An Artificial MnWO4 Cathode Electrolyte Interphase Enabling Enhanced Electrochemical Performance of δ-MnO2 Cathode for Aqueous Zinc Ion Battery
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Electrochemical Measurements
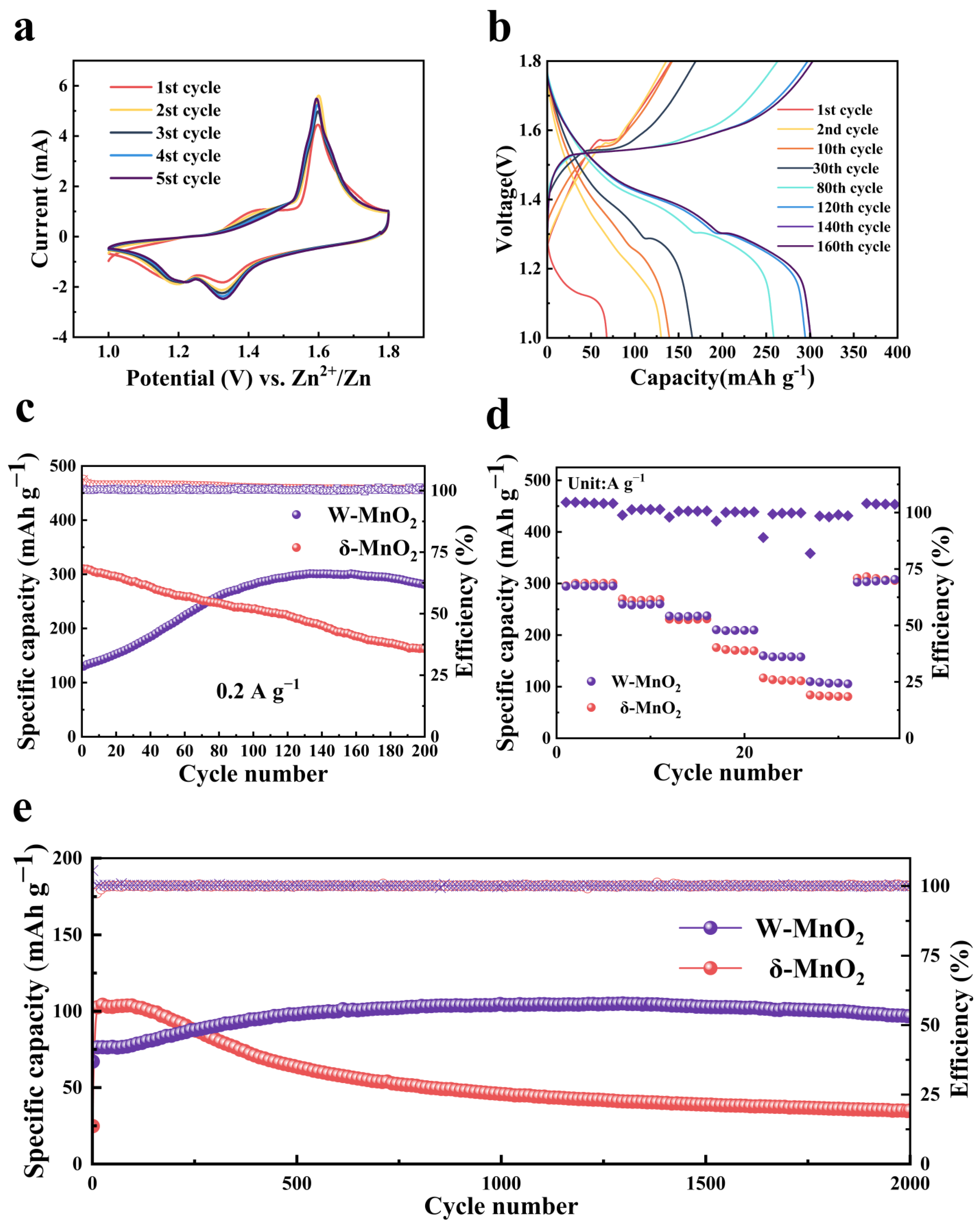
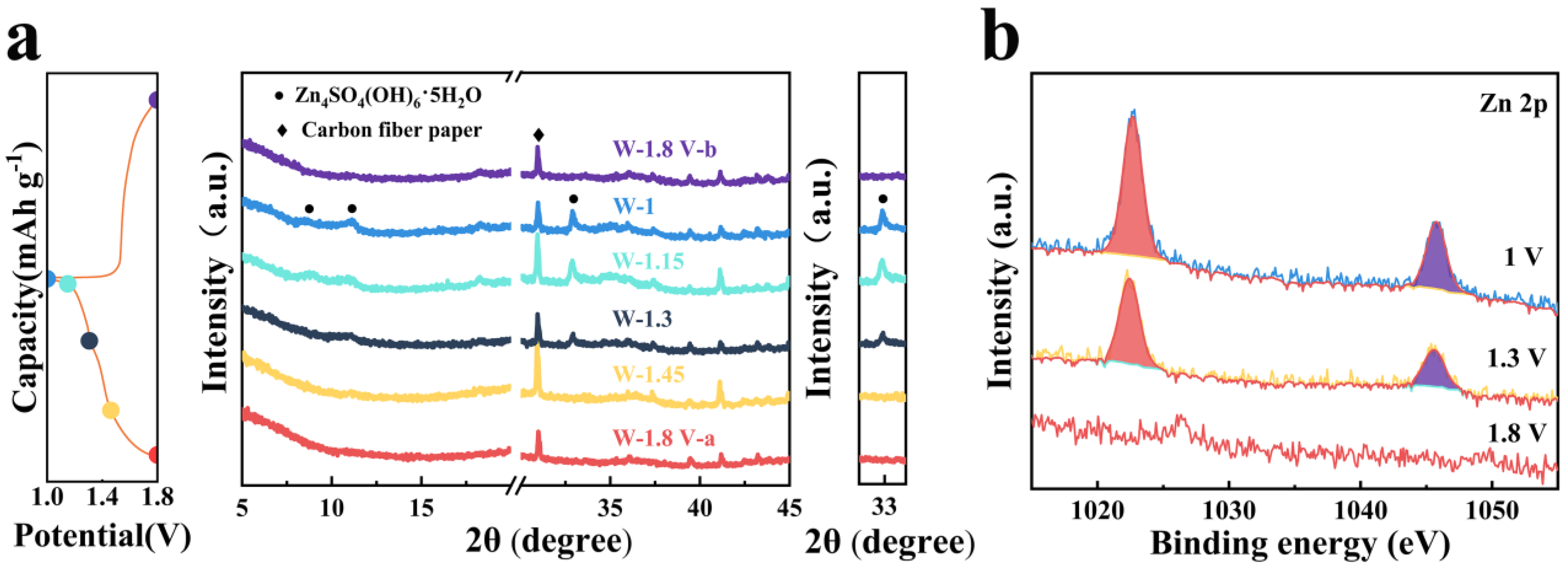
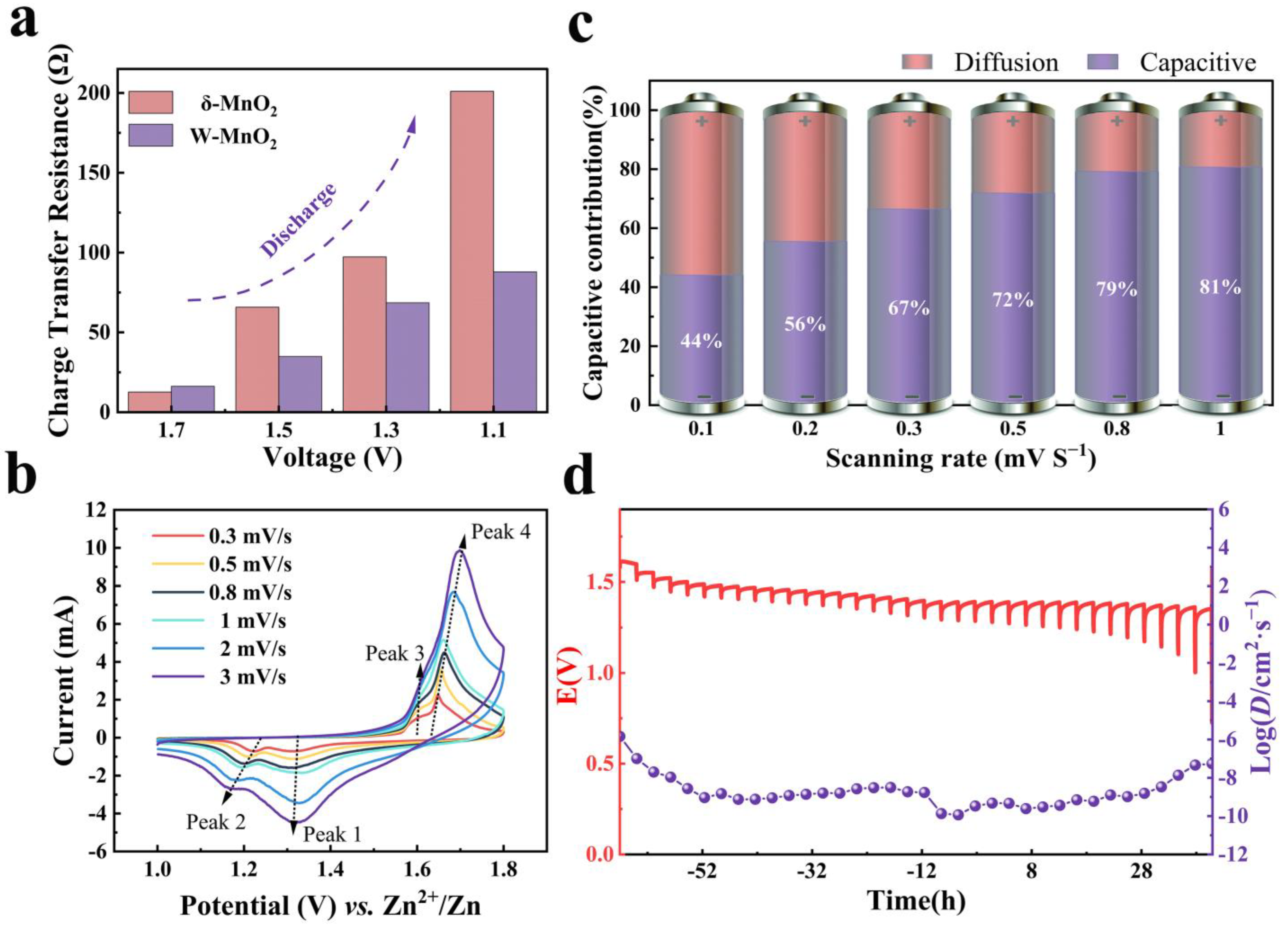
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macrelli, A.; Olivieri, M.; Lamperti, A.; Russo, V.; Bozzini, B.; Menegazzo, M.; Bussetti, G.; Casari, C.S.; Li Bassi, A. Nanostructured ZnxMn3−xO4 thin films by pulsed laser deposition: A spectroscopic and electrochemical study towards the application in aqueous Zn-ion batteries. Electrochim. Acta 2023, 442, 141909. [Google Scholar] [CrossRef]
- Zampardi, G.; La Mantia, F. Prussian blue analogues as aqueous Zn-ion batteries electrodes: Current challenges and future perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, L.; Cao, X.; Zhou, J.; Pan, A.; Fang, G.; Wang, S.; Liang, S. Ion migration and defect effect of electrode materials in multivalent-ion batteries. Prog. Mater. Sci. 2022, 125, 100911. [Google Scholar] [CrossRef]
- Tang, B.; Fang, G.; Zhou, J.; Wang, L.; Lei, Y.; Wang, C.; Lin, T.; Tang, Y.; Liang, S. Potassium vanadates with stable structure and fast ion diffusion channel as cathode for rechargeable aqueous zinc-ion batteries. Nano Energy 2018, 51, 579–587. [Google Scholar] [CrossRef]
- Chen, D.; Lu, M.; Cai, D.; Yang, H.; Han, W. Recent advances in energy storage mechanism of aqueous zinc-ion batteries. J. Energy Chem. 2021, 54, 712–726. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Fegade, U.; Jethave, G.; Khan, F.; Al Ahmed, A.; Karmouch, R.; Shariq, M.; Inamuddin; Ahmer, M.F. Recent development of aqueous zinc-ion battery cathodes and future challenges: Review. Int. J. Energ. Res. 2022, 46, 13152–13177. