Preparation of Titania–Silica Composite Aerogel at Atmospheric Pressure and Its Catalytic Performance in the Synthesis of Poly (Butylene Succinate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
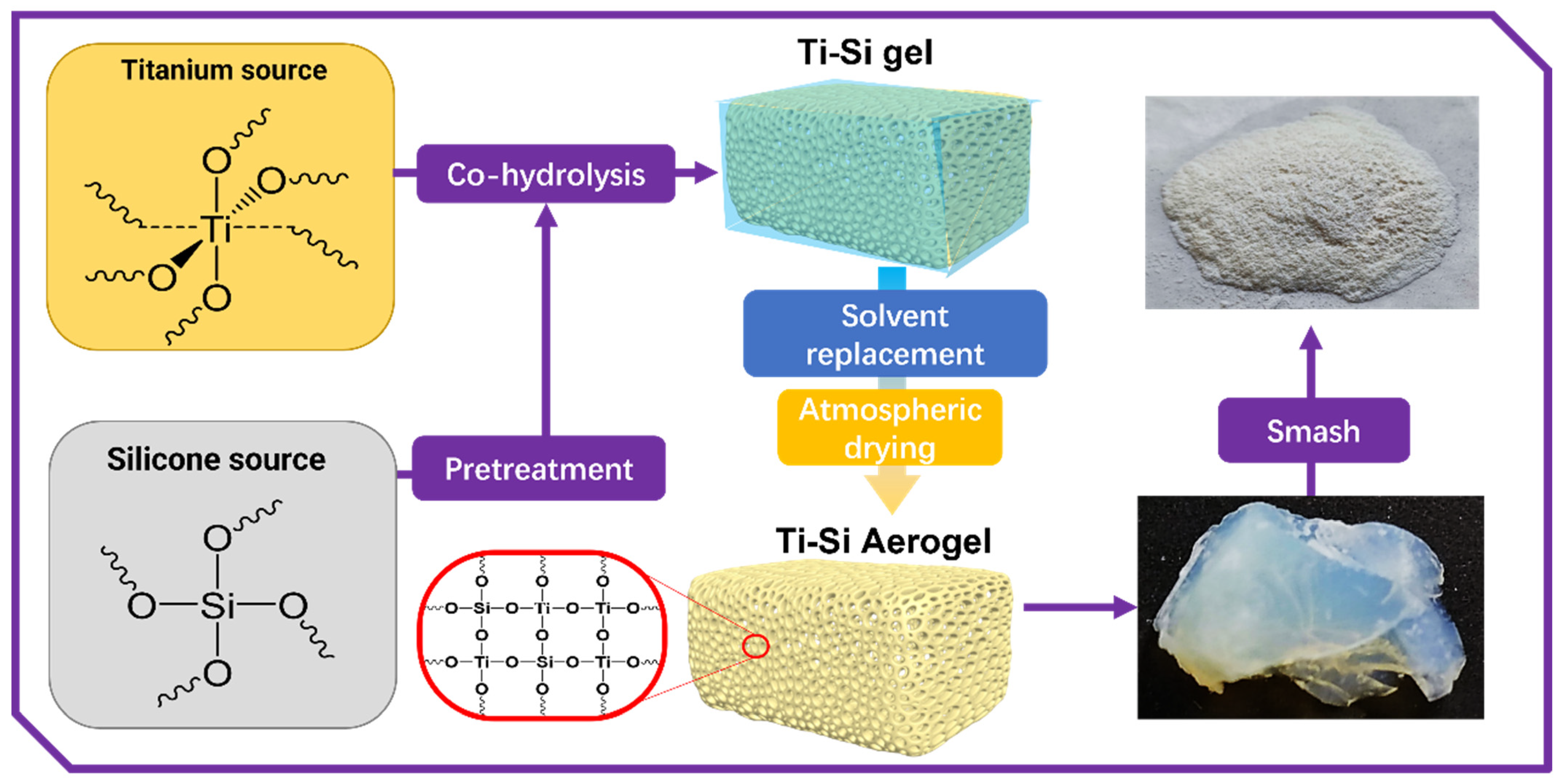
2.2. Preparation of Titania–Silica Composite Aerogels
2.2.1. Pre-Hydrolysis of Tetraethyl Silicate
2.2.2. Preparation of Titania–Silica Composite Alcohol Gels
2.2.3. Preparation of Titania–Silica Composite Aerogels
2.3. Preparation of PBS
2.4. Characterization and Measurement
3. Results and Discussion
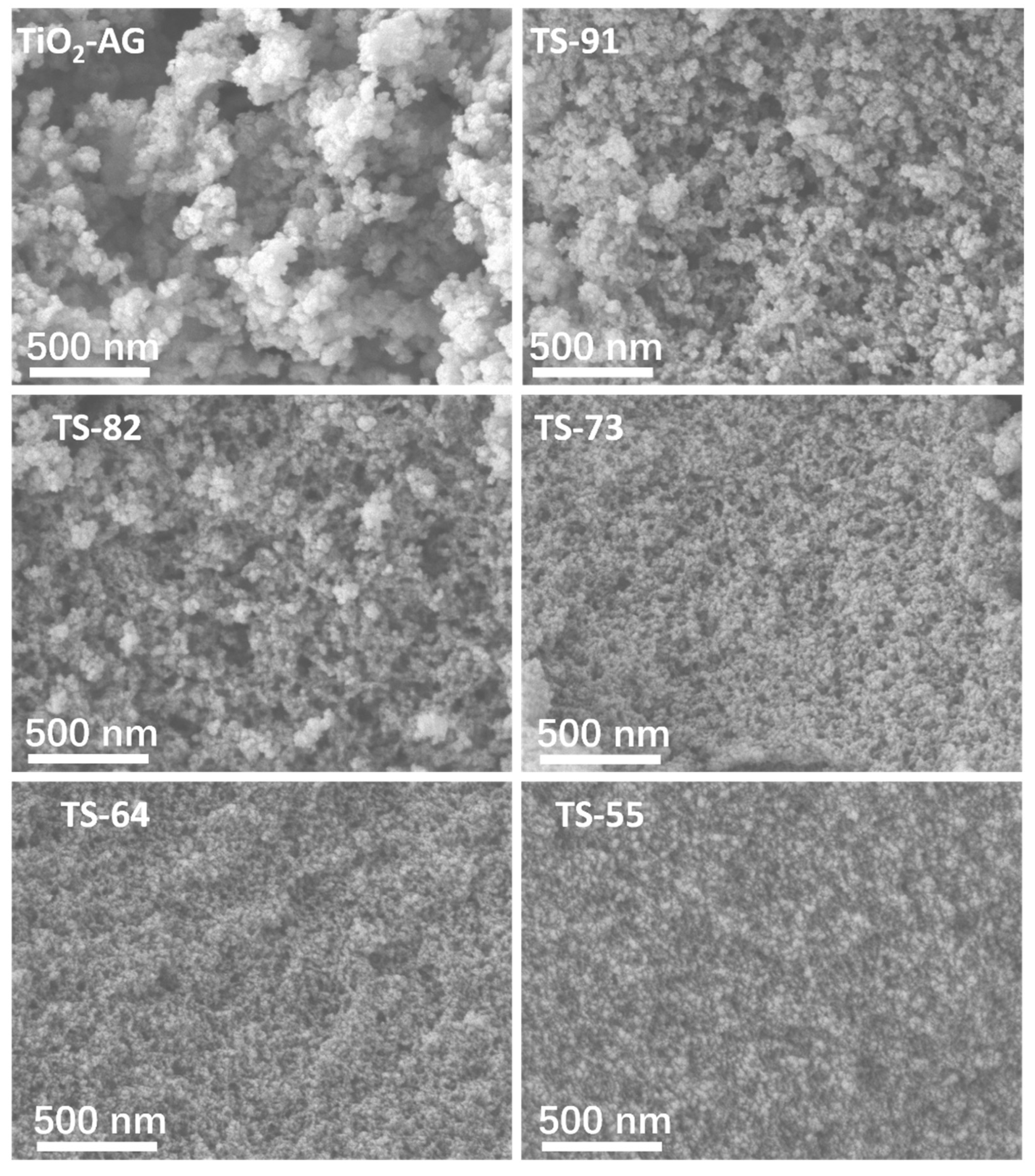
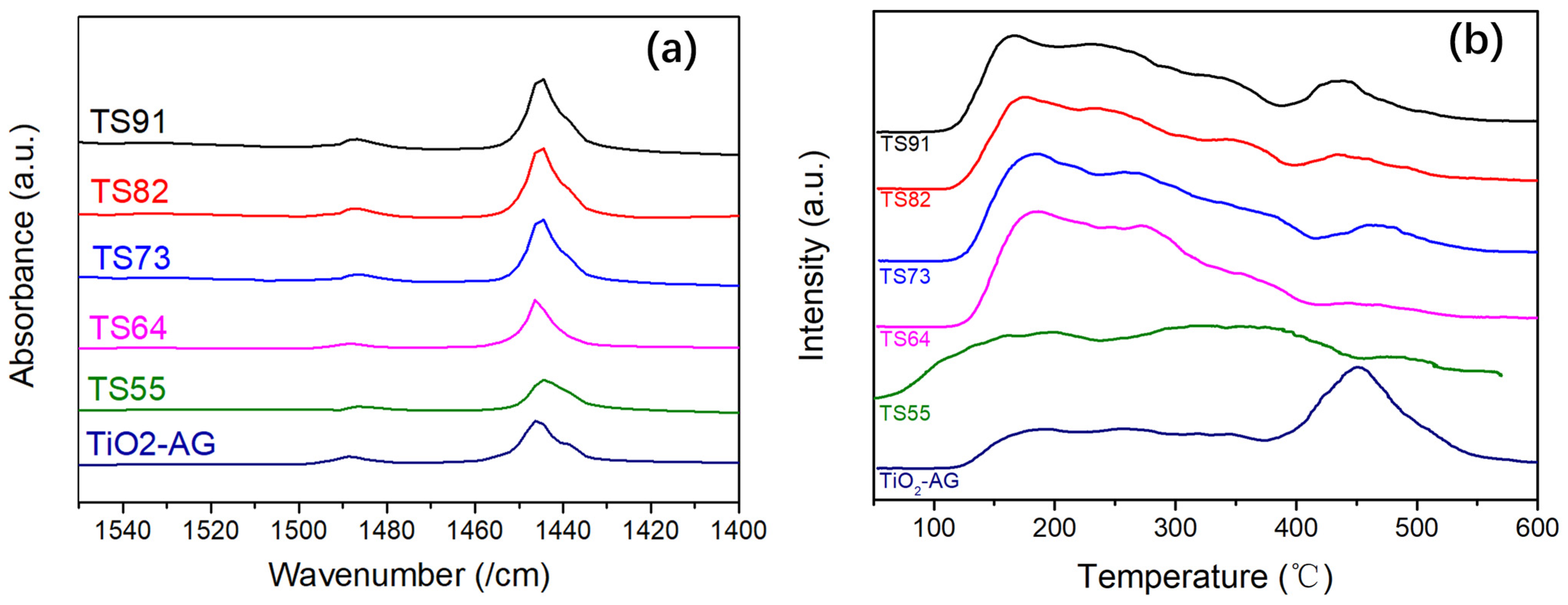
3.1. Surface Morphology and Structure of Titania–Silica Composite Aerogels
3.2. Catalytic Activity of Titania–Silica Composite Aerogels for the Polycondensation of PBS
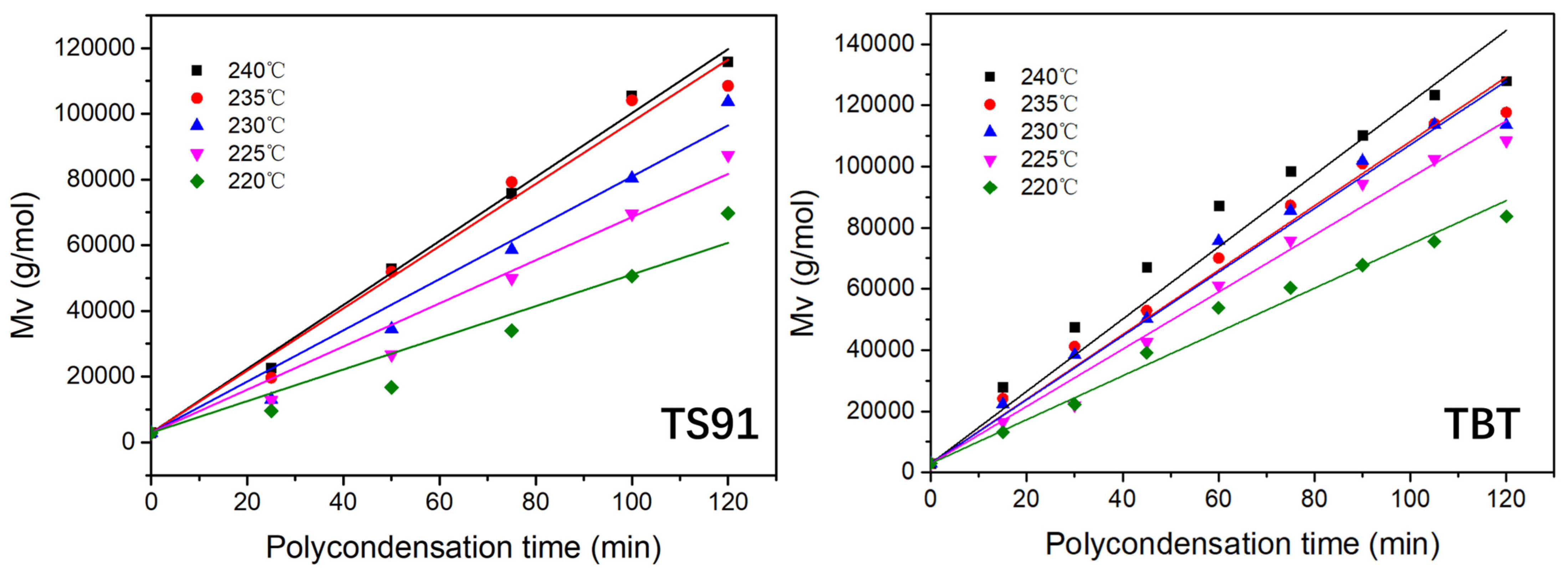
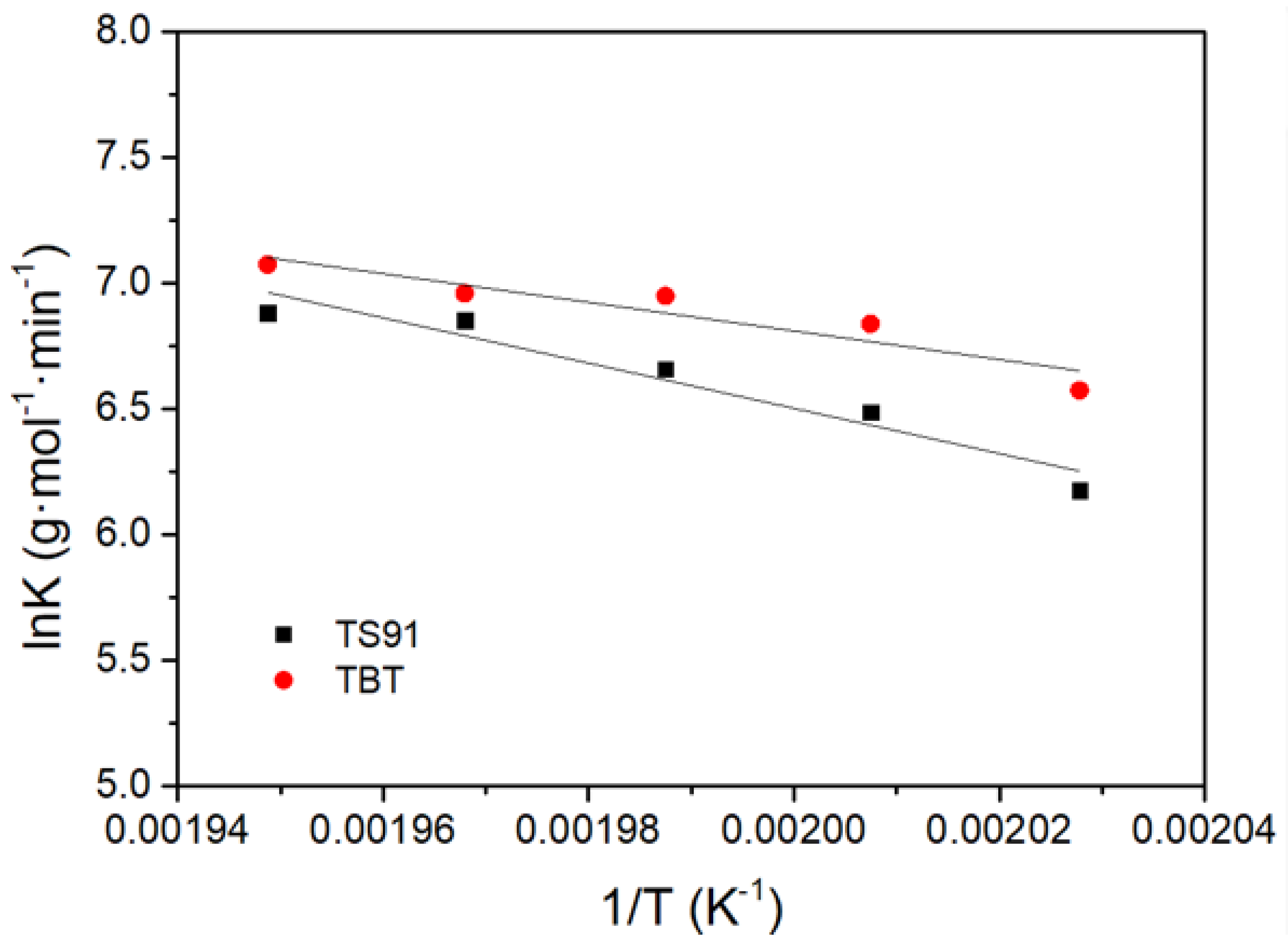
3.3. Reaction Kinetics of PBS Polycondensation Catalyzed by Titania–Silica Aerogel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nomadolo, N.; Dada, O.E.; Swanepoel, A.; Mokhena, T.; Muniyasamy, S. A Comparative Study on the Aerobic Biodegradation of the Biopolymer Blends of Poly (butylene succinate), Poly (butylene adipate terephthalate) and Poly (lactic acid). Polymers 2022, 14, 1894. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Bai, Z.; Su, T.; Wang, Z. Enzymatic degradation of poly (butylene succinate) with different molecular weights by cutinase. Int. J. Biol. Macromol. 2018, 111, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.-M.; Liu, P.; Wang, C.-X.; Meng, X.; Zhou, Q. Polymorphism regulation in Poly (hexamethylene succinate-co-hexamethylene fumarate): Altering the hydrogen bonds in crystalline lattice. Polymer 2017, 108, 272–280. [Google Scholar] [CrossRef]
- Duborper, C.; Samuel, C.; Akue-Asseko, A.C.; Loux, C.; Lacrampe, M.F.; Krawczak, P. Design of biobased poly (butylene succinate) foams by single-screw extrusion: Identification of relevant rheological parameters controlling foam morphologies. Polym. Eng. Sci. 2018, 58, 503–512. [Google Scholar] [CrossRef]
- Ratshoshi, B.K.; Farzad, S.; Görgens, J.F. Techno-economic assessment of polylactic acid and polybutylene succinate production in an integrated sugarcane biorefinery. Biofuels Bioprod. Biorefining 2021, 15, 1871–1887. [Google Scholar] [CrossRef]
- Xiao, F.; Fontaine, G.; Bourbigot, S. Recent developments in fire retardancy of polybutylene succinate. Polym. Degrad. Stab. 2021, 183, 109466. [Google Scholar] [CrossRef]
