A Novel Approach of Optimum Time Interval Estimation for Al-7.5Si/Al-18Si Liquid–Liquid Bimetal Casting in Sand and Metallic Moulds
Abstract
1. Introduction
2. Experimental
2.1. Martials
2.2. Bimetal Casting Process
2.3. Bimetal Casting Characterization
2.4. Total Solidification Time Estimation
3. Results and Discussion
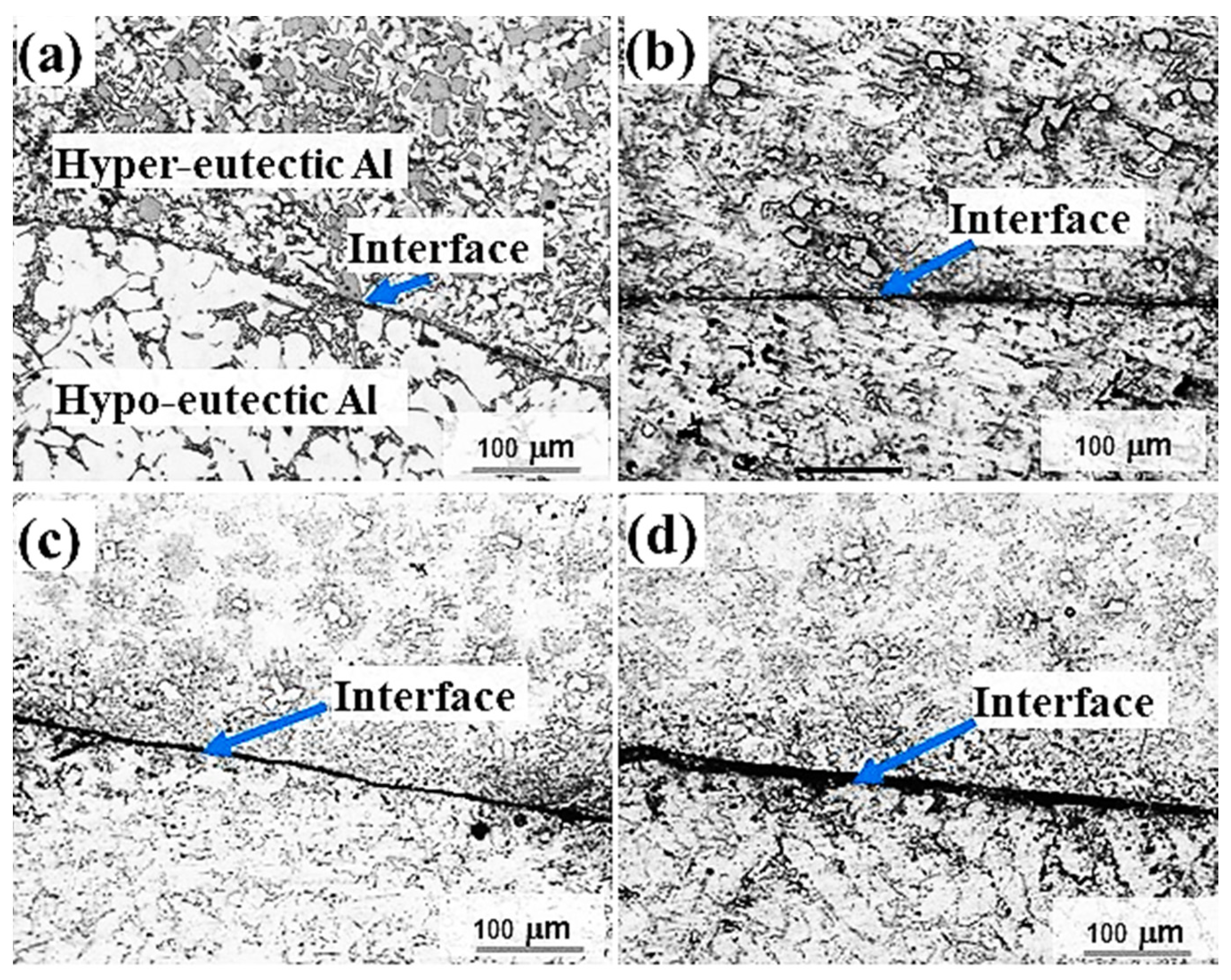
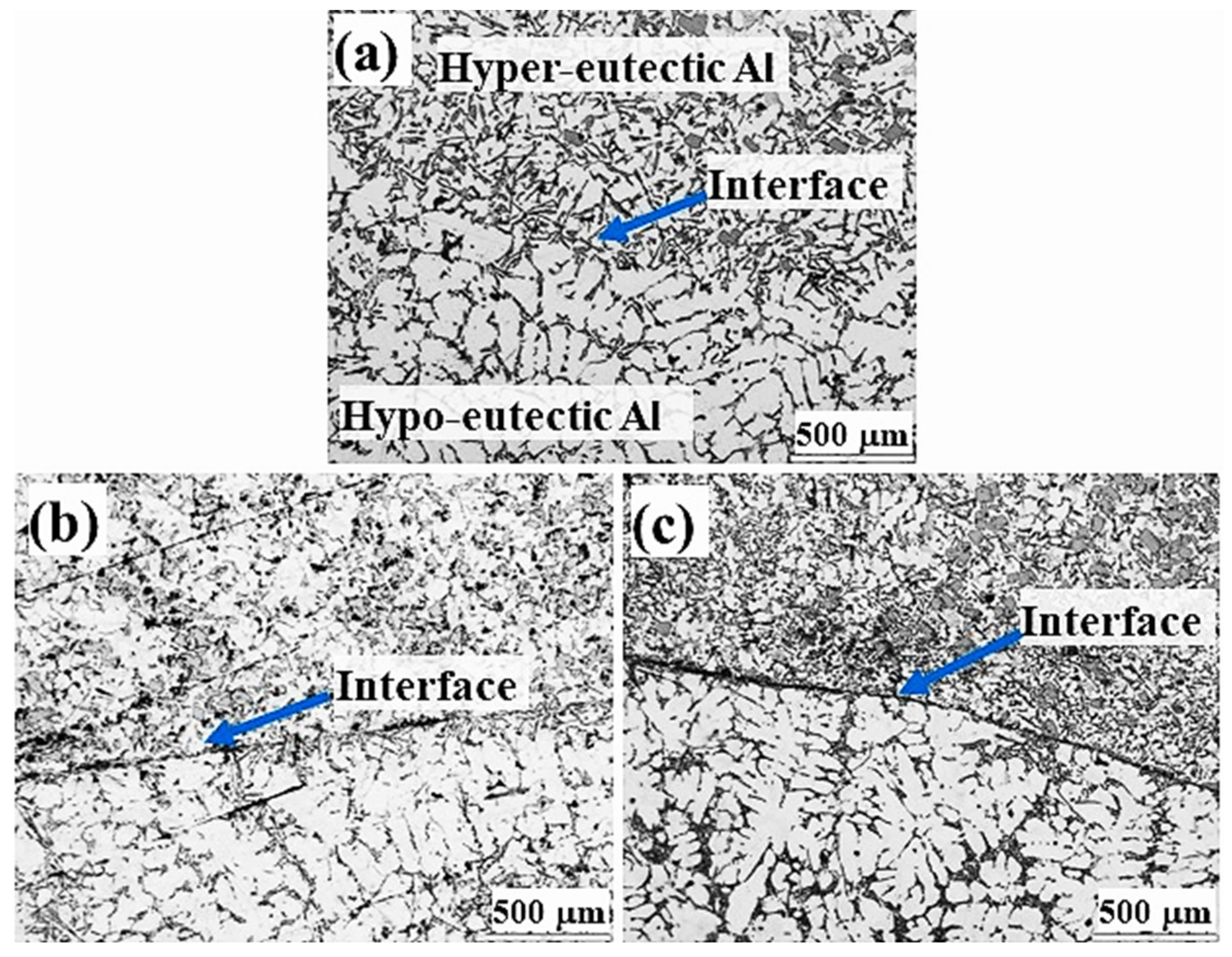
3.1. Interfacial Microstructure in Sand Mould Al/Al Bimetal Casting
3.2. Interfacial Microstructure in Metallic Mould Al/Al Bimetal Casting
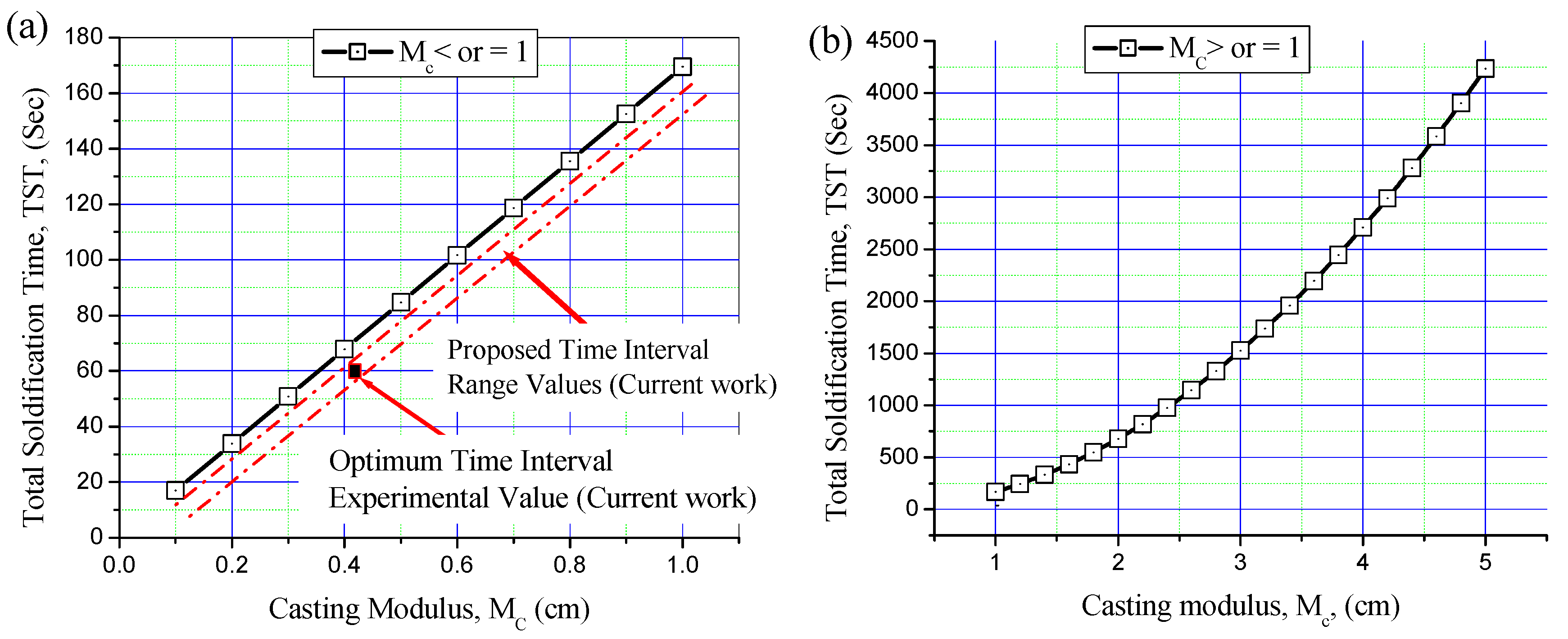
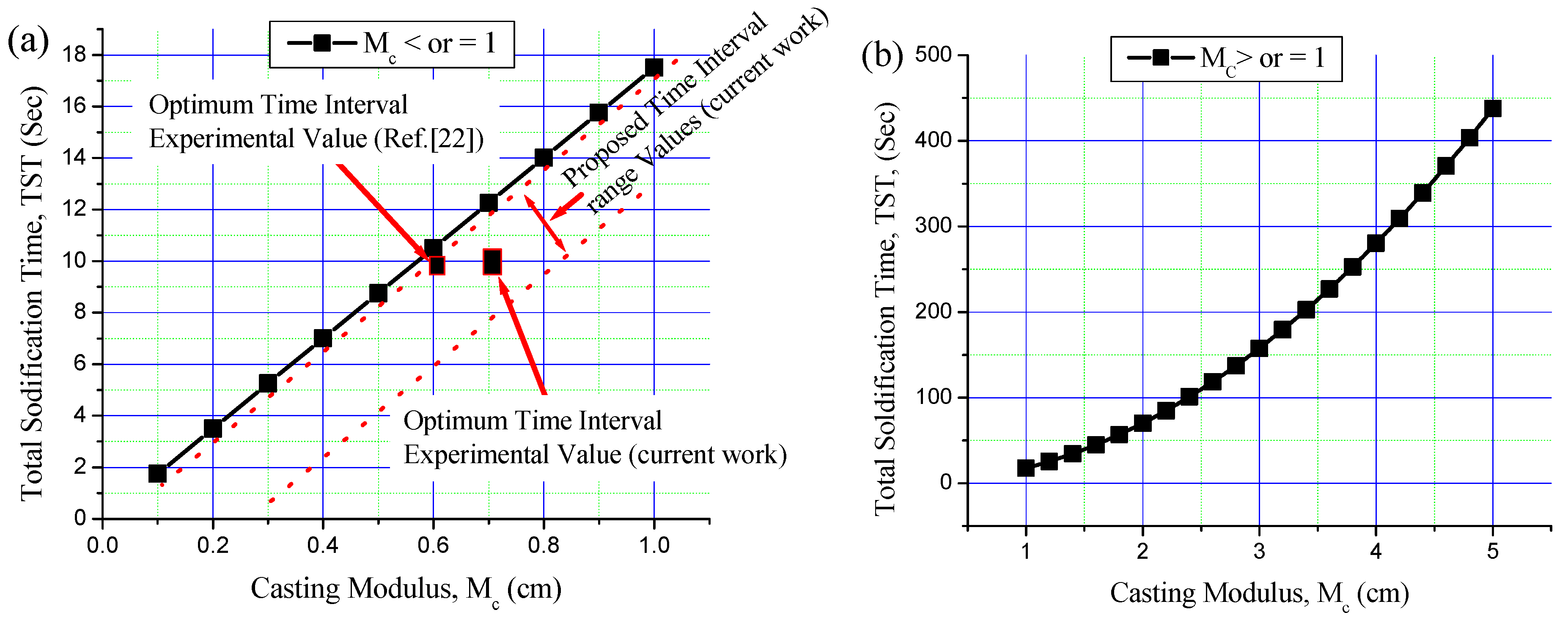
3.3. Time Interval Estimation for Al/Al Bimetal Casting in Sand and Metallic Moulds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.C.; Hu, J.; Nie, X.Y.; Li, H.X.; Du, Q.; Zhang, J.S.; Zhuang, L.Z. The interface bonding mechanism and related mechanical properties of Mg/Al compound materials fabricated by insert molding. Mater. Sci. Eng. A 2015, 635, 70–76. [Google Scholar] [CrossRef]
- Wróbel, T. Bimetallic layered castings alloy steel–grey cast iron. Arch. Mater. Sci. Eng. 2011, 48, 118–125. [Google Scholar] [CrossRef]
- Mao, F.; Zhang, P.; Wei, S.; Chen, C.; Zhang, G.; Xiong, M.; Wang, T.; Guo, J.; Wang, C. Interface Microstructure and Mechanical Properties of Al/Steel Bimetallic Composites Fabricated by Liquid-Solid Casting with Rare Earth Eu Additions. Materials 2022, 15, 6507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, W.; Qu, S.; Zhang, Y. Microstructures and mechanical properties of AZ91D/0Cr19Ni9 bimetal composite prepared by liquid-solid compound casting. Trans. Nonferrous Met. Soc. China 2019, 29, 51–58. [Google Scholar] [CrossRef]