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Yang, J.; Zhang, Y.; Cai, Z. Highly loaded and binder-free molybdenum trioxide cathode material prepared using multi-arc ion plating for aqueous zinc ion batteries. Materials 2022, 15, 5954. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Yuan, J.; Xia, Y.; Liu, Y.; Sun, A. Recent advance and modification strategies of transition metal dichalcogenides (TMDs) in aqueous zinc ion batteries. Materials 2022, 15, 2654. [Google Scholar] [CrossRef]
- Karapidakis, E.; Vernardou, D. Progress on V2O5 cathodes for multivalent aqueous batteries. Materials 2021, 14, 2310. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Liang, G.; Li, H.; Liu, Z.; Tang, Z.; Liang, J.; Zhi, C. A Superior δ-MnO2 cathode and a self-healing Zn-δ-MnO2 battery. ACS Nano 2019, 13, 10643–10652. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Mathew, V.; Song, J.; Kim, S.; Tung, D.P.; Jo, J.; Kim, S.; Baboo, J.P.; Xiu, Z.; et al. Ambient redox synthesis of vanadium-doped manganese dioxide nanoparticles and their enhanced zinc storage properties. Appl. Surf. Sci. 2017, 404, 435–442. [Google Scholar] [CrossRef]
- Chamoun, M.; Brant, W.R.; Tai, C.; Karlsson, G.; Noréus, D. Rechargeability of aqueous sulfate Zn/MnO2 batteries enhanced by accessible Mn2+ ions. Energy Storage Mater. 2018, 15, 351–360. [Google Scholar] [CrossRef]
- Zuo, Y.; Meng, T.; Tian, H.; Ling, L.; Zhang, H.; Zhang, H.; Sun, X.; Cai, S. Enhanced H+ storage of a MnO2 cathode via a MnO2 nanolayer interphase transformed from manganese phosphate. ACS Nano 2023, 17, 5600–5608. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, B.; Hu, C.; Chen, H.; Huang, J.; Yan, D.; Peng, S. Construction strategy of VO2 @V2C 1D/2D heterostructure and improvement of zinc-ion diffusion ability in VO2 (B). ACS Appl. Mater. Inter. 2022, 14, 28760–28768. [Google Scholar] [CrossRef]
