One-Step Solid-State Synthesis of Ni-Rich Cathode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis Procedures
2.2. Electrochemical Measurements
2.3. Material Characterization
3. Results and Discussion
3.1. Impact of Synthesis Conditions on the Electrochemical Performance
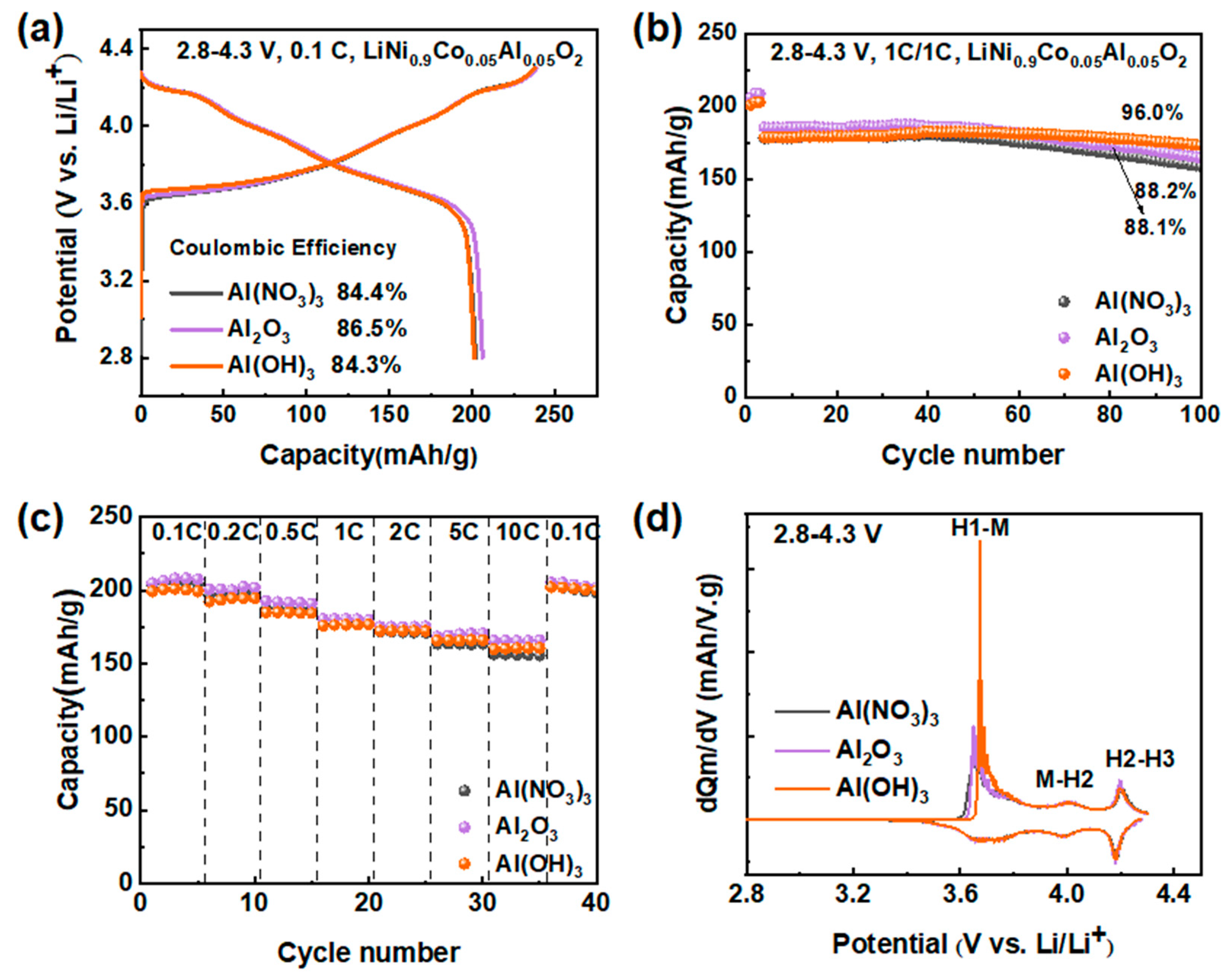
3.1.1. Impact of Aluminum Precursors
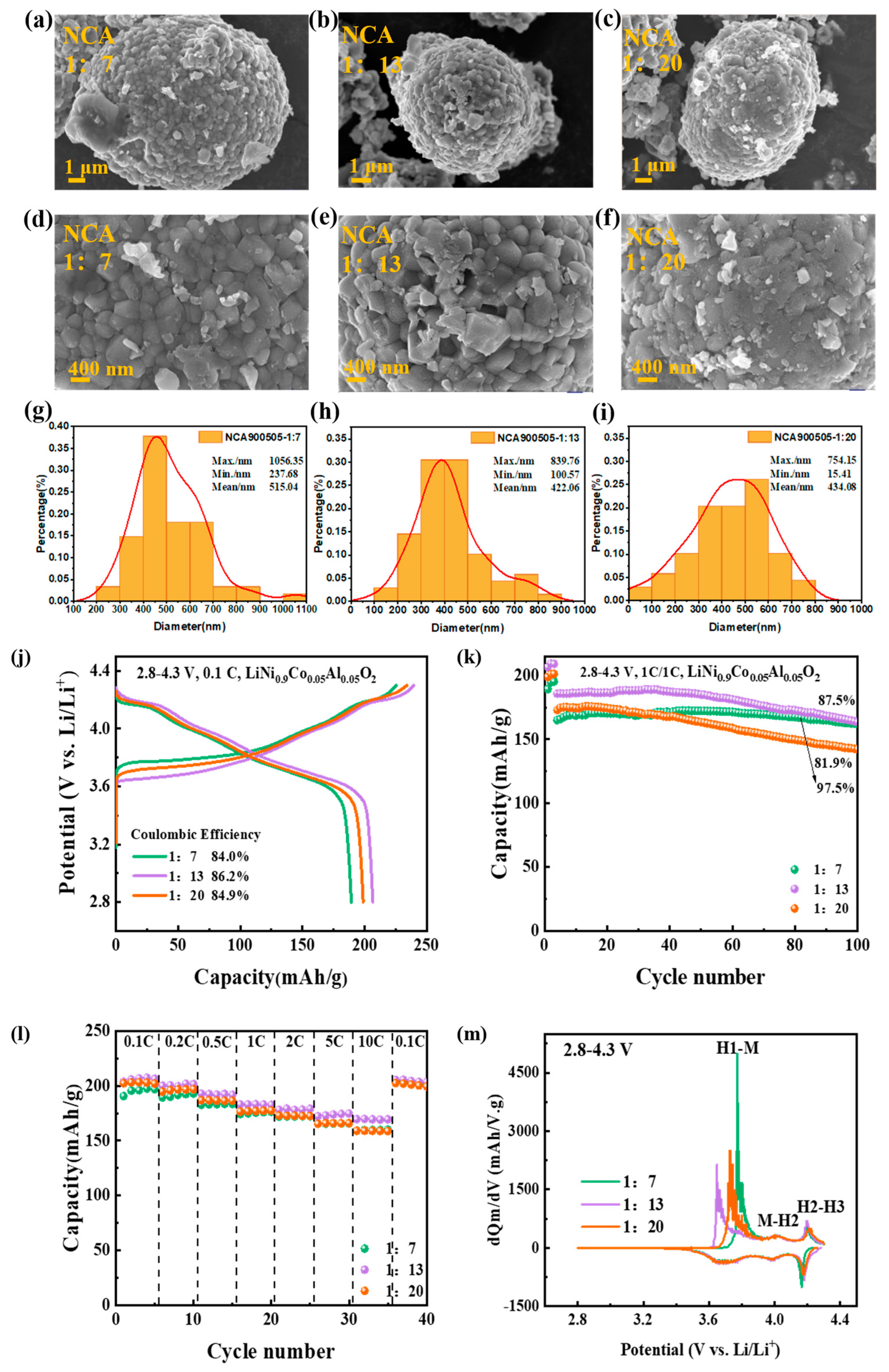
3.1.2. Impact of Ball Material Ratio
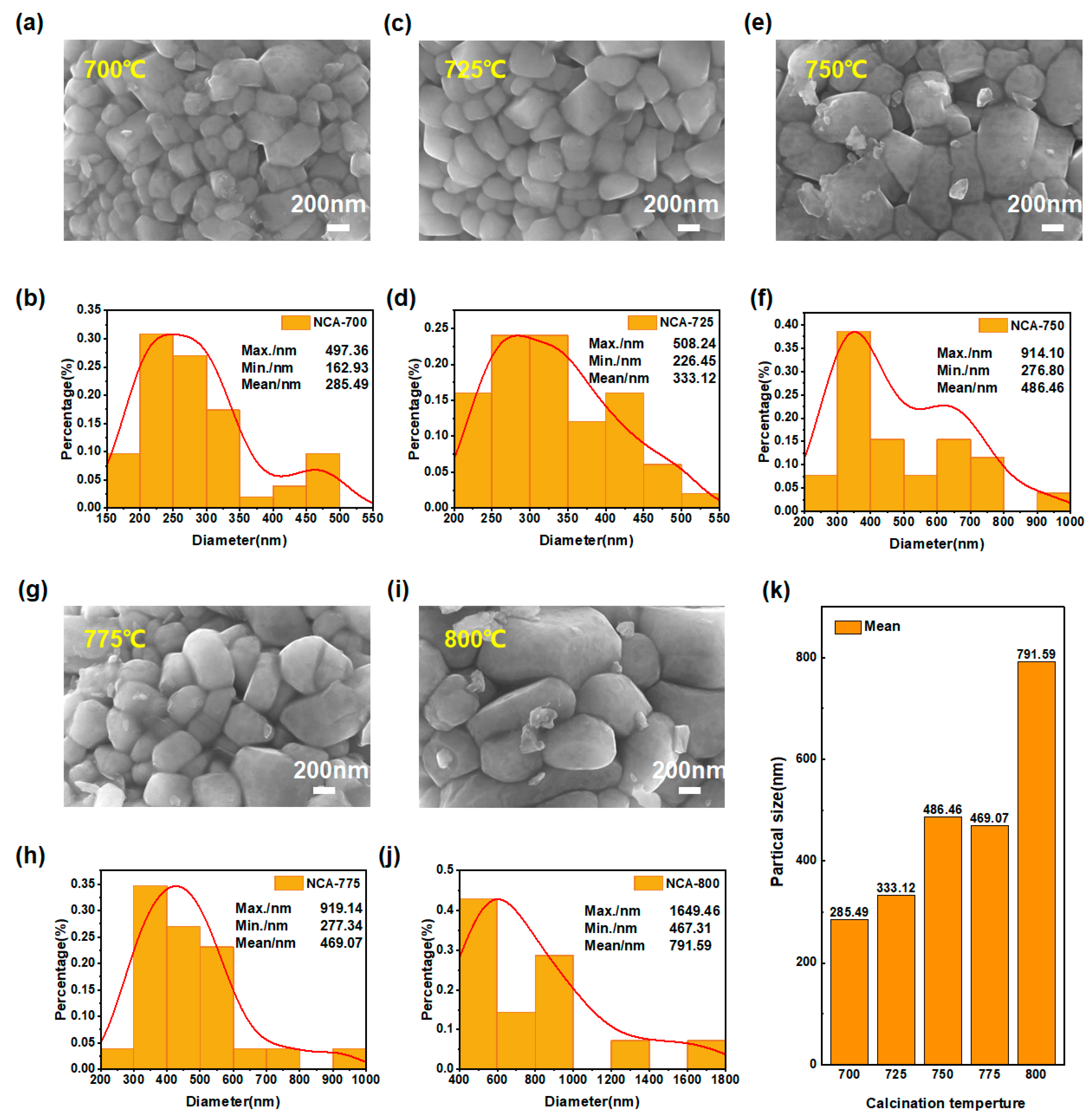
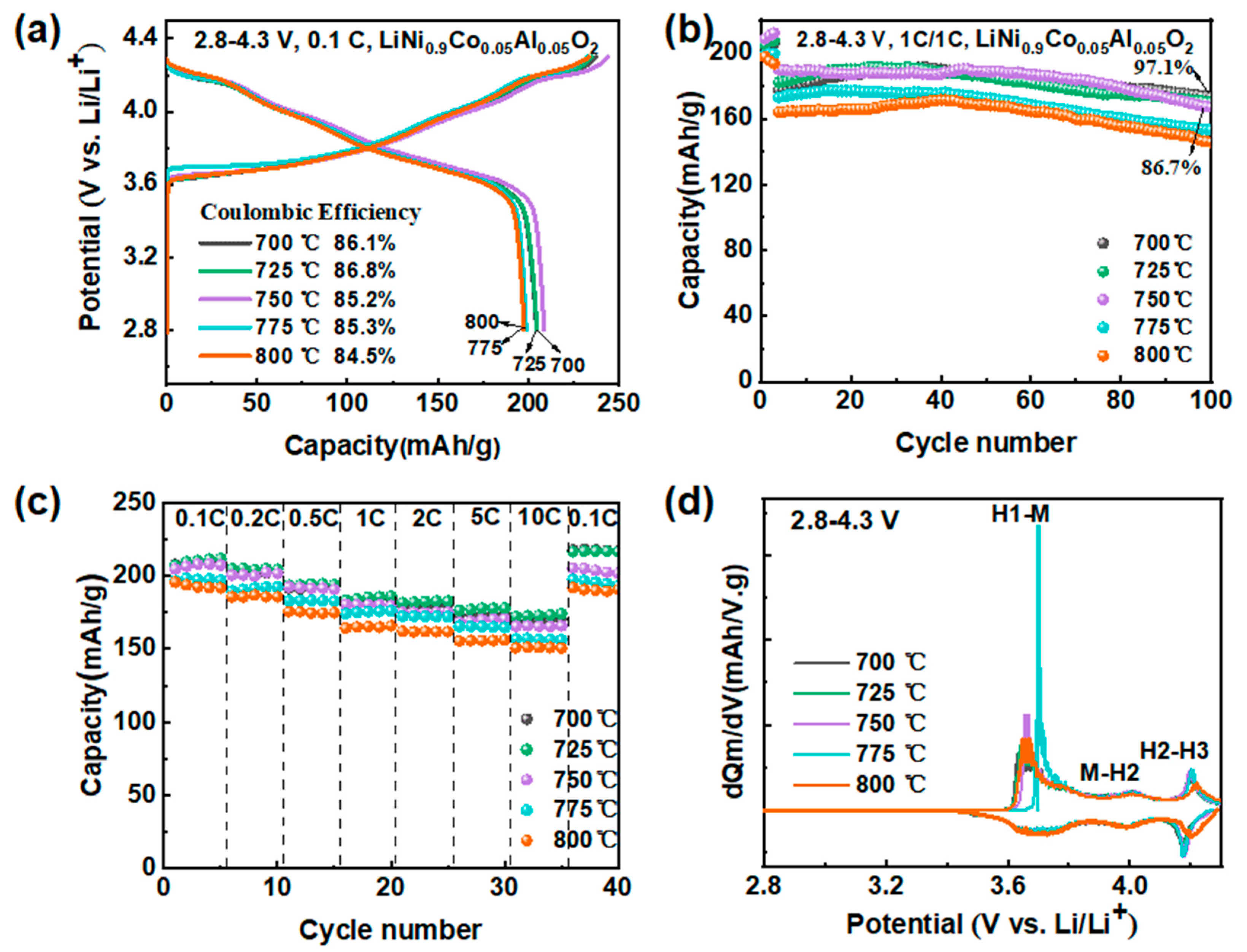
3.1.3. Impact of Calcination Temperature
3.2. Phase Composition and Surface Morphology of As-Prepared Cathode Materials
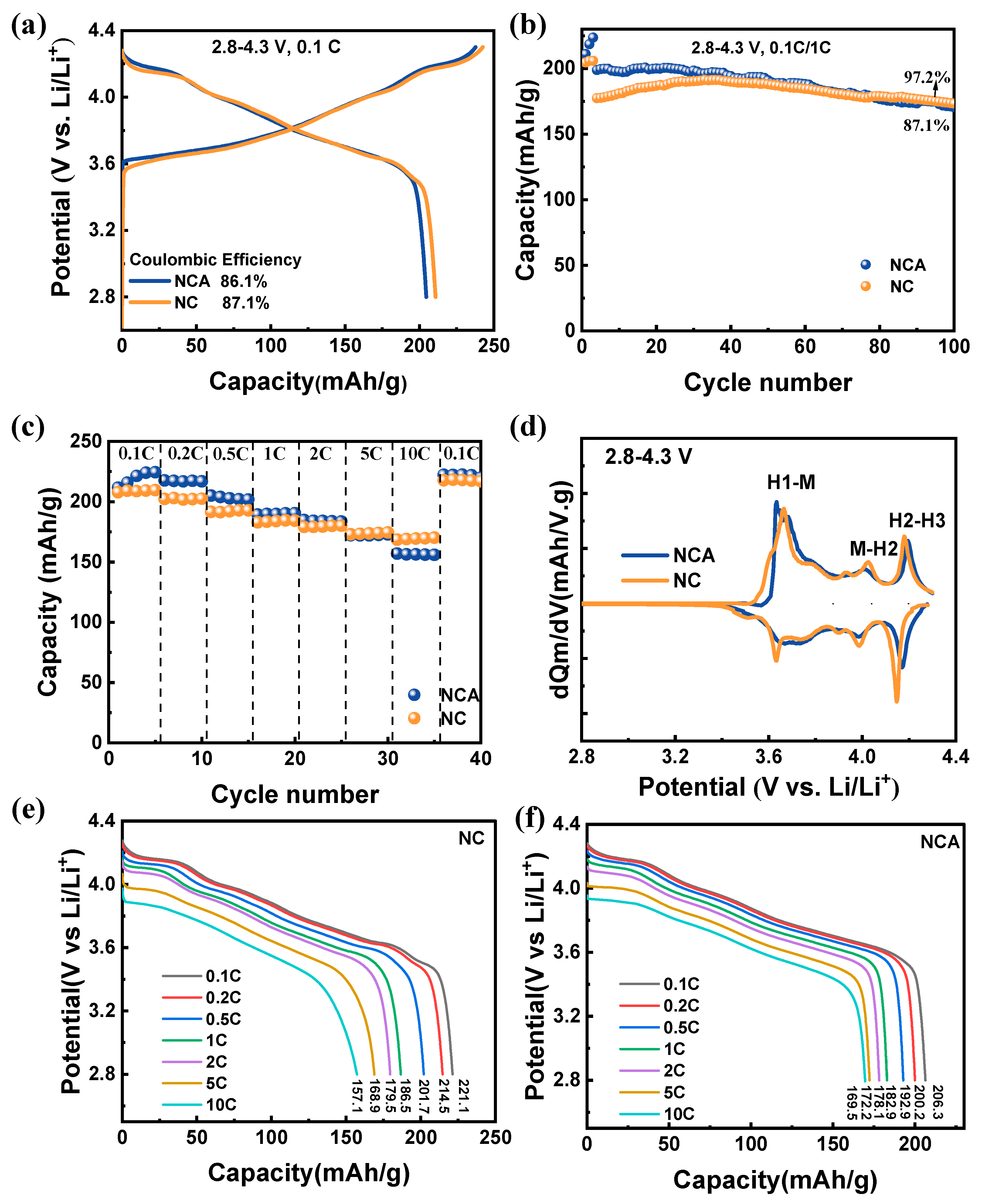
3.3. Enhanced Electrochemical Performance via the One-Step Solid-State Method
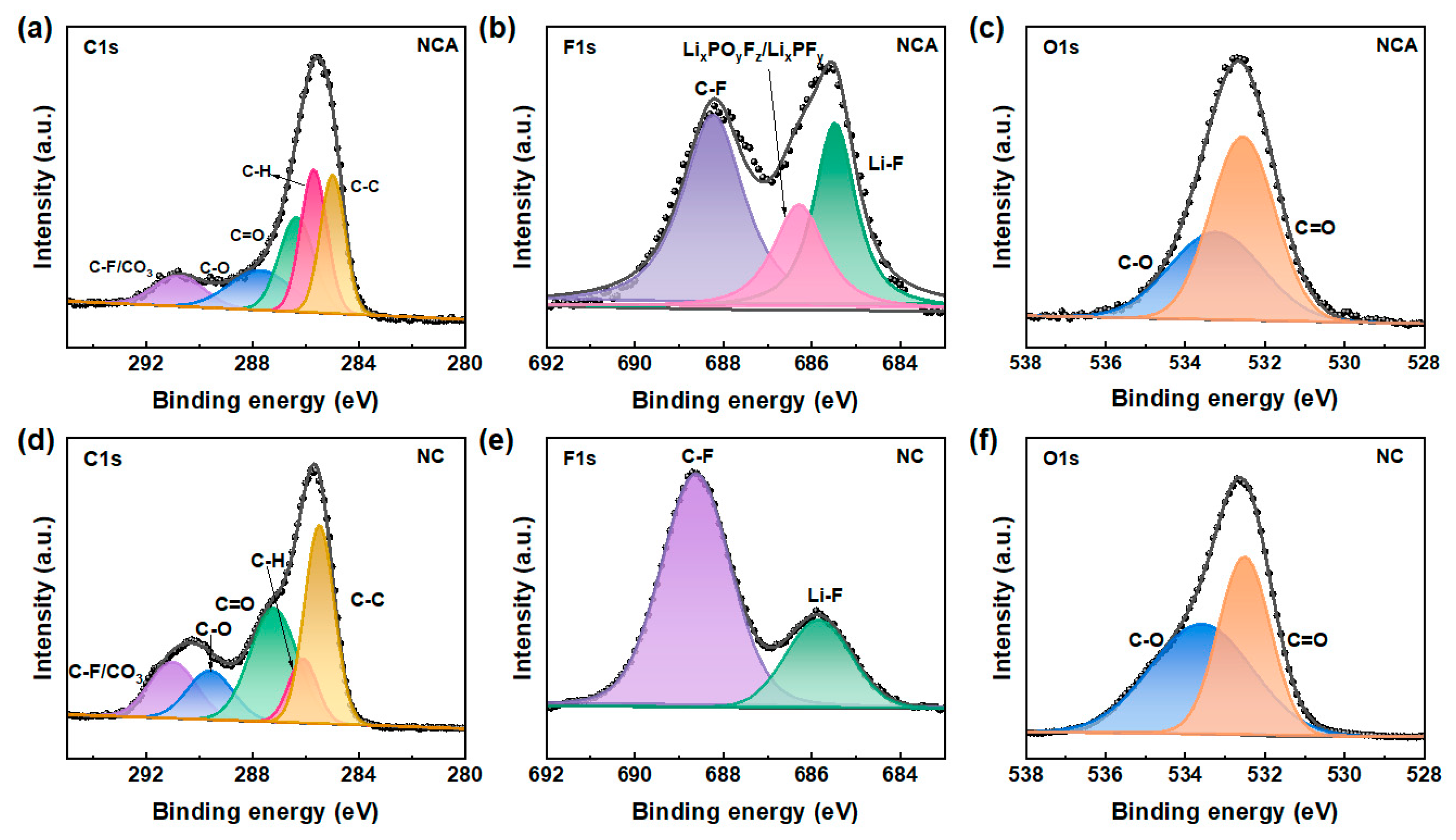
3.4. Phase Transition and Impedance Stabilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Biasi, L.; Schwarz, B.; Brezesinski, T.; Hartmann, P.; Janek, J.; Ehrenberg, H. Chemical, structural, and electronic aspects of formation and degradation behavior on different length scales of Ni-rich NCM and Li-rich HE-NCM cathode materials in Li-ion batteries. Adv. Mater. 