- Shin, J.; Kim, T.; Lim, K.; Cho, H.; Yang, D.; Jeong, C.; Yi, S. Effects of steel type and sandblasting pretreatment on the solid-liquid compound casting characteristics of zinc-coated steel/aluminum bimetals. J. Alloys Compd. 2019, 778, 170–185. [Google Scholar] [CrossRef]
- Aleshin, N.P.; Kobernik, N.V.; Mikheev, R.S.; Vaganov, V.E.; Reshetnyak, V.V.; Aborkin, A.V. Plasma—Powder Application of Antifrictional Babbitt Coatings Modified by Carbon Nanotubes. Russ. Eng. Res. 2016, 36, 46–52. [Google Scholar] [CrossRef]
- Belov, N.A.; Akopyan, T.K.; Gershman, I.; Stolyarova, O.O.; Yakovleva, A.O. Effect of Si and Cu additions on the phase composition, microstructure and properties of Al-Sn alloys. J. Alloys Compd. 2017, 695, 2730–2739. [Google Scholar] [CrossRef]
- Fathy, N.; Ramadan, M. Influence of volume ratio of liquid to solid and low pouring temperature on interface structure of cast Babbitt-steel bimetal composite. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 1966; Volume 2018, pp. 020028-1–020028-6. [Google Scholar] [CrossRef]
- Çam, G.; Koçak, M.; Dobi, D.; Heikinheimo, L.; Siren, M. Fracture behaviour of diffusion bonded bimaterial Ti–Al joints. Sci. Technol. Weld. Join. 1997, 2, 95–101. [Google Scholar] [CrossRef]
- Çam, G.; Özdemir, U.; Ventzke, V.; Koçak, M. Microstructural and mechanical characterization of diffusion bonded hybrid joints. J. Mater. Sci. 2008, 43, 3491–3499. [Google Scholar] [CrossRef]