- Dong, Y.; Jia, M.; Wang, Y.; Xu, J.; Liu, Y.; Jiao, L.; Zhang, N. Long-life zinc/vanadium pentoxide battery enabled by a concentrated aqueous ZnSO4 electrolyte with proton and zinc ion co-intercalation. ACS Appl. Energ. Mater. 2020, 3, 11183–11192. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y.; Qiu, M.; Xu, J.; Liu, Y.; Jiao, L.; Cheng, F. Rechargeable aqueous Zn-V2O5 battery with high energy density and long cycle life. ACS Energy Lett. 2018, 3, 1366–1372. [Google Scholar] [CrossRef]
- Chen, L.; Ruan, Y.; Zhang, G.; Wei, Q.; Jiang, Y.; Xiong, T.; He, P.; Yang, W.; Yan, M.; An, Q.; et al. Ultrastable and high-performance Zn/VO2 battery based on a reversible single-phase reaction. Chem. Mater. 2019, 31, 699–706. [Google Scholar] [CrossRef]
- Lu, K.; Song, B.; Zhang, J.; Ma, H. A rechargeable Na-Zn hybrid aqueous battery fabricated with nickel hexacyanoferrate and nanostructured zinc. J. Power Sources 2016, 321, 257–263. [Google Scholar] [CrossRef]
- Yang, Q.; Mo, F.; Liu, Z.; Ma, L.; Li, X.; Fang, D.; Chen, S.; Zhang, S.; Zhi, C. Activating C-coordinated iron of iron hexacyanoferrate for Zn hybrid-ion batteries with 10,000-cycle lifespan and superior rate capability. Adv. Mater. 2019, 31, 1901521. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Qin, R.; Ding, S.; Wang, Y.; Li, S.; Zhao, Q.; Pan, F. Structure and properties of Prussian blue analogues in energy storage and conversion applications. Adv. Funct. Mater. 2021, 31, 2006970. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system. Adv. Energy Mater. 2015, 5, 1400930. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. Infomat 2020, 2, 237–260. [Google Scholar] [CrossRef]
- Ren, Q.; Yuan, Y.; Wang, S. Interfacial strategies for suppression of Mn dissolution in rechargeable battery cathode materials. ACS Appl. Mater. Inter. 2022, 14, 23022–23032. [Google Scholar] [CrossRef]
