Lead-Free Ternary Glass for Radiation Protection: Composition and Performance Evaluation for Solar Cell Coverage
Abstract
:1. Introduction
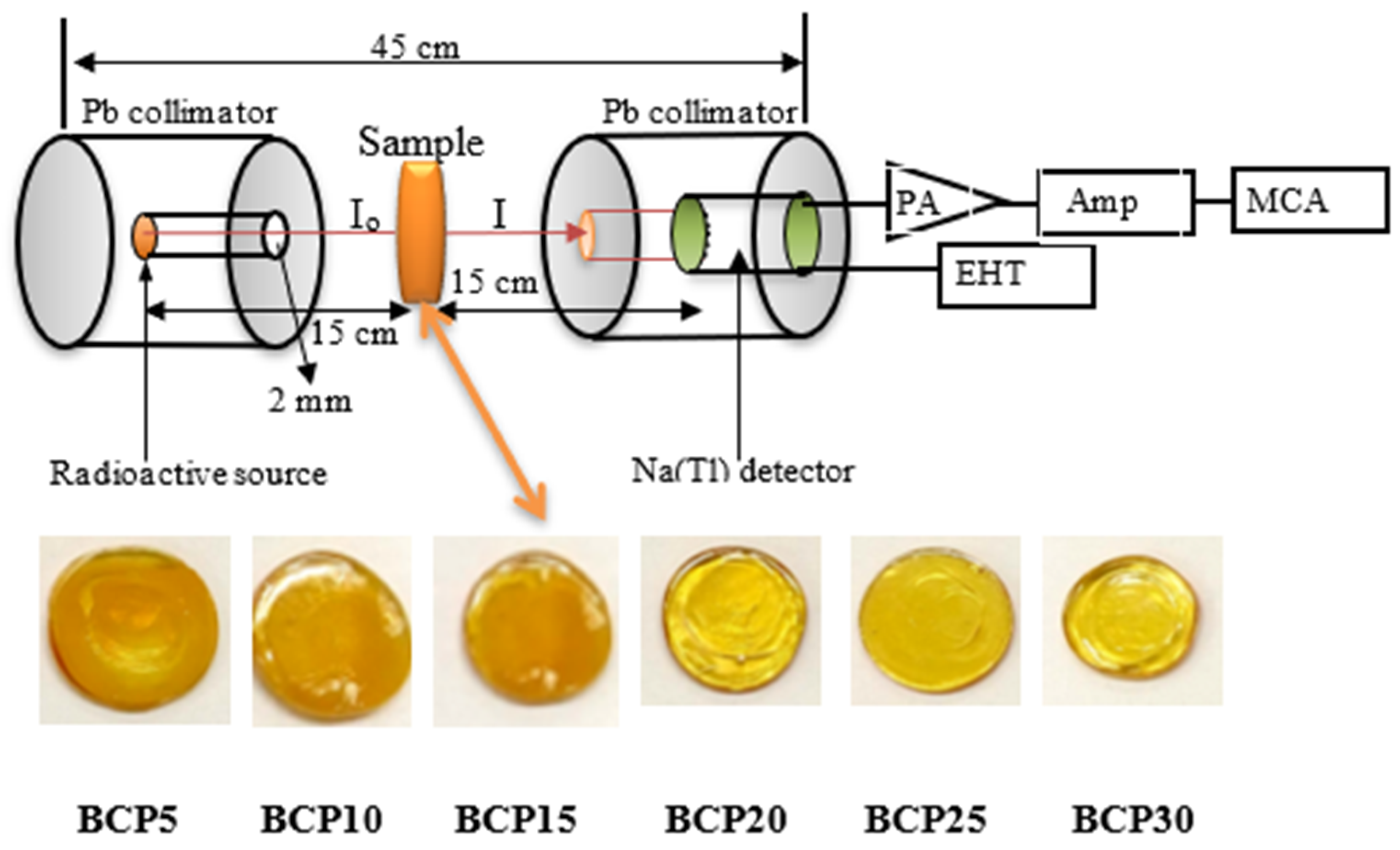
2. Experimental Procedure
3. Results and Discussions
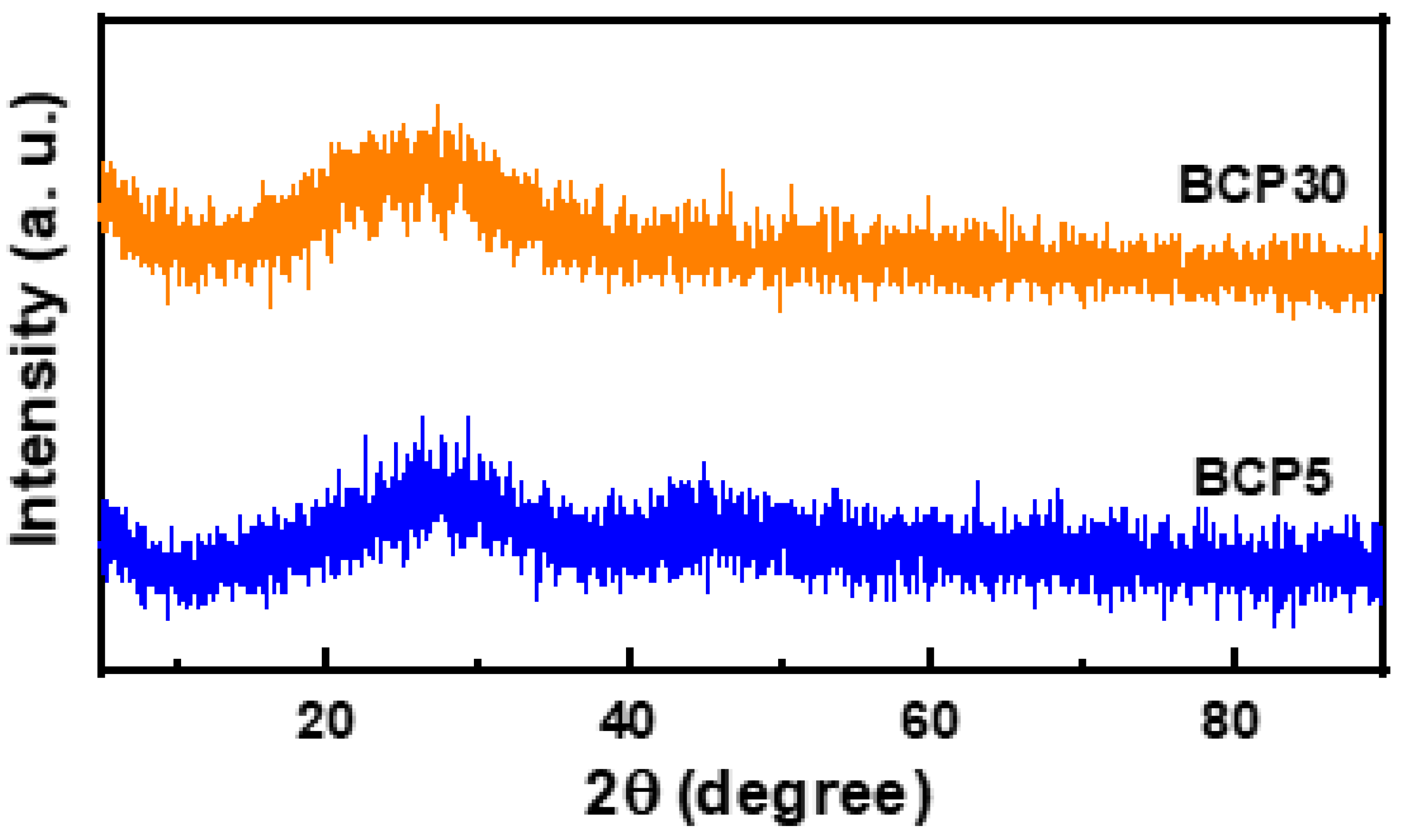
3.1. Structural Properties
3.2. Physical Properties
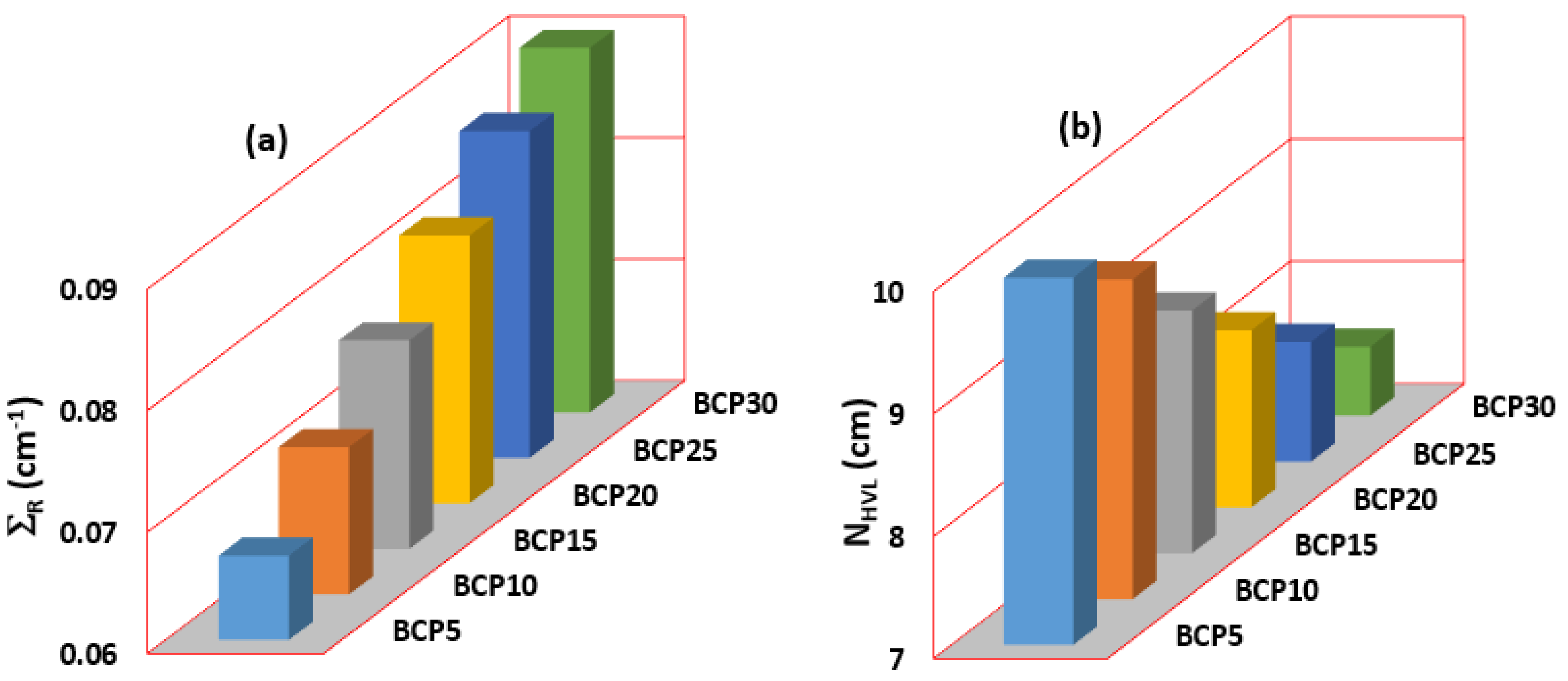
3.3. Shielding Parameter
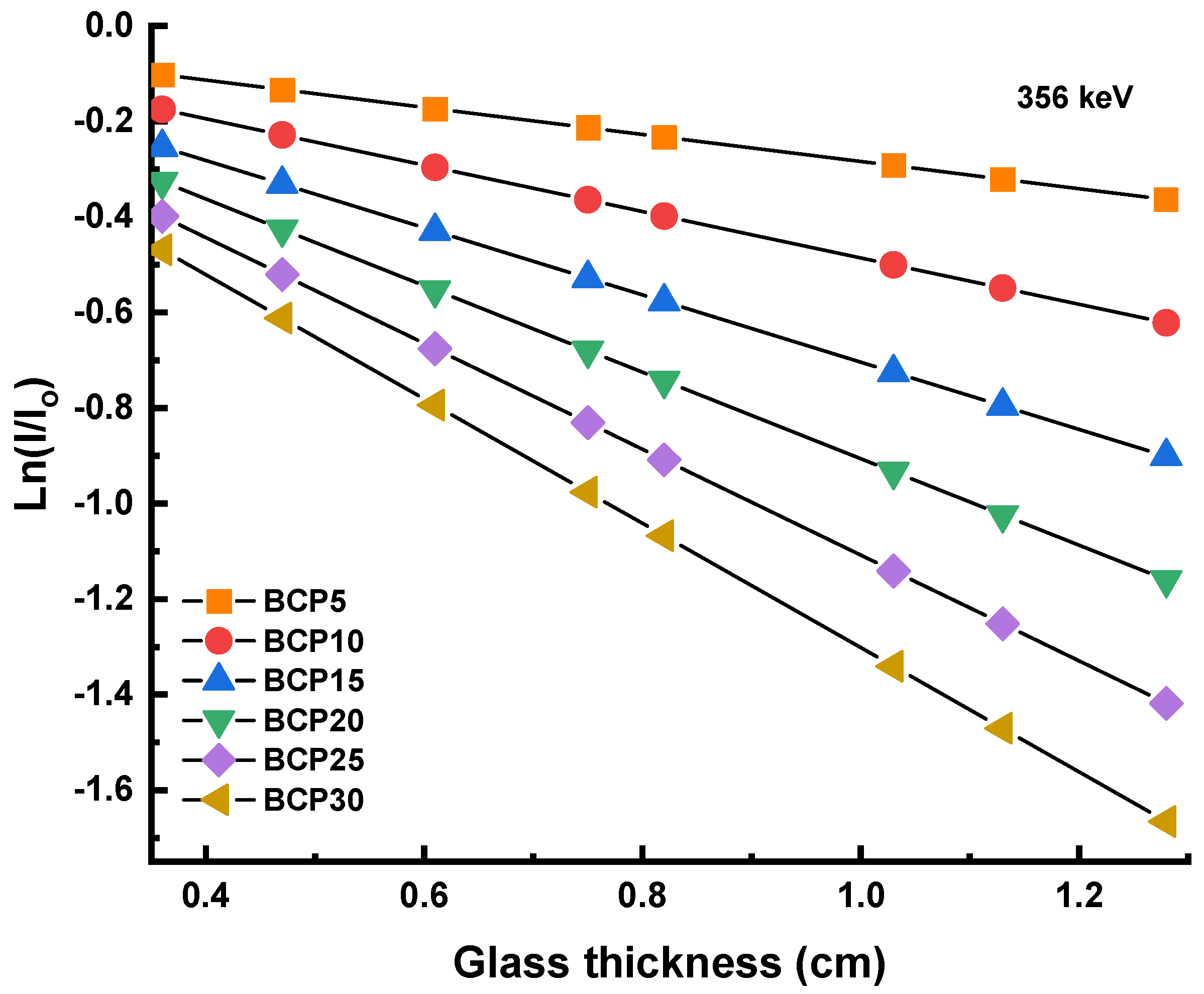
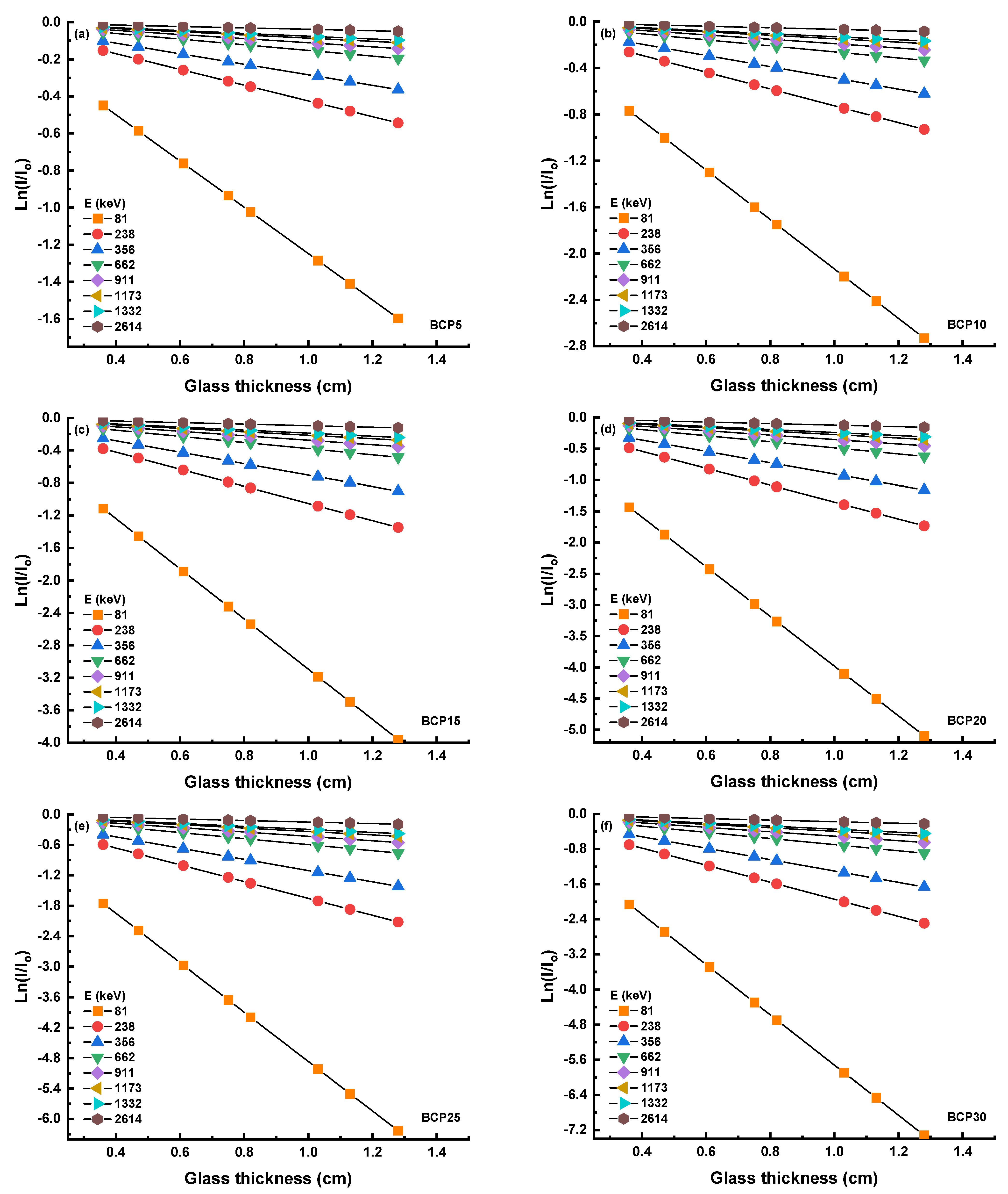
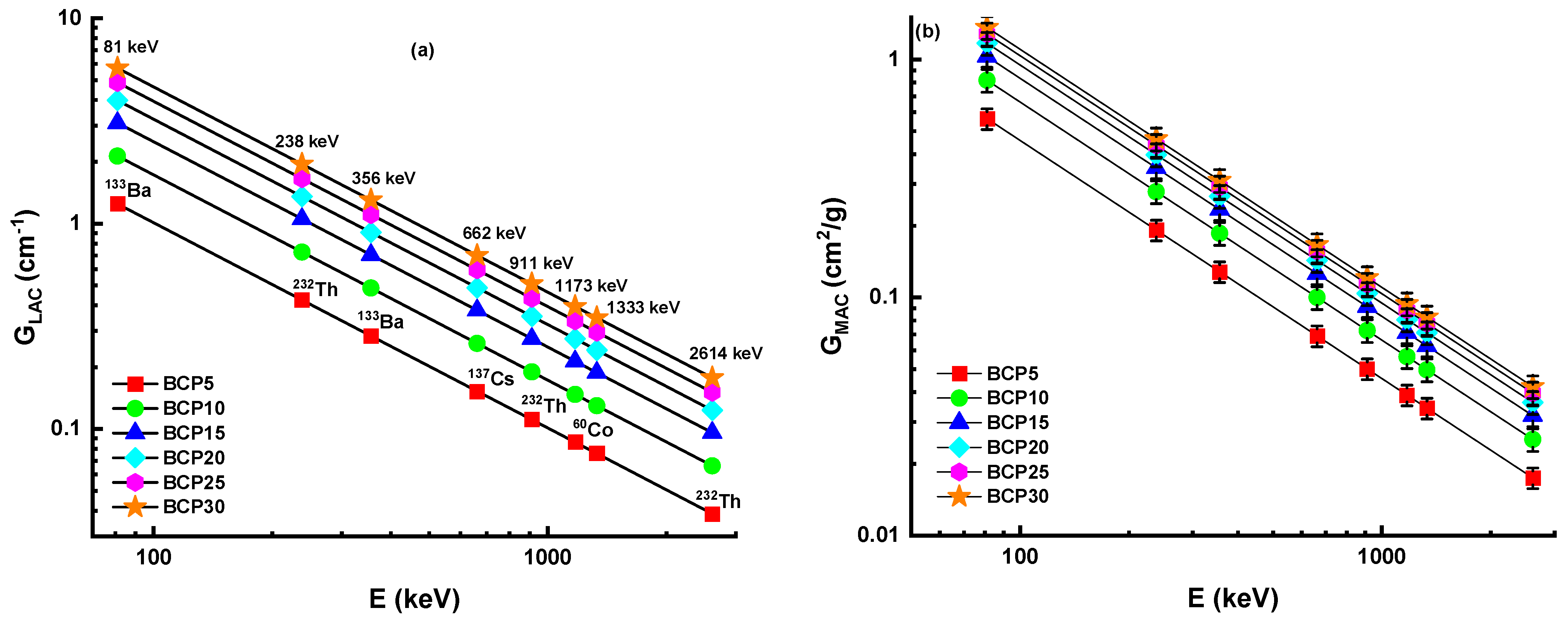
3.3.1. Linear and Mass Attenuation Coefficient
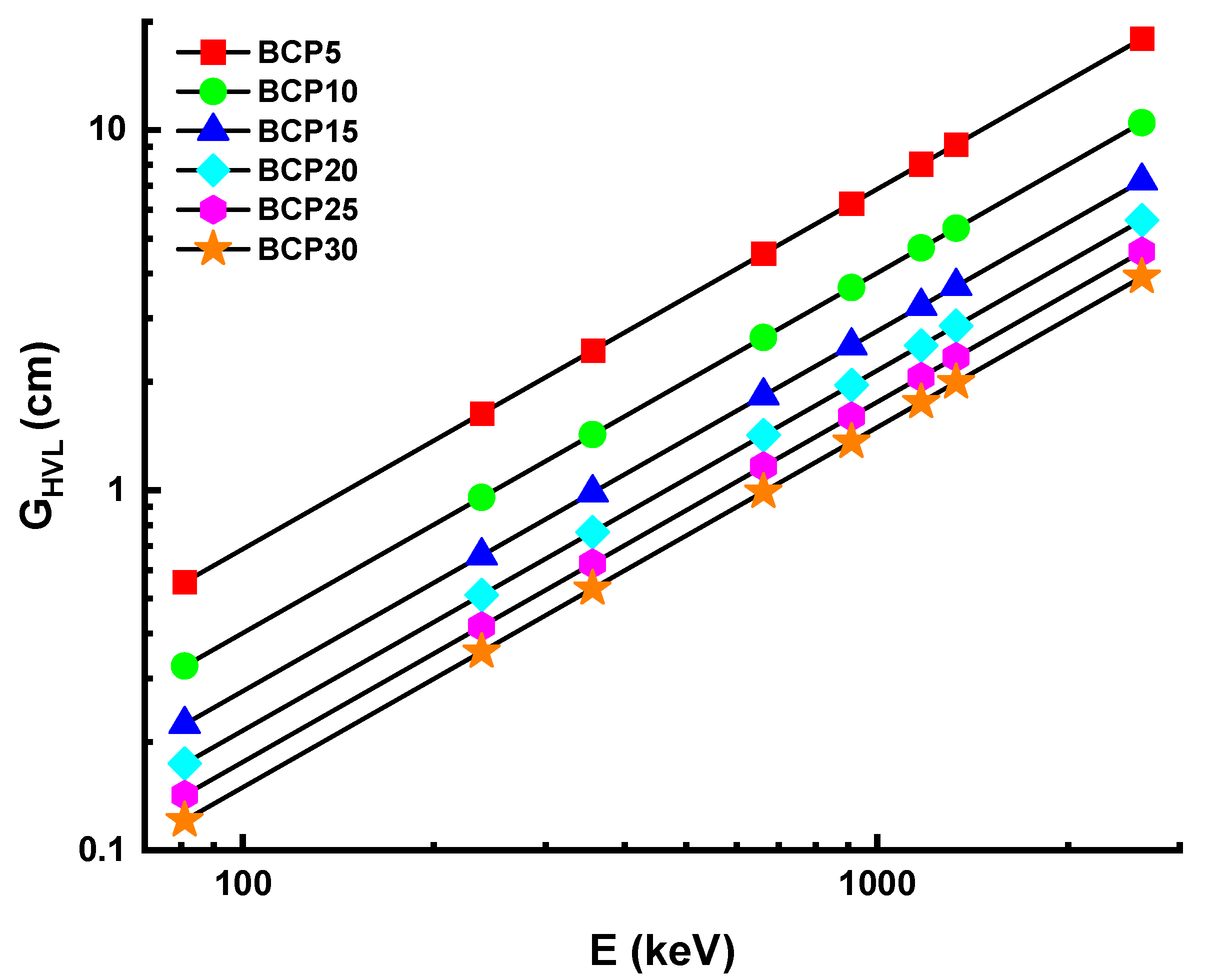
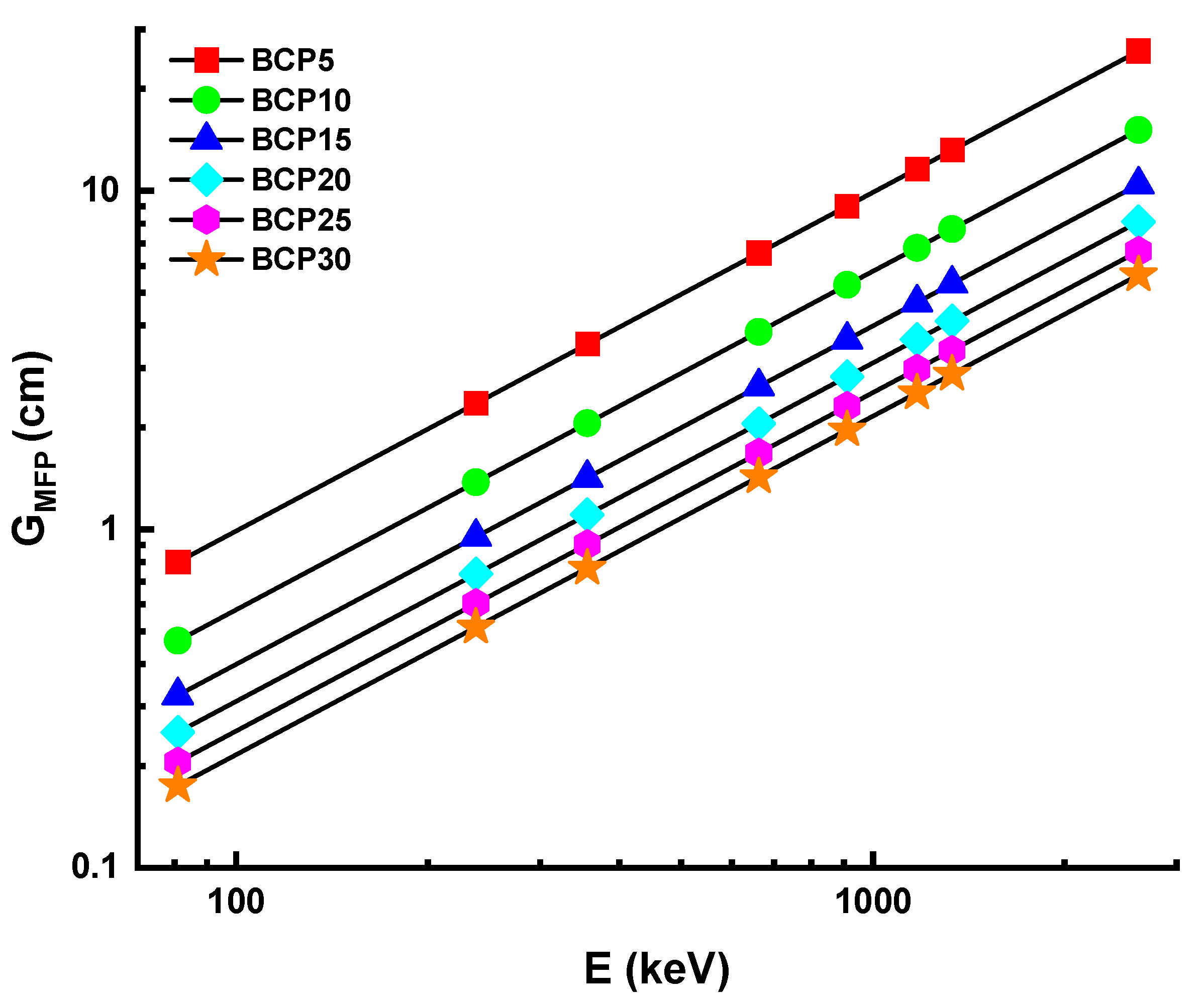
3.3.2. Half-Value Layer and Mean-Free Path
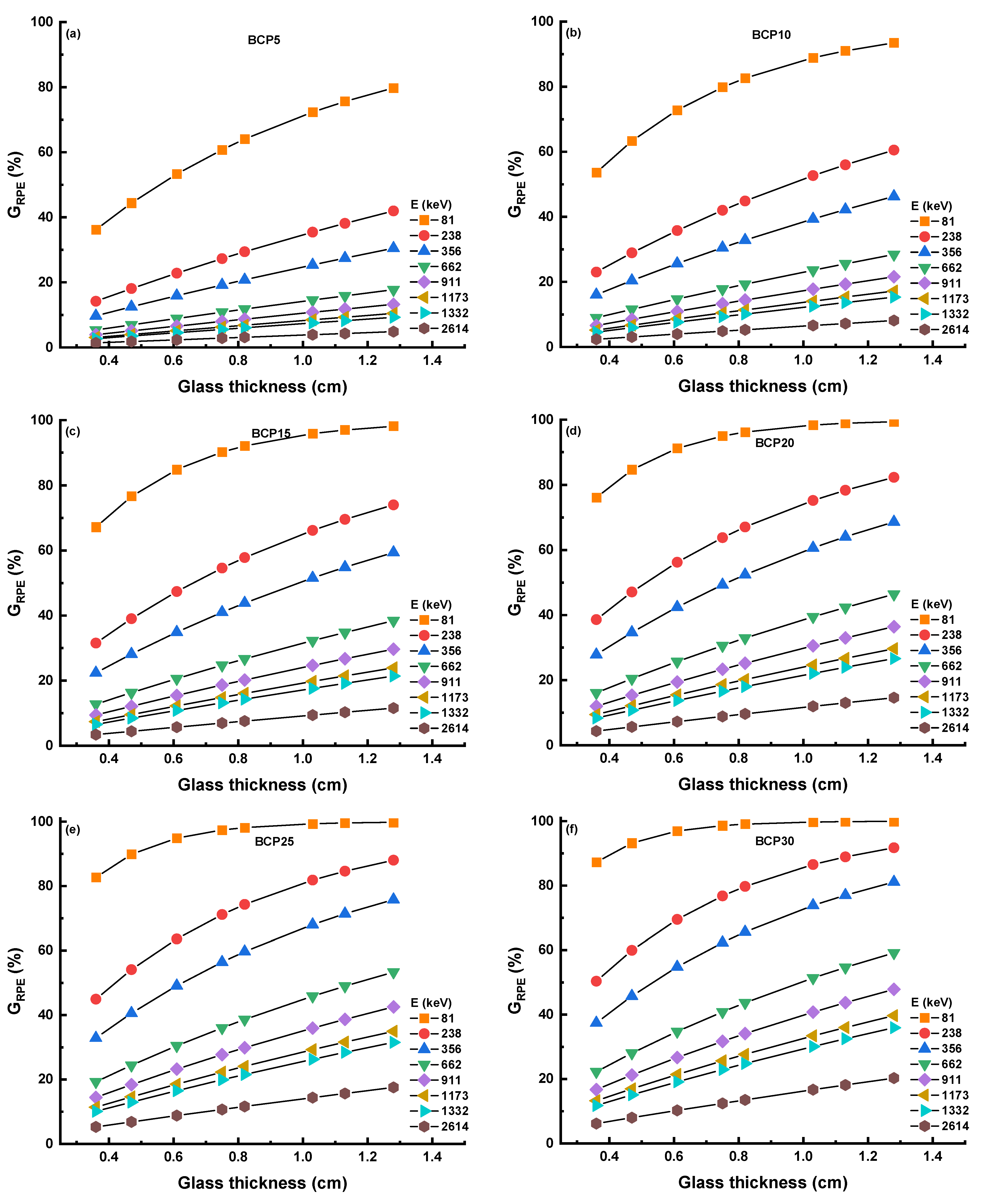
3.3.3. Radiation Protection Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Denglawey, A.; Zakaly, H.M.H.; Alshammari, K.; Issa, S.A.M.; Tekin, H.O.; AbuShanab, W.S.; Saddeek, Y.B. Prediction of mechanical and radiation parameters of glasses with high Bi2O3 concentration. Results Phys. 2021, 21, 103839. [Google Scholar] [CrossRef]
- Elazaka, A.I.; Zakaly, H.M.H.; Issa, S.A.M.; Rashad, M.; Tekin, H.O.; Saudi, H.A.; Gillette, V.H.; Erguzel, T.T.; Mostafa, A.G. New approach to removal of hazardous Bypass Cement Dust (BCD) from the environment: 20Na2O-20BaCl2-(60-x)B2O3-(x)BCD glass system and Optical, mechanical, structural and nuclear radiation shielding competences. J. Hazard. Mater. 2021, 403, 123738. [Google Scholar] [CrossRef] [PubMed]
