Development of Hydrophobic Coal-Fly-Ash-Based Ceramic Membrane for Vacuum Membrane Distillation
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
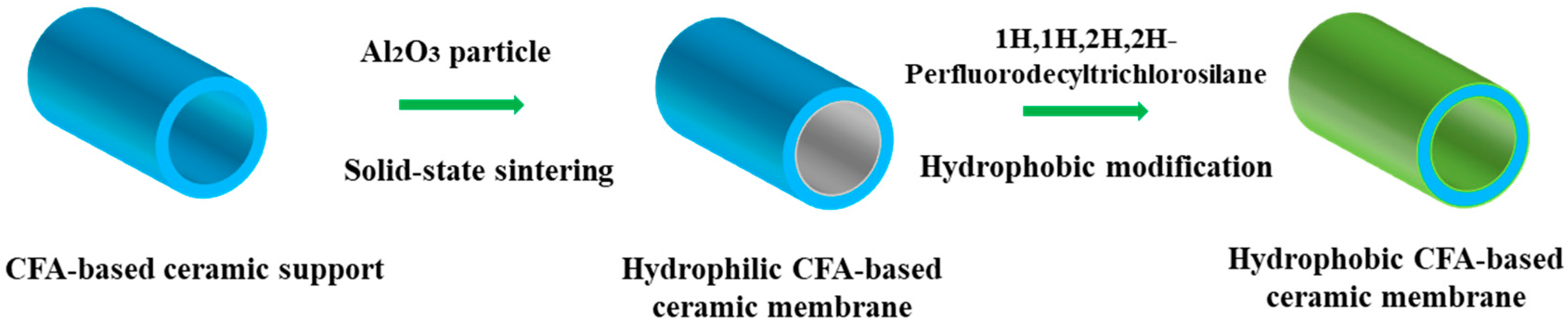
2.2. Preparation of CFA-Based Ceramic Membranes
2.3. Preparation of Hydrophobic Ceramic Membranes
2.4. Characterization
2.5. Membrane Distillation Test Using Hydrophobic Ceramic Membranes
3. Results and Discussion
3.1. Characterization of Fabricated Membranes
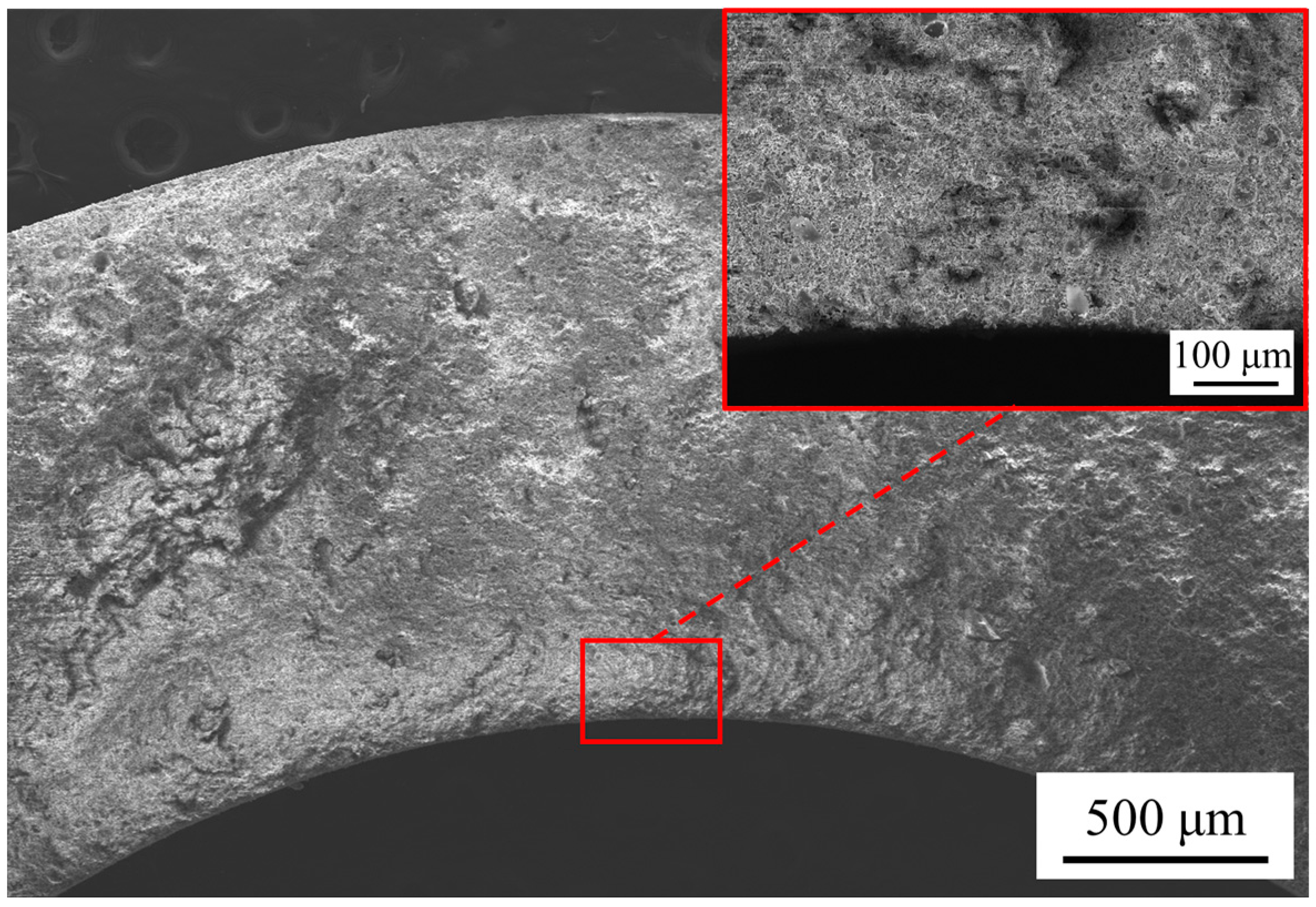
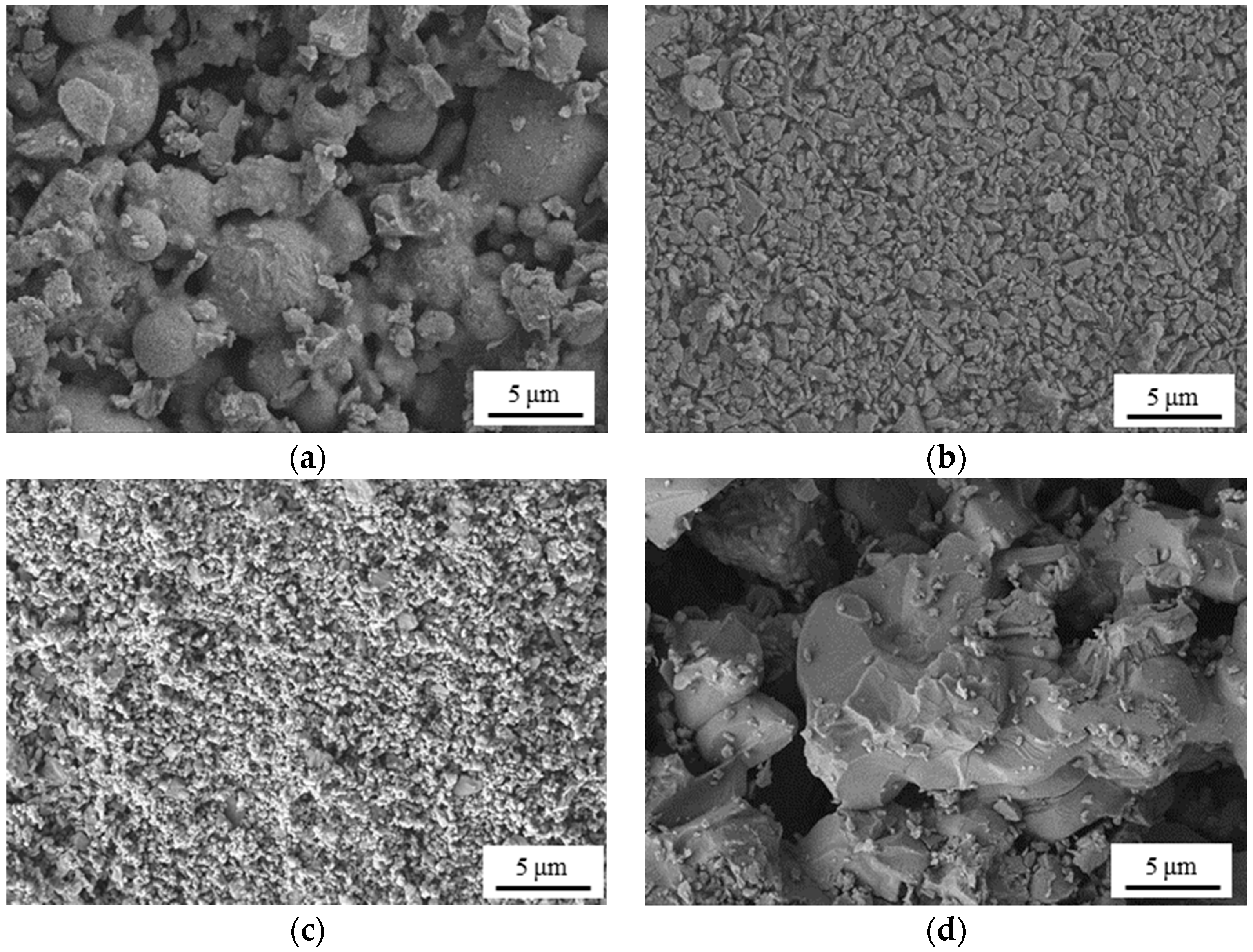
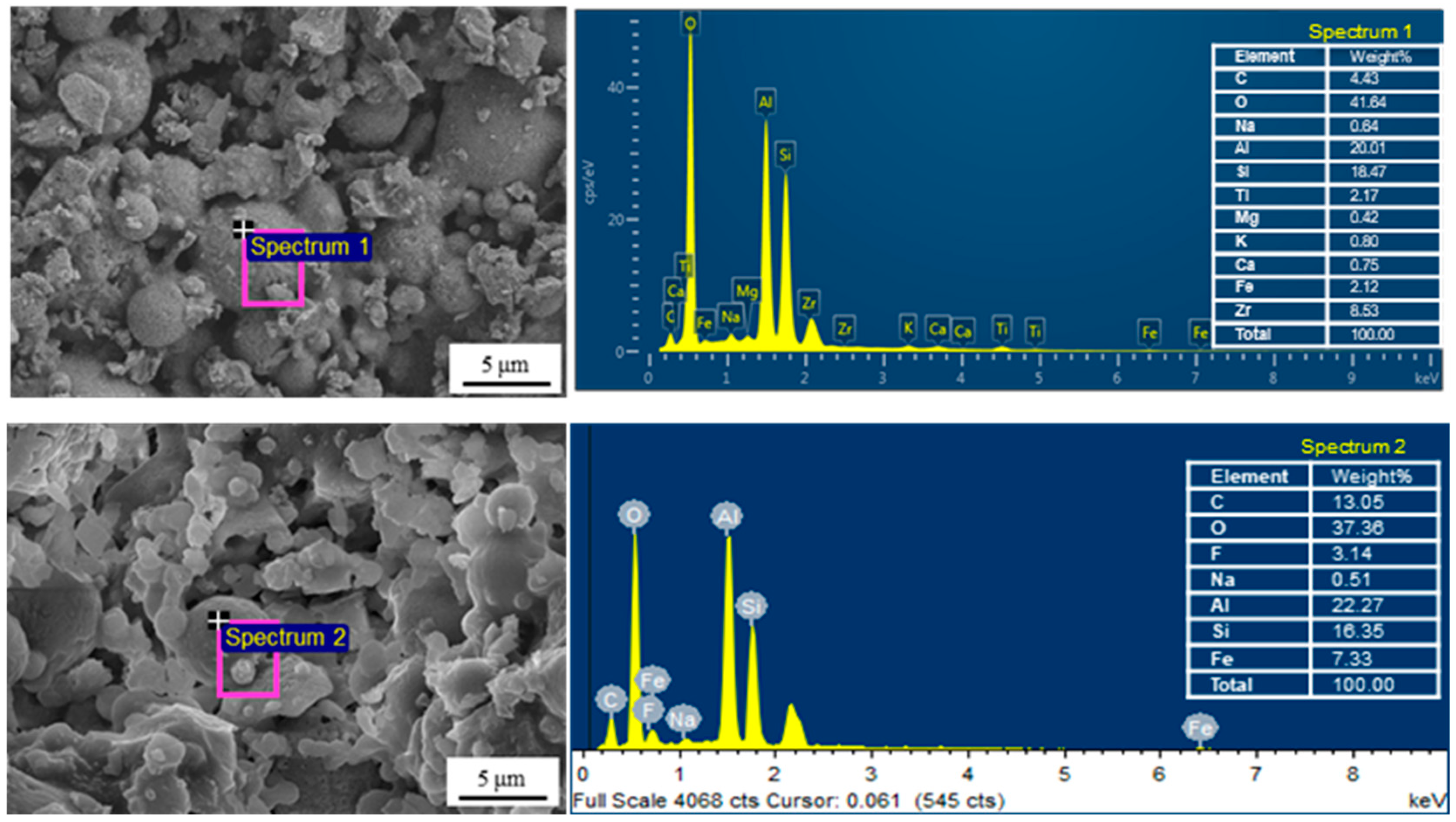
3.1.1. Morphology and Element Composition
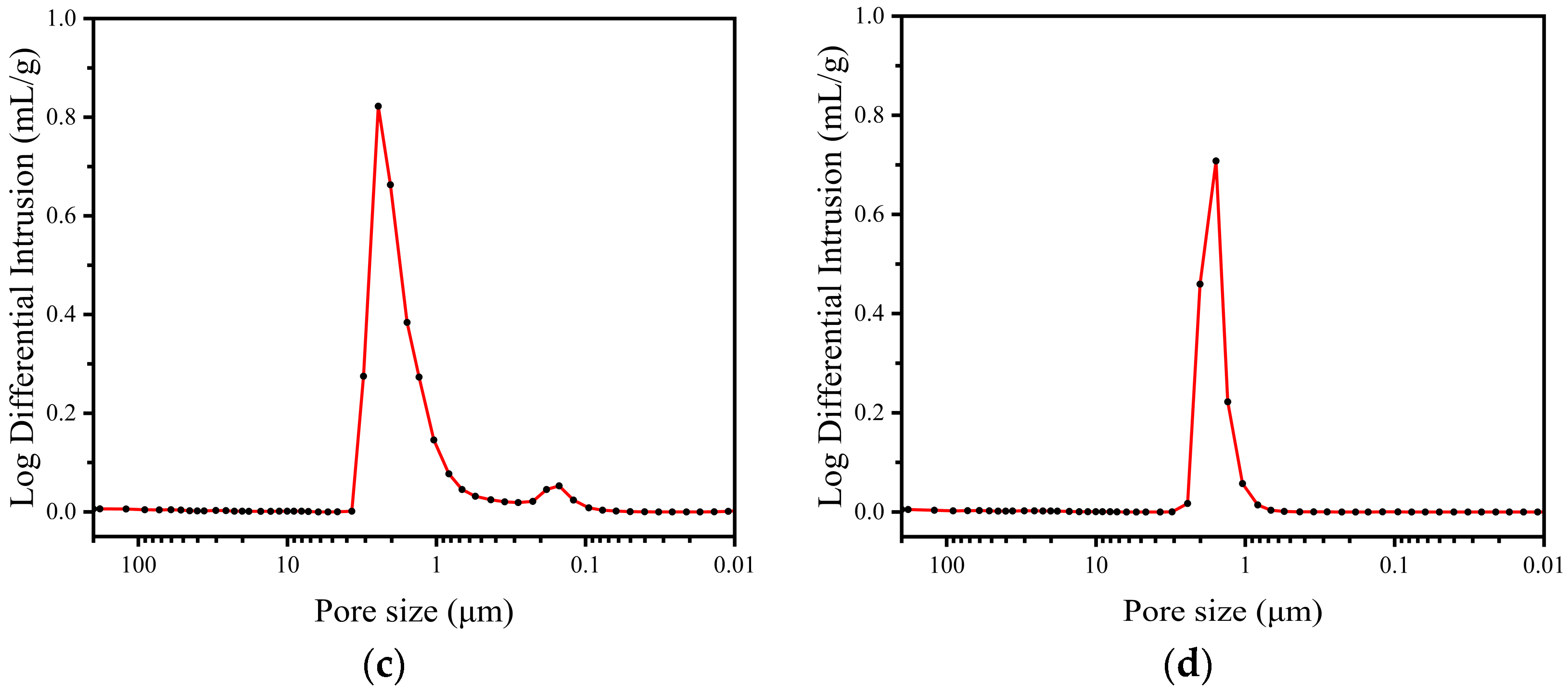
3.1.2. Pore Size Distribution
3.1.3. Pure Water Flux
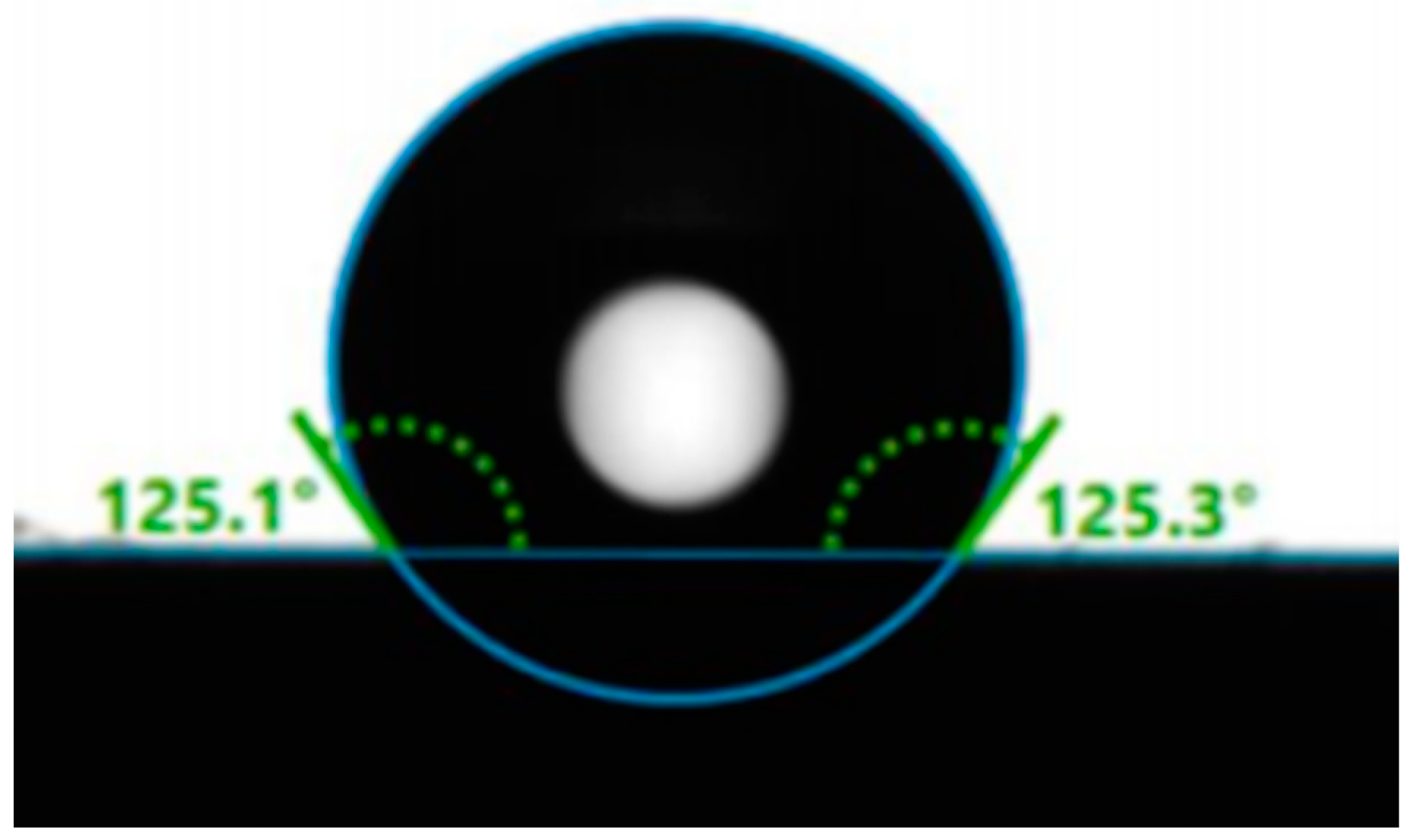
3.1.4. Wettability
3.1.5. Liquid Entry Pressure (LEP)
3.2. Vacuum Membrane Distillation Test
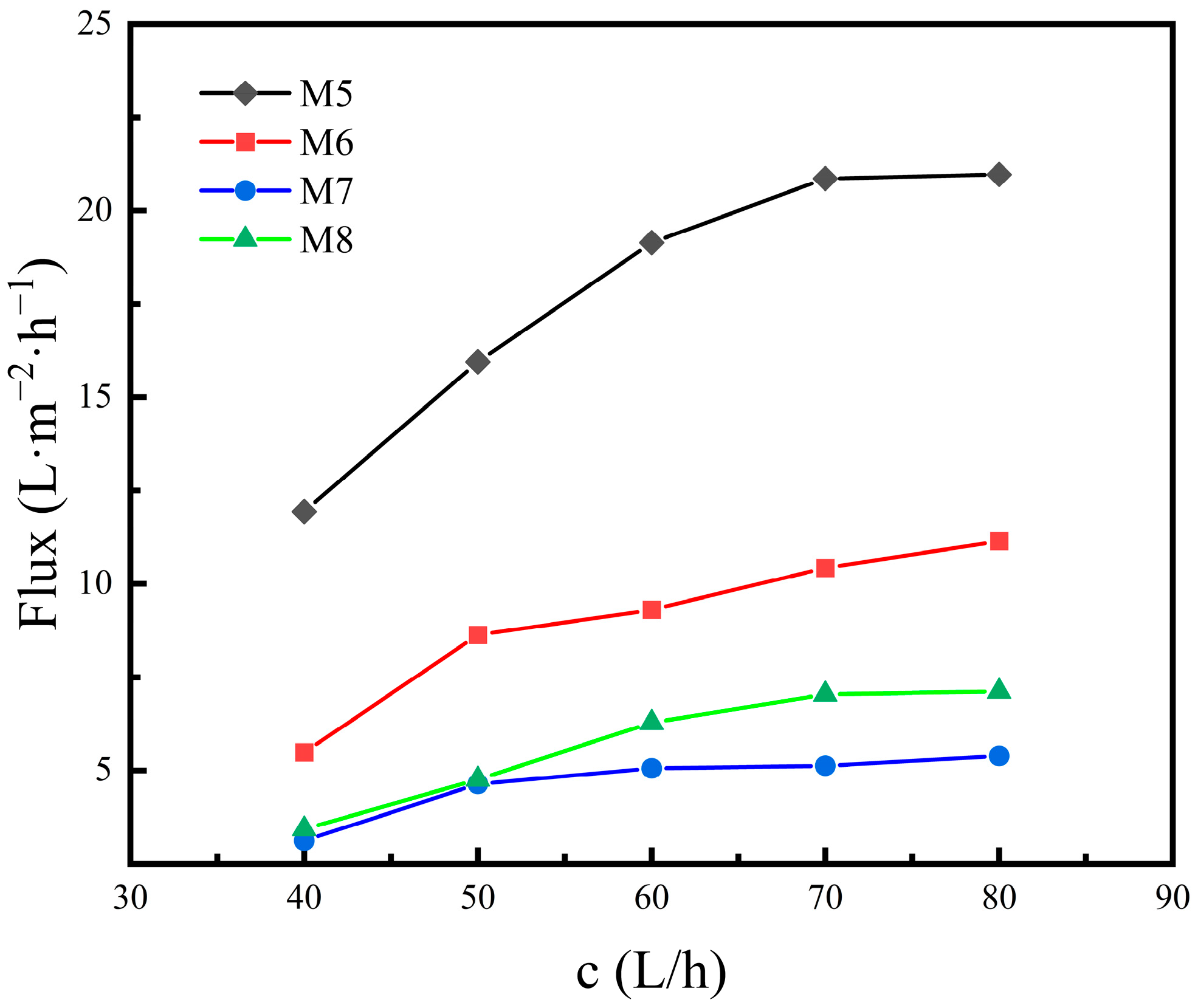
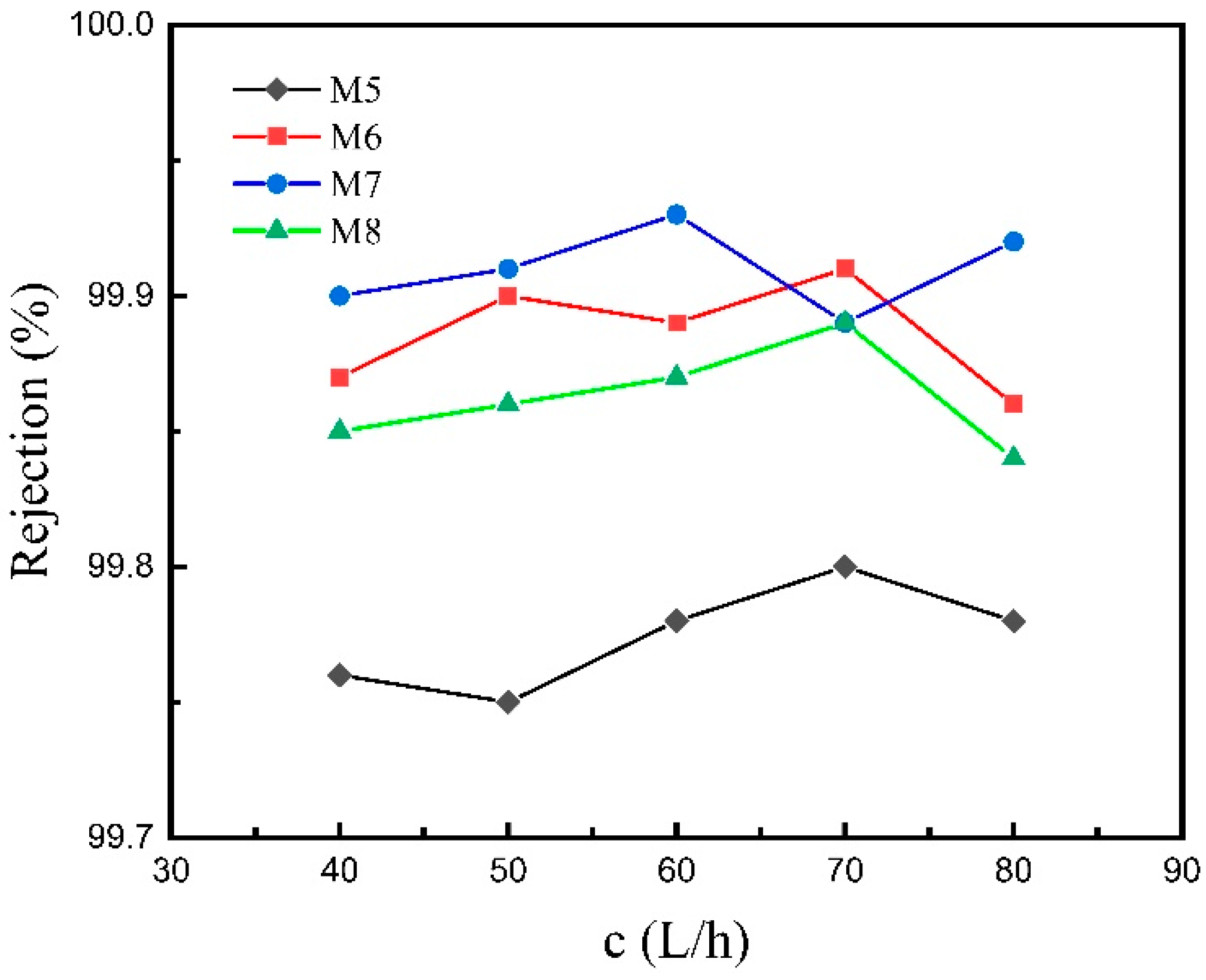
3.2.1. VMD Performance at Different Feed Flow Rates
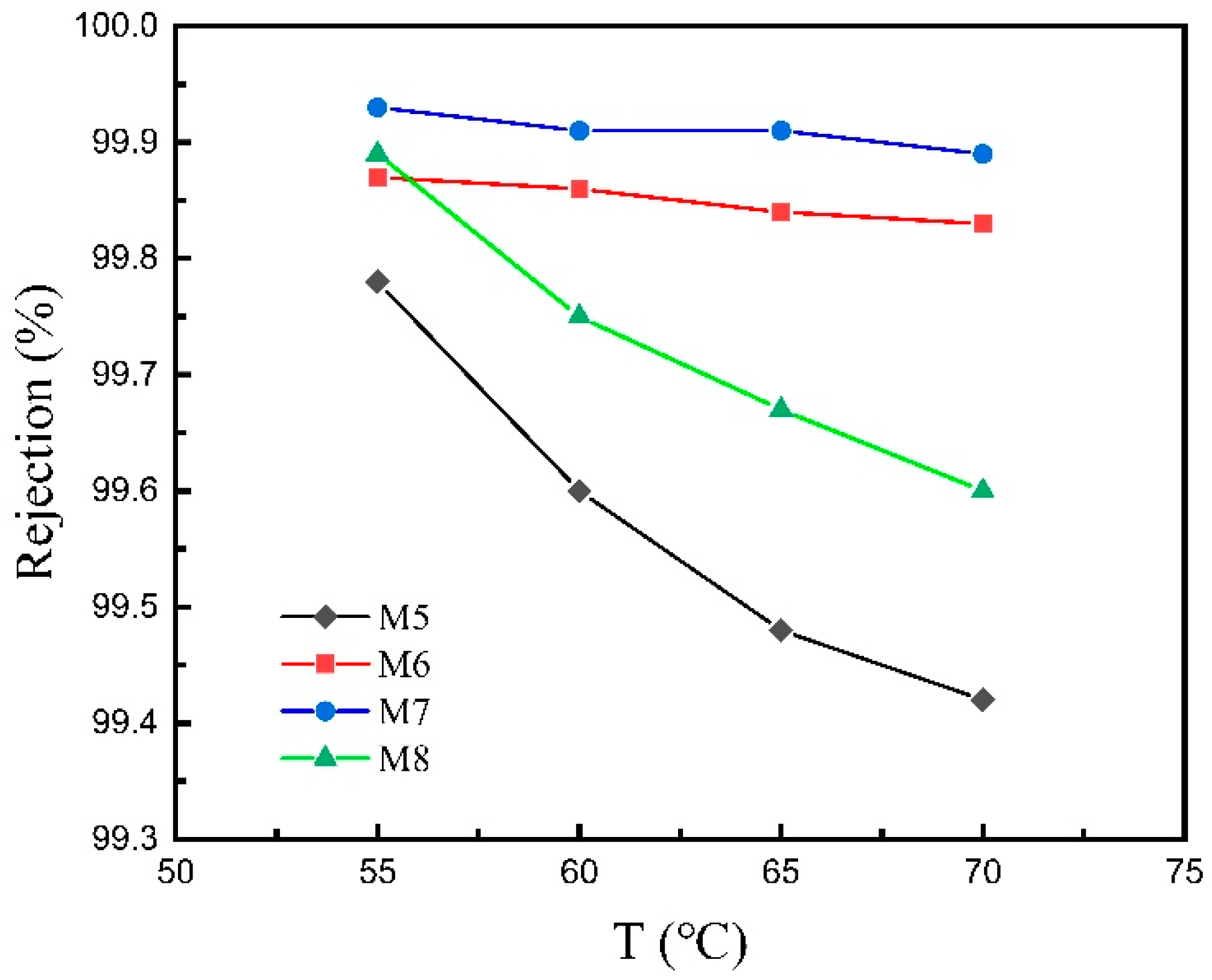
3.2.2. VMD Performance at Different Feed Temperatures
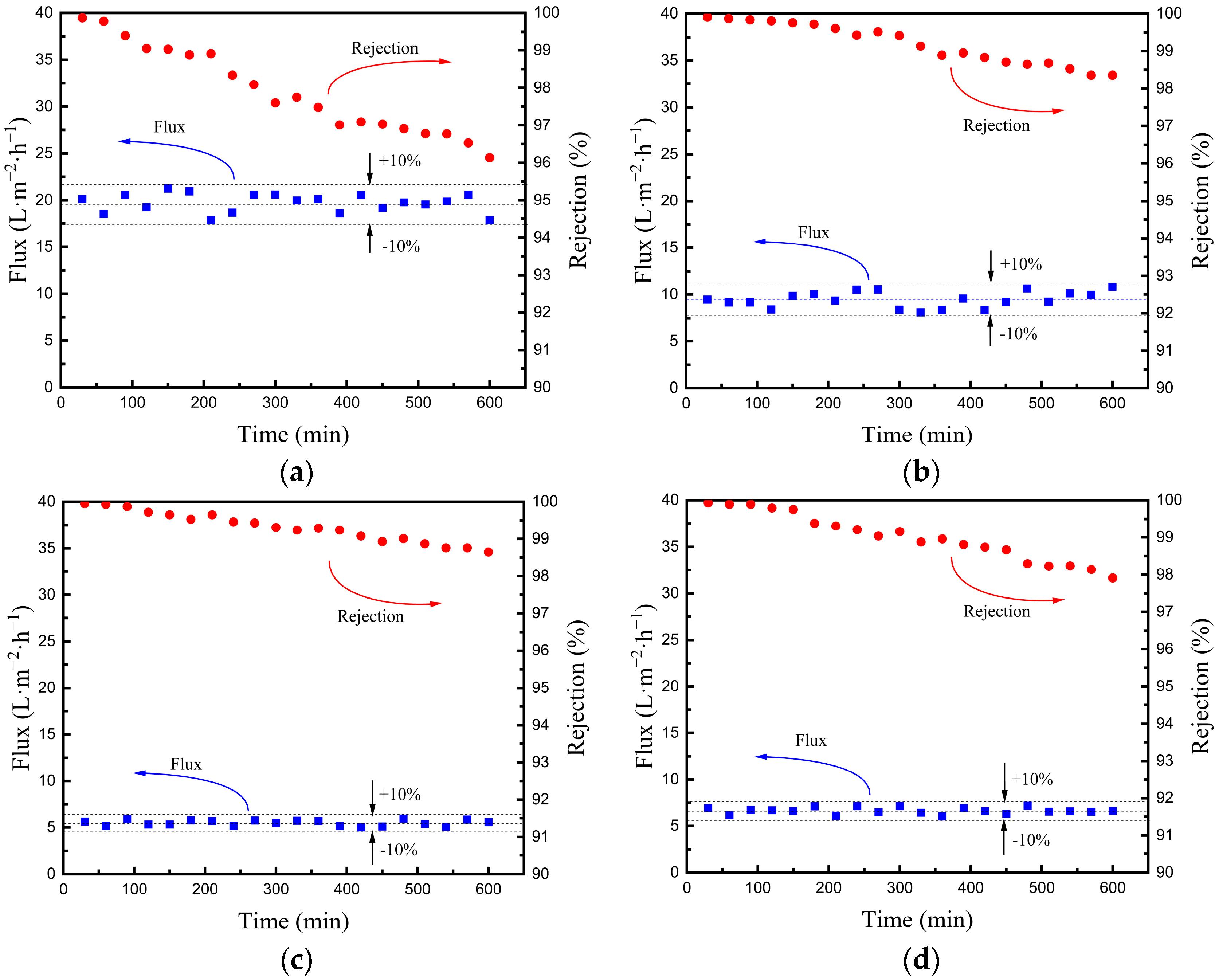
3.2.3. The Stability of Hydrophobic Ceramic Membranes in the VMD Test
4. Conclusions
- (1)
- In the vacuum membrane distillation process, with the increase in the mean pore size, the water flux through the membranes rises, but the salt rejection reduces, which is consistent with the trade-off effect. When the mean pore size increases from 0.15 μm to 1.57 μm, the water flux rises from 5.15 L·m−2·h−1 to 19.72 L·m−2·h−1, but the initial salt rejection reduces from 99.95% to 99.87%.
- (2)
- The performance of the CFA-based ceramic membrane in the VMD process is better than that of the alumina ceramic membrane. The alumina ceramic membrane possesses a larger mean pore size (1.57 μm) than that of the CFA-based membrane (0.18 μm), but exhibits a lower water flux during the VMD process of only 6.62 L·m−2·h−1, while the water flux of the CFA-based membrane reaches 9.54 L·m−2·h−1.
