A Novel Functionalized MoS2-Based Coating for Efficient Solar Desalination
Abstract
:1. Introduction
2. Results and Discussions
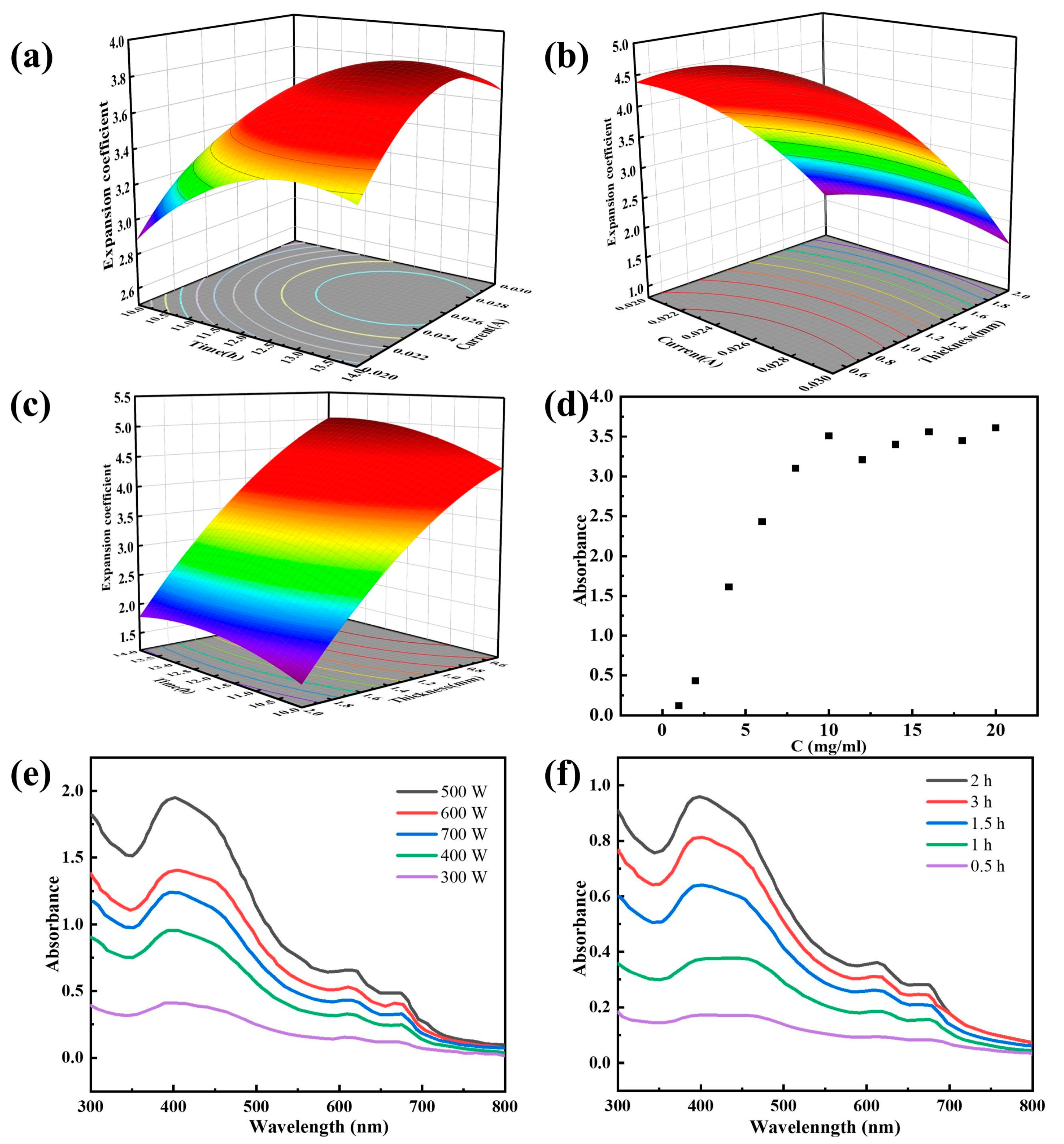
2.1. Optimization of Experimental Conditions for the Preparation of MoS2
2.2. Characterization
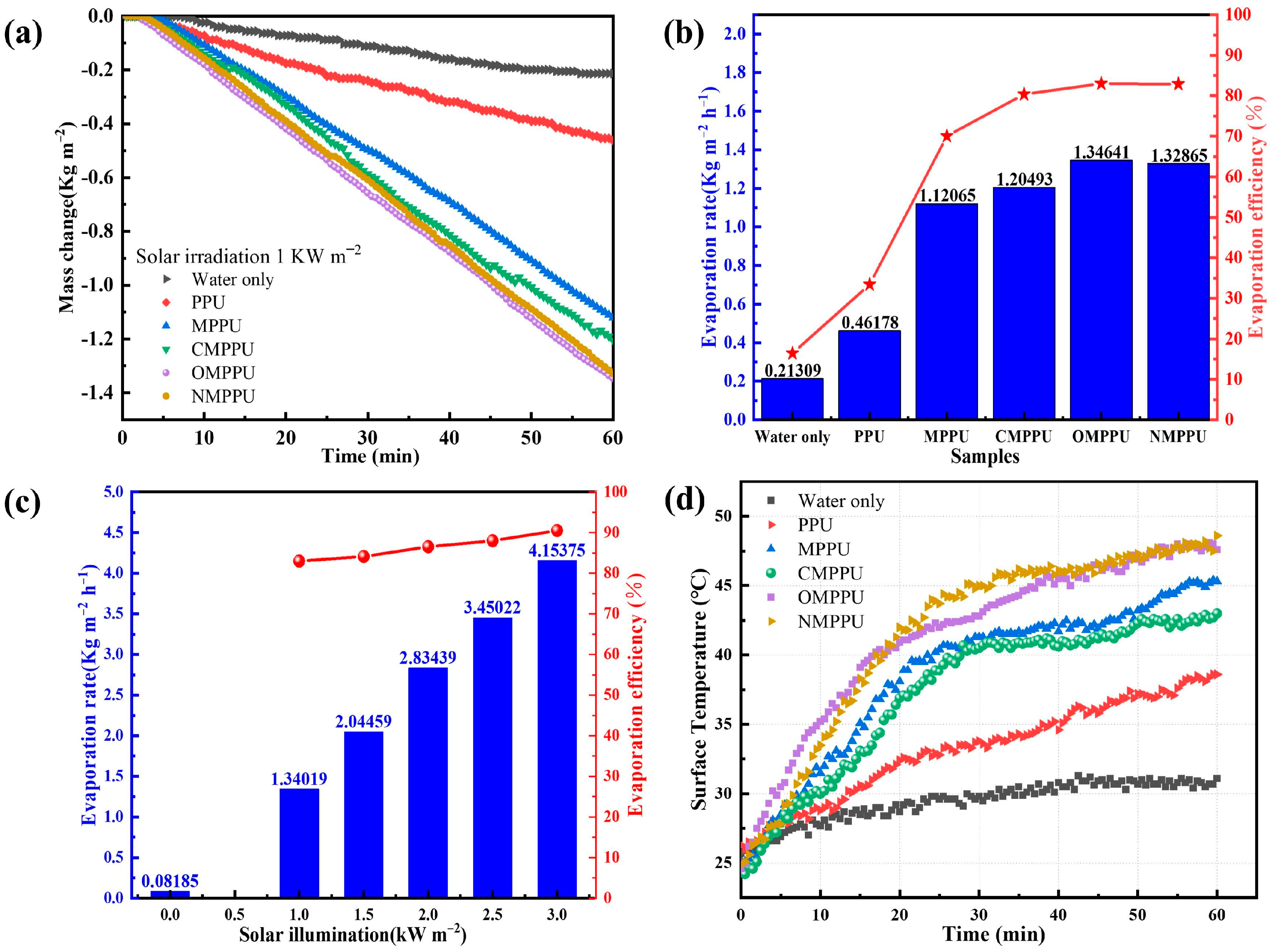
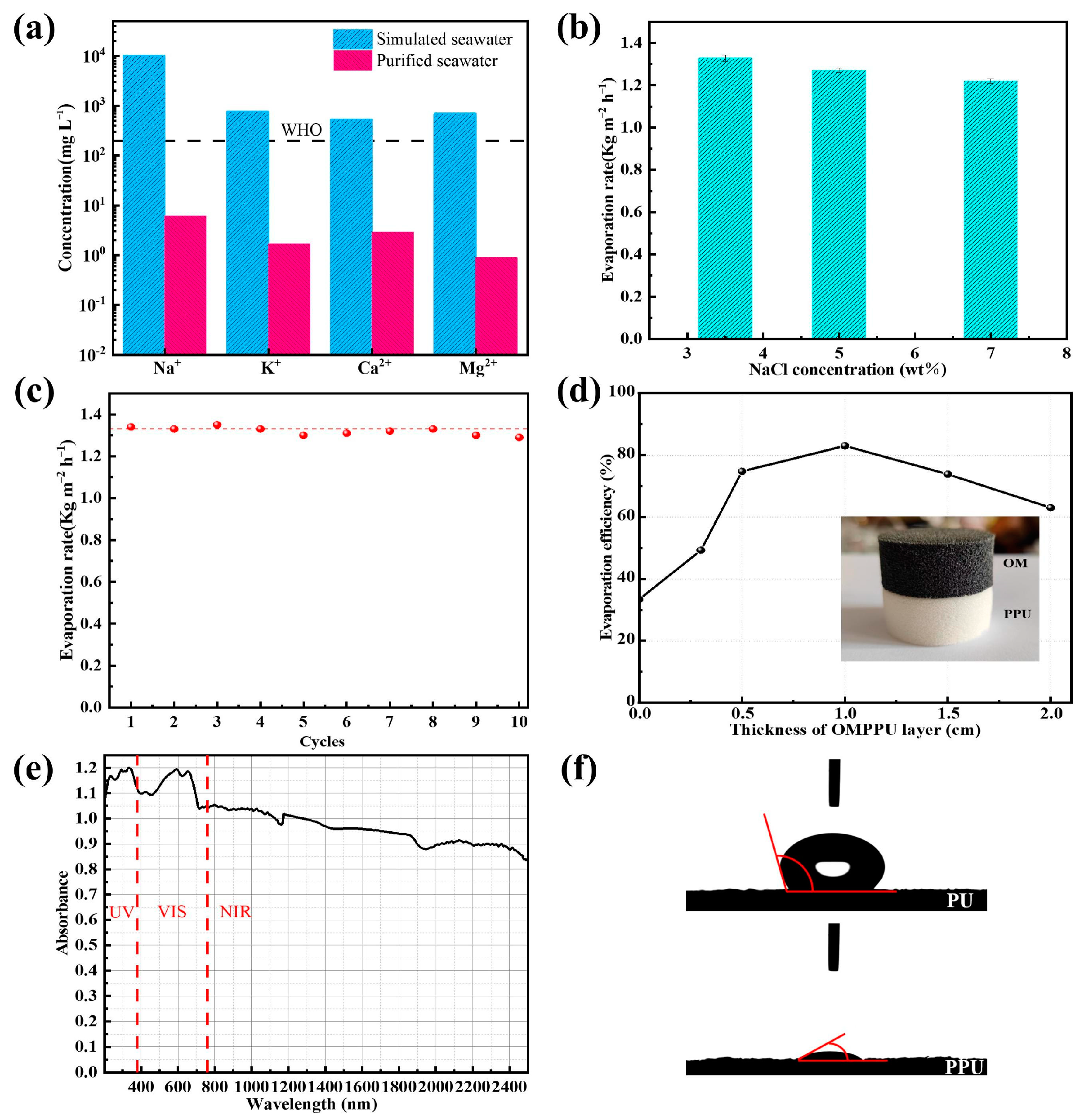
2.3. Solar Desalination Experiment
3. Experimental Sections
3.1. Materials
3.2. Exfoliation of Molybdenite
3.2.1. Electrochemical Method of Electrolysis of Molybdenite Flakes
3.2.2. Liquid Exfoliation of Expanded Molybdenite Flakes
3.2.3. Functionalization of MoS2
3.2.4. Functional MoS2 Coating Preparation
3.2.5. Evaporator Preparation
3.3. Characterization Methods
3.4. Experiments of Solar Steam Generation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Q.; Gao, L.; Huang, M.; Mersal, G.A.M.; Ibrahim, M.M.; El-Bahy, Z.M.; Shi, X.; Jiang, Q. Aminated lignin by ultrasonic method with enhanced arsenic (V) adsorption from polluted water. Adv. Compos. Hybrid Mater. 2022, 5, 1044–1053. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Metal-Organic frameworks (MOFs) in water filtration membranes for desalination and other applications. Appl. Mater. Today 2018, 11, 219–230. [Google Scholar] [CrossRef]
- Dai, J.; Wang, J.; Hou, X.; Ru, Q.; He, Q.; Srimuk, P.; Presser, V.; Chen, F. Dual-Zinc Electrode Electrochemical Desalination. Chemsuschem 2020, 13, 2792–2798. [Google Scholar] [CrossRef]
- Ahmed, F.; Umar, A.; Kumar, S.; Shaalan, N.M.; Arshi, N.; Alam, M.G.; Hasan, P.M.Z.; Ramay, S.M.; Khan, R.; Aljaafari, A.; et al. Manganese dioxide nanoparticles/reduced graphene oxide nanocomposites for hybrid capacitive desalination. Adv. Compos. Hybrid Mater. 2022, 6, 19. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, V.K.; Shukla, S.K.; Tirkey, J.V.; Rathore, P.K.S. A comprehensive review of direct solar desalination techniques and its advancements. J. Clean. Prod. 2020, 284, 124719. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.; Chen, X.; Chen, X.; Fu, Q.; Deng, H. Recent progress in solar photothermal steam technology for water purification and energy utilization. Chem. Eng. J. 2022, 448, 137603. [Google Scholar] [CrossRef]
