New Accelerated Corrosion Test Method Simulating Atmospheric Corrosion of Complex Phase Steel Combining Cyclic Corrosion Test and Electrochemically Accelerated Corrosion Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens and Cyclic Corrosion Test
2.2. Electrochemical Testing Setup
2.3. Surface Analysis
3. Results and Discussion
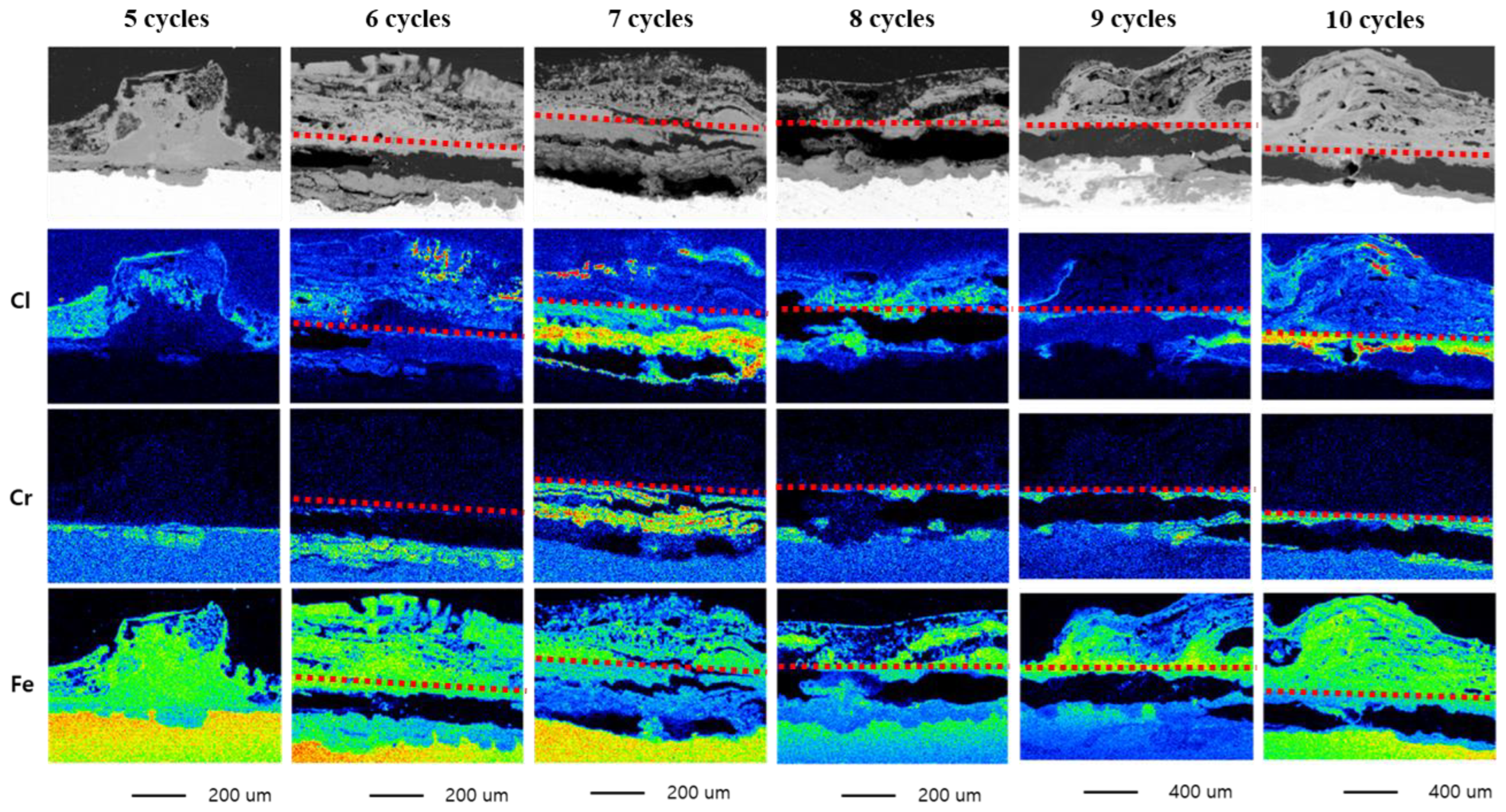
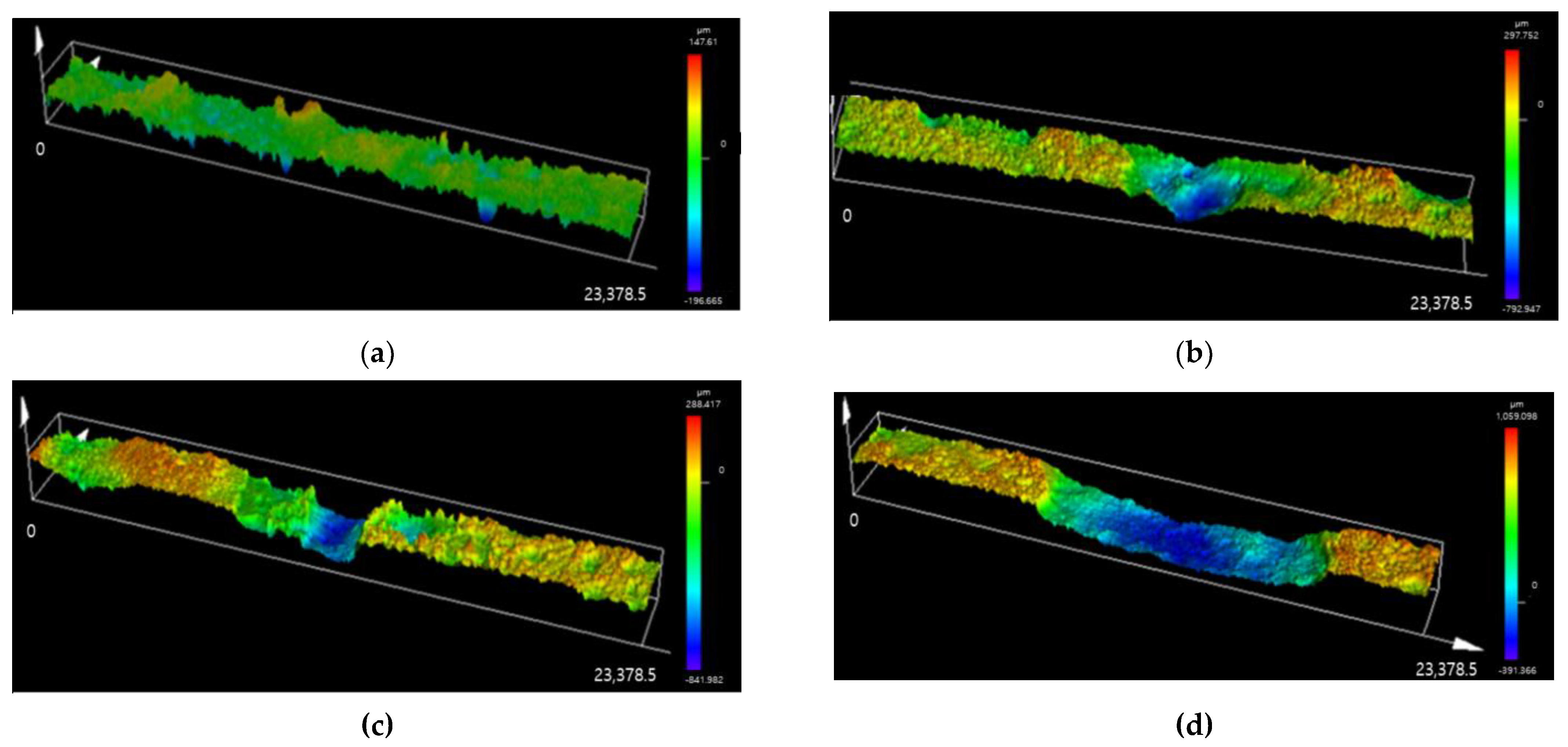
3.1. Minimum CCT Cycles for Localized Corrosion
3.2. E-CCT Cell Configuration
3.3. E-CCT
4. Conclusions
- A new electrochemical cyclic corrosion testing (E-CCT) method was developed to evaluate the localized corrosion penetration criteria for CP steel. This method shortens the material evaluation period by half.
- It has been confirmed that a minimum of seven CCT cycles is required to form a corrosion product, such as β-FeOOH, that can cause localized corrosion on the specimen’s surface. Considering the variability of the CCT, the minimum number of CCT cycles in which corrosion products are formed was set to ten.
