Optimized Morphology and Tuning the Mn3+ Content of LiNi0.5Mn1.5O4 Cathode Material for Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
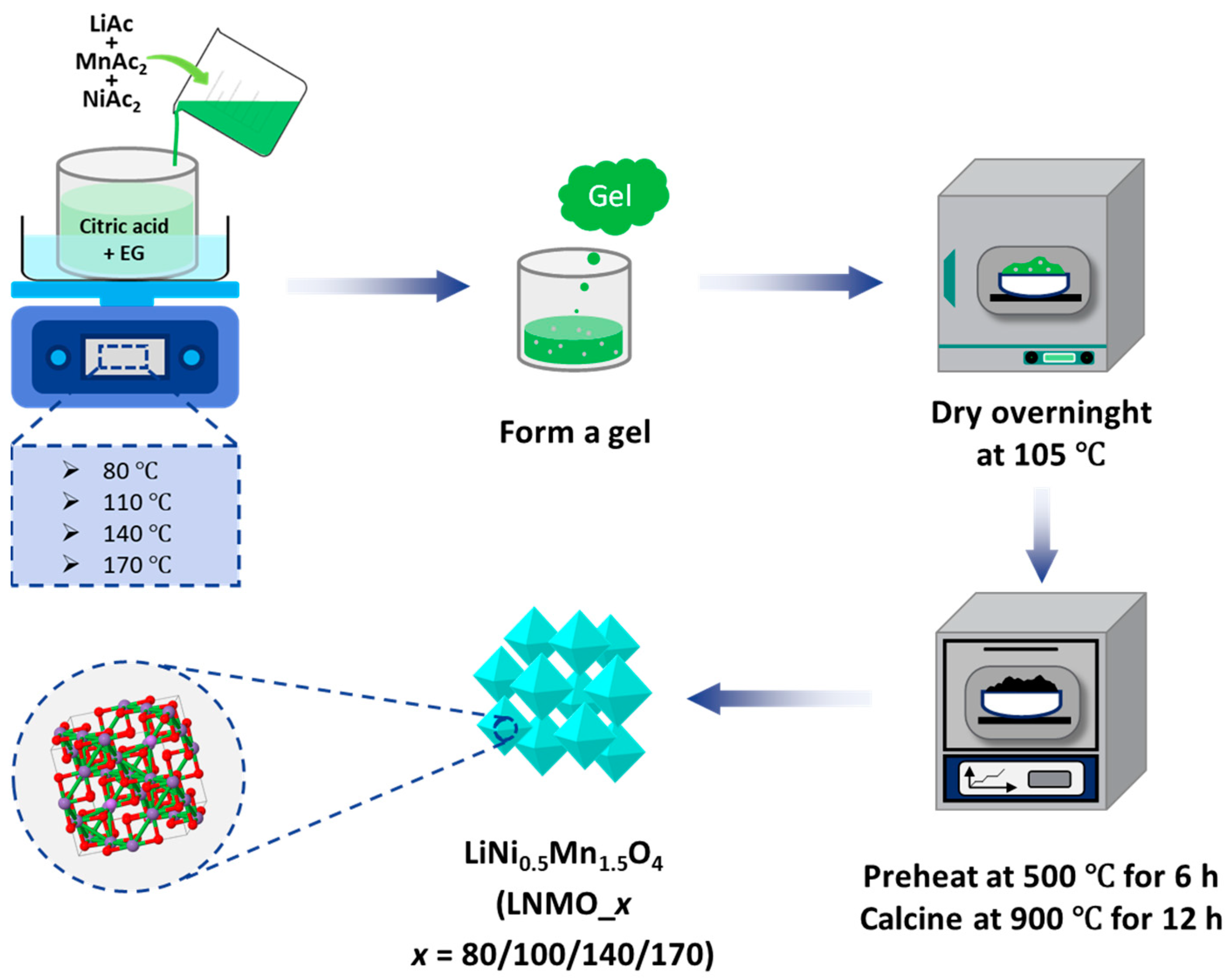
2.1. Materials and Synthesis
2.2. Characterizations
2.3. Electrochemical Tests
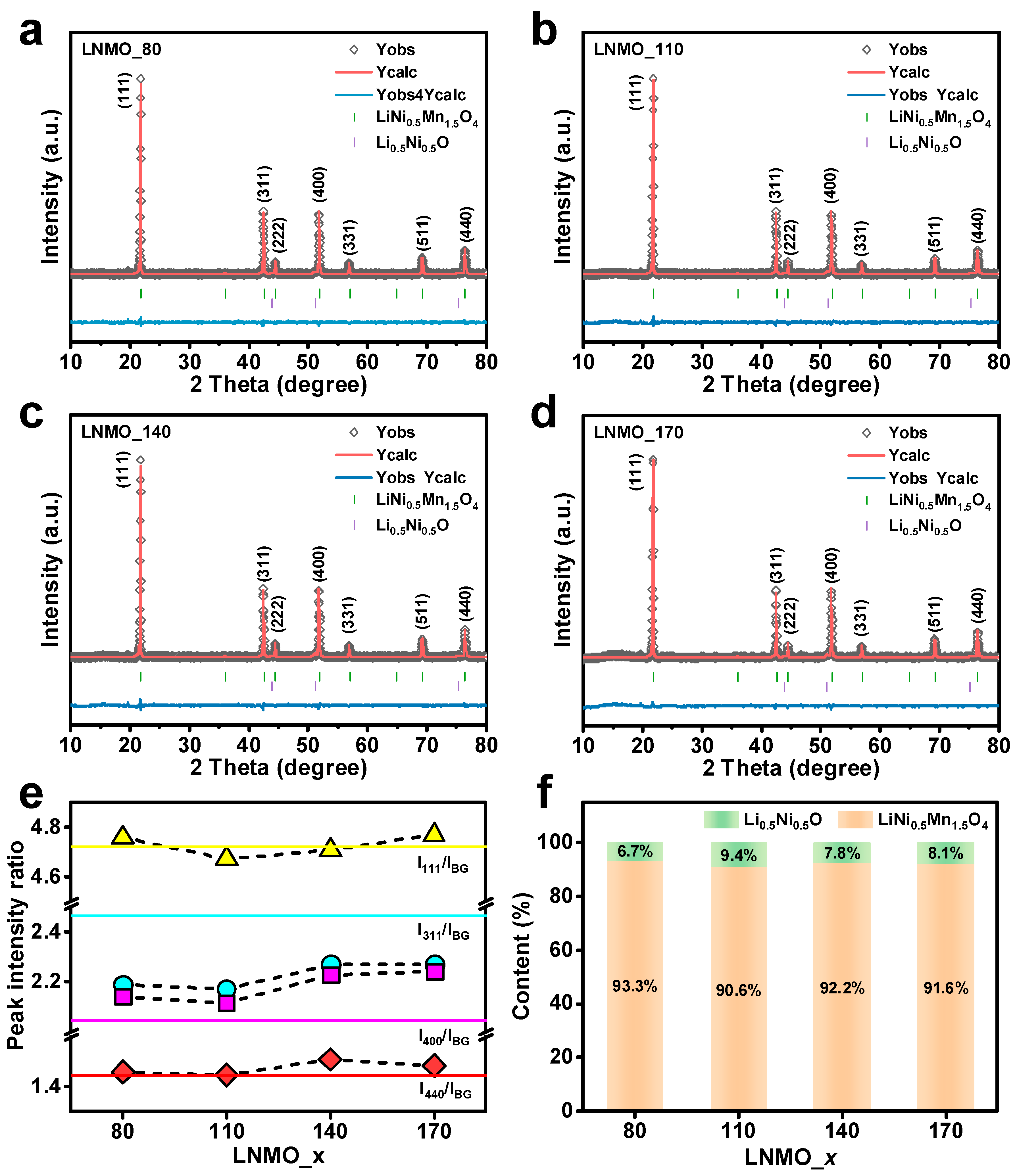
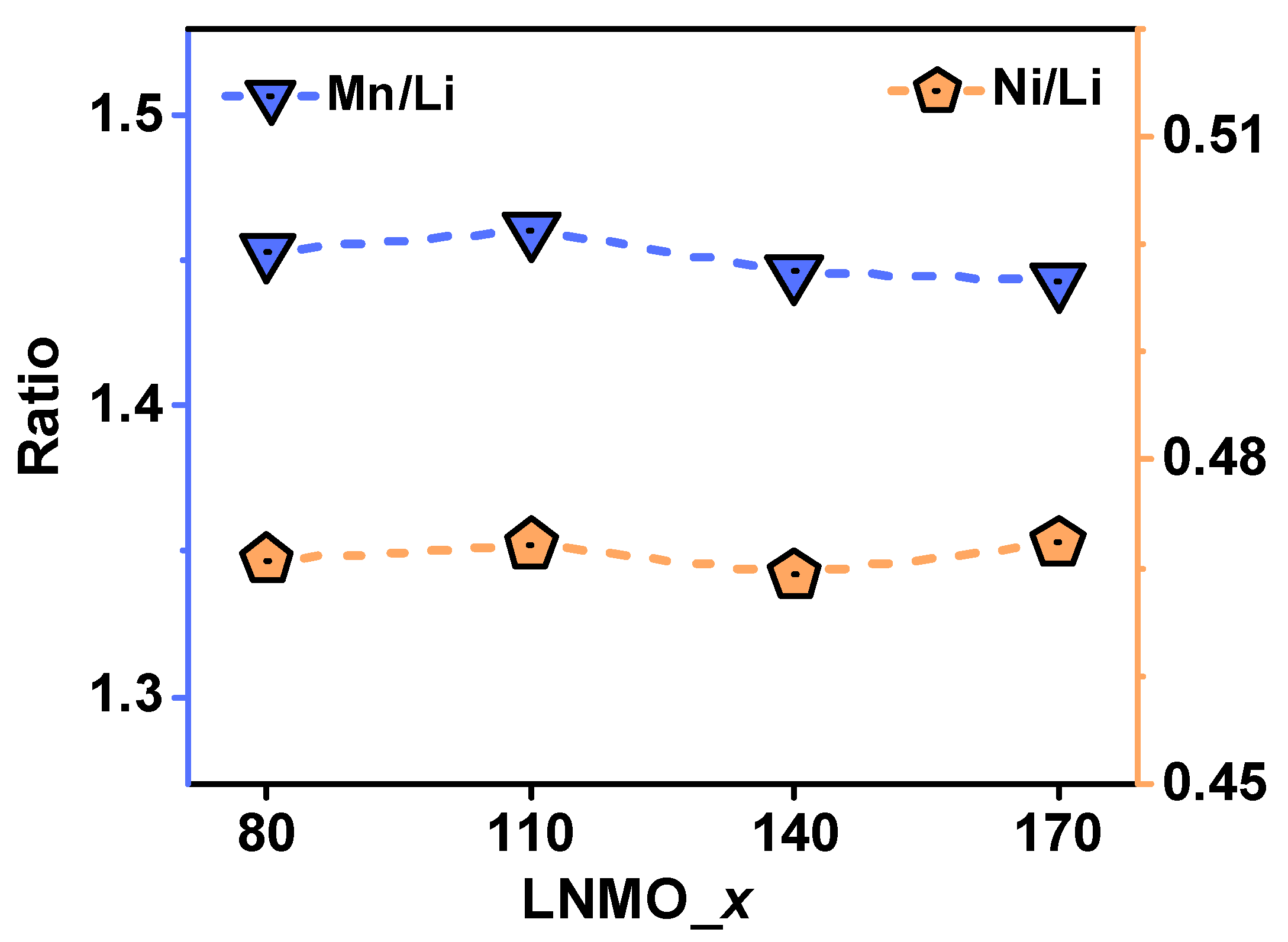
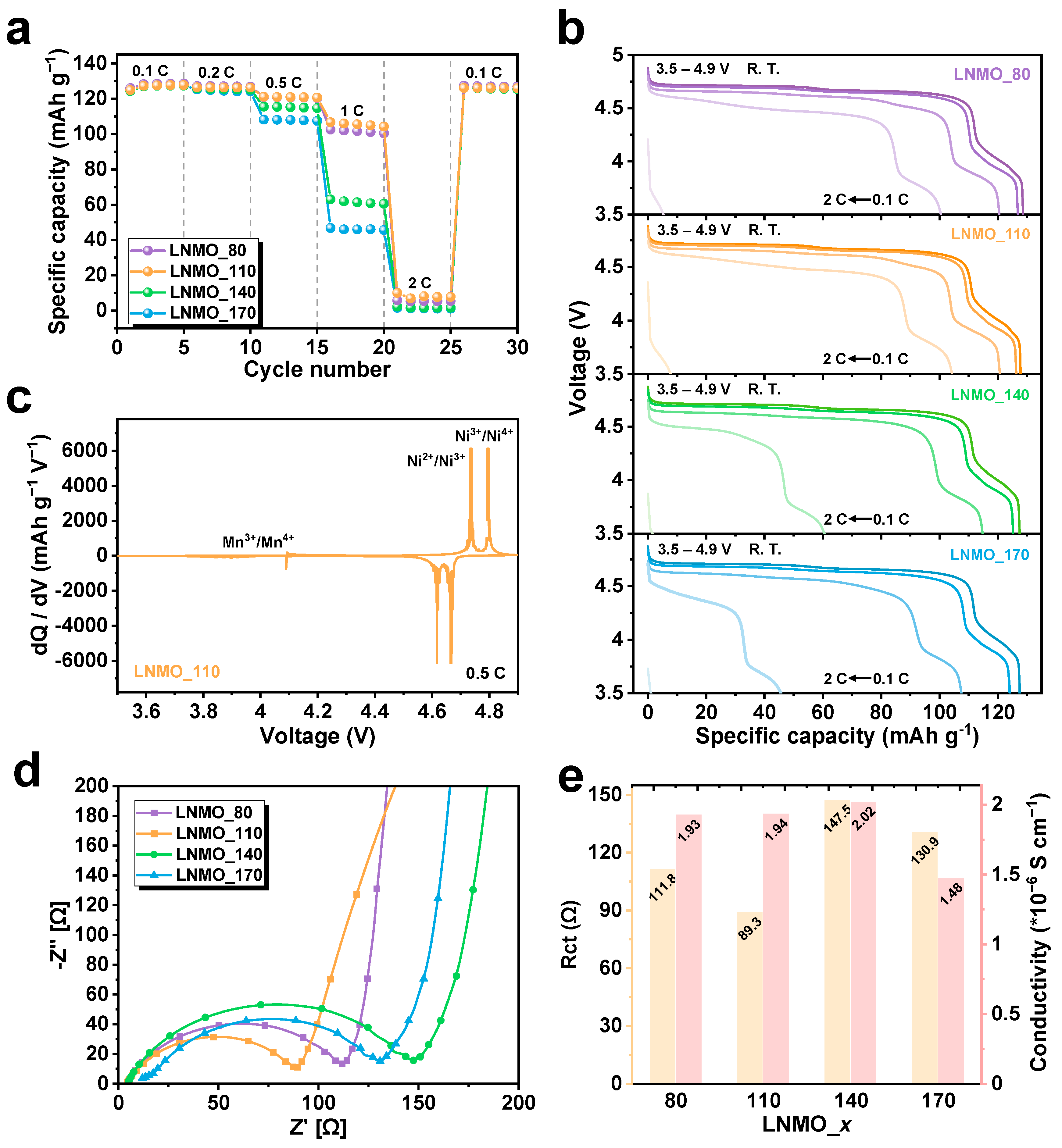
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Liu, T.; Bi, X.; Chen, Z.; Amine, K.; Zhong, C.; Lu, J. Cationic and Anionic Redox in Lithium-Ion Based Batteries. Chem. Soc. Rev. 2020, 49, 1688–1705. [Google Scholar] [CrossRef] [PubMed]
- Kuenzel, M.; Kim, G.-T.; Zarrabeitia, M.; Lin, S.D.; Schuer, A.R.; Geiger, D.; Kaiser, U.; Bresser, D.; Passerini, S. Crystal Engineering of TMPOx-Coated LiNi0.5Mn1.5O4 Cathodes for High-Performance Lithium-Ion Batteries. Mater. Today 2020, 39, 127–136. [Google Scholar] [CrossRef]
- Lin, Y.; Tian, H.; Qian, J.; Yu, M.; Hu, T.; Lassi, U.; Chen, Z.; Wu, Z. Biocarbon-Directed Vertical δ-MnO2 Nanoflakes for Boosting Lithium-Ion Diffusion Kinetics. Mater. Today Chem. 2022, 26, 101023. [Google Scholar] [CrossRef]
- Wang, T.; He, J.; Cheng, X.-B.; Zhu, J.; Lu, B.; Wu, Y. Strategies toward High-Loading Lithium–Sulfur Batteries. ACS Energy Lett. 2023, 8, 116–150. [Google Scholar] [CrossRef]
- Dai, P.; Kong, X.; Yang, H.; Li, J.; Zeng, J.; Zhao, J. Single-Crystal Ni-Rich Layered LiNi0.9Mn0.1O2 Enables Superior Performance of Co-Free Cathodes for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 4381–4390. [Google Scholar] [CrossRef]