- Fuzil, N.S.; Othman, N.H.; Alias, N.H.; Marpani, F.; Othman, M.H.D.; Ismail, A.F.; Lau, W.J.; Li, K.; Kusworo, T.D.; Ichinose, I.; et al. A review on photothermal material and its usage in the development of photothermal membrane for sustainable clean water production. Desalination 2021, 517, 115259. [Google Scholar] [CrossRef]
- Wang, P. Emerging investigator series: The rise of nano-enabled photothermal materials for water evaporation and clean water production by sunlight. Environ. Sci. Nano 2018, 5, 1078–1089. [Google Scholar] [CrossRef] [Green Version]
- Candreva, A.; De Rose, R.; Perrotta, I.D.; Guglielmelli, A.; La Deda, M. Light-Induced Clusterization of Gold Nanoparticles: A New Photo-Triggered Antibacterial against E. coli Proliferation. Nanomaterials 2023, 13, 746. [Google Scholar] [CrossRef]
- Fanizza, E.; Mastrogiacomo, R.; Pugliese, O.; Guglielmelli, A.; De Sio, L.; Castaldo, R.; Scavo, M.P.; Giancaspro, M.; Rizzi, F.; Gentile, G.; et al. NIR-Absorbing Mesoporous Silica-Coated Copper Sulphide Nanostructures for Light-to-Thermal Energy Conversion. Nanomaterials 2022, 12, 2545. [Google Scholar] [CrossRef]
- He, W.; Zhou, L.; Wang, M.; Cao, Y.; Chen, X.; Hou, X. Structure development of carbon-based solar-driven water evaporation systems. Sci. Bull. 2021, 66, 1472–1483. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, W.; Guan, G.; Song, G.; Zou, R.; Hu, J. Design and Functionalization of the NIR-Responsive Photothermal Semiconductor Nanomaterials for Cancer Theranostics. Accounts Chem. Res. 2017, 50, 2529–2538. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.S.; Arshad, N.; Wang, X. Nanoenabled Photothermal Materials for Clean Water Production. Glob. Chall. 2020, 5, 2000055. [Google Scholar] [CrossRef]
