Investigation and Characterization of Pickering Emulsion Stabilized by Alkali-Treated Zein (AZ)/Sodium Alginate (SA) Composite Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of ZS
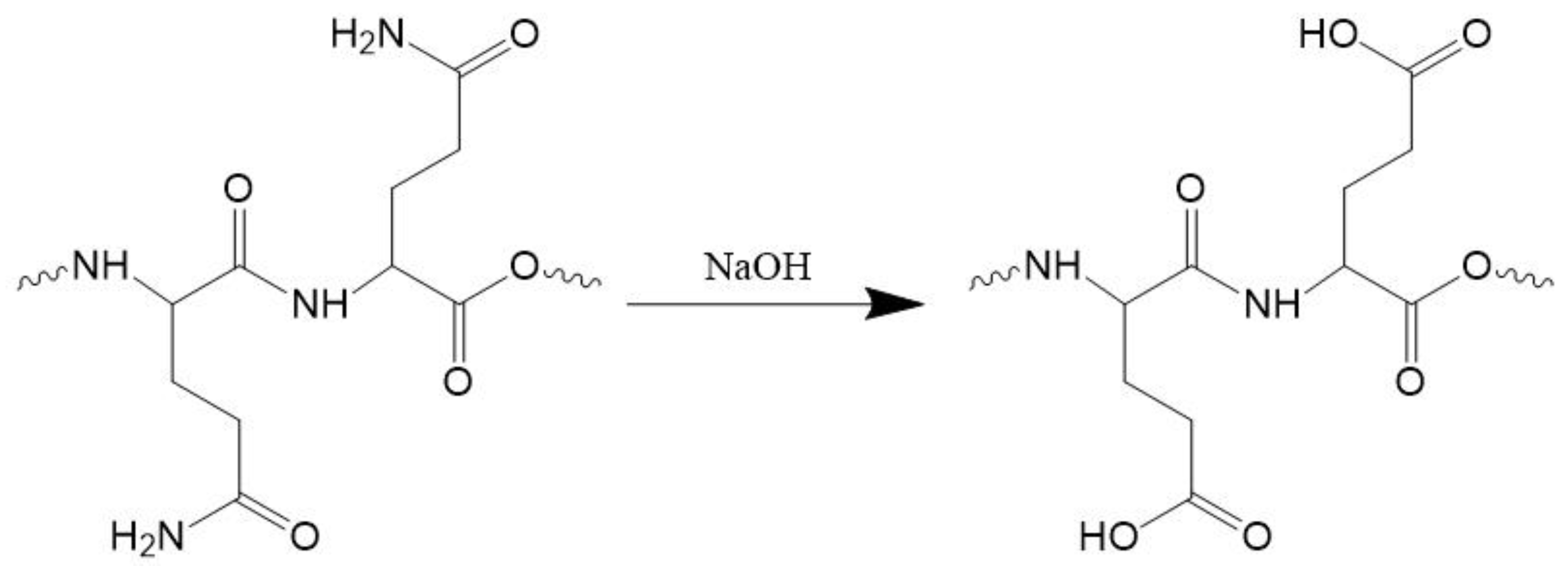
2.2.1. Preparation of AZ
2.2.2. Determination of the Degree of Deamidation (DD)
2.2.3. Measurement of the Degree of Hydrolysis (DH)
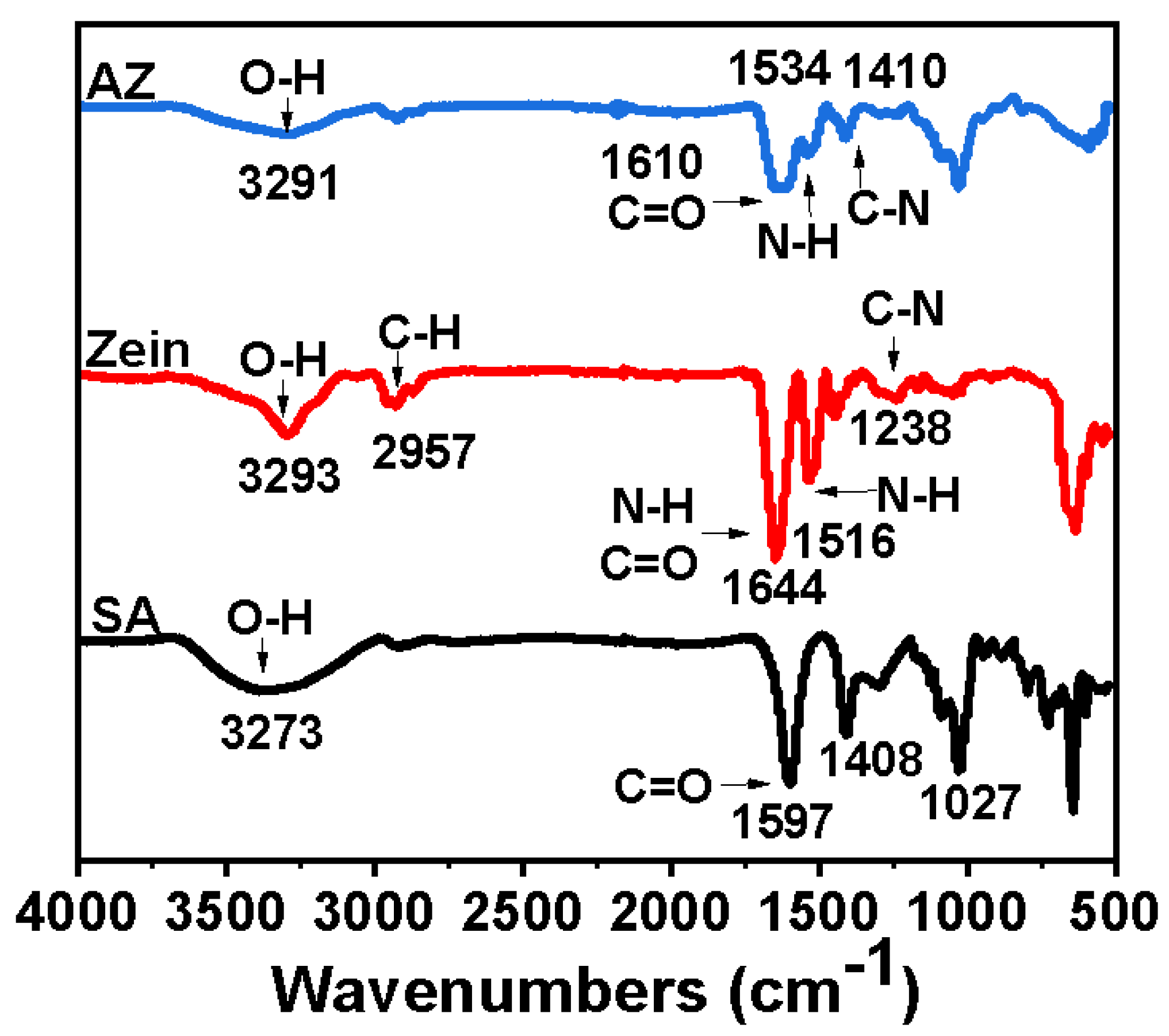
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR) Measurements
2.2.5. Preparation and Characterization of ZS Particles
2.2.6. Particle Size and Zeta-Potential
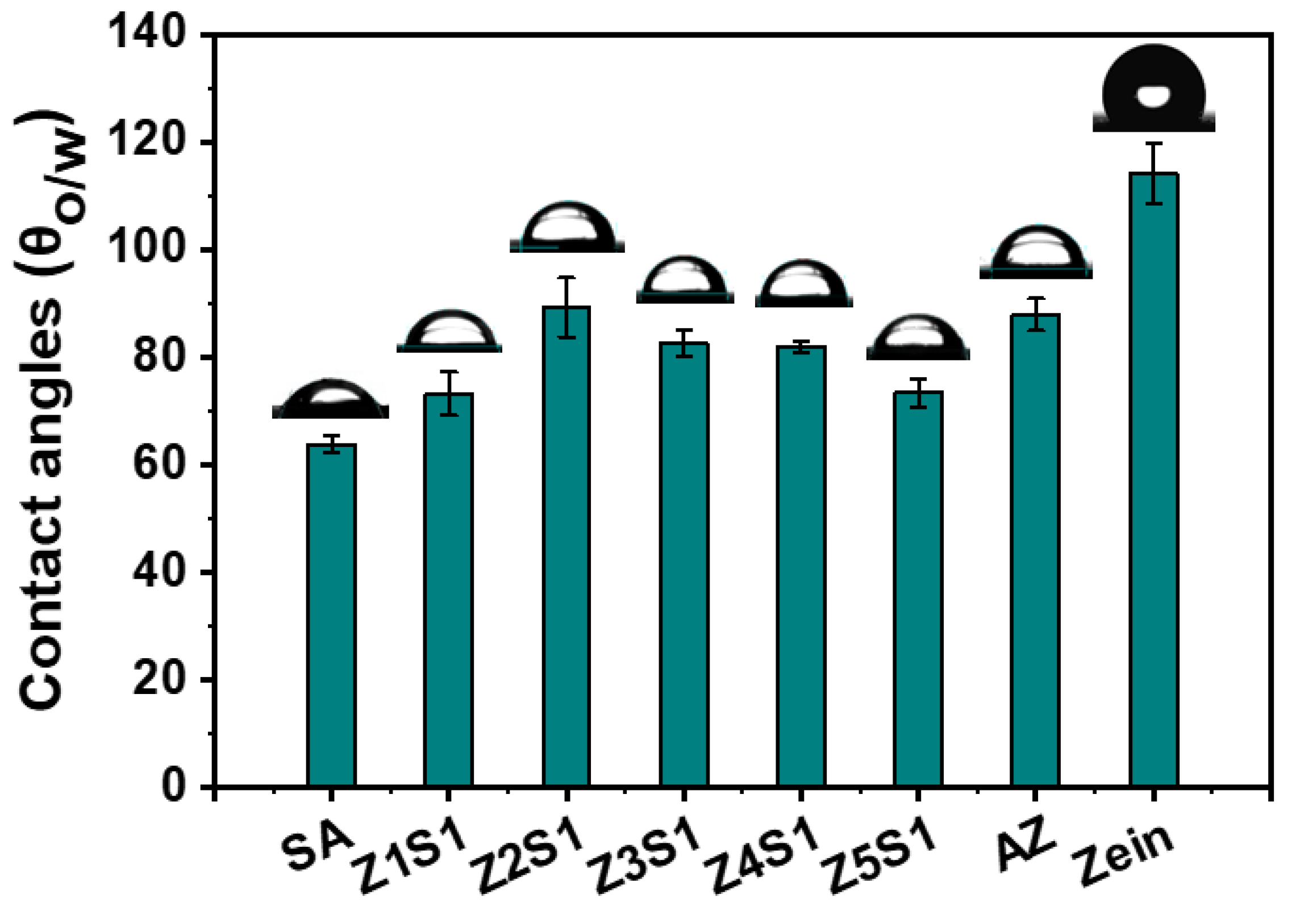
2.2.7. Surface Wettability
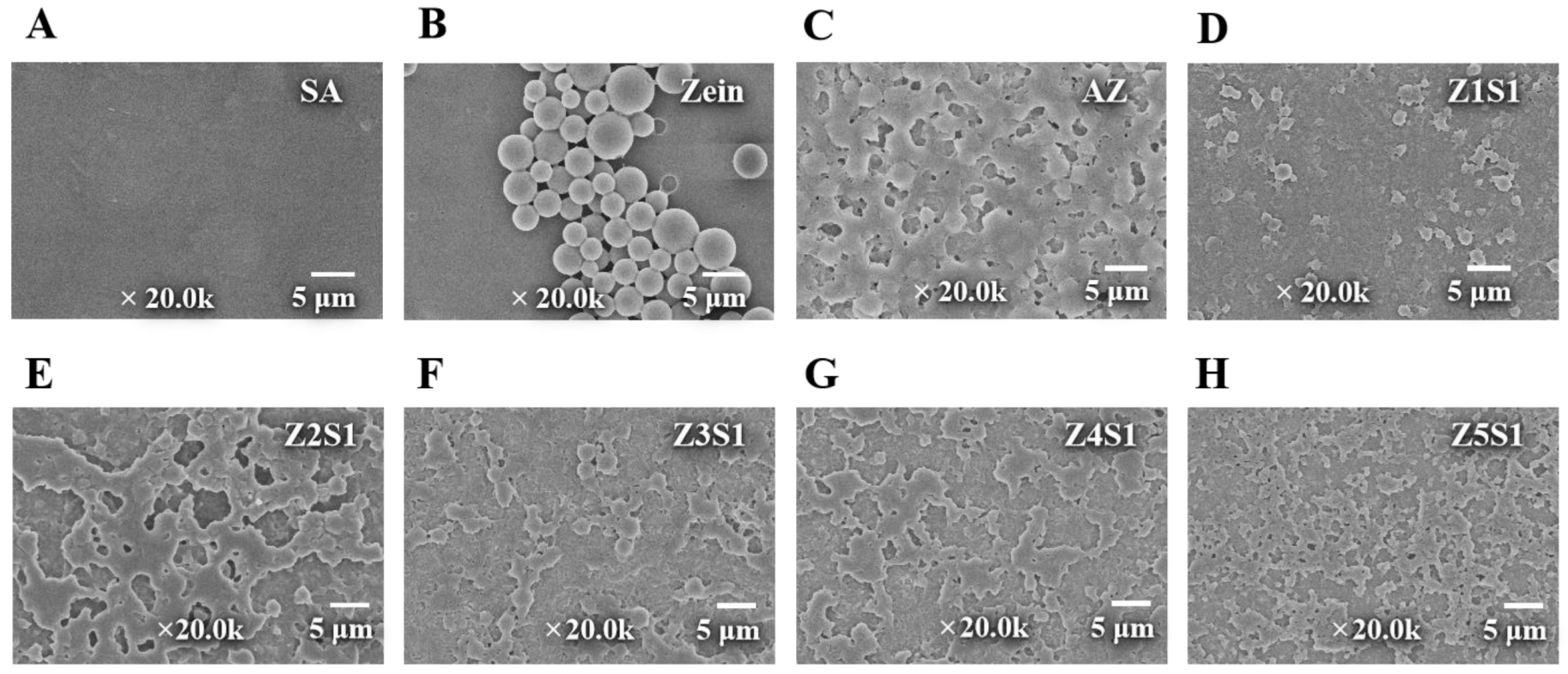
2.2.8. Field Emission Scanning Electron Microscope
2.2.9. Differential Scanning Calorimetry (DSC)
2.3. Preparation and Characterization of ZS-Stabilized Pickering Emulsion
2.3.1. Preparation of ZS-Stabilized Pickering Emulsion
2.3.2. Particle Size Distribution
2.3.3. Kinetic Stability-Turbiscan Stability Analyzer
2.3.4. Micromorphology
2.3.5. Rheological Property
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization
3.1.1. Determination of the DD and DH of AZ
3.1.2. FTIR Analysis
3.1.3. Particle Size and Zeta-Potential
3.1.4. Surface Wettability
3.1.5. Morphology
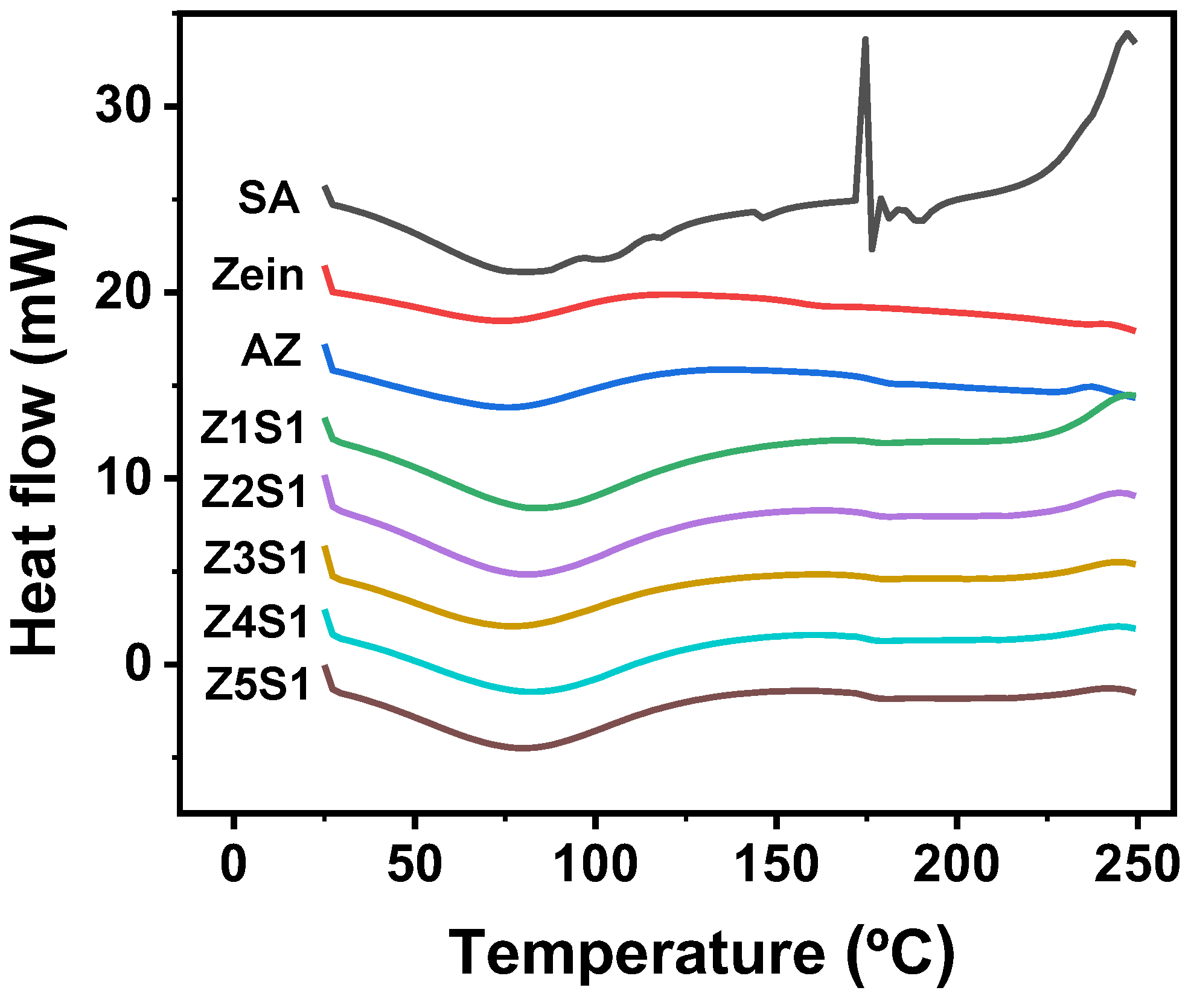
3.1.6. Differential Scanning Calorimetry (DSC)
3.2. Characterization of the Pickering Emulsion
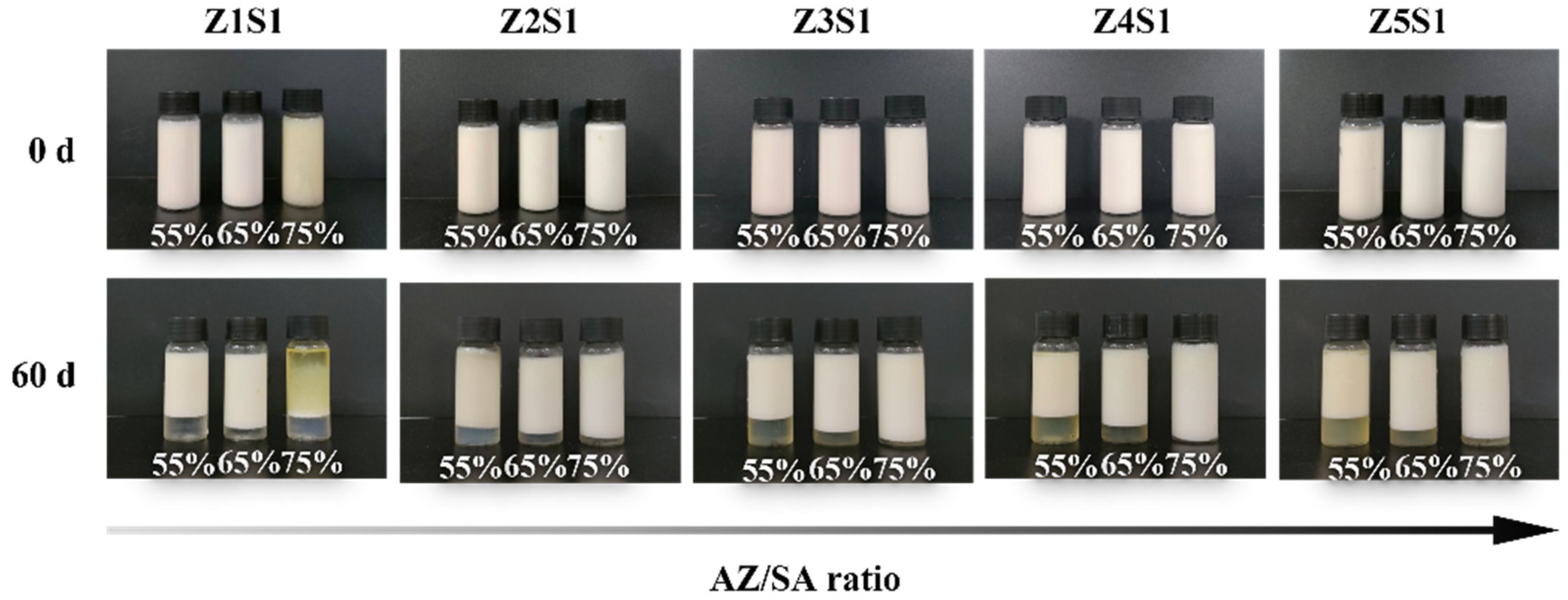
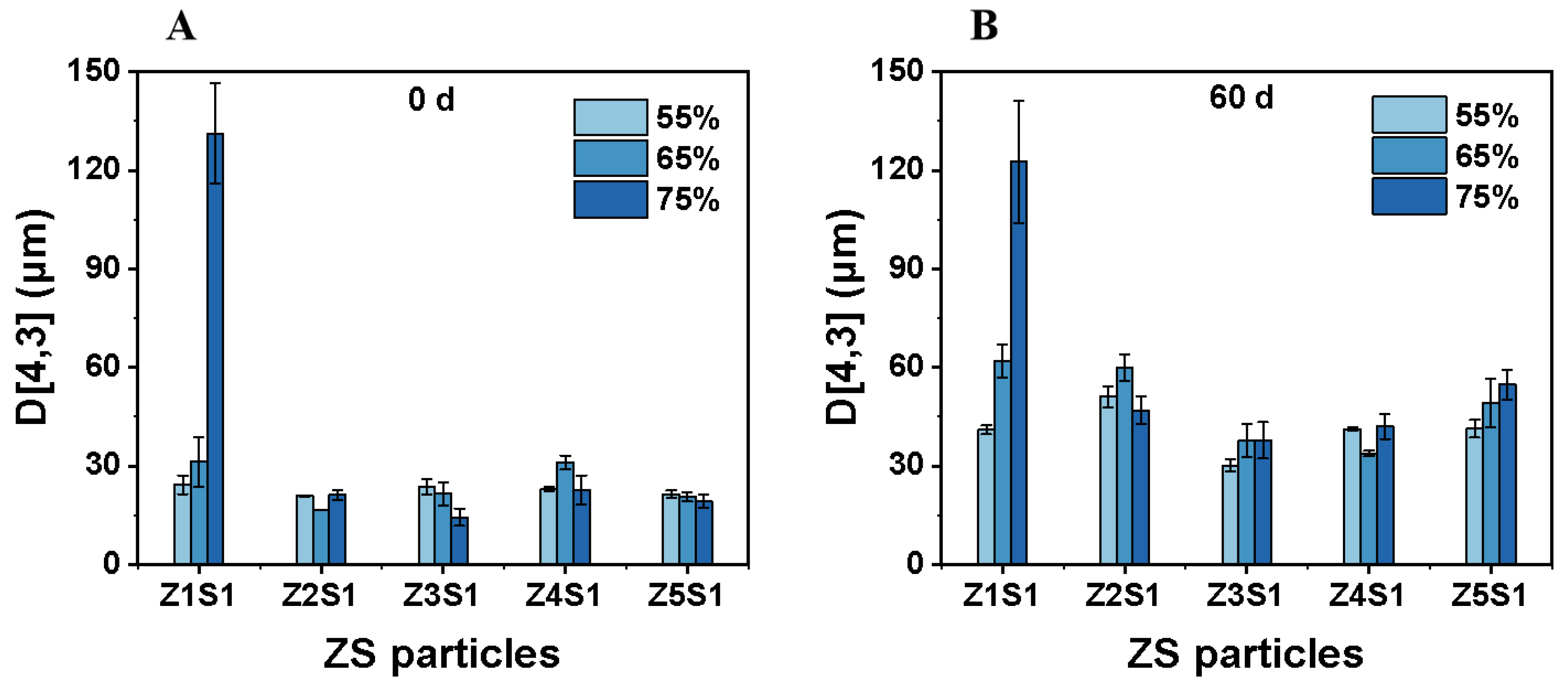
3.2.1. Droplet Size and Storage Stability
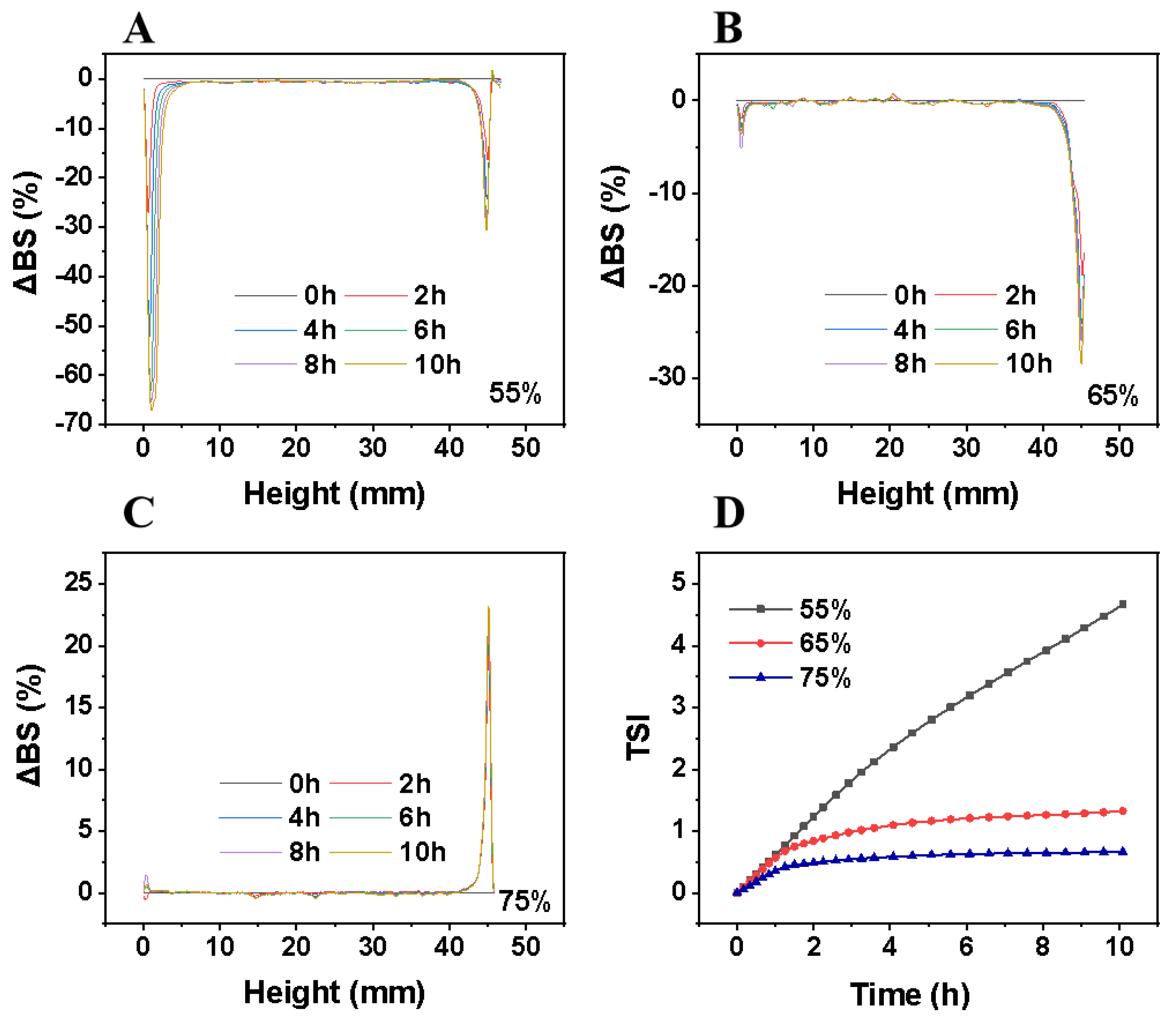
3.2.2. Kinetic Stability Analysis
3.2.3. Micromorphology