- Xing, Z.-G.; He, L.-X.; Liang, S.-X.; Chang, L.-B.; Xiao, Z.-X.; Xing, W.-L.; Shen, H.-B.; Cao, J.-J.; Liu, H.-J. Process Optimization of Dual-Liquid Casting and Interfacial Strength–Toughness of the Produced LAS/HCCI Bimetal. Materials 2023, 16, 2008. [Google Scholar] [CrossRef]
- Ramadan, M.; Alghamdi, A.S.; Subhani, T.; Halim, K.S.A. Fabrication and Characterization of Sn-Based Babbitt Alloy Nanocomposite Reinforced with Al2O3 Nanoparticles/Carbon Steel Bimetallic Material. Materials 2020, 13, 2759. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Fan, S.; Wang, J.; Li, G.; Zhang, Z.; Jiang, W. Effect of Vibration Acceleration on Interface Microstructure and Bonding Strength of Mg–Al Bimetal Produced by Compound Casting. Metals 2022, 12, 766. [Google Scholar] [CrossRef]
- Cholewa, M.; Wróbel, T.; Tenerowicz, S. Bimetallic layer castings. J. Achiev. Mater. Manuf. Eng. 2010, 43, 385–391. [Google Scholar]
- Wrobel, T. Characterization of Bimetallic Castings with an Austenitic Working Surface Layer and an Unalloyed Cast Steel Base. J. Mater. Eng. Perform. 2014, 23, 1711–1717. [Google Scholar] [CrossRef]
- Jiang, W.; Fana, Z.; Li, C. Improved steel/aluminum bonding in bimetallic castings by a compound casting process. J. Mater. Process. Technol. 2015, 226, 25–31. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, G.; Liu, X.; Liu, F. Effects of hot dipping galvanizing and aluminizing on interfacial microstructures and mechanical properties of aluminum/iron bimetallic composites. J. Alloys Compd. 2016, 25, 742–751. [Google Scholar] [CrossRef]
- Li, G.; Yang, W.; Jiang, W.; Guan, F.; Jiang, H.; Wu, Y.; Fan, Z. The Role of Vacuum Degree in the Bonding of Al/Mg Bimetal Prepared by a Compound Casting Process. J. Mater. Process. Technol. 2019, 265, 112–121. [Google Scholar] [CrossRef]
