1. Introduction
The development of advanced building-integrated photovoltaics (BIPVs) can address space constraint issues in high-rise buildings and meet their energy needs [
1]. In the BIPVs, the utilization of luminescent solar concentrators (LSCs) enables the realization of semi-transparent photovoltaic (PV) modules for converting the facades of the urban building into energy generators [
2]. LSCs are typically fabricated as waveguides consisting of transparent surfaces either coated with or matrices containing luminescent materials, such as organic dyes, quantum dots (QDs), or inorganic phosphors [
3,
4]. Although organic dyes have been used in the LSCs, they are hampered by poor stability and self-absorption loss due to their small Stokes shift [
3]. Recently, QDs have been explored as luminescent materials in LSCs owing to their higher stability and wider absorption band width than organic dyes [
5]. Nonetheless, QDs have small Stokes shifts and suffer from self-absorption loss [
3]. To overcome this issue, large Stokes shift-emitting QDs have been developed where host QD nanocrystals were doped with transition metal ions, such as Mn
2+, to emit long-wavelength visible light under short-wavelength ultraviolet (UV) light excitation [
6,
7].
On the other hand, lanthanide-doped inorganic phosphors are promising candidates for use in LSCs [
8,
9]. Compared to organic dyes and QDs, lanthanide-doped inorganic phosphors are more advantageous because they generally exhibit a large Stokes shift [
9]. For example, de Boer et al. applied SrB
4O
7:Sm,Eu phosphors to an LSC film, where the SrB
4O
7:Sm,Eu phosphor showed an absorption band below 600 nm and an emission peak at 685 nm [
9]. Liu et al. reported an LSC film consisting of a poly(methyl methacrylate) waveguide containing CaAlSiN
3:Eu
2+ phosphors with a Stokes shift of 112 nm [
10]. In addition, Weber and Lambe reported that Nd
3+-doped glass could be a luminescent medium because Nd
3+-ions have strong absorption bands in the 500–900 nm range and an emission peak at 1060 nm, which is a wavelength well suited for use in silicon solar cells [
11]. Thus, Nd
3+-doped phosphors appear desirable for LSCs due to the amelioration of the above drawbacks that plague organic dyes and QDs [
11]. However, micrometer-sized phosphors exhibit a scattering issue that increases non-emissive absorption and escape cone losses when these phosphors are utilized in LSCs [
8]. This scattering issue can be addressed using nanophosphors. According to Do’s group, light scattering decreases as the phosphor size decreases, and nanophosphors smaller than 50 nm can lead to transparent nanophosphor-based matrix [
12]. Thus, Nd
3+-doped nanophosphors, smaller than 50 nm, can be applied to a transparent LSCs coupled with silicon solar cells.
Although Nd
3+-doped nanophosphors have recently been reported, most of these studies focused on Nd
3+-doped upconversion nanophosphors for bio-related applications [
13,
14,
15,
16]. In contrast, herein, we focus on Nd
3+-doped downshifting nanophosphors (DSNPs) for transparent LSC applications. As described above, the Nd
3+ ions exhibit an emission peak at 1060 nm in Nd
3+-doped materials [
11,
16]. Additionally, the Nd
3+ ions can be deployed as sensitizers to enhance the luminescence of nanophosphors [
17,
18]. In particular, near-infrared (NIR) emission can be enhanced by Nd
3+–Yb
3+ energy transfer [
17]. This study prompted us to develop Nd
3+-sensitized and Yb
3+-activated DSNPs. In the current study, we synthesized NaYF
4:Nd
3+,Yb
3+-based DSNPs with a core/shell/shell (C/S/S) structure for strong NIR emission. The DSNP core was coated with an Nd
3+-doped active shell and a NaYF
4 inert shell. In addition, we fabricated a transparent polydimethylsiloxane (PDMS) composite encapsulating NaYF
4:Nd,Yb-based C/S/S DSNPs to investigate the feasibility of applying C/S/S DSNPs to LSCs.