3.2.4. Rheological Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Silva-Júnior, J. A Review of Pickering Emulsions: Perspectives and Applications. Pharmaceuticals 2022, 15, 1413. [Google Scholar]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends Food Sci. Technol. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Prog. Lipid Res. 2021, 81, 101081. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Feng, X.; Wang, Q.; Adhikari, B. Pickering and high internal phase Pickering emulsions stabilized by protein-based particles: A review of synthesis, application and prospective. Food Hydrocoll. 2020, 109, 106117. [Google Scholar] [CrossRef]
- Tao, S.; Guan, X.; Li, Y.; Jiang, H.; Gong, S.; Ngai, T. All-natural oil-in-water high internal phase Pickering emulsions featuring interfacial bilayer stabilization. J. Colloid Interface Sci. 2022, 607, 1491–1499. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Yu, Y.; Shan, Y.; Fu, F.; Jiang, B.; Zhang, D.; Ding, S. Zein/pullulan complex colloidal particle-stabilized Pickering emulsions for oral delivery of polymethoxylated flavones: Protection effect and in vitro digestion. J. Sci. Food Agric. 2022, 102, 3952–3963. [Google Scholar]
- Abdullah; Weiss, J.; Ahmad, T.; Zhang, C.; Zhang, H. A review of recent progress on high internal-phase Pickering emulsions in food science. Trends Food Sci. Technol. 2020, 106, 91–103. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, J.; Duan, Y.; Dai, L.; Wang, Y.; Sun, Q.; McClements, D.J.; Xu, X. Hydrolyzed rice glutelin nanoparticles as particulate emulsifier for Pickering emulsion: Structure, interfacial properties, and application for encapsulating curcumin. Food Hydrocoll. 2023, 134, 108105. [Google Scholar] [CrossRef]
- Fu, D.; Deng, S.; Mcclements, D.J.; Zhou, L.; Zou, L.; Yi, J.; Liu, C.; Liu, W. Encapsulation of β-carotene in wheat gluten nanoparticle-xanthan gum-stabilized Pickering emulsions: Enhancement of carotenoid stability and bioaccessibility. Food Hydrocoll. 2019, 89, 80–89. [Google Scholar] [CrossRef]