2019, 31, 1900985. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, T.; Zhang, Q.; Zheng, M.; Xu, K.; Yan, W. Enhanced electrochemical performance of La and F co-modified Ni-rich cathode. Ionics 2020, 26, 1165–1171. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Croguennec, L.; Palacin, M.R. Recent achievements on inorganic electrode materials for lithium-ion batteries. J. Am. Chem. Soc. 2015, 137, 3140–3156. [Google Scholar] [CrossRef]
- Nam, G.W.; Park, N.Y.; Park, K.J.; Yang, J.; Liu, J.; Yoon, C.S.; Sun, Y.K. Capacity fading of Ni-rich NCA cathodes: Effect of microcracking extent. ACS Energy Lett. 2019, 4, 2995–3001. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Lee, Y.M.; Nam, K.M.; Hwang, E.H.; Kwon, Y.G.; Kang, D.H.; Kim, S.S.; Song, S.W. Interfacial origin of performance improvement and fade for 4.6 V LiNi0.5Co0.2Mn0.3O2 battery cathodes. J. Phys. Chem. C 2014, 118, 10631–10639. [Google Scholar] [CrossRef]
- Guilmard, M.; Rougier, A.; Grüne, M.; Croguennec, L.; Delmas, C. Effects of aluminum on the structural and electrochemical properties of LiNiO2. J. Power Sources 2003, 115, 305–314. [Google Scholar] [CrossRef]