- Tan, C.; Yang, J.; Pan, Q.; Li, Y.; Li, Y.; Cui, L.; Fan, X.; Zheng, F.; Wang, H.; Li, Q. Optimizing Interphase Structure to Enhance Electrochemical Performance of High Voltage LiNi0.5Mn1.5O4 Cathode via Anhydride Additives. Chem. Eng. J. 2021, 410, 128422. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.; Lee, J.-H.; Kim, B.-K.; Shin, H.; Kim, J.; Park, S. Nano-Interface Engineering in All-Solid-State Lithium Metal Batteries: Tailoring Exposed Crystal Facets of Epitaxially Grown LiNi0.5Mn1.5O4 Films. Nano Energy 2021, 79, 105480. [Google Scholar] [CrossRef]
- Sun, J.; Ye, L.; Zhao, X.; Zhang, P.; Yang, J. Electronic Modulation and Structural Engineering of Carbon-Based Anodes for Low-Temperature Lithium-Ion Batteries: A Review. Molecules 2023, 28, 2108. [Google Scholar] [CrossRef]
- Payandeh, S.; Strauss, F.; Mazilkin, A.; Kondrakov, A.; Brezesinski, T. Tailoring the LiNbO3 Coating of Ni-Rich Cathode Materials for Stable and High-Performance All-Solid-State Batteries. Nano Res. Energy 2022, 1, e9120016. [Google Scholar] [CrossRef]
- Hietaniemi, M.; Hu, T.; Välikangas, J.; Niittykoski, J.; Lassi, U. Effect of Precursor Particle Size and Morphology on Lithiation of Ni0.6Mn0.2Co0.2(OH)2. J. Appl. Electrochem. 2021, 51, 1545–1557. [Google Scholar] [CrossRef]
- Välikangas, J.; Laine, P.; Hietaniemi, M.; Hu, T.; Tynjälä, P.; Lassi, U. Precipitation and Calcination of High-Capacity LiNiO2 Cathode Material for Lithium-Ion Batteries. Appl. Sci. 2020, 10, 8988. [Google Scholar] [CrossRef]
- Kraft, L.; Zünd, T.; Schreiner, D.; Wilhelm, R.; Günter, F.J.; Reinhart, G.; Gasteiger, H.A.; Jossen, A. Comparative Evaluation of LMR-NCM and NCA Cathode Active Materials in Multilayer Lithium-Ion Pouch Cells: Part II. Rate Capability, Long-Term Stability, and Thermal Behavior. J. Electrochem. Soc. 2021, 168, 020537. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Zhong, J.; Wang, T.; Wang, L.; Xu, H.; Cao, J.; Li, J.; Zhang, G.; Fei, H.; et al. Hierarchically Micro/Nanostructured Current Collectors Induced by Ultrafast Femtosecond Laser Strategy for High-Performance Lithium-ion Batteries. Energy Environ. Mater. 2022, 5, 969–976. [Google Scholar] [CrossRef]