- Ramadan, M.; Alghamdi, A.S.; Hafez, K.M.; Subhani, T.; Abdel Halim, K.S. Development and Optimization of Tin/Flux Mixture for Direct Tinning and Interfacial Bonding in Aluminum/Steel Bimetallic Compound Casting. Materials 2020, 13, 5642. [Google Scholar] [CrossRef]
- Cholewa, M.; Tenerowicz, S.; Wróbel, T. Quality of the joint between cast steel and cast iron in bimetallic castings. Arch. Foundry Eng. 2008, 3, 37–40. [Google Scholar]
- Ramadan, M. Interface Structure and Elements Diffusion of As-Cast and Annealed Ductile Iron/Stainless Steel Bimetal Castings. Eng. Technol. Appl. Sci. Res. 2018, 8, 2709–2714. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Li, G.; Yang, L.; Liu, X. Effects of Melt-to-Solid Insert Volume Ratio on the Microstructures and Mechanical Properties of Al/Mg Bimetallic Castings Produced by Lost Foam Casting. Metall. Mater. Trans. A 2016, 47, 6487–6497. [Google Scholar] [CrossRef]
- Jiang, W.; Jiang, Z.; Li, G.; Wu, Y.; Fan, Z. Microstructure of Al/Al bimetallic composites by lost foam casting with Zn interlayer. Mater. Sci. Technol. 2017, 34, 487–492. [Google Scholar] [CrossRef]
- Ramadan, M.; Alghamdi, A.S. Interfacial microstructures and properties of hyper-eutectic Al–21Si/hypo-eutectic Al–7.5Si bimetallic material fabricated by liquid–liquid casting route. Mech. Sci. 2020, 11, 371–379. [Google Scholar] [CrossRef]
- Parihar, R.S.; Setti, S.G.; Sahu, R.K. Recent advances in the manufacturing processes of functionally graded materials: A review. Sci. Eng. Compos. Mater. 2018, 25, 309–336. [Google Scholar] [CrossRef]