2. Materials and Methods
For the syntheses of core, core/shell (C/S), and C/S/S DSNPs, YCl3∙6H2O (99.99%), NdCl3∙6H2O (99.9%), YbCl3∙6H2O (99.9%), NaOH (99.99%), NH4F (≥99.99%), 1-Octadecene (ODE, 90%, technical grade), and oleic acid (OA, 90%, technical grade) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
We synthesized Nd
3+ and Yb
3+-doped NaYF
4 core DSNPs using a co-precipitation method [
19]. To synthesize the core DSNPs, YCl
3·6H
2O (0.9 − x mmol), NdCl
3·6H
2O (x mmol, x = 0.1, 0.2, 0.25, 0.3, 0.4 and 0.5), YbCl
3·6H
2O (0.1 mmol), 6 mL of OA, and 15 mL of ODE were heat-treated at 150 °C for 40 min. After cooling the reaction solution to room temperature, 10 mL of methanol (MeOH) solution comprising NaOH (2.5 mmol) and NH
4F (4 mmol) was added to it, and the solution was stirred for 40 min. MeOH was removed, and core DSNPs were synthesized by heat treatment at 320 °C for 1 h. The core DSNPs were washed with MeOH, ethanol, and hexane, and then dispersed in 10 mL of chloroform.
To synthesize the C/S DSNPs, YCl3·6H2O (0.6 − x mmol), NdCl3·6H2O (x mmol, x = 0, 0.06, 0.12, 0.18, 0.24), 6 mL of OA, and 15 mL of ODE were loaded into a 3-neck flask and heat-treated at 150 °C. After the reaction solution was cooled to room temperature, core DSNPs and MeOH solution containing NaOH and NH4F were added to it. After removing MeOH, C/S DSNPs were synthesized by heat treatment at 320 °C for 1 h. The synthesized C/S DSNPs were washed in the same manner as the core DSNPs and dispersed in 10 mL of chloroform.
To synthesize C/S/S DSNPs, YCl3·6H2O (1 mmol), OA (6 mL), and ODE (15 mL) were mixed and heat-treated at 150 °C for 40 min. After the heat treatment, the reaction mixture was cooled to room temperature, followed by the addition of C/S DSNPs solution and MeOH solution containing NaOH (2.5 mmol) and NH4F (4 mmol) to it. The subsequent synthetic process was the same as that used to synthesize C/S DSNPs.
To prepare the PDMS-based LSC film, the C/S/S DSNP solution (0.5 mL) was mixed with SYLGARD 184 silicone elastomer (10 mL, Dow Chemical Company, Midland, MI, USA). The curing agent (1 mL) was then added to the mixture of the C/S/S DSNPs and silicone elastomer. Subsequently, the mixture was poured into a mold and baked at 80 °C for 1 h.
Transmission electron microscopy (TEM) images were obtained using a Tecnai F20 G2 transmission electron microscope (FEI Co., Hillsboro, OR, USA) at 200 kV. Energy-dispersive X-ray spectroscopy (EDS) was conducted using an FEI Talos F200X (FEI Co., Hillsboro, OR, USA) instrument at 200 kV. Structural analysis based on X-ray diffraction (XRD) was performed using a Bruker D8 Advance X-ray diffractometer with Cu-Kα radiation. Photoluminescence (PL) spectra were obtained using a PL/PLE-500 (PSI Trading Co., Ltd, Suwon, Republic of Korea) coupled with an 800 nm NIR diode laser. Time-resolved PL spectra were obtained by monitoring at 978 nm under pulsed irradiation with 800 nm NIR light using an InGaAs detector (IGA-030-TE2-H Photodetector, Dongwoo Optron Co., Ltd., Gwangju-si, Republic of Korea) and a monochromator (Monora323i, Dongwoo Optron Co., LTD., Republic of Korea). Absorption spectra were recorded using a Perkin-Elmer Lambda25 UV/vis spectrophotometer (Perkin-Elmer, Waltham, MA, USA). Transmittance spectra were obtained using a Shimadzu UV-3600 plus a UV-VIS-NIR spectrophotometer (Shimadzu, Kyoto, Japan).