- Tomon, C.; Sarawutanukul, S.; Phattharasupakun, N.; Duangdangchote, S.; Chomkhuntod, P.; Joraleechanchai, N.; Bunyanidhi, P.; Sawangphruk, M. Core-Shell Structure of LiMn2O4 Cathode Material Reduces Phase Transition and Mn Dissolution in Li-Ion Batteries. Commun. Chem. 2022, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Deiner, L.J.; Tsao, B.H.; Fellner, J.P. Aerosol Jet-Printed LFP Cathodes with Bimodal Pore Distribution Improve the Rate Capability of LIB Cells. ACS Appl. Energy Mater. 2021, 4, 9507–9512. [Google Scholar] [CrossRef]
- Sliz, R.; Molaiyan, P.; Fabritius, T.; Lassi, U. Printed Electronics to Accelerate Solid-State Battery Development. Nano Express 2022, 3, 021002. [Google Scholar] [CrossRef]
- Hong, J.; Gent, W.E.; Xiao, P.; Lim, K.; Seo, D.-H.; Wu, J.; Csernica, P.M.; Takacs, C.J.; Nordlund, D.; Sun, C.-J.; et al. Metal–Oxygen Decoordination Stabilizes Anion Redox in Li-Rich Oxides. Nat. Mater. 2019, 18, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Zhang, G.; Wu, D.; Sun, F.; Tao, S.; Chu, S.; Qian, B.; Chu, W.; Song, L. Insight into the Cation Migration and Surface Structural Evolution of Spinel LiNi0.5Mn1.5O4 Cathode Material for Lithium-Ion Batteries. Chem. Eng. J. 2023, 451, 138708. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Yu, F.; Deng, L.; Ke, W.; Zhang, S.; Que, L.; Wu, B.; Ding, F.; Zhao, L.; et al. Addressing Mn Dissolution in High-Voltage LiNi0.5Mn1.5O4 Cathodes via Interface Phase Modulation. Adv. Funct. Mater. 2022, 32, 2207285. [Google Scholar] [CrossRef]