- Barrino, F.; De La Rosa-Ramírez, H.; Schiraldi, C.; López-Martínez, J.; Samper, M.D. Preparation and Characterization of New Bioplastics Based on Polybutylene Succinate (PBS). Polymers 2023, 15, 1212. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhao, L.; Gao, X.; Xu, Z.; Liu, Z.; Hu, D. Engineering of polybutylene succinate with long-chain branching toward high foamability and degradation. Polym. Degrad. Stab. 2021, 194, 109745. [Google Scholar] [CrossRef]
- Arrigo, R.; D’Anna, A.; Frache, A. Fully bio-based ternary polymer blends: Structural characterization and mechanical behavior. Mater. Today Sustain. 2023, 21, 100314. [Google Scholar] [CrossRef]
- Rogers, M.E.; Long, T.E.; Turner, S.R. Introduction to Synthetic Methods in Step-Growth Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 1–16. [Google Scholar]
- Jacquel, N.; Freyermouth, F.; Fenouillot, F.; Rousseau, A.; Pascault, J.P.; Fuertes, P.; Saint-Loup, R. Synthesis and properties of poly (butylene succinate): Efficiency of different transesterification catalysts. J. Polym. Sci. Pol. Chem. 2011, 49, 5301–5312. [Google Scholar] [CrossRef]
- Finelli, L.; Lorenzetti, C.; Messori, M.; Sisti, L.; Vannini, M. Comparison between titanium tetrabutoxide and a new commercial titanium dioxide based catalyst used for the synthesis of poly (ethylene terephthalate). J. Appl. Polym. Sci. 2004, 92, 1887–1892. [Google Scholar] [CrossRef]