- Li, W.; Tekell, M.C.; Huang, Y.; Bertelsmann, K.; Lau, M.; Fan, D. Synergistic High-Rate Solar Steaming and Mercury Removal with MoS2/C@ Polyurethane Composite Sponges. Adv. Energy Mater. 2018, 8, 1802108. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Tao, P.; Shen, Q.; Yi, N.; Zhang, F.; Liu, Q.; Song, C.; Zhang, D.; Shang, W.; et al. Bio-Inspired Evaporation Through Plasmonic Film of Nanoparticles at the Air-Water Interface. Small 2014, 10, 3234–3239. [Google Scholar] [CrossRef] [PubMed]
- Djellabi, R.; Noureen, L.; Dao, V.-D.; Meroni, D.; Falletta, E.; Dionysiou, D.D.; Bianchi, C.L. Recent advances and challenges of emerging solar-driven steam and the contribution of photocatalytic effect. Chem. Eng. J. 2022, 431, 134024. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Fu, L.; Zou, M.; Li, Z.; Cao, A.; Yuan, Q. An Ultrathin Flexible 2D Membrane Based on Single-Walled Nanotube-MoS2 Hybrid Film for High-Performance Solar Steam Generation. Adv. Funct. Mater. 2017, 28, 1704505. [Google Scholar] [CrossRef]
- Yin, R.; Jing, B.; He, S.; Hu, J.; Lu, G.; Ao, Z.; Wang, C.; Zhu, M. Near-infrared light to heat conversion in peroxydisulfate activation with MoS2: A new photo-activation process for water treatment. Water Res. 2020, 190, 116720. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Song, S.; Li, Y.; Jia, F.; Feng, T.; Hu, N. Flexible 2D@3D Janus evaporators for high-performance and continuous solar desalination. Desalination 2022, 525, 115483. [Google Scholar] [CrossRef]
- Ju, P.; Hao, L.; Zhang, Y.; Sun, J.; Dou, K.; Lu, Z.; Liao, D.; Zhai, X.; Sun, C. Facile fabrication of a novel spindlelike MoS2/BiVO4 Z-scheme heterostructure with superior visible-light-driven photocatalytic disinfection performance. Sep. Purif. Technol. 2022, 299, 121706. [Google Scholar] [CrossRef]
- Ghim, D.; Jiang, Q.; Cao, S.; Singamaneni, S.; Jun, Y.-S. Mechanically interlocked 1T/2H phases of MoS2 nanosheets for solar thermal water purification. Nano Energy 2018, 53, 949–957. [Google Scholar] [CrossRef]