- Albrecht, S.; Kümpers, J.; Kruft, M.; Malcus, S.; Vogler, C.; Wahl, M.; Wohlfahrt-Mehrens, M. Electrochemical and thermal behavior of aluminum- and magnesium-doped spherical lithium nickel cobalt mixed oxides Li1−x(Ni1−y−zCoyMz)O2 (M = Al, Mg). J. Power Sources 2003, 119, 178–183. [Google Scholar] [CrossRef]
- Lee, W.; Muhammad, S.; Kim, T.; Kim, H.; Lee, E.; Jeong, M.; Son, S.; Ryou, J.H.; Yoon, W.S. New insight into Ni-rich layered structure for next-generation Li rechargeable batteries. Adv. Energy Mater. 2018, 8, 1701788. [Google Scholar] [CrossRef]
- Wang, D.F.; Li, Y.X.; Wang, W.L.; Yang, Y. Effects of Al-doping on properties of Ni-rich cathode materials employing different aluminum sources. J. Electrochem. 2019, 25, 660–668. [Google Scholar] [CrossRef]
- Chen, C.H.; Liu, J.; Stoll, M.E.; Henriksen, G.; Vissers, D.R.; Amine, K. Aluminum-doped lithium nickel cobalt oxide electrodes for high-power lithium-ion batteries. J. Power Sources 2004, 128, 278–285. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Li, H.; Liu, A.; Wang, Y.; Yin, S.; Wu, H.; Dahn, J.R. Impact of the synthesis conditions on the performance of LiNixCoyAlzO2 with high Ni and low Co content. J. Electrochem. Soc. 2018, 165, A3544–A3557. [Google Scholar] [CrossRef]