- Fang, G.; Zhu, C.; Chen, M.; Zhou, J.; Tang, B.; Cao, X.; Zheng, X.; Pan, A.; Liang, S. Suppressing manganese dissolution in potassium manganate with rich oxygen defects engaged high-energy-density and durable aqueous zinc-ion battery. Adv. Funct. Mater. 2019, 29, 1808375. [Google Scholar] [CrossRef]
- Poyraz, A.S.; Laughlin, J.; Zec, Z. Improving the cycle life of cryptomelane type manganese dioxides in aqueous rechargeable zinc ion batteries: The effect of electrolyte concentration. Electrochim. Acta 2019, 305, 423–432. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, Y.; Fang, G.; Liang, S. Electrolyte/electrode interfacial electrochemical behaviors and optimization strategies in aqueous zinc-ion batteries. Energy Storage Mater. 2022, 45, 618–646. [Google Scholar] [CrossRef]
- Verma, V.; Kumar, S.; Manalastas, W.; Srinivasan, M. Undesired reactions in aqueous rechargeable zinc ion batteries. ACS Energy Lett. 2021, 6, 1773–1785. [Google Scholar] [CrossRef]
- Guo, S.; Liang, S.; Zhang, B.; Fang, G.; Ma, D.; Zhou, J. Cathode interfacial layer formation via in situ electrochemically charging in aqueous zinc-ion battery. ACS Nano 2019, 13, 13456–13464. [Google Scholar] [CrossRef]
- Luo, S.; Cao, X.; Su, Q.; Zhang, Y.; Liu, S.; Xie, X.; Liang, S.; Pan, A. Layered barium vanadate cathodes for aqueous zinc batteries: Enhancing cycling stability through inhibition of vanadium dissolution. ACS Appl. Energ. Mater. 2021, 4, 6197–6204. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, G.; Yan, M.; Xiong, T.; He, P.; He, L.; Xu, X.; Mai, L. Graphene scroll-coated α-MnO2 nanowires as high-performance cathode materials for aqueous Zn-ion battery. Small 2018, 14, 1703850. [Google Scholar] [CrossRef]
- Guo, J.; Ming, J.; Lei, Y.; Zhang, W.; Xia, C.; Cui, Y.; Alshareef, H.N. Artificial solid electrolyte interphase for suppressing surface reactions and cathode dissolution in aqueous zinc ion batteries. ACS Energy Lett. 2019, 4, 2776–2781. [Google Scholar] [CrossRef]