- Elshami, W.; Tekin, H.O.; Issa, S.A.M.; Abuzaid, M.M.; Zakaly, H.M.H.; Issa, B.; Ene, A. Impact of Eye and Breast Shielding on Organ Doses During Cervical Spine Radiography: Design and Validation of MIRD Computational Phantom. Front. Public Health 2021, 9, 1580. [Google Scholar] [CrossRef]
- Boodaghi Malidarre, R.; Akkurt, I.; Ekmekci, I.; Zakaly, H.M.H.; Mohammed, H. The role of La2O3 rare earth (RE) material in the enhancement of the radiation shielding, physical, mechanical and acoustic properties of the tellurite glasses. Radiat. Eff. Defects Solids 2022. [Google Scholar] [CrossRef]
- Khanna, A.; Bhatti, S.S.; Singh, K.J.; Thind, K.S. Gamma-ray attenuation coefficients in some heavy metal oxide borate glasses at 662 keV. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1996, 114, 217–220. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Zakaly, H.M.H.; Issa, S.A.M.; Rashad, M.; Pyshkina, M.; Tekin, H.O.; El-Mallawany, R.; Mostafa, M.Y.A. Synthesis, physical, optical, mechanical, and radiation attenuation properties of TiO2–Na2O–Bi2O3–B2O3 glasses. Ceram. Int. 2020, 47, 185–204. [Google Scholar] [CrossRef]
- Singh, K.J.; Kaur, S.; Kaundal, R.S. Comparative study of gamma ray shielding and some properties of PbO-SiO2-Al2O3 and Bi2O3-SiO2-Al2O3 glass systems. Radiat. Phys. Chem. 2014, 96, 153–157. [Google Scholar] [CrossRef]
- Mostafa, A.M.A.; Zakaly, H.M.H.; Pyshkina, M.; Issa, S.A.M.; Tekin, H.O.; Sidek, H.A.A.; Matori, K.A.; Zaid, M.H.M. Multi-objective optimization strategies for radiation shielding performance of BZBB glasses using Bi2O3: A FLUKA Monte Carlo code calculations. J. Mater. Res. Technol. 2020, 9, 12335–12345. [Google Scholar] [CrossRef]