- Ma, C.; Wen, Y.; Qiao, Q.; He, P.; Ren, S.; Li, M.; Zhao, P.; Qiu, J.; Tang, G. Improving Electrochemical Performance of High-Voltage Spinel LiNi0.5Mn1.5O4 Cathodes by Silicon Oxide Surface Modification. ACS Appl. Energy Mater. 2021, 4, 12201–12210. [Google Scholar] [CrossRef]
- Gao, C.; Liu, H.; Bi, S.; Wang, Y.; Wang, Q.; Fan, S.; Meng, X. Insights for the New Function of N,N-Dimethylpyrrolidone in Preparation of a High-Voltage Spinel LiNi0.5Mn1.5O4 Cathode. ACS Appl. Mater. Inter. 2021, 13, 20014–20023. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hua, W.; Zhou, S.; He, X.; Liu, L. In Situ Synchrotron Radiation Diffraction Study of the Li+ de/Intercalation Behavior in Spinel LiNi0.5Mn1.5O4−δ. Chem. Eng. J. 2020, 400, 125998. [Google Scholar] [CrossRef]
- Wei, L.; Tao, J.; Yang, Y.; Fan, X.; Ran, X.; Li, J.; Lin, Y.; Huang, Z. Surface Sulfidization of Spinel LiNi0.5Mn1.5O4 Cathode Material for Enhanced Electrochemical Performance in Lithium-Ion Batteries. Chem. Eng. J. 2020, 384, 123268. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Li, W.; Qiao, Y.; Shang, H.; Ge, W.; Qu, M.; Fan, W.; Xie, Z. The Synergetic Effect of LiMg0.5Mn1.5O4 Coating and Mg2+ Doping on Improving Electrochemical Performances of High-Voltage LiNi0.5Mn1.5O4 by Sol-Gel Self-Combustion Method. ChemistrySelect 2020, 5, 2593–2601. [Google Scholar] [CrossRef]
- Guo, X.; Yang, C.; Chen, J.; Tian, Q.; Zhang, H.; Huang, G. Facile Synthesis of Spinel LiNi0.5Mn1.5O4 as 5.0 V-Class High-Voltage Cathode Materials for Li-Ion Batteries. Chin. J. Chem. Eng. 2021, 39, 247–254. [Google Scholar] [CrossRef]