- Dai, L.; Yang, S.; Wei, Y.; Sun, C.; McClements, D.J.; Mao, L.; Gao, Y. Development of stable high internal phase emulsions by pickering stabilization: Utilization of zein-propylene glycol alginate-rhamnolipid complex particles as colloidal emulsifiers. Food Chem. 2019, 275, 246–254. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; An, S.; Jiang, Q.; Gao, D.; Li, X. Construction of porous materials from Pickering high internal-phase emulsions stabilized by zein-Hohenbuehelia serotina polysaccharides nanoparticles and their adsortion performances. Food Hydrocoll. 2023, 134, 108101. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.; Xie, Q.T.; Cheng, J.S.; Zhang, B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem. 2021, 340, 127893. [Google Scholar] [CrossRef]
- Donsi, F.; Voudouris, P.; Veen, S.J.; Velikov, K.P. Zein-based colloidal particles for encapsulation and delivery of epigallocatechin gallate. Food Hydrocoll. 2017, 63, 508–517. [Google Scholar] [CrossRef]
- Khalil, A.A.; Deraz, S.F.; Elrahman, S.A.; El-Fawal, G. Enhancement of Mechanical Properties, Microstructure, and Antimicrobial Activities of Zein Films Cross-Linked Using Succinic Anhydride, Eugenol, and Citric Acid. Prep. Biochem. Biotech. 2015, 45, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Kanerva, P.; Brinck, O.; Sontag-Strohm, T.; Salovaara, H.; Loponen, J. Deamidation of gluten proteins and peptides decreases the antibody affinity in gluten analysis assays. J. Cereal Sci. 2011, 53, 335–339. [Google Scholar] [CrossRef]
- Chen, Y.; Shu, M.; Yao, X.; Wu, K.; Zhang, K.; He, Y.; Nishinari, K.; Phillips, G.O.; Yao, X.; Jiang, F. Effect of zein-based microencapsules on the release and oxidation of loaded limonene. Food Hydrocoll. 2018, 84, 330–336. [Google Scholar] [CrossRef]