3. Results and Discussion
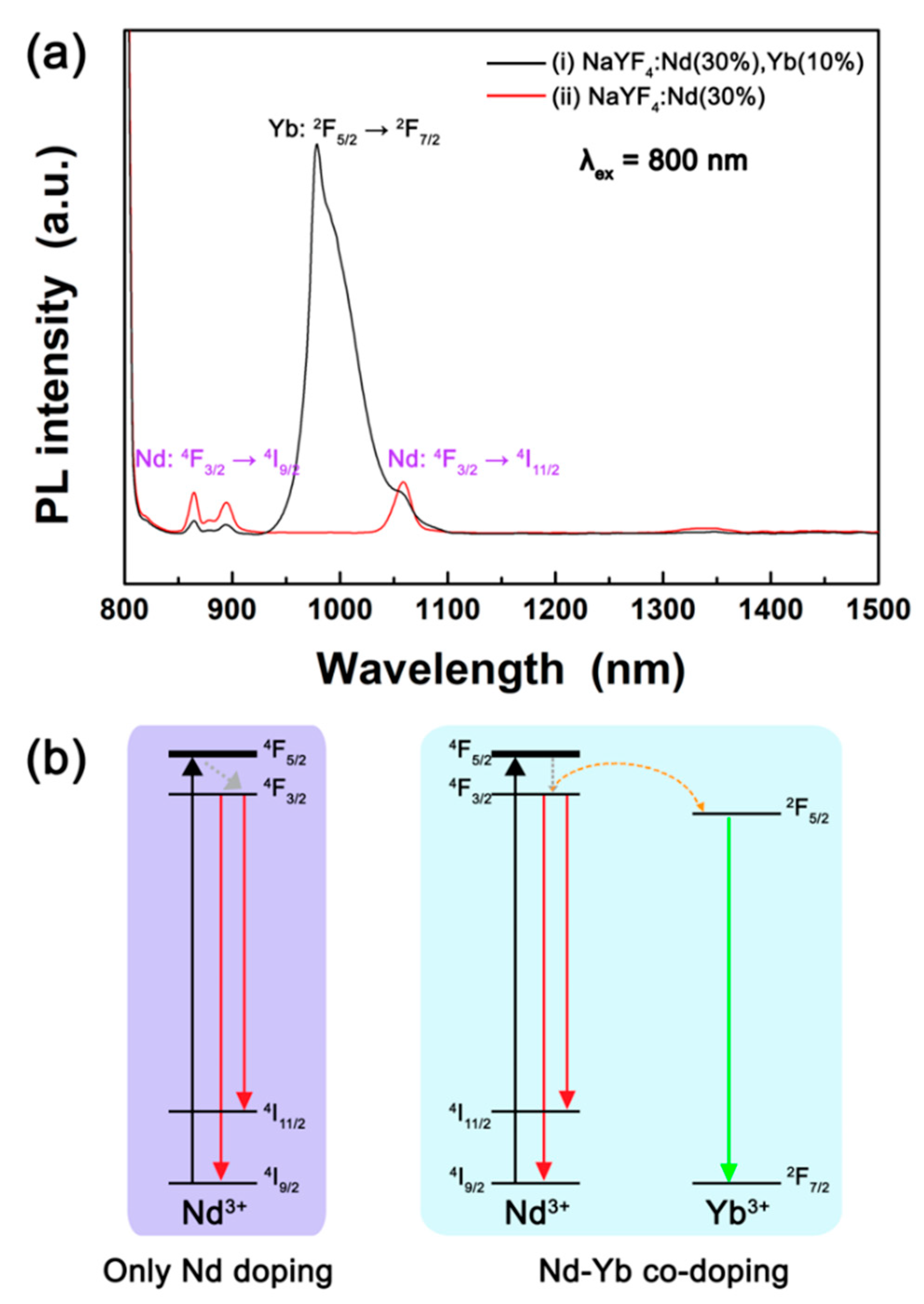
Figure 1 shows the PL spectra of NaYF
4:Nd (30%),Yb (10%) and NaYF
4:Nd (30%) core DSNPs under 800 nm NIR light. The NaYF
4:Nd (30%),Yb (10%) core DSNPs exhibited strong Yb
3+ emission band peaking at 978 nm and weak Nd
3+ peaks at 862 nm and 1060 nm. In contrast, the NaYF
4:Nd (30%) core DSNPs only showed Nd
3+ emission peaks at 862 nm and 1060 nm. As shown in
Figure 1a, the emission intensity of Yb
3+ ions is much stronger than that of Nd
3+ ions, indicating that the Nd
3+ and Yb
3+ co-doped DSNPs have the potential to achieve strong NIR-to-NIR emission.