- Yu, J.; Cao, C.; Liu, S.; Pan, Y. Eco-friendly magneto-photothermal sponge for the fast recovery of highly viscous crude oil spill. Sep. Purif. Technol. 2022, 298, 121668. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, F.; Huang, A.; Qin, Y.; Song, S.; Li, Y.; Arroyo, M.A.C. MoS2@sponge with double layer structure for high-efficiency solar desalination. Desalination 2020, 481, 114359. [Google Scholar] [CrossRef]
- Chen, X.; Berner, N.C.; Backes, C.; Duesberg, G.S.; McDonald, A.R. Functionalization of two-Dimensional MoS2: On the reaction between MoS2 and organic thiols. Angew. Chem. 2016, 128, 5897–5902. [Google Scholar] [CrossRef]
- Ries, L.; Petit, E.; Michel, T.; Diogo, C.C.; Gervais, C.; Salameh, C.; Bechelany, M.; Balme, S.; Miele, P.; Onofrio, N.; et al. Enhanced sieving from exfoliated MoS2 membranes via covalent functionalization. Nat. Mater. 2019, 18, 1112–1117. [Google Scholar] [CrossRef]
- Presolski, S.; Pumera, M. Covalent functionalization of MoS2. Mater. Today 2015, 19, 140–145. [Google Scholar] [CrossRef]
- Nguyen, E.P.; Carey, B.J.; Ou, J.Z.; van Embden, J.; Della Gaspera, E.; Chrimes, A.F.; Spencer, M.J.S.; Zhuiykov, S.; Kalantar-Zadeh, K.; Daeneke, T. Electronic Tuning of 2D MoS2 through Surface Functionalization. Adv. Mater. 2015, 27, 6225–6229. [Google Scholar] [CrossRef]
- Stergiou, A.; Tagmatarchis, N. Molecular functionalization of two-dimensional MoS2 nanosheets. Chem. A Eur. J. 2018, 24, 18246–18257. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Ling, C.; Yuan, S.; Chen, Q.; Wang, J. Towards a Comprehensive Understanding of the Reaction Mechanisms Between Defective MoS2 and Thiol Molecules. Angew. Chem. Int. Ed. 2017, 56, 10501–10505. [Google Scholar] [CrossRef]
- Chou, S.S.; De, M.; Kim, J.; Byun, S.; Dykstra, C.; Yu, J.; Huang, J.; Dravid, V.P. Ligand conjugation of chemically exfoliated MoS2. J. Am. Chem. Soc. 2013, 135, 4584–4587. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; He, B.; Yang, Y.; He, Y. Facile approach to surface functionalized MoS2 nanosheets. RSC Adv. 2014, 4, 32570–32578. [Google Scholar] [CrossRef]