- Luan, J.; Wu, K.; Li, C.; Liu, J.; Ni, X.; Xiao, M.; Xu, Y.; Kuang, Y.; Jiang, F. pH-Sensitive drug delivery system based on hydrophobic modified konjac glucomannan. Carbohyd. Polym. 2017, 171, 9–17. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Yang, Y.; Lei, L.; Lei, X.; Zhao, J.; Zhang, Y.; Li, L.; Wang, Q.; Ming, J. The interaction mechanisms, and structural changes of the interaction between zein and ferulic acid under different pH conditions. Food Hydrocoll. 2022, 124, 107251. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, N.; Yang, J.; Hu, J.; Zhang, K.; Nishinari, K.; Phillips, G.O.; Fang, Y. Protein/polysaccharide intramolecular electrostatic complex as superior food-grade foaming agent. Food Hydrocoll. 2020, 101, 105474. [Google Scholar] [CrossRef]
- Xu, X.; Luo, L.; Liu, C.; Zhang, Z.; McClements, D.J. Influence of electrostatic interactions on behavior of mixed rice glutelin and alginate systems: pH and ionic strength effects. Food Hydrocoll. 2017, 63, 301–308. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, L.; Liu, Y.; Li, J. Mayonnaise-like high internal phase Pickering emulsions stabilized by co-assembled phosphorylated perilla protein isolate and chitosan for extrusion 3D printing application. Food Hydrocoll. 2023, 135, 108133. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.F.; Lin, F.; Su, C.R.; Zeng, Q.Z.; Su, D.X.; He, S.; Wang, Q.; Zhang, J.L.; Yuan, Y. Fabrication and characterization of bi-crosslinking Pickering emulsions stabilized by gliadin/alginate coacervate particles. J. Food Eng. 2021, 291, 110318. [Google Scholar] [CrossRef]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Yan, J.; Liang, X.; Ma, C.; McClements, D.J.; Liu, X.; Liu, F. Design and characterization of double-cross-linked emulsion gels using mixed biopolymers: Zein and sodium alginate. Food Hydrocoll. 2021, 113, 106473. [Google Scholar] [CrossRef]