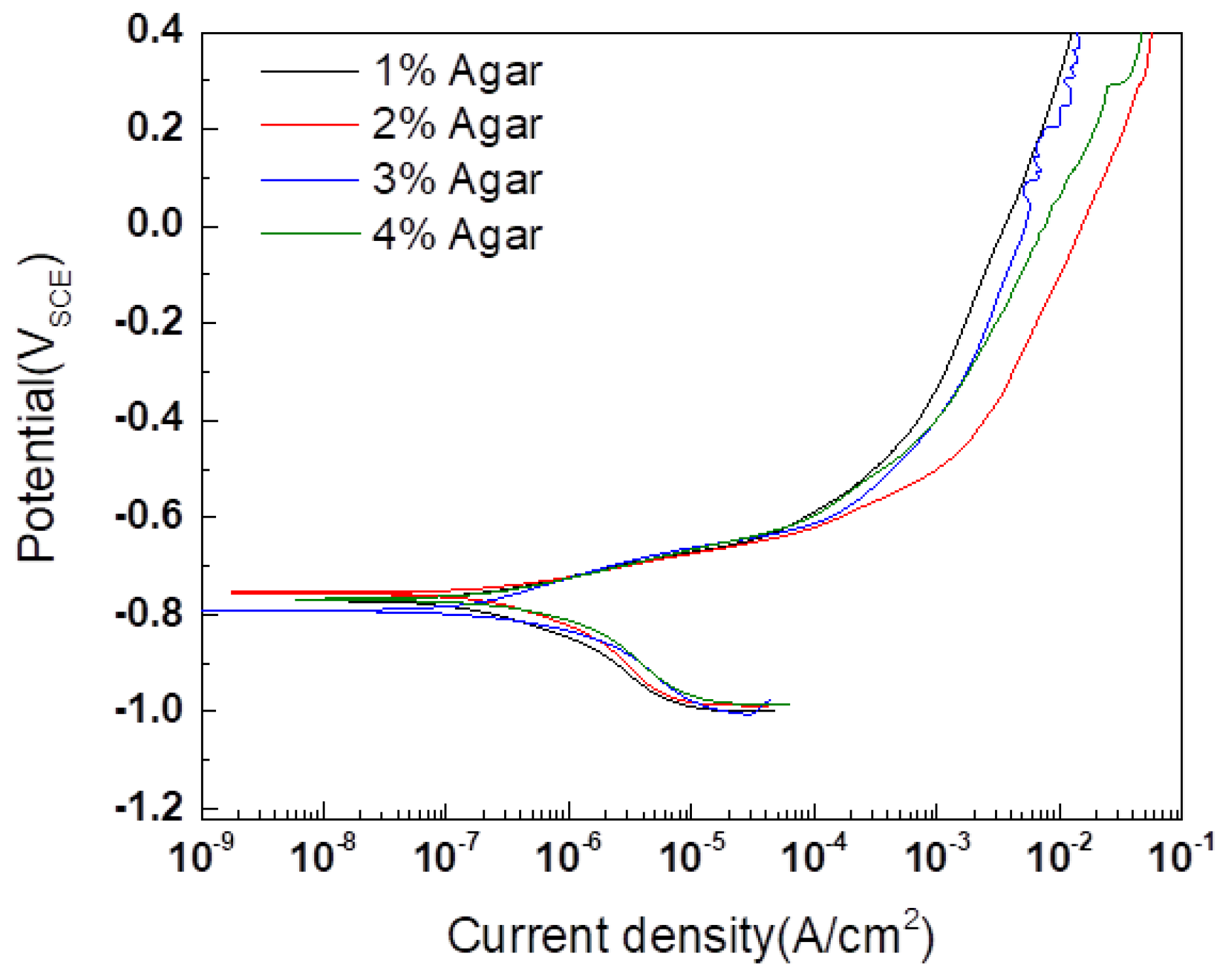
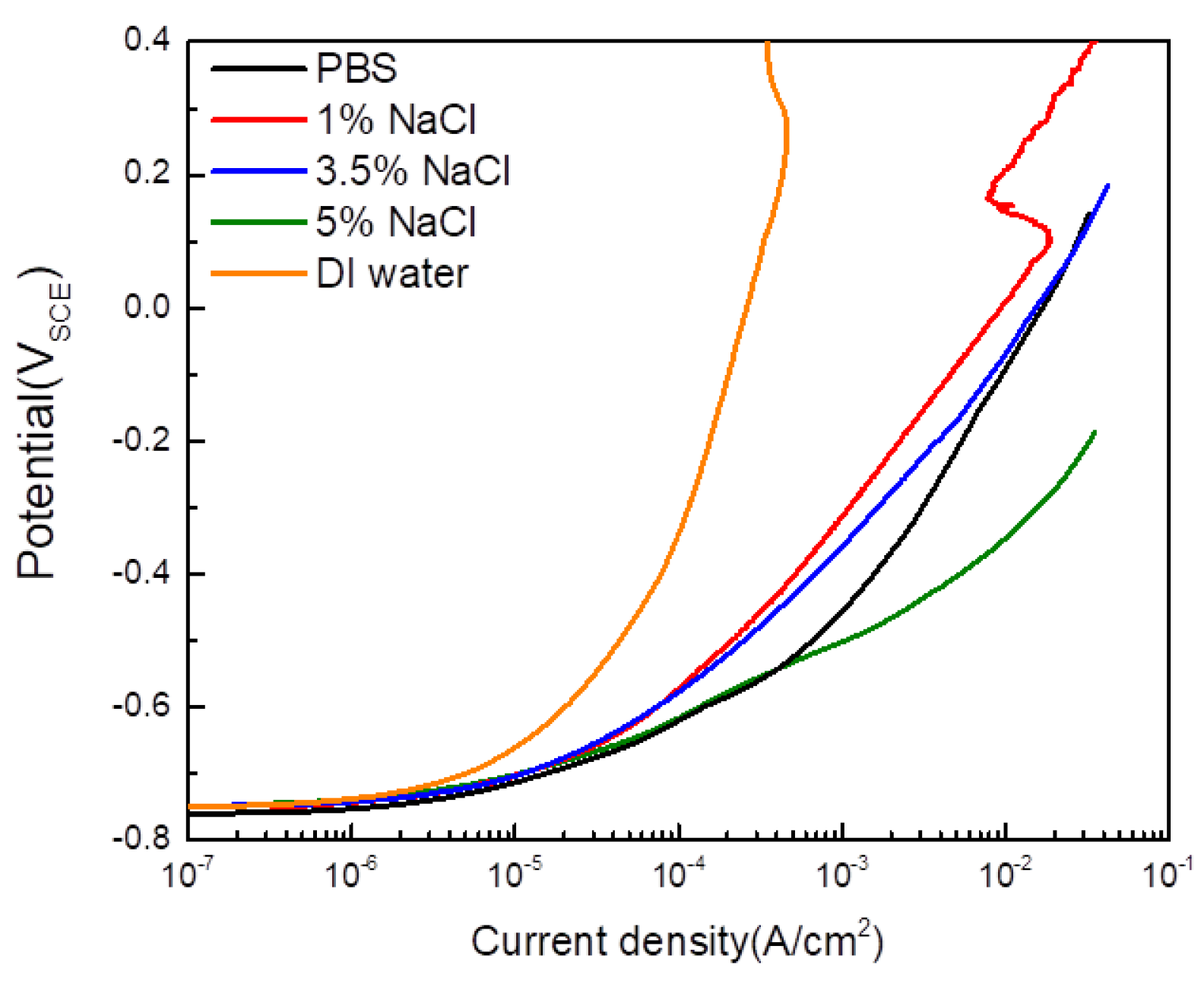
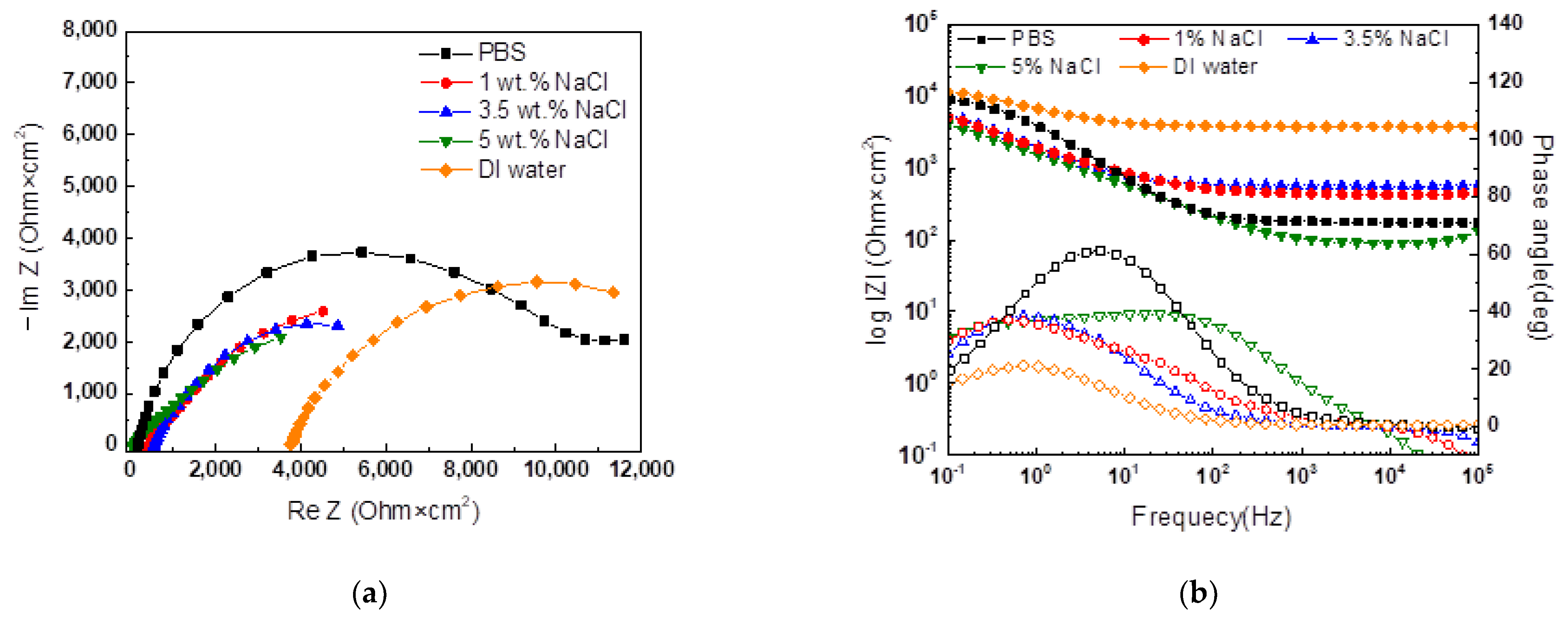
- An experimental cell was designed to preserve the corrosion product formed by CCT and to proceed with electrochemical acceleration. The electrolyte used was composed of agar and PBS. The agar electrolyte provided an adequate amount of water to the specimen through its internal matrix and properties of syneresis, preventing the corrosion products’ deformation and enabling electrochemical acceleration. The optimal agar concentration, confirmed at 2%, showed sufficient ion conductivity and a low corrosion rate. PBS was used as an external electrolyte positioned on top of the agar, applied to suppress environmental changes through its pH buffer characteristics and to serve as a source of water for the reduction reaction on the counter electrode during acceleration.
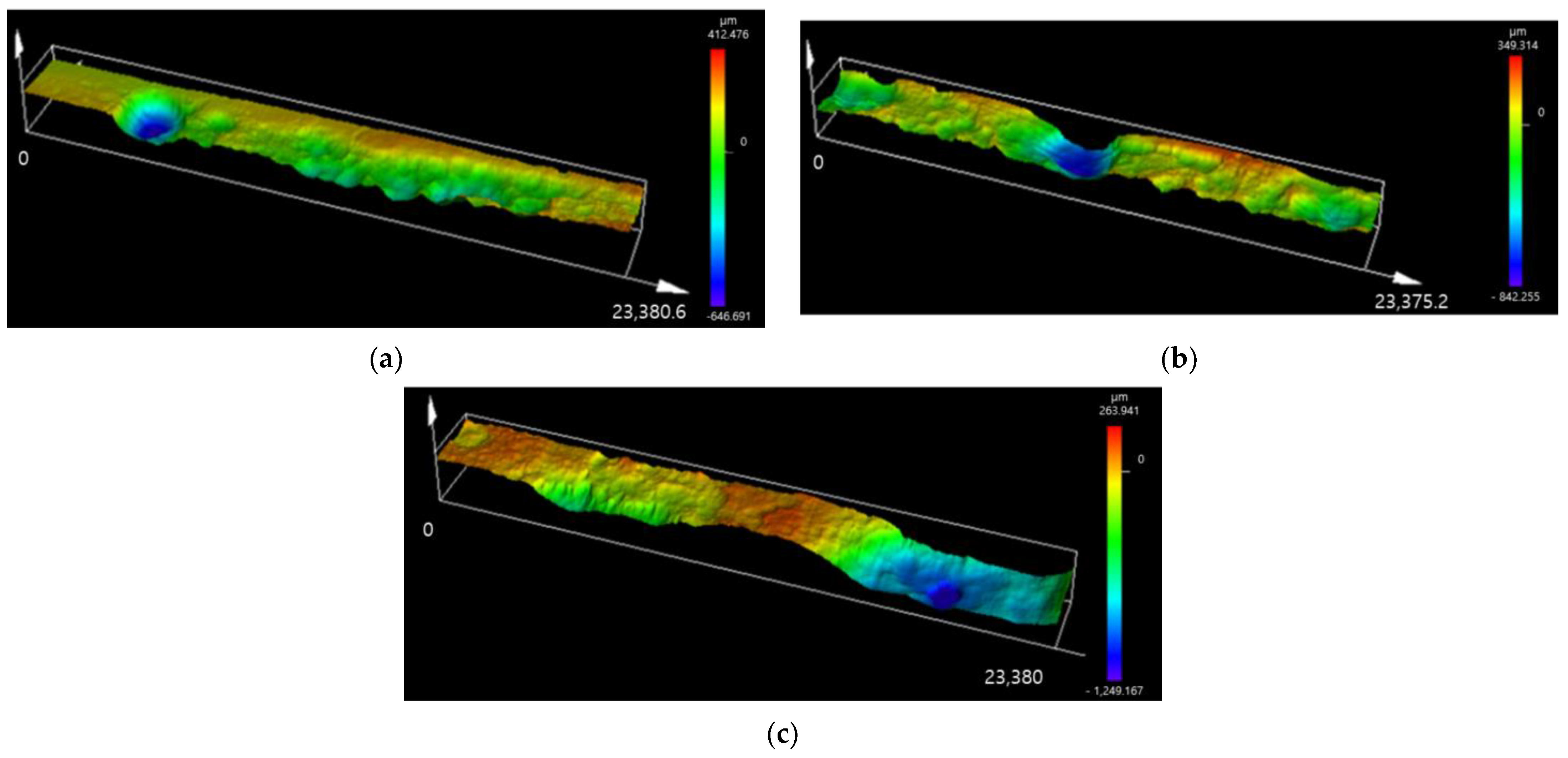
- The surfaces of E-CCT specimens subjected to 20, 25, and 30 cycles of the CCT were analyzed. The results confirmed that the ratio of the area where localized corrosion occurred to the total reaction area and the maximum localized corrosion depth were similar. Considering the shortened evaluation period and these results, it is judged that the E-CCT has potential as an alternative to the CCT. However, differences were observed in the distribution and shape of localized corrosion, as well as in the depth of uniform corrosion, when compared to the CCT. These differences are judged to be due to minimizing corrosion factors other than corrosion products formed through the CCT in the E-CCT.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, S.; Zhang, Y.; Qi, L.; Kang, Y. Effect of single tensile overload on fatigue crack growth behavior in DP780 dual phase steel. Int. J. Fatigue 2018, 106, 49–55. [Google Scholar] [CrossRef]