- Wu, G.; Wu, Z.; Liu, L.; Cui, W.; Du, D.; Xue, Y. NIR light responsive MoS2 nanomaterials for rapid sterilization: Optimum photothermal effect via sulfur vacancy modulation. Chem. Eng. J. 2021, 427, 132007. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Wang, Z.; Song, L.; Feng, Y.; Yao, J. Chinese ink enabled wood evaporator for continuous water desalination. Desalination 2020, 496, 114727. [Google Scholar] [CrossRef]
- Jiang, H.; Fang, H.; Wang, D.; Sun, J. Spray-coated commercial PTFE membrane from MoS2/LaF3/PDMS ink as solar absorber for efficient solar steam generation. Sol. RRL 2020, 4, 2000126. [Google Scholar] [CrossRef]
- Chen, H.; Shen, T.; Li, Y.; Chen, X.; Yang, H.; Li, W. Green Photothermal Ink for 0D to 3D Solar-Driven Devices. Adv. Mater. Interfaces 2021, 8, 2101639. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; He, X.; Song, X.; Li, Z.; Zhang, X.; Zhong, W.; Du, Y. Effects of ultrasonic cavitation intensity on the efficient liquid-exfoliation of MoS2 nanosheets. RSC Adv. 2014, 4, 50981–50987. [Google Scholar] [CrossRef]
- Dong, H.; Chen, D.; Wang, K.; Zhang, R. High-Yield Preparation and Electrochemical Properties of Few-Layer MoS2 Nanosheets by Exfoliating Natural Molybdenite Powders Directly via a Coupled Ultrasonication-Milling Process. Nanoscale Res. Lett. 2016, 11, 409. [Google Scholar] [CrossRef] [Green Version]
- Jawaid, A.; Nepal, D.; Park, K.; Jespersen, M.; Qualley, A.; Mirau, P.; Drummy, L.F.; Vaia, R.A. Mechanism for liquid phase exfoliation of MoS2. Chem. Mater. 2016, 28, 337–348. [Google Scholar] [CrossRef]
- Majd, S.M.; Salimi, A.; Ghasemi, F. An ultrasensitive detection of miRNA-155 in breast cancer via direct hybridization assay using two-dimensional molybdenum disulfide field-effect transistor biosensor. Biosens. Bioelectron. 2018, 105, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and Applications of Mechanically Exfoliated Single-Layer and Multilayer MoS2 and WSe2 Nanosheets. Accounts Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, C.; Chiaula, V.; Yu, L.; Pinelo, M.; Woodley, J.M.; Daugaard, A.E. Simple Preparation of Thiol-Ene Particles in Glycerol and Surface Functionalization by Thiol-Ene Chemistry (TEC) and Surface Chain Transfer Free Radical Polymerization (SCT-FRP). Macromol. Rapid Commun. 2017, 39, 1700394. [Google Scholar] [CrossRef]