- Fu, C.; Li, G.; Luo, D.; Li, Q.; Fan, J.; Li, L. Nickel-rich layered microspheres cathodes: Lithium/nickel disordering and electrochemical performance. ACS Appl. Mater. Interfaces 2014, 6, 15822–15831. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.H.; Kang, Y.C. Fine-sized LiNi0.8Co0.15Mn0.05O2 cathode particles prepared by spray pyrolysis from the polymeric precursor solutions. Ceram. Int. 2009, 35, 1633–1639. [Google Scholar] [CrossRef]
- Shim, J.H.; Kim, C.Y.; Cho, S.W.; Missiul, A.; Kim, J.K.; Ahn, Y.J.; Lee, S. Effects of heat-treatment atmosphere on electrochemical performances of Ni-rich mixed-metal oxide (LiNi0.80Co0.15Mn0.05O2) as a cathode material for lithium ion battery. Electrochim. Acta 2014, 138, 15–21. [Google Scholar] [CrossRef]
- Idris, M.S.; West, A.R. The effect on cathode performance of oxygen non-stoichiometry and interlayer mixing in layered rock salt LiNi0.8Mn0.1Co0.1O2−δ. J. Electrochem. Soc. 2012, 159, A396–A401. [Google Scholar] [CrossRef]
- Dearden, C.; Zhu, M.; Wang, B.; Castro, R.H.R. Synthesis, size reduction, and delithiation of carbonate-free nanocrystalline lithium nickel oxide. J. Mater. Sci. 2013, 48, 1740–1745. [Google Scholar] [CrossRef]
- Rougier, A.; Gravereau, P.; Delmas, C. Optimization of the composition of the Li1−zNi1+zO2 electrode materials: Structural, magnetic, and electrochemical studies. J. Electrochem. Soc. 1996, 143, 1168–1175. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 1993, 140, 1862–1870. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, X.; Bommel, A.; Rowe, A.; Dahn, J. Coprecipitation synthesis of NixMn1−x(OH)2 mixed hydroxids. Chem. Mater. 2010, 22, 1015–1021. [Google Scholar] [CrossRef]
- Luo, W.; Dahn, J.R. Preparation of Co1−zAlz(OH)2(NO3)z layered double hydroxides and Li(Co1−zAlz)O2. Chem. Mater. 2009, 21, 56–62. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D. Synthesis of high-density nickel cobalt aluminum hydroxide by continuous coprecipitation method. ACS Appl. Mater. Interfaces 2012, 4, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Q.; Zhang, Q.; Huang, J.Q.; Wei, F. Hierarchical nanocomposites derived from nanocarbons and layered double hydroxides—Properties, synthesis, and applications. Adv. Funct. Mater. 