- Sharifimehr, S.; Fatemi, A.; Cha, S.C.; Bae, M.-K.; Hong, S.-H. Fatigue behavior of AHSS lap shear and butt arc welds including the effect of periodic overloads and underloads. Int. J. Fatigue 2016, 87, 6–14. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Yang, F.; Chen, H.; Liang, N.; Wu, Y.; Zhang, J.; Ma, H.; Klu, E.E.; Gao, B.; et al. Corrosion behavior and mechanism of Cr–Mo alloyed steel: Role of ferrite/bainite duplex microstructure. J. Alloys Compd. 2019, 809, 151787. [Google Scholar] [CrossRef]
- LeBozec, N.; Blandin, N.; Thierry, D. Accelerated corrosion tests in the automotive industry: A comparison of the performance towards cosmetic corrosion. Mater. Corros. 2018, 59, 889–894. [Google Scholar] [CrossRef]
- Alcántara, J.; de la Fuente, D.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine atmospheric corrosion of carbon steel: A review. Materials 2017, 10, 406–472. [Google Scholar] [CrossRef]
- Yu, J.; Wang, H.; Yu, Y.; Luo, Z.; Liu, W.; Wang, C. Corrosion behavior of X65 pipeline steel: Comparison of wet–dry cycle and full immersion. Corros. Sci. 2018, 133, 276–287. [Google Scholar] [CrossRef]
- Morcillo, M.; González-Calbet, J.M.; Jiménez, J.A.; Díaz, I.; Alcántara, J.; Chico, B.; Mazario-Fernandez, A.; Gomez-Herrero, A.; Liorente, I.; De la Fuente, D. Environmental conditions for akaganeite formation in marine atmosphere mild steel corrosion products and its characterization. Corrosion 2015, 71, 872–886. [Google Scholar] [CrossRef]
- Cho, S.W.; Ko, S.J.; Yoo, J.S.; Yoo, Y.H.; Song, Y.K.; Kim, J.G. Effect of Cr on Aqueous and Atmospheric Corrosion of Automotive Carbon Steel. Materials 2021, 14, 2444. [Google Scholar] [CrossRef] [PubMed]