- Wen, Z.; McBride, M.K.; Zhang, X.; Han, X.; Martinez, A.M.; Shao, R.; Zhu, C.; Visvanathan, R.; Clark, N.; Wang, Y.; et al. Reconfigurable LC Elastomers: Using a Thermally Programmable Monodomain to Access Two-Way Free-Standing Multiple Shape Memory Polymers. Macromolecules 2018, 51, 5812–5819. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Du, K.; Wang, S.; Huang, Z.; Yuan, L.; Li, Z.; Wang, H.; Zheng, L.; Chai, Z.; et al. Effective removal of U(VI) and Eu(III) by carboxyl functionalized MXene nanosheets. J. Hazard. Mater. 2020, 396, 122731. [Google Scholar] [CrossRef]
- Zhang, S.; Song, S.; Gu, P.; Ma, R.; Wei, D.; Zhao, G.; Wen, T.; Jehan, R.; Hu, B.; Wang, X. Visible-light-driven activation of persulfate over cyano and hydroxyl group co-modified mesoporous g-C3N4 for boosting bisphenol A degradation. J. Mater. Chem. A 2019, 7, 5552–5560. [Google Scholar] [CrossRef]
- Suručić, L.; Janjić, G.; Marković, B.; Tadić, T.; Vuković, Z.; Nastasović, A.; Onjia, A. Speciation of Hexavalent Chromium in Aqueous Solutions Using a Magnetic Silica-Coated Amino-Modified Glycidyl Methacrylate Polymer Nanocomposite. Materials 2023, 16, 2233. [Google Scholar] [CrossRef]
- Clifford, A.F. The electronegativity of groups. J. Phys. Chem. 1959, 63, 1227–1231. [Google Scholar] [CrossRef]
- Jiang, Q.; Xu, P.; Feng, J.; Sun, M. Application of Covalent Organic Porous Polymers-Functionalized Basalt Fibers for in-Tube Solid-Phase Microextraction. Molecules 2020, 25, 5788. [Google Scholar] [CrossRef]
- Yang, J.; Pang, Y.; Huang, W.; Shaw, S.K.; Schiffbauer, J.; Pillers, M.A.; Mu, X.; Luo, S.; Zhang, T.; Huang, Y.; et al. Functionalized Graphene Enables Highly Efficient Solar Thermal Steam Generation. ACS Nano 2017, 11, 5510–5518. [Google Scholar] [CrossRef]