- Li, S.; Yu, D.; Liu, L.; Yao, S.; Wang, X.; Jin, X.; Zhang, D.; Du, F. In-situ electrochemical induced artificial solid electrolyte interphase for MnO@C nanocomposite enabling long-lived aqueous zinc-ion batteries. Chem. Eng. J. 2022, 430, 132673. [Google Scholar] [CrossRef]
- Liu, Y.; Zhi, J.; Hoang, T.K.A.; Zhou, M.; Han, M.; Wu, Y.; Shi, Q.; Xing, R.; Chen, P. Paraffin Based cathode-electrolyte interface for highly reversible aqueous zinc-ion battery. ACS Appl. Energ. Mater. 2022, 5, 4840–4849. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, B.; Hu, J.; Liu, J.; Miao, L.; Jiang, J. An in situ artificial cathode electrolyte interphase strategy for suppressing cathode dissolution in aqueous zinc ion batteries. Small Methods 2021, 5, 2100094. [Google Scholar] [CrossRef]
- Chen, X.; Cao, Z.; Xing, L.; Liao, Y.; Qiu, Y.; Li, W. Improved Li-storage performance with PEDOT-decorated MnO2 nanoboxes. Nanoscale 2017, 9, 18467–18473. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, Q.; Liu, H.; Tian, S.; Chen, B.; Zhao, J.; Li, J. A case study of β- and δ-MnO2 with different crystallographic forms on ion-storage in rechargeable aqueous zinc ion battery. Electrochim. Acta 2019, 324, 134867. [Google Scholar] [CrossRef]
- Wu, P.; Dai, S.; Chen, G.; Zhao, S.; Xu, Z.; Fu, M.; Chen, P.; Chen, Q.; Jin, X.; Qiu, Y.; et al. Interfacial effects in hierarchically porous α-MnO2/Mn3O4 heterostructures promote photocatalytic oxidation activity. Appl. Catal. B Environ. 2020, 268, 118418. [Google Scholar] [CrossRef]
- Puttaswamy, R.; Nagaraj, R.; Kulkarni, P.; Beere, H.K.; Upadhyay, S.N.; Balakrishna, R.G.; Sanna Kotrappanavar, N.; Pakhira, S.; Ghosh, D. Constructing a high-performance aqueous rechargeable zinc-ion battery cathode with self-assembled mat-like packing of intertwined Ag(I) pre-inserted V3O7·H2O microbelts with reduced graphene oxide core. ACS Sustain. Chem. Eng. 2021, 9, 3985–3995. [Google Scholar] [CrossRef]
