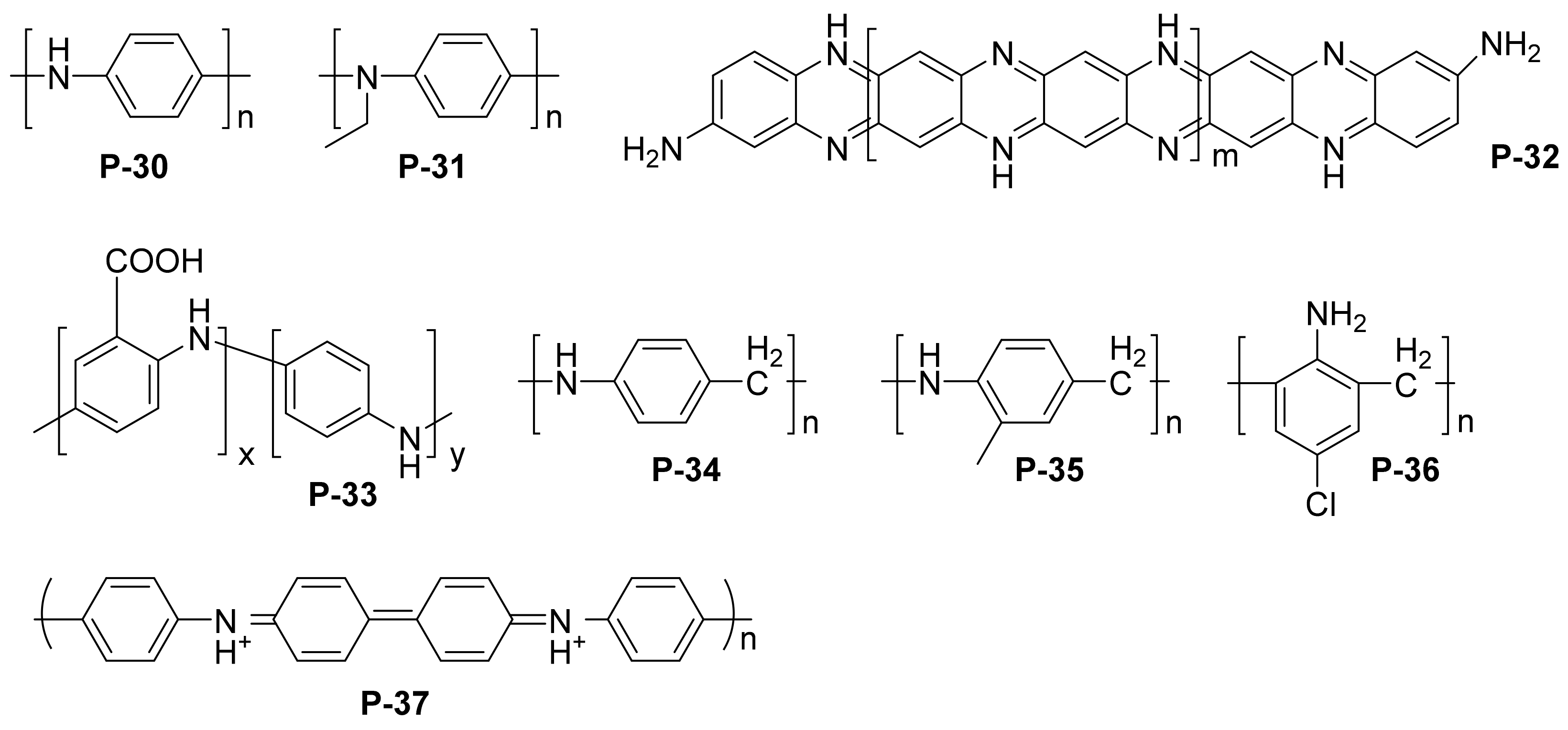
| P-30 | iron | 3.5% NaCl/0.1 M HCl | PDP, | / | [78] |
| P-31 | copper | 0.1 M H2SO4 | PDP, EIS | / | [83] |
| P-32 | iron | 1 M HCl | PDP, EIS | 92.7 | [84] |
| P-33 | carbon steel | 1 M HCl | weight loss, PDP, EIS | 90.5 | [86] |
| P-34/P-35/P-36 | mild steel | 1 M HCl | weight loss, PDP, EIS | P-34: 97.23 P-35: 98.46 P-36: 98.96 | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, S.; Yan, J.; Zhang, J.; Zhang, Q.; Yan, Y. Recent Progress of Polymeric Corrosion Inhibitor: Structure and Application. Materials 2023, 16, 2954. https://doi.org/10.3390/ma16082954
Wang X, Liu S, Yan J, Zhang J, Zhang Q, Yan Y. Recent Progress of Polymeric Corrosion Inhibitor: Structure and Application. Materials. 2023; 16(8):2954. https://doi.org/10.3390/ma16082954
Chicago/Turabian StyleWang, Xuanyi, Shuang Liu, Jing Yan, Junping Zhang, Qiuyu Zhang, and Yi Yan. 2023. "Recent Progress of Polymeric Corrosion Inhibitor: Structure and Application" Materials 16, no. 8: 2954. https://doi.org/10.3390/ma16082954
APA StyleWang, X., Liu, S., Yan, J., Zhang, J., Zhang, Q., & Yan, Y. (2023). Recent Progress of Polymeric Corrosion Inhibitor: Structure and Application. Materials, 16(8), 2954. https://doi.org/10.3390/ma16082954