- Chico, B.; Alcántara, J.; Pino, E.; Díaz, I.; Simancas, J.; Torres-Pardo, A.; de la Fuente, D.; Antonio Jimenez, J.; Marco, J.F.; Maria Gonzalez-calbet, J.; et al. Rust exfoliation on carbon steels in chloride-rich atmospheres. Corros. Rev. 2015, 33, 263–282. [Google Scholar] [CrossRef]
- Yang, W.; Ni, R.-C.; Hua, H.-Z.; Pourbaix, A. The behavior of chromium and molybdenum in the propagation process of localized corrosion of steels. Corros. Sci. 1984, 24, 691–707. [Google Scholar] [CrossRef]
- Barat, B.R.; Cano, E. The use of agar gelled electrolyte for in situ electrochemical measurements on metallic cultural heritage. Electrochim. Acta 2015, 182, 751–762. [Google Scholar] [CrossRef]
- Valet, S.; Burkert, A.; Ebell, G.; Babutzka, M. Determination of the corrosion product layer resistance on zinc and electrolytically galvanized steel samples by using gel electrolytes. Electrochim. Acta 2021, 385, 138191. [Google Scholar] [CrossRef]
- Monrrabal, G.; Bautista, A.; Velasco, F. Use of innovative gel electrolytes for electrochemical corrosion measurements on carbon and galvanized steel surfaces. Corrosion 2019, 75, 1502–1512. [Google Scholar] [CrossRef]
- Bai, Z.; Dan, W.; Yu, G.; Wang, Y.; Chen, Y.; Huang, Y.; Yang, C.; Dan, N. Tough and tissue-adhesive polyacrylamide/collagen hydrogel with dopamine-grafted oxidized sodium alginate as crosslinker for cutaneous wound healing. RSC Adv. 2018, 8, 42123–42132. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.G.; Kim, G.P.; Lee, M.; Song, H.D.; Yi, J. A biodegradable gel electrolyte for use in high-performance flexible supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3503–3511. [Google Scholar] [CrossRef]
- Bennett, D. NaCl doping and the conductivity of agar phantoms. Mater. Sci. Eng. C 2011, 31, 494–498. [Google Scholar] [CrossRef]