- (3)
- Considering the water flux, the salt rejection and the stability, the CFA-based membrane with a mean pore size of 0.18 μm exhibits the best performance in the VMD process, with a water flux of 9.54 L·m−2·h−1 and a salt rejection of higher than 98.36%.
- (4)
- The stability test of the four hydrophobic membranes in the VMD process is carried out. The results show that the water flux fluctuates slightly and the salt rejection declines mildly after operating for 10 h, which indicates that the hydrophobicity of the fabricated hydrophobic membranes is stable in the VMD process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Distefano, T.; Kelly, S. Are we in deep water? Water scarcity and its limits to economic growth. Ecol. Econ. 2017, 142, 130–147. [Google Scholar] [CrossRef]
- Anwer, S.; Anjum, D.H.; Luo, S.; Abbas, Y.; Li, B.; Iqbal, S.; Liao, K. 2D Ti3C2Tx MXene nanosheets coated cellulose fibers based 3D nanostructures for efficient water desalination. Chem. Eng. J. 2021, 406, 126827. [Google Scholar] [CrossRef]
- Qamar, M.A.; Javed, M.; Shahid, S.; Iqbal, S.; Abubshait, S.A.; Abubshait, H.A.; Ramay, S.M.; Mahmood, A.; Ghaithan, H.M. Designing of highly active g-C3N4/Co@ZnO ternary nanocomposites for the disinfection of pathogens and degradation of the organic pollutants from wastewater under visible light. J. Environ. Chem. Eng. 2021, 9, 105534. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Sun, X.; Duddu, R.; Lin, S. Mechanism of pore wetting in membrane distillation with alcohol vs. surfactant. J. Membr. Sci. 2018, 559, 183–195. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, L.; El-Bourawi, M.S.; Ma, R. Analysis of a solar-powered membrane distillation system. Desalination 2005, 172, 27–40. [Google Scholar] [CrossRef]