2012, 22, 675–694. [Google Scholar] [CrossRef]
- Liu, A.; Dahn, J. The formation of layered double hydroxide phases in the coprecipitation syntheses of [Ni0.80Co0.15](1−x)/0.95Alx(OH)2(anionn−) (x = 0–0.2, n = 1, 2). ChemEngineering 2019, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Weigel, T.; Schipper, F.; Erickson, E.; Susai, F.; Markovsky, B.; Aurbach, D. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations. ACS Energy Lett. 2019, 4, 508–516. [Google Scholar] [CrossRef]
- Zheng, L.; Bennett, J.C.; Obrovac, M.N. All-dry synthesis of single crystal NMC cathode materials for Li-ion batteries. J. Electrochem. Soc. 2020, 167, 130536. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Q.; Wang, Z.; Wang, S.; Zhou, Y.; Song, D.; Zhang, H.; Shi, X.; Li, C.; Zhang, L.; et al. Improved interfacial chemistry and enhanced high voltage-resistance capability of an in situ polymerized electrolyte for LiNi0.8Co0.15Al0.05O2–Li batteries. J. Mater. Chem. A 2021, 9, 3597–3604. [Google Scholar] [CrossRef]
- Makimura, Y.; Zheng, S.; Ikuhara, Y.; Ukyo, Y. Microstructural observation of LiNi0.8Co0.15Al0.05O2 after charge and discharge by scanning transmission electron microscopy. J. Electrochem. Soc. 2012, 159, A1070–A1073. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, H.; Svensson, A.M.; Fossdal, A.; Sheridan, E.; Lu, S.; Vullum-Bruer, F. High capacity Li[Ni0.8Co0.1Mn0.1]O2 synthesized by sol–gel and co-precipitation methods as cathode materials for lithium-ion batteries. Solid State Ion. 2013, 249, 105–111. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, N.; Stark, J.E.; Arab, P.; Li, H.; Dahn, J.R. Synthesis of Co-Free Ni-Rich Single Crystal Positive Electrode Materials for Lithium Ion Batteries: Part I. Two-Step Lithiation Method for Al- or Mg-Doped LiNiO2. J. Electrochem. Soc. 2021, 168, 040531. [Google Scholar] [CrossRef]
- Cui, J.; Ding, X.; Luo, D.; Xie, H.; Zhang, Z.; Zhang, B.; Tan, F.; Liu, C.; Lin, Z. Effect of Cationic Uniformity in Precursors on Li/Ni Mixing of Ni-Rich Layered Cathodes. Energy Fuels 2021, 35, 1842–1850. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Q.; Wu, X.; Chen, Z.