- Hunold, A.; Machts, R.; Haueisen, J. Head phantoms for bioelectromagnetic applications: A material study. BioMed. Eng. OnLine 2020, 19, 87. [Google Scholar] [CrossRef]
- Rahbani, J.; Behzad, A.R.; Khashab, N.M.; Al-Ghoul, M. Characterization of internal structure of hydrated agar and gelatin matrices by cryo-SEM. Electrophoresis 2013, 34, 405–408. [Google Scholar] [CrossRef]
- Pernodet, N.; Maaloum, M.; Tinland, B. Pore size of agarose gels by atomic force microscopy. Electrophoresis 1997, 18, 55–58. [Google Scholar] [CrossRef]
- Maaloum, M.; Pernodet, N.; Tinland, B. Agarose gel structure using atomic force microscopy: Gel concentration and ionic strength effects. Electrophoresis 1998, 19, 1606–1610. [Google Scholar] [CrossRef]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar gel strength: A correlation study between chemical composition and rheological properties. Eur. Polym. J. 2020, 123, 109442. [Google Scholar] [CrossRef]
- Liew, S.Y.; Juan, J.C.; Lai, C.W.; Pan, G.T.; Yang, T.C.K.; Lee, T.K. An eco-friendly water-soluble graphene-incorporated agar gel electrolyte for magnesium-air batteries. Ionics 2019, 25, 1291–1301. [Google Scholar] [CrossRef]
- Heyn, A. Comparison of liquid and gel electrolytes for the investigation of pitting corrosion on stainless steels. IOP Conf. Ser. Mater. Sci. Eng. 2020, 882, 012010. [Google Scholar] [CrossRef]
- Pasquini, L.; Wacrenier, O.; Vona, M.D.; Knauth, P. Hydration and ionic conductivity of model cation and anion-conducting ionomers in buffer solutions (Phosphate, Acetate, Citrate). J. Phys. Chem. B 2018, 122, 12009–12016. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.V.; Muñoz, A.I. Electrochemical characterisation of biomedical alloys for surgical implants in simulated body fluids. Corros. Sci. 2008, 50, 1954–1961. [Google Scholar] [CrossRef]







| wt.% | C | Si | Mn | Cr | Ni | Al | Nb | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|