- Ardelean, I.; Cora, S.; Ciceo Lucacel, R.; Hulpus, O. EPR and FT-IR spectroscopic studies of B2O3Bi2O3MnO glasses. Solid State Sci. 2005, 7, 1438–1442. [Google Scholar] [CrossRef]
- Rashad, M.; Saudi, H.A.; Zakaly, H.M.H.; Issa, S.A.M.; Abd-Elnaiem, A.M. Control optical characterizations of Ta+5–doped B2O3–Si2O–CaO–BaO glasses by irradiation dose. Opt. Mater. 2021, 112, 110613. [Google Scholar] [CrossRef]
- Liu, W.; Sanz, J.; Pecharromán, C.; Sobrados, I.; Lopez-Esteban, S.; Torrecillas, R.; Wang, D.-Y.; Moya, J.S.; Cabal, B. Synthesis, characterization and applications of low temperature melting glasses belonging to P2O5CaO Na2O system. Ceram. Int. 2019, 45, 12234–12242. [Google Scholar] [CrossRef]
- Chromčíková, M.; Hruška, B.; Nowicka, A.; Svoboda, R.; Liška, M. Role of modifiers in the structural interpretation of the glass transition behavior in MgO/BaO-Al2O3-P2O5 glasses. J. Non-Cryst. Solids 2021, 573, 121114. [Google Scholar] [CrossRef]
- Kucuk, N.; Manohara, S.R.; Hanagodimath, S.M.; Gerward, L. Modeling of gamma ray energy-absorption buildup factors for thermoluminescent dosimetric materials using multilayer perceptron neural network: A comparative study. Radiat. Phys. Chem. 2013, 86, 10–22. [Google Scholar] [CrossRef]
- Un, A.; Demir, F. Determination of mass attenuation coefficients, effective atomic numbers and effective electron numbers for heavy-weight and normal-weight concretes. Appl. Radiat. Isot. 2013, 80, 73–77. [Google Scholar] [CrossRef]