- Ulu Okudur, F.; Mylavarapu, S.K.; Safari, M.; De Sloovere, D.; D’Haen, J.; Joos, B.; Kaliyappan, P.; Kelchtermans, A.-S.; Samyn, P.; Van Bael, M.K.; et al. LiNi0.5Mn1.5O4−δ (LNMO) as Co-Free Cathode for Lithium Ion Batteries via Solution-Gel Synthesis: Particle Size and Morphology Investigation. J. Alloy. Compd. 2022, 892, 162175. [Google Scholar] [CrossRef]
- Liang, W.; Wang, P.; Ding, H.; Wang, B.; Li, S. Granularity Control Enables High Stability and Elevated-Temperature Properties of Micron-Sized Single-Crystal LiNi0.5Mn1.5O4 Cathodes at High Voltage. J. Mater. 2021, 7, 1049–1060. [Google Scholar] [CrossRef]
- Lin, F.; Guo, J.; Wang, L.; Zhou, Y.; Wu, H.; Zhou, D. Synergistic Effect of Mg and Y Co-Dopants on Enhancement of Electrochemical Properties of LiNi0.5Mn1.5O4 Spinel. Electrochim. Acta 2021, 399, 139433. [Google Scholar] [CrossRef]
- Nisar, U.; Al-Hail, S.A.J.A.; Petla, R.K.; Shakoor, R.A.; Essehli, R.; Kahraman, R.; AlQaradawi, S.Y.; Kim, D.K.; Belharouak, I.; Amin, M.R. Understanding the Origin of the Ultrahigh Rate Performance of a SiO2-Modified LiNi0.5Mn1.5O4 Cathode for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 7263–7271. [Google Scholar] [CrossRef]
- Laine, P.; Hietaniemi, M.; Välikangas, J.; Kauppinen, T.; Tynjälä, P.; Hu, T.; Wang, S.; Singh, H.; Ulla, L. Co-Precipitation of Mg-Doped Ni0.8Co0.1Mn0.1(OH)2: Effect of Magnesium Doping and Washing on the Battery Cell Performance. Dalton Trans. 2023, 52, 1413–1424. [Google Scholar] [CrossRef]
- Nisar, U.; Al-Hail, S.A.J.A.; Kumar, P.R.; Abraham, J.J.; Mesallam, S.M.A.; Shakoor, R.A.; Amin, R.; Essehli, R.; Al-Qaradawi, S. Fast and Scalable Synthesis of LiNi0.5Mn1.5O4 Cathode by Sol–Gel-assisted Microwave Sintering. Energy Technol. 2021, 9, 2100085. [Google Scholar] [CrossRef]
- Yin, C.; Bao, Z.; Tan, H.; Zhou, H.; Li, J. Metal-Organic Framework-Mediated Synthesis of LiNi0.5Mn1.5O4: Tuning the Mn3+ Content and Electrochemical Performance by Organic Ligands. Chem. Eng. J. 2019, 372, 408–419. [Google Scholar] [CrossRef]