- Bundschuh, J.; Ghaffour, N.; Mahmoudi, H.; Goosen, M.; Mushtaq, S.; Hoinkis, J. Low-cost low-enthalpy geothermal heat for freshwater production: Innovative applications using thermal desalination processes. Renew. Sustain. Energy Rev. 2015, 43, 196–206. [Google Scholar] [CrossRef]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef]
- Pal, P.; Manna, A.K. Removal of arsenic from contaminated groundwater by solar-driven membrane distillation using three different commercial membranes. Water Res. 2010, 44, 5750–5760. [Google Scholar] [CrossRef]
- Alsaadi, A.S.; Ghaffour, N.; Li, J.D.; Gray, S.; Francis, L.; Maab, H.; Amy, G.L. Modeling of air-gap membrane distillation process: A theoretical and experimental study. J. Membr. Sci. 2013, 445, 53–65. [Google Scholar] [CrossRef][Green Version]
- Kudelova, T.; Bartuli, E.; Strunga, A.; Hvozda, J.; Dohnal, M. Fully Polymeric Distillation Unit Based on Polypropylene Hollow Fibers. Polymers 2021, 13, 1031. [Google Scholar] [CrossRef] [PubMed]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Cabassud, C.; Wirth, D. Membrane distillation for water desalination: How to choose an appropriate membrane? Desalination 2003, 157, 307–314. [Google Scholar] [CrossRef]
- Chen, X.; Chen, T.; Li, J.; Qiu, M.; Fu, K.; Cui, Z.; Fan, Y.; Drioli, E. Ceramic nanofiltration and membrane distillation hybrid membrane processes for the purification and recycling of boric acid from simulative radioactive waste water. J. Membr. Sci. 2019, 579, 294–301. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. A low-cost hydrophobic kaolin hollow fiber membrane (h-KHFM) for arsenic removal from aqueous solution via direct contact membrane distillation. Sep. Purif. Technol. 2019, 214, 31–39. [Google Scholar] [CrossRef]