- Bagheri, R.; Moghaddam, A.K.; Shirmardi, S.P.; Azadbakht, B.; Salehi, M. Determination of gamma-ray shielding properties for silicate glasses containing Bi2O3, PbO, and BaO. J. Non-Cryst. Solids 2018, 479, 62–71. [Google Scholar] [CrossRef]
- Gaikwad, D.K.; Pawar, P.P.; Selvam, T.P. Mass attenuation coefficients and effective atomic numbers of biological compounds for gamma ray interactions. Radiat. Phys. Chem. 2017, 138, 75–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Hao, Z.; Yang, A.; Xu, H.; Jiang, F. Investigation of the P2O5–Bi2O3–CaO system: Glass forming region, structure, properties. J. Non-Cryst. Solids 2023, 600, 122022. [Google Scholar] [CrossRef]
- Chaitanya, Y.; Yusub, S.; Ramesh Babu, A.; Aruna, V.; Sree Ram, N.; Linga Raju, C. Impact of copper ions on physical, structural, spectroscopic, and dielectric properties of Bi2O3–CaO–P2O5–B2O3 glasses. Mater. Chem. Phys. 2022, 290, 126584. [Google Scholar] [CrossRef]
- Elqahtani, Z.M.; Sayyed, M.I.; Kumar, A.; Jecong, J.F.M.; Almuqrin, A.H. Impact of Bi2O3 on optical properties and radiation attenuation characteristics of Bi2O3-Li2O-P2O5 glasses. Optik 2021, 248, 168081. [Google Scholar] [CrossRef]
- Li, X.; Tao, X.; Xia, Y.; Luo, M.; Zeng, X.; Shi, J.; Xiao, Z.; Kong, L.B. Preparation and characterization of glassy waste forms based on SrF2-Fe2O3-PbO/Bi2O3-P2O5 system. J. Non-Cryst. Solids 2022, 581, 121303. [Google Scholar] [CrossRef]