- Rosedhi, N.D.; Idris, N.H.; Rahman, M.M.; Din, M.F.M.; Wang, J. Disordered Spinel LiNi0.5Mn1.5O4 Cathode with Improved Rate Performance for Lithium-Ion Batteries. Electrochim. Acta 2016, 206, 374–380. [Google Scholar] [CrossRef]
- Hallot, M.; Roussel, P.; Lethien, C. Sputtered LiNi0.5Mn1.5O4 Thin Films for Lithium-Ion Microbatteries. ACS Appl. Energy Mater. 2021, 4, 3101–3109. [Google Scholar] [CrossRef]
- Lahiru Sandaruwan, R.D.; Cong, L.; Ma, L.; Ma, S.; Wang, H. Tackling the Interfacial Issues of Spinel LiNi0.5Mn1.5O4 by Room-Temperature Spontaneous Dediazonation Reaction. ACS Appl. Mater. Inter. 2021, 13, 13264–13272. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, J.; Wei, Z.; Yang, H.; Ma, Z.; Ma, R.; Zhou, J.; Yang, Y.; Peng, L.; Fei, H.; et al. Bacteria-Derived Biological Carbon Building Robust Li–S Batteries. Nano Lett. 2019, 19, 4384–4390. [Google Scholar] [CrossRef]
- Spence, S.L.; Xu, Z.; Sainio, S.; Nordlund, D.; Lin, F. Tuning the Morphology and Electronic Properties of Single-Crystal LiNi0.5Mn1.5O4−δ: Exploring the Influence of LiCl–KCl Molten Salt Flux Composition and Synthesis Temperature. Inorg. Chem. 2020, 59, 10591–10603. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; He, Y.; Lu, K. Li4Ti5O12 Epitaxial Coating on LiNi0.5Mn1.5O4 Surface for Improving the Electrochemical Performance through Solvothermal-Assisted Processing. J. Alloy. Compd. 2019, 779, 978–984. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.-S.; Xia, Y.; Deng, L.; Que, L.-F.; Yu, F.-D.; Wang, Z.-B. Suppressed Phase Separation in Spinel LiNi0.5Mn1.5O4 Cathode via Interstitial Sites Modulation. Nano Energy 2022, 91, 106636. [Google Scholar] [CrossRef]