- Xiao, X.; Pan, L.; Chen, B.; Chen, T.; Wang, Y.; Zhang, Q.; Ren, L.; Xu, W. A Janus and superhydrophilic design for stable and efficient high-salinity brine solar interfacial desalination. Chem. Eng. J. 2023, 455, 140777. [Google Scholar] [CrossRef]
- World Health Organization. Safe Drinking-Water from Desalination. Available online: https://apps.who.int/iris/bitstream/handle/10665/70621/WHO_HSE_WSH_11.03_eng.pdf;jsessionid=9ADC4D2503BF733B24203DABE04D3D1B?sequence=1 (accessed on 10 October 2022).
- Gupta, A.; Arunachalam, V.; Vasudevan, S. Liquid-Phase Exfoliation of MoS2 Nanosheets: The Critical Role of Trace Water. J. Phys. Chem. Lett. 2016, 7, 4884–4890. [Google Scholar] [CrossRef] [PubMed]
- Salavagione, H.J.; Sherwood, J.; De Bruyn, M.; Budarin, V.L.; Ellis, G.J.; Clark, J.H.; Shuttleworth, P.S. Identification of high performance solvents for the sustainable processing of graphene. Green Chem. 2017, 19, 2550–2560. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ni, G.; Cooper, T.; Xu, N.; Li, J.; Zhou, L.; Hu, X.; Zhu, B.; Yao, P.; Zhu, J. Measuring Conversion Efficiency of Solar Vapor Generation. Joule 2019, 3, 1798–1803. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Wang, G.; Ming, X.; Mei, T.; Wang, J.; Li, J.; Qian, J.; Wang, X. PEGylated Self-Growth MoS2 on a Cotton Cloth Substrate for High-Efficiency Solar Energy Utilization. ACS Appl. Mater. Interfaces 2018, 10, 24583–24589. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Wang, Q.; Feng, T.; Wang, L.; Fan, Z. A Novel Functionalized MoS2-Based Coating for Efficient Solar Desalination. Materials 2023, 16, 3105. https://doi.org/10.3390/ma16083105
Yu Q, Wang Q, Feng T, Wang L, Fan Z. A Novel Functionalized MoS2-Based Coating for Efficient Solar Desalination. Materials. 2023; 16(8):3105. https://doi.org/10.3390/ma16083105
Chicago/Turabian StyleYu, Qinghong, Qingmiao Wang, Tao Feng, Li Wang, and Zhixuan Fan. 2023. "A Novel Functionalized MoS2-Based Coating for Efficient Solar Desalination" Materials 16, no. 8: 3105. https://doi.org/10.3390/ma16083105