- Gao, N.; Li, M.; Jing, W.; Fan, Y.; Xu, N. Improving the filtration performance of ZrO2 membrane in non-polar organic solvents by surface hydrophobic modification. J. Membr. Sci. 2011, 375, 276–283. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Misra, B.N. Grafting: A versatile means to modify polymers: Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Sah, A.; Castricum, H.L.; Bliek, A.; Blank, D.H.A.; Ten Elshof, J.E. Hydrophobic modification of γ-alumina membranes with organochlorosilanes. J. Membr. Sci. 2004, 243, 125–132. [Google Scholar] [CrossRef][Green Version]
- Larbot, A.; Gazagnes, L.; Krajewski, S.; Bukowska, M.; Kujawski, W. Water desalination using ceramic membrane distillation. Desalination 2004, 168, 367–372. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Koter, S.; Kujawski, W. Highly Efficient Hydrophobic Titania Ceramic Membranes for Water Desalination. ACS Appl. Mater. Interfaces 2014, 6, 14223–14230. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W.; Bryjak, M.; Kujawski, J.K. How To Functionalize Ceramics by Perfluoroalkylsilanes for Membrane Separation Process? Properties and Application of Hydrophobized Ceramic Membranes. ACS Appl. Mater. Interfaces 2018, 8, 7564. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, W.; Kujawa, J.; Wierzbowska, E.; Cerneaux, S.; Bryjak, M.; Kujawski, J. Influence of hydrophobization conditions and ceramic membranes pore size on their properties in vacuum membrane distillation of water–organic solvent mixtures. J. Membr. Sci. 2016, 499, 442–451. [Google Scholar] [CrossRef]
- Matsuura, T.; Khayet, M. Membrane Distillation: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Alobaidani, S.; Curcio, E.; Macedonio, F.; Diprofio, G.; Alhinai, H.; Drioli, E. Potential of membrane distillation in seawater desalination: Thermal efficiency, sensitivity study and cost estimation. J. Membr. Sci. 2008, 323, 85–98. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Zhang, J.; Xu, X.; Chen, C. β-Sialon ceramic hollow fiber membranes with high strength and low thermal conductivity for membrane distillation. J. Eur. Ceram. Soc. 2016, 36, 59–65. [Google Scholar] [CrossRef]