- Li, C.; Liu, B.; Jiang, N.; Ding, Y. Elucidating the Charge-Transfer and Li-Ion-Migration Mechanisms in Commercial Lithium-Ion Batteries with Advanced Electron Microscopy. Nano Res. Energy 2022, 1, e9120031. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, G.; Han, Y.; Yu, F.; Qin, X.; Shao, G.; Wang, Z. Boosted Electrochemical Performance of LiNi0.5Mn1.5O4 via Synergistic Modification of Li+-Conductive Li2ZrO3 Coating Layer and Superficial Zr-Doping. Electrochim. Acta 2020, 343, 136105. [Google Scholar] [CrossRef]
- Ren, S.; Wen, Y.; Chen, H.; He, P.; Ma, C.; Zhang, C.; Li, M.; Han, D. Bioinspired PDA@TiO2 Modification on High-Voltage LiNi0.5Mn1.5O4 toward Enhancing Electrochemical Performance. J. Alloy. Compd. 2021, 889, 161690. [Google Scholar] [CrossRef]
- Siqin, G.; Qilu; Tian, W. Scalable Synthesis of High-Voltage LiNi0.5Mn1.5O4 with High Electrochemical Performances by a Modified Solid-State Method for Lithium Ion Batteries. Inorg. Chem. Commun. 2021, 134, 109067. [Google Scholar] [CrossRef]
- Lee, B.-Y.; Chu, C.-T.; Krajewski, M.; Michalska, M.; Lin, J.-Y. Temperature-Controlled Synthesis of Spinel Lithium Nickel Manganese Oxide Cathode Materials for Lithium-Ion Batteries. Ceram. Int. 2020, 46, 20856–20864. [Google Scholar] [CrossRef]
- Lu, X.; Liu, C.; Zhu, W.; Lu, Z.; Li, W.; Yang, Y.; Yang, G. Synthesis of Micron-Sized LiNi0.5Mn1.5O4 Single Crystals through in Situ Microemulsion/Coprecipitation and Characterization of Their Electrochemical Capabilities. Powder Technol. 2019, 343, 445–453. [Google Scholar] [CrossRef]
- Wei, A.; Chang, L.; Luo, S.; Cao, S.; Bi, X.; Yang, W.; Liu, J.; Zhang, F. Preparation of LiNi0.5Mn1.5O4 Cathode Materials by Non-Constant Temperature Calcination and Research on Its Performance. Ionics 2021, 28, 555–565. [Google Scholar] [CrossRef]