- Greß, T.; Mittler, T.; Volk, W. Casting methods for the production of rotationally symmetric copper bimetals. Mater. Sci. Technol. 2020, 36, 906–916. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Wei, Z.J.; Rong, S.F.; Wang, H.W.; Zou, C.M. Formation mechanism of bimetal composite layer between LCS and HCCI. China Foundry 2016, 13, 396–401. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, Z.L.; Jiang, W.M.; Li, G.Y.; Guan, F.; Jiang, H.X.; Fan, Z.T. Interface characteristics of Mg/Al bimetal produced by a novel liquid-liquid compound casting process with an Al interlayer. Int. J. Adv. Manuf. Technol. 2019, 101, 1125–1132. [Google Scholar] [CrossRef]
- Tiryakioglu, M.; Tiryakioglu, E.; Askeland, D.R. Statistical Investigation of the Effects of Shape, Size and Superheat on Solidification Times of Castings. AFS Trans. 1997, 95, 907–913. [Google Scholar]
- Groover, M.P. Fundamentals of Modern Manufacturing: Materials, Processes and Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; 223p. [Google Scholar]
- Wang, H.; Hamed, M.S.; Shankar, S. Interaction between primary dendrite arm spacing and velocity of fluid flow during solidification of Al–Si binary alloys. J. Mater. Sci. 2018, 53, 9771–9789. [Google Scholar] [CrossRef]
- Luo, A.; William, V.Y.; Wang, W. Measurement of Thermal Properties for Sand Molds—A Heat Balance Approach. Can. Metall. Q. 1992, 31, 73–77. [Google Scholar] [CrossRef]
- Miettinen, J. Calculation of solidification-related thermophysical properties for steels. Metall. Mater. Trans. B 1997, 28, 281–297. [Google Scholar] [CrossRef]