- Rachniyom, W.; Chaiphaksa, W.; Limkitjaroeanporn, P.; Tuschaoen, S.; Sangwaranatee, N.; Kaewkhao, J. Effect of Bi2O3 on radiation shielding properties of glasses from coal fly ash. Mater. Today Proc. 2018, 5, 14046–14051. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Abulyazied, D.E.; Alrowaily, A.W.; Saudi, H.A.; Ali, E.S.; Henaish, A.M.A.; Zakaly, H.M.H. Improving the electrical, optical and radiation shielding properties of polyvinyl alcohol yttrium oxide composites. J. Rare Earths 2023, in press. [Google Scholar] [CrossRef]
- Abulyazied, D.E.; Issa, S.A.M.; Alrowaily, A.W.; Saudi, H.A.; Zakaly, H.M.H.; Ali, E.S. Polylactic acid tungsten trioxide reinforced composites: A study of their thermal, optical, and gamma radiation attenuation performance. Radiat. Phys. Chem. 2023, 205, 110705. [Google Scholar] [CrossRef]
- Gaafar, M.S.; Shaarany, I.; Alharbi, T. Structural investigations on some cadmium-borotellurate glasses using ultrasonic, FT-IR and X-ray techniques. J. Alloys Compd. 2014, 616, 625–632. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Mostafa, A.M.A. Effect of Bi2O3 in borate-tellurite-silicate glass system for development of gamma-rays shielding materials. J. Alloys Compd. 2017, 695, 302–331. [Google Scholar] [CrossRef]
- Bagheri, R.; Khorrami Moghaddam, A.; Yousefnia, H. Gamma Ray Shielding Study of Barium–Bismuth–Borosilicate Glasses as Transparent Shielding Materials using MCNP-4C Code, XCOM Program, and Available Experimental Data. Nucl. Eng. Technol. 2017, 49, 216–223. [Google Scholar] [CrossRef]
- Çelikbilek Ersundu, M.; Ersundu, A.E.; Sayyed, M.I.; Lakshminarayana, G.; Aydin, S. Evaluation of physical, structural properties and shielding parameters for K2O–WO3–TeO2 glasses for gamma ray shielding applications. J. Alloys Compd. 2017, 714, 278–286. [Google Scholar] [CrossRef]