| CP steel | 0.11–0.18 | 0.4–1.2 | 1.8–2.6 | 0.5–1.5 | 0.8–1.2 | 0.01–0.03 | 0.01–0.02 | 0.01–0.03 | Bal. |
| Ecorr (VSCE) | icorr (μA/cm2) | βa (V/Decade) | βc (V/Decade) | |
|---|---|---|---|---|
| 1% agar | −0.773 | 0.518 | 0.072 | −0.195 |
| 2% agar | −0.754 | 0.354 | 0.052 | −0.146 |
| 3% agar | −0.790 | 1.150 | 0.092 | −0.213 |
| 4% agar | −0.769 | 0.871 | 0.083 | −0.200 |
| Rs (Ω∙cm2) | CPE1 | Ragar (Ω∙cm2) | CPE2 | Rct (Ω∙cm2) | χ2 | |||
|---|---|---|---|---|---|---|---|---|
| Crust (F/cm2) | n1 | Crust (F/cm2) | n2 | |||||
| 1% agar | 133.6 | 3.00 × 10−5 | 0.2558 | 134.4 | 3.95 × 10−5 | 0.8678 | 16,580 | 2.215 × 10−4 |
| 2% agar | 176.5 | 4.45 × 10−5 | 0.8395 | 8374 | 5.62 × 10−5 | 0.323 | 20,850 | 3.028 × 10−5 |
| 3% agar | 381 | 5.56 × 10−5 | 0.7494 | 10,820 | 1.25 × 10−2 | 1 | 1448 | 5.812 × 10−4 |
| 4% agar | 474.5 | 4.93 × 10−5 | 0.7926 | 7669 | 1.25 × 10−3 | 0.4603 | 9773 | 1.630 × 10−5 |
| Ecorr (VSCE) | βa (V/Decade) | |
|---|---|---|
| 5% NaCl | −0.747 | 0.153 |
| 3.5% NaCl | −0.748 | 0.270 |
| 1% NaCl | −0.754 | 0.319 |
| PBS | −0.762 | 0.289 |
| DI water | −0.748 | 0.898 |
| Rs (Ω∙cm2) | CPE1 | Ragar (Ω∙cm2) | CPE2 | Rct (Ω∙cm2) | χ2 | |||
|---|---|---|---|---|---|---|---|---|
| Crust (F/cm2) | n1 | Crust (F/cm2) | n2 | |||||
| PBS | 176.5 | 4.45 × 10−5 | 0.8395 | 8374 | 5.62 × 10−4 | 0.323 | 20,850 | 3.028 × 10−5 |
| 1% NaCl | 434.1 | 5.16 × 10−5 | 0.7691 | 798.7 | 1.61 × 10−4 | 0.638 | 8809 | 3.396 × 10−5 |
| 3.5% NaCl | 567.3 | 8.07 × 10−5 | 0.8124 | 1561 | 1.21 × 10−4 | 0.717 | 5701 | 1.246 × 10−5 |
| 5% NaCl | 102.9 | 6.98 × 10−6 | 1 | 114.3 | 2.52 × 10−4 | 0.4887 | 13,460 | 3.576 × 10−4 |
| DI water | 3772 | 4.78 × 10−5 | 0.7393 | 5967 | 1.35 × 10−4 | 0.6063 | 5587 | 4.400 × 10−5 |
| Cycles (x) | Uniform Corrosion | Localized Corrosion |