- Copur, M.; Turan, A.; Eruslu, M.N. Effects of Chills on the Solidification Pattern of an Axial Steel Cast Impeller. Metalurgija 2015, 54, 515–518. [Google Scholar]
- Wang, G.; Huang, H.; Yang, Z.; Shi, X.; He, X. Numerical Simulation on the Die Filling Process of the Thixo-Forging of Al-7 wt pct Si/Al-22 wt pct Si Bimetal Composite. Metall. Mater. Trans. B 2015, 46, 2121–2128. [Google Scholar] [CrossRef]
- Wei, G.; Huang, P.; Xu, C.; Liu, D.; Ju, X.; Du, X.; Xing, L.; Yang, Y. Thermophysical property measurements and thermal energy storage capacity analysis of aluminum alloys. Solar Energy 2016, 137, 66–72. [Google Scholar] [CrossRef]
- Hafeez, F.; Ahmed, N.; Ali, M.A.; Farooq, M.U.; AlFaify, A.Y.; Rehman, A.U. A comprehensive efficiency evaluation of conventional and ablation sand casting on the example of the AlSi7Mg alloy impeller. Int. J. Adv. Manuf. Technol. 2022, 121, 3653–3672. [Google Scholar] [CrossRef]
- Dudareva, N.Y.; Ivashin, P.V.; Gallyamova, R.F.; Tverdokhlebov, A.Y.; Krishtal, M.M. Structure And Thermophysical Properties Of Oxide Layer Formed By Micro arc Oxidation on Ak12D Al–Si Alloy. Met. Sci. Heat Treat. 2021, 62, 11–12. [Google Scholar] [CrossRef]




| Materials | Chemical Compositions (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Si | Cu | Mg | Ni | Fe | Zn | Ti | Mn | Al | |
| Al-7.5Si (M1) | 7.45 | 0.86 | 0.21 | 0.10 | 0.71 | 0.10 | 0.05 | - | Bal. |
| Al-18Si (M2) | 18.80 | 0.63 | 0.66 | 1.80 | 0.63 | 0.07 | 0.08 | 0.10 | Bal. |
| Sand Mould | Metallic Mould | |
|---|---|---|
| Melting temperature of the liquid (K), Tm | 913 | |
| Superheat (K), ΔTs | 80 | |
| Temperature of the mould (in K), To | 288 | 543 |