- Garofalo, A.; Donato, L.; Drioli, E.; Criscuoli, A.; Carnevale, M.C.; Alharbi, O.; Aljlil, S.A.; Algieri, C. Supported MFI zeolite membranes by cross flow filtration for water treatment. Sep. Purif. Technol. 2014, 137, 28–35. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Yang, J.; Du, Z.; Zhang, H.; Li, Z. Enhancing Performance of Ceramic Membranes for Recovering Water and Heat from Flue Gas. Chem. Eng. Res. Des. 2023, 192, 208–222. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pakshirajan, K.; Pugazhenthi, G. Process intensification through waste fly ash conversion and application as ceramic membranes: A review. Sci. Total Environ. 2022, 808, 151968. [Google Scholar] [CrossRef]
- Liang, D.; Huang, J.; Zhang, Y.; Zhang, Z.; Chen, H.; Zhang, H. Influence of dextrin content and sintering temperature on the properties of coal fly ash-based tubular ceramic membrane for flue gas moisture recovery. J. Eur. Ceram. Soc. 2021, 41, 5696–5710. [Google Scholar] [CrossRef]
- Fu, H.; Li, Z.; Zhang, Y.; Zhang, H.; Chen, H. Preparation, characterization and properties study of a superhydrophobic ceramic membrane based on fly ash. Ceram. Int. 2022, 48, 11573–11587. [Google Scholar] [CrossRef]
- Devi, S.; Ray, P.; Singh, K.; Singh, P.S. Preparation and characterization of highly micro-porous PVDF membranes for desalination of saline water through vacuum membrane distillation. Desalination 2014, 346, 9–18. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhou, J.; Miao, Y.; Yang, S.; Zhou, M.; Zhong, Z.; Xing, W. Lower-temperature preparation of SiC ceramic membrane using zeolite residue as sintering aid for oil-in-water separation. J. Membr. Sci. 2020, 610, 118238. [Google Scholar] [CrossRef]