Figure 1b shows the energy level diagram of the Nd
3+ and Yb
3+ ions. When the NaYF
4:Nd core DSNPs are irradiated with 800 nm NIR light, the energy is absorbed by the Nd
3+ ions, resulting in
4I
9/2 →
4F
5/2 transition, and then the excited energy is relaxed to the
4F
3/2 level [
20]. As a result, Nd
3+ shows PL peaks at 862 nm and 1060 nm owing to
4F
3/2 →
4I
9/2 and
4F
3/2 →
4I
11/2 electronic transitions, respectively [
20]. In NaYF
4:Nd,Yb core DSNPs, the energy absorbed by Nd
3+ ions is transferred to the
2F
5/2 level of the adjacent Yb
3+ ions, resulting in a broad NIR emission band peaking at 978 nm due to the
2F
5/2 →
2F
7/2 transition [
20]. Because NaYF
4:Nd,Yb emits strong and broad emission under 800 nm NIR light, we adopted NaYF
4:Nd,Yb as the core DSNP and optimized the Nd
3+ concentration for further enhancement of the NIR-to-NIR emission.
Figure 2 shows TEM images of NaYF
4:Nd (x%),Yb (10%) core DSNPs with various Nd
3+ concentrations. The sizes of NaYF
4:Nd (10%),Yb (10%), NaYF
4:Nd (20%),Yb (10%), NaYF
4:Nd (25%),Yb (10%), NaYF
4:Nd (30%),Yb (10%), NaYF
4:Nd (40%),Yb (10%), and NaYF
4:Nd (50%),Yb (10%) core DSNPs were found to be 20.8 ± 0.9 nm, 19.6 ± 0.7 nm, 19.1 ± 0.7 nm, 20.0 ± 0.9 nm, 14.1 ± 0.8 nm, and 13.4 ± 0.9 nm (average size ± standard deviation), respectively (
Figure S1).
Figure 2 and
Figure S1 showed that DSNPs with uniform size and morphology were synthesized. The size of the synthesized core DSNPs decreased with increasing Nd
3+ concentrations. This result suggests that some of the Y
3+ ions (r = 1.159 Å) in the NaYF
4 host lattice were replaced with Nd
3+ ions (r = 1.249 Å) because doping with lanthanide ions larger than the Y
3+ ion leads to a decrease in particle size [
21]. The XRD patterns of the synthesized core DSNPs are shown in
Figure S2. The XRD patterns exhibited that the core DSNPs with a single hexagonal phase were synthesized without impurities even when the concentration of Nd
3+ ions in the core increased to 50%. In addition, the XRD patterns show that the (110) and (101) diffraction peaks observed at approximately 29° and 30° shifted to lower angles as the concentration of Nd
3+ ions increased. The XRD peak shift is due to the replacement of Y
3+ ions with Nd
3+ ions, which are comparatively larger than Y
3+ ions [
22].
Figure S3 shows EDS map images and EDS spectrum obtained for the NaYF
4:Nd (30%),Yb (10%) core DSNPs. Nd Lα, Yb Lα, and Y Kα peaks were observed in the EDS spectrum, and the EDS maps for the dopants Nd and Yb overlapped with the Y Kα map, confirming that NaYF
4 was co-doped with Nd
3+ and Yb
3+ ions.
Figure 3a shows the absorption spectra of the NaYF
4:Nd (x%),Yb (10%) core DSNPs with various Nd
3+ concentrations. The absorption peaks are attributed to the electronic transitions of
4I
9/2 →
2H
11/2 (623 nm),
4I
9/2 →
4F
9/2 (680 nm),
4I
9/2 →
4F
7/2 (740 nm),
4I
9/2 →
4F
5/2 (794 nm), and
4I
9/2 →
4F
3/2 (865 nm) [
23,
24,
25,
26]. In contrast, the absorption peak observed at 976 nm is due to the
2F
7/2 →
2F
5/2 electronic transition of Yb
3+ ions [
20,
23,
24]. The inset of
Figure 3a shows that the absorbance of the NaYF
4:Nd,Yb core DSNPs at 794 nm systematically increased as the Nd
3+ concentration increased.