- Li, Y.; Zou, Y.; Que, F.; Zhang, H. Recent advances in fabrication of edible polymer oleogels for food applications. Curr. Opin. Food Sci. 2022, 43, 114–119. [Google Scholar] [CrossRef]
- Yin, B.; Wang, C.; Liu, Z.; Yao, P. Peptide-polysaccharide conjugates with adjustable hydrophilicity/hydrophobicity as green and pH sensitive emulsifiers. Food Hydrocoll. 2017, 63, 120–129. [Google Scholar] [CrossRef]
- Jin, J.; Ma, H.; Wang, K.; Yagoub, A.E.-G.A.; Owusu, J.; Qu, W.; He, R.; Zhou, C.; Ye, X. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015, 24, 55–64. [Google Scholar] [CrossRef]
- Williams, A.P. Enzymic hydrolysis of food proteins. Food Chem. 1987, 26, 81–82. [Google Scholar] [CrossRef]
- Li, Z.; Hu, W.; Dong, J.; Azi, F.; Xu, X.; Tu, C.; Tang, S.; Dong, M. The use of bacterial cellulose from kombucha to produce curcumin loaded Pickering emulsion with improved stability and antioxidant properties. Food Sci. Hum. Wellness 2023, 12, 669–679. [Google Scholar] [CrossRef]
- Espinosa Solis, V.; Garcia Tejeda, Y.V.; Portilla Rivera, O.M.; Chavez Murillo, C.E.; Barrera-Figueroa, V. Effect of mixed particulate emulsifiers on spray dried avocado oil-in-water pickering emulsions. Polymers 2022, 14, 3064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Luo, Y.; Wang, Q. Effect of acid and base treatments on structural, rheological, and antioxidant properties of a-zein. Food Chem. 2011, 124, 210–220. [Google Scholar] [CrossRef]