- Wei, Y.; Xie, Z.; Qi, H. Superhydrophobic-superoleophilic SiC membranes with micro-nano hierarchical structures for high-efficient water-in-oil emulsion separation. J. Membr. Sci. 2020, 601, 117842. [Google Scholar] [CrossRef]
- Zou, D.; Qiu, M.; Chen, X.; Drioli, E.; Fan, Y. One step co-sintering process for low-cost fly ash based ceramic microfiltration membrane in oil-in-water emulsion treatment. Sep. Purif. Technol. 2019, 210, 511–520. [Google Scholar] [CrossRef]
- Boulkrinat, A.; Bouzerara, F.; Harabi, A.; Harrouche, K.; Stelitano, S.; Russo, F.; Galiano, F.; Figoli, A. Synthesis and characterization of ultrafiltration ceramic membranes used in the separation of macromolecular proteins. J. Eur. Ceram. Soc. 2020, 40, 5967–5973. [Google Scholar] [CrossRef]
- Wu, Z.; Faiz, R.; Li, T.; Kingsbury, B.F.K.; Li, K. A controlled sintering process for more permeable ceramic hollow fibre membranes. J. Membr. Sci. 2013, 446, 286–293. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Dong, Y.; Dong, X.; Hampshire, S.; Zhu, L.; Zhu, Z.; Li, L. Feasible recycling of industrial waste coal fly ash for preparation of anorthite-cordierite based porous ceramic membrane supports with addition of dolomite. J. Eur. Ceram. Soc. 2016, 36, 1059–1071. [Google Scholar] [CrossRef]
- Cerneaux, S.; Strużyńska, I.; Kujawski, W.M.; Persin, M.; Larbot, A. Comparison of various membrane distillation methods for desalination using hydrophobic ceramic membranes. J. Membr. Sci. 2009, 337, 55–60. [Google Scholar] [CrossRef]
- Bin Bandar, K.; Alsubei, M.D.; Aljlil, S.A.; Bin Darwish, N.; Hilal, N. Membrane distillation process application using a novel ceramic membrane for Brackish water desalination. Desalination 2021, 500, 114906. [Google Scholar] [CrossRef]
- Krajewski, S.R.; Kujawski, W.; Bukowska, M.; Picard, C.; Larbot, A. Application of fluoroalkylsilanes (FAS) grafted ceramic membranes in membrane distillation process of NaCl solutions. J. Membr. Sci. 2006, 281, 253–259. [Google Scholar] [CrossRef]