Figure 3b illustrates the PL spectra of NaYF
4:Nd (x%),Yb (10%) core DSNPs with various Nd
3+ concentrations. Characteristic emission peaks of Yb
3+ and Nd
3+ were observed at 978 nm and 862 nm, respectively. The emission intensity of Yb
3+ increased as the concentration of Nd
3+ in the core increased from 10% to 30%, whereas the emission intensity of Yb
3+ decreased when the Nd
3+ concentration was higher than 30%. Within the synthetic conditions applied in this study, NaYF
4:Nd (30%),Yb (10%) showed the highest PL intensity under 800 nm excitation.
It is well known that the efficiency of nanophosphors is sensitive to surface defects [
27,
28]. Passivation of the surface of nanophosphors is an efficient way to improve their luminescent properties [
29]. Thus, the NaYF
4:Nd (30%),Yb (10%)/NaYF
4 C/S DSNPs were synthesized to enhance the emission intensity of Yb
3+ ions at 978 nm under 800 nm irradiation. Previously, Vetrone et al. reported that the active shell, where the shell was doped with sensitizer ions, was beneficial for further PL enhancement of nanophosphors [
30]. Thus, the NaYF
4 shell was doped with Nd
3+ ions, and the C/S DSNPs could absorb more 800 nm NIR light than the core DSNPs.
Figure 4 shows the TEM images of NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (x%) C/S DSNPs. The concentration of Nd
3+ ion in the shell was adjusted to 0%, 10%, 20%, 30%, and 40%, and the corresponding C/S DSNPs sizes were measured to be 34.3 nm ± 1.2 nm × 26.7 nm ± 1.2 nm, 32.3 nm ± 1.0 nm × 26.2 nm ± 1.2 nm, 27.5 nm ± 1.2 nm, 26.3 nm ± 1.2, 26.2 nm ± 1.4 nm, respectively. The TEM results showed that NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (x%) C/S DSNPs with uniform sizes were synthesized. High resolution (HR)-TEM images of the C/S DSNPs are shown in
Figure 4a insets. The HR-TEM images show that the NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (x%) C/S DSNPs exhibited high crystallinity and a hexagonal structure, judging from clear lattice fringes and the measured lattice spacing (0.52 nm), which coincides with
d-spacing between the (10
0) planes of hexagonal NaYF
4 [
31]. The crystal structures of the NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (x%) C/S DSNPs were also investigated using the XRD patterns (
Figure 4b). The XRD patterns show that the NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (x%) C/S DSNPs exhibited a single hexagonal phase without any impurity.
Figure 4c shows PL spectra of the NaYF
4:Nd (30%),Yb (10%) core and NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (x%) C/S DSNPs under 800 nm NIR light. After the shell formation on the NaYF
4:Nd (30%),Yb (10%) core, the C/S DSNPs showed higher PL intensity than the core DSNPs. The Yb
3+ emission intensity of the C/S DSNPs was further enhanced by doping the shell with Nd
3+ ions. In the PL spectra, we observed a weak Nd
3+ emission peak at 862 nm (
4F
3/2 →
4I
9/2) and a strong Yb
3+ emission band at 978 nm (
2F
5/2 →
2F
7/2) [
15,
20]. When the shell was doped with 10% Nd
3+ ions, the NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%) C/S DSNPs exhibited the highest emission intensity. In contrast, when the Nd
3+ concentration in the shell was higher than 10%, the Yb
3+ emission intensity decreased as the Nd
3+ concentration increased.