- Cook, J.B.; Kim, H.S.; Yan, Y.; Ko, J.S.; Robbennolt, S.; Dunn, B.; Tolbert, S.H. Mesoporous MoS2 as a transition metal dichalcogenide exhibiting pseudocapacitive Li and Na-ion charge storage. Adv. Energy Mater. 2016, 6, 1501937. [Google Scholar] [CrossRef]
- Deiss, E. Spurious chemical diffusion coefficients of Li+ in electrode materials evaluated with GITT. Electrochim. Acta 2005, 50, 2927–2932. [Google Scholar] [CrossRef]
- Chen, W.; Li, G.; Pei, A.; Li, Y.; Liao, L.; Wang, H.; Wan, J.; Liang, Z.; Chen, G.; Zhang, H.; et al. A manganese-hydrogen battery with potential for grid-scale energy storage. Nat. Energy 2018, 3, 428–435. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Gao, X.; Wu, H.; Li, W.; Tian, Y.; Zhang, Y.; Wu, H.; Yang, L.; Zou, G.; Hou, H.; Ji, X. H+-insertion boosted α-MnO2 for an Aqueous Zn-ion battery. Small 2020, 16, 1905842. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Liu, N.; Wu, X.; Fan, L.; Gong, S.; Guo, Z.; Chen, A.; Zhao, C.; Mao, Y.; Zhang, N.; Sun, K. Intercalation pseudocapacitive Zn2+ storage with hydrated vanadium dioxide toward ultrahigh rate performance. Adv. Mater. 2020, 32, 1908420. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, P.; Yang, H.; Min, H.; Niu, M.; Jin, S.; Jiang, Y.; Pan, Z.; Yan, J.; Shen, X.; et al. Manipulating intercalation-extraction mechanisms in structurally modulated δ-MnO2 nanowires for high-performance aqueous zinc-ion batteries. Chem. Eng. J. 2022, 433, 133687. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, P.; Ling, L.; Tian, M.; Wang, Z.; Tian, H.; Meng, T.; Sun, X.; Cai, S. Boosted H+ intercalation enables ultrahigh rate performance of the δ-MnO2 cathode for aqueous zinc batteries. ACS Appl. Mater. Inter. 2022, 14, 26653–26661. [Google Scholar] [CrossRef]