- Chen, X.; Fu, W.Y.; Luo, Y.C.; Cui, C.; Suppavorasatit, I.; Liang, L. Protein deamidation to produce processable ingredients and engineered colloids for emerging food applications. Compr. Rev. Food Sci. Food 2021, 20, 3788–3817. [Google Scholar] [CrossRef]
- Dong, S.R.; Xu, H.H.; Ma, J.Y.; Gao, Z.W. Enhanced molecular flexibility of α-zein in different polar solvents. J. Cereal Sci. 2020, 96, 103097. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, Z.; Chen, L. Effects of deamidation on structure and functional properties of barley hordein. J. Agric. Food Chem. 2010, 58, 11448–11455. [Google Scholar] [CrossRef]
- Cabra, V.; Arreguin, R.; Vazquez-Duhalt, R.; Farres, A. Effect of Alkaline Deamidation on the Structure, Surface Hydrophobicity, and Emulsifying Properties of the Z19 α-Zein. J. Agric. Food Chem. 2007, 55, 439–445. [Google Scholar] [CrossRef]
- Lei, F.; Hu, C.; Zhang, N.; He, D. The specificity of an aminopeptidase affects its performance in hydrolyzing peanut protein isolate and zein. LWT 2019, 102, 37–44. [Google Scholar] [CrossRef]
- Chang, Y.; Jiao, Y.; Li, D.J.; Liu, X.L.; Han, H. Glycosylated zein as a novel nanodelivery vehicle for lutein. Food Chem. 2022, 376, 131927. [Google Scholar] [CrossRef]
- Ni, X.; Wang, K.; Wu, K.; Corke, H.; Nishinari, K.; Jiang, F. Stability, microstructure and rheological behavior of konjac glucomannan-zein mixed systems. Carbohyd. Polym. 2018, 188, 260–267. [Google Scholar] [CrossRef]
- Li, F.; Li, X.; Huang, K.; Luo, Y.; Mei, X. Preparation and characterization of pickering emulsion stabilized by hordein-chitosan complex particles. J. Food Eng. 2021, 292, 110275. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, P.; Zhang, T.; Wang, Z.; Cai, D.; Chen, X.; Shen, Y.; Xu, J.; Song, C.; Goff, D. Effect of carboxymethyl chitosan on the storage stability of frozen dough: State of water, protein structures and quality attributes. Food Res. Int. 2022, 151, 110863. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, A.; Costa, A.; Cunha, R.L. Cellulose nanocrystals from ultrasound process stabilizing O/W Pickering emulsion. Int. J. Biol. Macromol. 2020, 158, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, L.; Yang, F.; Yao, J.; Ma, Y.; Liu, J. Construction of high internal phase Pickering emulsions stabilized by bamboo fungus protein gels with the effect of pH. Food Chem. 2022, 369, 130954. [Google Scholar] [CrossRef]
- Zhou, F.Z.; Wu, X.N.; Yin, Z.L.; Zhu, S.W.; Tang, J.H.; Yang, C.H.; Yang, X.Q. Fabrication of Zein/Pectin Hybrid Particle-Stabilized Pickering High Internal Phase Emulsions with Robust and Ordered Interface Architecture. J. Agric. Food Chem. 2018, 66, 11113–11123. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Feng, Y.; Su, C.; Wang, Q.; Zhang, Y.; Wei, Y.; Zhao, M.; Nishinari, K.; Fang, Y. Structure and tribology of κ-carrageenan gels filled with natural oil bodies. Food Hydrocoll. 2020, 107, 105945. [Google Scholar] [CrossRef]
- Tisserand, C.; Brambilla, G.; Meunier, G.; Parker, A. Predicting the long-term stability of depletion-flocculated emulsions by static multiple light scattering (SMLS). J. Dispers. Sci. Technol. 2019, 41, 648–655. [Google Scholar] [CrossRef]
- Choi, S.J.; Won, J.W.; Park, K.M.; Chang, P.S. A new method for determining the emulsion stability index by backscattering light detection. J. Food Process Eng. 2014, 37, 229–236. [Google Scholar] [CrossRef]
- Liu, B.; Liu, B.; Wang, R.; Li, Y. α-Lactalbumin self-assembled nanoparticles with various morphologies, stiffnesses, and sizes as pickering stabilizers for oil-in-water emulsions and delivery of curcumin. J. Agric. Food Chem. 2021, 69, 2485–2492. [Google Scholar] [CrossRef]