Figure 4d shows a schematic illustration of the C/S DSNP and an energy level diagram of Yb
3+ and Nd
3+. Further enhancement of the Yb
3+ emission intensity can be explained by energy transfer from Nd
3+ in the shell to Nd
3+/Yb
3+ in the core [
20,
32]. When the C/S DSNPs are illuminated with 800 nm NIR light, it is absorbed by Nd
3+ ions in the shell in addition to those in the core, and the excited energy is transferred to the Nd
3+ ions in the core, followed by energy transfer from Nd
3+ to Yb
3+. As the concentration of Nd
3+ ions increases in the shell, the energy loss can increase due to energy migration to the surface [
28,
33]. Consequently, the emission from the NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (x%) C/S DSNPs decreased when the concentration of Nd
3+ ions in the shell was greater than 10% (
Figure 4c).
Because Nd
3+ ions doped in the shell are affected by surface defects, the second shell was formed around the NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%) C/S DSNPs. As shown in
Scheme 1, NaYF
4:Nd (10%) shell was grown on the NaYF
4:Nd (30%),Yb (10%) core DSNPs followed by the formation of NaYF
4 shell. Since the crystal structure of NaYF
4 is hexagonal, anisotropic shell growth on the NaYF
4-based nanoparticles can occur [
34,
35]. Faster growth rate along [0001] direction rather than other crystallographic axes yields rod-like nanoparticles [
35]. After growth of the NaYF
4:Nd (10%) first shell on the core, the DSNPs showed rod-like morphology where the length along the [0001] direction was slightly larger than that along short axis. After the formation of the second shell on the C/S DSNPs, particle size increased and the C/S/S DSNPs apparently exhibits a rod shape.
Figure 5a shows the TEM image of NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs. The NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs showed a uniform rod shape with the size of 45.9 nm ± 1.4 nm × 29.9 nm ± 1.3 nm. As shown in
Figure S4, NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs with a single hexagonal phase were successfully synthesized without impurities.
Figure 5b shows the HR-TEM image and the corresponding fast Fourier transformation (FFT) pattern of NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs. The NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs exhibited clear lattice fringes with a lattice spacing of 0.52 nm, which coincides with
d-spacing between the (10
0) planes of hexagonal NaYF
4. This result indicates that highly crystalline hexagonal-structured NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs were synthesized.
Figure 5c shows a schematic illustration and EDS map images of the NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs. In the high-angle annular dark-field scanning TEM (HAADF-STEM) image, the core-shell structure in the DSNPs was roughly observed by brightness contrast. However, the C/S/S structure was not observed. Therefore, EDS analysis was conducted to investigate the C/S/S structure of DSNPs. The EDS map images show that Yb
3+ and Nd
3+ ions were present in the inner region of NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs, whereas Y
3+ ions were present in all of the regions. A comparison of the Nd Lα and Yb Lα maps revealed that Nd
3+ was observed in a wider area than Yb
3+ doped in the core, indicating Nd
3+ doping of the first shell. Magnified EDS maps also directly show the formation of core/shell/shell structure (
Figure S5). Consequently, EDS analysis confirmed the successful synthesis of the C/S/S-structured DSNPs.
Figure 5d shows the PL spectra of the NaYF
4:Nd (30%),Yb (10%) core, NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%) C/S, and NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs under 800 nm NIR light. The NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs showed 3.0 times higher Yb
3+ emission intensity than the core DSNPs. Moreover, the NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs exhibited 10% higher PL intensity than the C/S DSNPs.
When we investigated time-resolved PL properties of the NaYF
4:Nd (30%),Yb (10%)-based DSNPs, PL lifetime of the DSNPs increased as the shell was grown on the core (
Figure S6). The PL lifetimes of the DSNPs could be obtained by fitting the time-resolved PL profiles using an exponential decay function [
36], and in our study, the time-resolved PL profiles were well fitted with bi-exponential and single exponential decay functions (
Figure S6). The PL lifetime of the DSNPs increased from 611 μs for NaYF
4:Nd (30%),Yb (10%) core DSNPs to 803 μs for NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%) C/S DSNPs. The NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs showed the longest PL lifetime (816 μs) among the core, C/S, and C/S/S DSNPs. The increase in the PL lifetime in the NaYF
4:Nd (30%),Yb (10%)-based DSNPs indicates that the luminescence quenching is inhibited by the reduction of non-radiative pathway after shell formation [
37]. Additionally, we investigated the stability of the NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs. As shown in
Figure S7, the synthesized NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs showed high photostability and thermal stability. The C/S/S DSNPs exhibited stable PL properties against continuous irradiation of NIR light and ultraviolet light, respectively (
Figure S7a–c). In addition, the C/S/S DSNPs showed stable PL characteristics after heat treatment at 100 °C for 1 h. These results indicate that the C/S/S DSNPs are beneficial for their practical application.