- Huang, C.; Ko, C.; Chen, L.; Huang, C.; Tung, K.; Liao, Y. A simple coating method to prepare superhydrophobic layers on ceramic alumina for vacuum membrane distillation. Sep. Purif. Technol. 2018, 198, 79–86. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Wang, H.; Ding, F.; Jin, G.; Li, C.; Meng, H. Superhydrophobic modification of ceramic membranes for vacuum membrane distillation. Chin. J. Chem. Eng. 2017, 25, 1395–1401. [Google Scholar] [CrossRef]
- Li, Y.; Nomura, T.; Sakoda, A.; Suzuki, M. Fabrication of carbon coated ceramic membranes by pyrolysis of methane using a modified chemical vapor deposition apparatus. J. Membr. Sci. 2002, 197, 23–35. [Google Scholar] [CrossRef]
- Ren, C.; Fang, H.; Gu, J.; Winnubst, L.; Chen, C. Preparation and characterization of hydrophobic alumina planar membranes for water desalination. J. Eur. Ceram. Soc. 2015, 35, 723–730. [Google Scholar] [CrossRef]
- Gu, J.; Ren, C.; Zong, X.; Chen, C.; Winnubst, L. Preparation of alumina membranes comprising a thin separation layer and a support with straight open pores for water desalination. Ceram. Int. 2016, 42, 12427–12434. [Google Scholar] [CrossRef]






| Membranes | Support | Separation Layer | |||
|---|---|---|---|---|---|
| Material | Internal/External Diameter (mm) | Length (m) | Number of Layers | Thickness (μm) | |
| M1 | CFA | 8/12 ± 0.23 | 0.5 ± 2.88 × 10−3 | 0 | 0 |
| M2 | CFA | 1 | 40 ± 4.96 | ||
| M3 | CFA | 2 | 100 ± 7.93 | ||
| M4 | Al2O3 | 0 | 0 | ||
| Membranes | CA (°, Left) | CA (°, Right) | CA (°, Average) |
|---|---|---|---|
| M5 | 123.0 | 123.2 | 123.1 |
| M6 | 127.9 | 127.9 | 127.9 |
| M7 | 125.1 | 125.3 | 125.2 |
| M8 | 125.5 | 125.4 | 125.5 |
| Membranes | Maximum Pore Size (×10−6 m) | LEP (MPa) |
|---|---|---|
| M5 | 2.12 | 0.066 |
| M6 | 2.41 | 0.065 |