- Li, Z.; Wu, H.; Yang, M.; Xu, D.; Chen, J.; Feng, H.; Lu, Y.; Zhang, L.; Yu, Y.; Kang, W. Stability mechanism of O/W Pickering emulsions stabilized with regenerated cellulose. Carbohyd. Polym. 2018, 181, 224–233. [Google Scholar] [CrossRef]
- Fang, X.; Zhao, X.; Yu, G.; Zhang, L.; Li, J. Effect of molecular weight and pH on the self-assembly microstructural and emulsification of amphiphilic sodium alginate colloid particles. Food Hydrocoll. 2020, 103, 105593. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, M.; He, D.; Li, X.; Shi, L.; Wang, L.; Xu, J.; Zheng, Y.; Yin, T. Cryoprotective Roles of Carboxymethyl Chitosan during the Frozen Storage of Surimi: Protein Structures, Gel Behaviors and Edible Qualities. Foods 2022, 11, 356. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Y.; Xiao, Q.; Yang, Y.; Liu, M.; Wang, X.; Deng, P.; Wu, K.; Liu, Y.; Peng, B.; Jiang, F.; et al. Investigation and Characterization of Pickering Emulsion Stabilized by Alkali-Treated Zein (AZ)/Sodium Alginate (SA) Composite Particles. Materials 2023, 16, 3164. https://doi.org/10.3390/ma16083164
Kuang Y, Xiao Q, Yang Y, Liu M, Wang X, Deng P, Wu K, Liu Y, Peng B, Jiang F, et al. Investigation and Characterization of Pickering Emulsion Stabilized by Alkali-Treated Zein (AZ)/Sodium Alginate (SA) Composite Particles. Materials. 2023; 16(8):3164. https://doi.org/10.3390/ma16083164
Chicago/Turabian StyleKuang, Ying, Qinjian Xiao, Yichen Yang, Menglong Liu, Xiaosa Wang, Pengpeng Deng, Kao Wu, Yi Liu, Bo Peng, Fatang Jiang, and et al. 2023. "Investigation and Characterization of Pickering Emulsion Stabilized by Alkali-Treated Zein (AZ)/Sodium Alginate (SA) Composite Particles" Materials 16, no. 8: 3164. https://doi.org/10.3390/ma16083164