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Synthesis of Ni-Rich Layered-Oxide Nanomaterials with Enhanced Li-Ion Diffusion Pathways as High-Rate Cathodes for Li-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 6583–6590. [Google Scholar] [CrossRef]
- Nam, G.; Hwang, J.; Kang, D.; Oh, S.; Chae, S.; Yoon, M.; Ko, M. Mechanical densification synthesis of single-crystalline Ni-rich cathode for high-energy lithium-ion batteries. J. Energy Chem. 2023, 79, 562–568. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Shi, Q.; Zhan, C.; Liu, G. One-Step Solid-State Synthesis of Ni-Rich Cathode Materials for Lithium-Ion Batteries. Materials 2023, 16, 3079. https://doi.org/10.3390/ma16083079
Wang L, Shi Q, Zhan C, Liu G. One-Step Solid-State Synthesis of Ni-Rich Cathode Materials for Lithium-Ion Batteries. Materials. 2023; 16(8):3079. https://doi.org/10.3390/ma16083079
Chicago/Turabian StyleWang, Lifan, Qinling Shi, Chun Zhan, and Guicheng Liu. 2023. "One-Step Solid-State Synthesis of Ni-Rich Cathode Materials for Lithium-Ion Batteries" Materials 16, no. 8: 3079. https://doi.org/10.3390/ma16083079