- Liang, C.; Li, L.; Fang, H. New Molten Salt Synthesis of LiNi0.5Mn1.5O4 Cathode Material. Mater. Res. Express 2018, 5, 075511. [Google Scholar] [CrossRef]


| Sample | Mn | Ni | |||||
|---|---|---|---|---|---|---|---|
| Mn3+/% | Mn4+/% | Valence | Ni2+/% | Ni3+/% | Ni4+/% | Valence | |
| LNMO_80 | 32.2 | 67.8 | 3.678 | 48.9 | 28.1 | 23.0 | 2.741 |
| LNMO_110 | 27.7 | 72.3 | 3.723 | 23.8 | 50.1 | 26.1 | 3.023 |
| LNMO_140 | 29.1 | 70.9 | 3.709 | 39.2 | 17.6 | 43.2 | 3.040 |
| LNMO_170 | 34.1 | 65.9 | 3.659 | 44.8 | 39.7 | 15.4 | 2.706 |
| Sample Name | Current Density (C) | Specific Capacity (mAh g−1) | Cycle no. | Ref. |
|---|---|---|---|---|
| LiNi0.5Mn1.5O4 | 0.3 | 130.6 | 300 | [43] |
| LNMO-900 | 0.1 | 135 | 50 | [44] |
| LNM-0.25 | 1 | 119 | 200 | [45] |
| LiNi0.5Mn1.5O4- 5.0 °C/min | 1 | 102 | 100 | [46] |
| 1000 °C-40 h | 0.2 | 134 | 50 | [47] |
| D-700 | 0.1 | 101.4 | 50 | [31] |
| LNMO_110 | 0.1 | 116.8 | 100 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Välikangas, J.; Sliz, R.; Molaiyan, P.; Hu, T.; Lassi, U. Optimized Morphology and Tuning the Mn3+ Content of LiNi0.5Mn1.5O4 Cathode Material for Li-Ion Batteries. Materials 2023, 16, 3116. https://doi.org/10.3390/ma16083116
Lin Y, Välikangas J, Sliz R, Molaiyan P, Hu T, Lassi U. Optimized Morphology and Tuning the Mn3+ Content of LiNi0.5Mn1.5O4 Cathode Material for Li-Ion Batteries. Materials. 2023; 16(8):3116. https://doi.org/10.3390/ma16083116
Chicago/Turabian StyleLin, Yan, Juho Välikangas, Rafal Sliz, Palanivel Molaiyan, Tao Hu, and Ulla Lassi. 2023. "Optimized Morphology and Tuning the Mn3+ Content of LiNi0.5Mn1.5O4 Cathode Material for Li-Ion Batteries" Materials 16, no. 8: 3116. https://doi.org/10.3390/ma16083116