- Aktas, B.; Yalcin, S.; Dogru, K.; Uzunoglu, Z.; Yilmaz, D. Structural and radiation shielding properties of chromium oxide doped borosilicate glass. Radiat. Phys. Chem. 2019, 156, 144–149. [Google Scholar] [CrossRef]
- Yalcin, S.; Aktas, B.; Yilmaz, D. Radiation shielding properties of Cerium oxide and Erbium oxide doped obsidian glass. Radiat. Phys. Chem. 2019, 160, 83–88. [Google Scholar] [CrossRef]
- Mhareb, M.H.A.; Alajerami, Y.S.M.; Sayyed, M.I.; Dwaikat, N.; Alqahtani, M.; Alshahri, F.; Saleh, N.; Alonizan, N.; Ghrib, T.; Al-Dhafar, S.I. Radiation shielding, structural, physical, and optical properties for a series of borosilicate glass. J. Non-Cryst. Solids 2020, 550, 120360. [Google Scholar] [CrossRef]
- Al-Yousef, H.A.; Sayyed, M.I.; Alotiby, M.; Kumar, A.; Alghamdi, Y.S.; Alotaibi, B.M.; Alsaif, N.A.M.; Mahmoud, K.A.; Al-Hadeethi, Y. Evaluation of optical, and radiation shielding features of New phosphate-based glass system. Optik 2021, 242, 167220. [Google Scholar] [CrossRef]
- Al-Harbi, F.F.; Prabhu, N.S.; Sayyed, M.I.; Almuqrin, A.H.; Kumar, A.; Kamath, S.D. Evaluation of structural and gamma ray shielding competence of Li2O-K2O-B2O3-HMO (HMO = SrO/TeO2/PbO/Bi2O3) glass system. Optik 2021, 248, 168074. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, R.; Rani, S.; Sidhu, B.S. Physical, structural and nuclear radiation shielding behaviour of xBaO-(0.30-x)MgO-0.10Na2O-0.10Al2O3-0.50B2O3 glass matrix. Mater. Chem. Phys. 2022, 276, 125415. [Google Scholar] [CrossRef]