| M7 | 2.46 | 0.062 |
| M8 | 2.44 | 0.061 |
| Membrane | MD Type | Feed Solution | Pore Size (μm) | Water Flux (L·m−2·h−1) | Salt Rejection (%) | Ref. |
|---|---|---|---|---|---|---|
| Zirconia | VMD | NaCl (17,000 ppm) | 0.05 | 7.5 | >99 | [38] |
| Titania | VMD | NaCl (17,000 ppm) | 0.005 | 6.08 | >99 | [38] |
| Zeolite membrane | VMD | NaCl (35,000 ppm) | 0.07 | 5.2 | 99 | [26] |
| Red clay ceramic | VMD | NaCl (10,000 ppm) | 0.035 | 6.39 | >80 | [39] |
| Zirconia | AGMD | NaCl (0.1 mol·L−1) | 0.05 0.2 | ~6 | ~100 | [40] |
| Alumina | VMD | NaCl (3.5 wt%) | 0.4 | 29.3 | 99.9 | [41] |
| Alumina | VMD | NaCl (1 wt%) | 0.2 | 27.28 | 99.99 | [42] |
| Alumina-silica | AGMD | Seawater | 0.1 | 3.3–7.5 | 98.5–99 | [43] |
| Alumina | DCMD | NaCl (2 wt%) | 0.76 | 19.1 | >99.5 | [44] |
| Alumina | SGMD | NaCl (4 wt%) | ~0.8 | 21 | >99 | [45] |
| CFA | VMD | NaCl (10,000 ppm) | 0.18 | 9.54 | 98.36 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yang, J.; Qi, R.; Huang, J.; Chen, H.; Zhang, H. Development of Hydrophobic Coal-Fly-Ash-Based Ceramic Membrane for Vacuum Membrane Distillation. Materials 2023, 16, 3153. https://doi.org/10.3390/ma16083153
Zhang Z, Yang J, Qi R, Huang J, Chen H, Zhang H. Development of Hydrophobic Coal-Fly-Ash-Based Ceramic Membrane for Vacuum Membrane Distillation. Materials. 2023; 16(8):3153. https://doi.org/10.3390/ma16083153
Chicago/Turabian StyleZhang, Zheng, Jihao Yang, Run Qi, Jiguang Huang, Haiping Chen, and Heng Zhang. 2023. "Development of Hydrophobic Coal-Fly-Ash-Based Ceramic Membrane for Vacuum Membrane Distillation" Materials 16, no. 8: 3153. https://doi.org/10.3390/ma16083153
APA StyleZhang, Z., Yang, J., Qi, R., Huang, J., Chen, H., & Zhang, H. (2023). Development of Hydrophobic Coal-Fly-Ash-Based Ceramic Membrane for Vacuum Membrane Distillation. Materials, 16(8), 3153. https://doi.org/10.3390/ma16083153