To investigate the feasibility of LSC applications of the NaYF
4:Nd (30%), Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs, we fabricated NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNP-PDMS composites.
Figure 6a shows a schematic illustration of bare PDMS- and DSNP-PDMS composite-coupled solar cells. In a bare PDMS-coupled solar cell, incident sunlight directly passes through the PDMS polymer so that most sunlight cannot reach the solar cell. In contrast, in the DSNP-PDMS composite-coupled solar cell, NIR photons of incident sunlight are absorbed by the DSNPs, and the DSNPs emit NIR light in all directions. The emitted light is directed to the silicon solar cell attached to the edge of the DSNP-PDMS composite, which can result in electricity generation from the silicon solar cell.
Figure 6b shows the transmittance spectra of the bare PDMS and the C/S/S DSNP-PDMS composite. The average transmittance values of the bare PDMS and the C/S/S DSNP-PDMS composite in the visible region (λ = 380–750 nm) were 92.1 and 79.4%, respectively. Although the transmittance of the C/S/S DSNP-PDMS composite was lower than that of the bare PDMS polymer, it was still highly transparent, as shown in the inset of
Figure 6b. Therefore, we believe that the C/S/S DSNP-PDMS composite can be applied to transparent PV modules. Additionally, in the transmittance spectrum of the C/S/S DSNP-PDMS composite, peaks were observed at 574, 740, and 794 nm, which were attributed to the light absorption via
4I
9/2 →
4G
5/2,
4I
9/2 →
4F
7/2, and
4I
9/2 →
4F
5/2 transitions of Nd
3+ ions in the NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs, indicating the presence of NaYF
4:Nd (30%),Yb (10%)/NaYF
4:Nd (10%)/NaYF
4 C/S/S DSNPs in the PDMS composite [
25].
Figure S8a shows the current density versus voltage curves of the bare PDMS- and C/S/S DSNP-PDMS composite-coupled silicon solar cells. The C/S/S DSNP-PDMS composite-coupled silicon solar cell showed increased short-circuit current density compared with the bare PDMS-coupled silicon solar cell. The efficiencies of the bare PDMS- and the C/S/S DSNP-PDMS composite-coupled silicon solar cells were measured to be 0.92 and 1.96%, respectively, under Air Mass (AM) 1.5G illumination using a solar simulator. Since the transmittance of the C/S/S DSNP-PDMS composite is lower than the bare PDMS, the light scattering effect may contribute to the increase in solar cell efficiency. Since the absorption of the C/S/S DSNP-PDMS composite was low (
Figure 6b), the contribution of light scattering to the increase in solar cell efficiency seems to be larger than that of the luminescence from the C/S/S DSNPs in the DSNP-PDMS composite to the increase in solar cell efficiency. However, when the C/S/S DSNP-PDMS composite was excited with weak 800 nm NIR light (1 mW), it showed clear emission band in the NIR spectral region (
Figure S8b). Thus, it seems that both light scattering and luminescence due to the C/S/S DSNPs in the DSNP-PDMS composite contribute to the increase in solar cell efficiency, although the contribution of the luminescence from the C/S/S DSNPs may be low. Since the Nd
3+ ions exhibit strong absorption peaks in NIR spectral region (
Figure 3a), the absorption of the C/S/S DSNP-PDMS composite in the NIR spectral region can be enhanced after further optimization of the C/S/S DSNP-PDMS composite preparation. The transmittance of the C/S/S DSNP-PDMS composite can be further increased via further optimization of the DSNP-PDMS composite preparation. The contribution of the luminescence from the C/S/S DSNPs in the DSNP-PDMS composite will then increase, and the light scattering effect will decrease for the increase in solar cell efficiency. The optimized DSNP-PDMS composites can be suitable as LSCs for transparent PV modules.