|---|---|---|
| Depth (y) (mm) | Maximum Depth (y) (mm) | |
| 0 | 0.000 | 0.000 |
| 10 | 0.093 | 0.353 |
| 20 | 0.258 | 1.083 |
| 30 | 0.339 | 1.204 |
| Corrosion depth equation | y = 0.0107x | y = 0.0444x |
| Impressed Current Density (mA/cm2) | Time (Hour) | Charge per Unit Area (C/cm2) | Weight Loss (g/cm2) |
|---|---|---|---|
| 2.140 | 120 | 924.458 | 0.257 |
| CCT | E-CCT | |
|---|---|---|
| 20 cycles | 17.4 | 21.1 |
| 25 cycles | 31.2 | 30.5 |
| 30 cycles | 33.7 | 34.3. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.M.; Kim, G.-i.; Son, G.-H.; Yoo, Y.-H.; Hong, S.; Kim, J.-G. New Accelerated Corrosion Test Method Simulating Atmospheric Corrosion of Complex Phase Steel Combining Cyclic Corrosion Test and Electrochemically Accelerated Corrosion Test. Materials 2023, 16, 3132. https://doi.org/10.3390/ma16083132
Kim KM, Kim G-i, Son G-H, Yoo Y-H, Hong S, Kim J-G. New Accelerated Corrosion Test Method Simulating Atmospheric Corrosion of Complex Phase Steel Combining Cyclic Corrosion Test and Electrochemically Accelerated Corrosion Test. Materials. 2023; 16(8):3132. https://doi.org/10.3390/ma16083132
Chicago/Turabian StyleKim, Kyung Min, Geon-il Kim, Gyeong-Ho Son, Yun-Ha Yoo, Sujik Hong, and Jung-Gu Kim. 2023. "New Accelerated Corrosion Test Method Simulating Atmospheric Corrosion of Complex Phase Steel Combining Cyclic Corrosion Test and Electrochemically Accelerated Corrosion Test" Materials 16, no. 8: 3132. https://doi.org/10.3390/ma16083132
APA StyleKim, K. M., Kim, G.-i., Son, G.-H., Yoo, Y.-H., Hong, S., & Kim, J.-G. (2023). New Accelerated Corrosion Test Method Simulating Atmospheric Corrosion of Complex Phase Steel Combining Cyclic Corrosion Test and Electrochemically Accelerated Corrosion Test. Materials, 16(8), 3132. https://doi.org/10.3390/ma16083132