- Pang, Q.; Sun, C.; Yu, Y.; Zhao, K.; Zhang, Z.; Voyles, P.M.; Chen, G.; Wei, Y.; Wang, X. H2V3O8 nanowire/graphene electrodes for aqueous rechargeable zinc ion batteries with high rate capability and large capacity. Adv. Energy Mater. 2018, 8, 1800144. [Google Scholar] [CrossRef]
- Wang, X.; Xi, B.; Ma, X.; Feng, Z.; Jia, Y.; Feng, J.; Qian, Y.; Xiong, S. Boosting zinc-ion storage capability by effectively suppressing vanadium dissolution based on robust layered barium vanadate. Nano Lett. 2020, 20, 2899–2906. [Google Scholar] [CrossRef]
- Cook, J.B.; Kim, H.S.; Lin, T.C.; Lai, C.H.; Dunn, B.; Tolbert, S.H. Pseudocapacitive charge storage in thick composite MoS2 Nanocrystal-based electrodes. Adv. Energy Mater. 2017, 7, 1601283. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, H.; Zhang, H.; Zuo, Y.; Ling, L.; Meng, T.; Zhang, H.; Sun, X.; Cai, S. An Artificial MnWO4 Cathode Electrolyte Interphase Enabling Enhanced Electrochemical Performance of δ-MnO2 Cathode for Aqueous Zinc Ion Battery. Materials 2023, 16, 3228. https://doi.org/10.3390/ma16083228
Tian H, Zhang H, Zuo Y, Ling L, Meng T, Zhang H, Sun X, Cai S. An Artificial MnWO4 Cathode Electrolyte Interphase Enabling Enhanced Electrochemical Performance of δ-MnO2 Cathode for Aqueous Zinc Ion Battery. Materials. 2023; 16(8):3228. https://doi.org/10.3390/ma16083228
Chicago/Turabian StyleTian, Hao, Huanlin Zhang, You Zuo, Lei Ling, Tengfei Meng, Hang Zhang, Xiaohong Sun, and Shu Cai. 2023. "An Artificial MnWO4 Cathode Electrolyte Interphase Enabling Enhanced Electrochemical Performance of δ-MnO2 Cathode for Aqueous Zinc Ion Battery" Materials 16, no. 8: 3228. https://doi.org/10.3390/ma16083228
APA StyleTian, H., Zhang, H., Zuo, Y., Ling, L., Meng, T., Zhang, H., Sun, X., & Cai, S. (2023). An Artificial MnWO4 Cathode Electrolyte Interphase Enabling Enhanced Electrochemical Performance of δ-MnO2 Cathode for Aqueous Zinc Ion Battery. Materials, 16(8), 3228. https://doi.org/10.3390/ma16083228