| Latent heat of fusion (J kg−1), L | 4.45 × 105 [31] | |
| Thermal conductivity of the mould (Wm−1 K−1), k | 1.4 [32] | 19.54 [33,34] |
| Density of the mould (kg m−3), ρ | 1570 [32] | 7800 [33,34] |
| Specific heat of the mould (J kg−1 K−1), C | 1153 [32] | 522 [33,34] |
| Density of the metal (kg m−3), ρm | 2.76 × 103 [35] | |
| Specific heat of the metal (J kg−1 K−1), Cm | 1058 [36] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathy, N.; Ramadan, M.; Hafez, K.M.; Abdulaziz, F.; Ayadi, B.; Alghamdi, A.S. A Novel Approach of Optimum Time Interval Estimation for Al-7.5Si/Al-18Si Liquid–Liquid Bimetal Casting in Sand and Metallic Moulds. Materials 2023, 16, 3004. https://doi.org/10.3390/ma16083004
Fathy N, Ramadan M, Hafez KM, Abdulaziz F, Ayadi B, Alghamdi AS. A Novel Approach of Optimum Time Interval Estimation for Al-7.5Si/Al-18Si Liquid–Liquid Bimetal Casting in Sand and Metallic Moulds. Materials. 2023; 16(8):3004. https://doi.org/10.3390/ma16083004
Chicago/Turabian StyleFathy, Naglaa, Mohamed Ramadan, Khalid M. Hafez, Fahad Abdulaziz, Badreddine Ayadi, and Abdulaziz S. Alghamdi. 2023. "A Novel Approach of Optimum Time Interval Estimation for Al-7.5Si/Al-18Si Liquid–Liquid Bimetal Casting in Sand and Metallic Moulds" Materials 16, no. 8: 3004. https://doi.org/10.3390/ma16083004
APA StyleFathy, N., Ramadan, M., Hafez, K. M., Abdulaziz, F., Ayadi, B., & Alghamdi, A. S. (2023). A Novel Approach of Optimum Time Interval Estimation for Al-7.5Si/Al-18Si Liquid–Liquid Bimetal Casting in Sand and Metallic Moulds. Materials, 16(8), 3004. https://doi.org/10.3390/ma16083004