- Bashter, I.I. Calculation of radiation attenuation coefficients for shielding concretes. Ann. Nucl. Energy 1997, 24, 1389–1401. [Google Scholar] [CrossRef]
- Özkalaycı, F.; Kaçal, M.R.; Agar, O.; Polat, H.; Sharma, A.; Akman, F. Lead(II) chloride effects on nuclear shielding capabilities of polymer composites. J. Phys. Chem. Solids 2020, 145, 109543. [Google Scholar] [CrossRef]
- Ozel, F.; Akman, F.; Kaçal, M.R.; Ozen, A.; Arslan, H.; Polat, H.; Yurtcan, S.; Agar, O. Production of microstructured BaZrO3 and Ba2P2O7-based polymer shields for protection against ionizing photons. J. Phys. Chem. Solids 2021, 158, 110238. [Google Scholar] [CrossRef]
- Tekin, H.O.; Kaçal, M.R.; Issa, S.A.M.; Polat, H.; Susoy, G.; Akman, F.; Kilicoglu, O.; Gillette, V.H. Sodium dodecatungstophosphate hydrate-filled polymer composites for nuclear radiation shielding. Mater. Chem. Phys. 2020, 256, 123667. [Google Scholar] [CrossRef]
- Akman, F.; Ogul, H.; Ozkan, I.; Kaçal, M.R.; Agar, O.; Polat, H.; Dilsiz, K. Study on gamma radiation attenuation and non-ionizing shielding effectiveness of niobium-reinforced novel polymer composite. Nucl. Eng. Technol. 2021, 54, 283–292. [Google Scholar] [CrossRef]
- Falahatkar Gashti, M.; Hosein Ghasemzadeh Mousavinejad, S.; Jalal Khaleghi, S. Evaluation of gamma and neutron radiation shielding properties of the GGBFS based geopolymer concrete. Constr. Build. Mater. 2023, 367, 130308. [Google Scholar] [CrossRef]

| Elements | BCP5 | BCP10 | BCP15 | BCP20 | BCP25 | BCP30 |
|---|---|---|---|---|---|---|
| Bi | 0.163149 | 0.281292 | 0.370796 | 0.440947 | 0.497411 | 0.543837 |
| O | 0.43717 | 0.38764 | 0.350117 | 0.320707 | 0.297035 | 0.277572 |
| Ca | 0.10951 | 0.080919 | 0.059259 | 0.042282 | 0.028618 | 0.017383 |
| P | 0.290171 | 0.250148 | 0.219828 | 0.196063 | 0.176936 | 0.161208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uosif, M.A.M.; Issa, S.A.M.; Ene, A.; Mostafa, A.M.A.; Atta, A.; El Agammy, E.F.; Zakaly, H.M.H. Lead-Free Ternary Glass for Radiation Protection: Composition and Performance Evaluation for Solar Cell Coverage. Materials 2023, 16, 3036. https://doi.org/10.3390/ma16083036
Uosif MAM, Issa SAM, Ene A, Mostafa AMA, Atta A, El Agammy EF, Zakaly HMH. Lead-Free Ternary Glass for Radiation Protection: Composition and Performance Evaluation for Solar Cell Coverage. Materials. 2023; 16(8):3036. https://doi.org/10.3390/ma16083036
Chicago/Turabian StyleUosif, Mohamed A. M., Shams A. M. Issa, Antoaneta Ene, Ahmed M. A. Mostafa, Ali Atta, Emam F. El Agammy, and Hesham M. H. Zakaly. 2023. "Lead-Free Ternary Glass for Radiation Protection: Composition and Performance Evaluation for Solar Cell Coverage" Materials 16, no. 8: 3036. https://doi.org/10.3390/ma16083036
APA StyleUosif, M. A. M., Issa, S. A. M., Ene, A., Mostafa, A. M. A., Atta, A., El Agammy, E. F., & Zakaly, H. M. H. (2023). Lead-Free Ternary Glass for Radiation Protection: Composition and Performance Evaluation for Solar Cell Coverage. Materials, 16(8), 3036. https://doi.org/10.3390/ma16083036