- Han, Y.-K.; Kim, S.-R.; Kim, J. Preparation and characterization of high molecular weight poly (butylene succinate). Macromol. Res. 2002, 10, 108–114. [Google Scholar] [CrossRef]
- MacDonald, W.A. New advances in poly (ethylene terephthalate) polymerization and degradation. Polym. Int. 2002, 51, 923–930. [Google Scholar] [CrossRef]
- Ste, K.; Miles, C.; McClain, A.; Wis, E.; Sobolewski, P.; Kohn, J.; Puskas, J.; Wagner, H.D.; El Fray, M. Biocopolyesters of poly (butylene succinate) containing long-chain biobased glycol synthesized with heterogeneous titanium dioxide catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10623–10632. [Google Scholar]
- Hwang, S.Y.; Yoo, E.S.; Im, S.S. Effects of TS-1 zeolite structures on physical properties and enzymatic degradation of poly (butylene succinate)(PBS)/TS-1 zeolite hybrid composites. Polymer 2011, 52, 965–975. [Google Scholar] [CrossRef]
- Thakur, A.; Hamamoto, T.; Ikeda, T.; Chammingkwan, P.; Wada, T.; Taniike, T. Microwave-assisted polycondensation for screening of organically-modified TiO2/SiO2 catalysts. Appl. Catal. A Gen. 2020, 595, 117508. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Wang, Y.; Li, Z. Thermal stability of anatase TiO2 aerogels. Surf. Interface Anal. 2017, 49, 173–176. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Baskakova, Y.V. Granulated Graphene Oxide/Titanium Dioxide Composite Aerogel as a High-Performance Photocatalyst. High Energy Chem. 2020, 54, 95–101. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Feizbakhshan, M.; Amdebrhan, B.T.; Shi, S.; Xin, W.; Nguyen, T.; Chen, M.; Zhou, X. TiO2-SiO2 nanocomposite aerogel loaded in melamine-impregnated paper for multi-functionalization: Formaldehyde degradation and smoke suppression. Constr. Build. Mater. 2018, 161, 381–388. [Google Scholar] [CrossRef]
- Hutter, R.; Mallat, T.; Peterhans, A.; Baiker, A. Control of acidity and selectivity of titania–silica aerogel for the epoxidation of β-isophorone. J. Mol. Catal. A-Chem. 1999, 138, 241–247. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Achilias, D.S. Synthesis of poly (alkylene succinate) biodegradable polyesters, Part II: Mathematical modelling of the polycondensation reaction. Polymer 2008, 49, 3677–3685. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels—Airy materials: Chemistry, structure, and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef]
- Fricke, J.; Emmerling, A. Aerogels—Recent progress in production techniques and novel applications. J. Sol-Gel Sci. Technol. 1998, 13, 299–303. [Google Scholar] [CrossRef]
- Aravind, P.R.; Shajesh, P.; Mukundan, P.; Warrier, K.G.K. Silica–titania aerogel monoliths with large pore volume and surface area by ambient pressure drying. J. Sol-Gel Sci. Technol. 2009, 52, 328–334. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, S.; Abbas, Y.; Liu, W.; Zhang, Y.; Wu, Z.; Xu, B. Structurally designed heterochain polymer derived porous carbons with high surface area for high-performance supercapacitors. Appl. Surf. Sci. 2020, 530, 147296. [Google Scholar] [CrossRef]
- Sinha, P.; Datar, A.; Jeong, C.; Deng, X.; Chung, Y.G.; Lin, L.-C. Surface area determination of porous materials using the Brunauer–Emmett–Teller (BET) method: Limitations and improvements. J. Phys. Chem. C 2019, 123, 20195–20209. [Google Scholar] [CrossRef]
- Naderi, M. Chapter Fourteen—Surface Area: Brunauer–Emmett–Teller (BET). In Progress in Filtration and Separation; Tarleton, S., Ed.; Academic Press: Oxford, UK, 2015; pp. 585–608. [Google Scholar]
- Yue, X.; Xiang, J.; Chen, J.; Li, H.; Qiu, Y.; Yu, X. High surface area, high catalytic activity titanium dioxide aerogels prepared by solvothermal crystallization. J. Mater. Sci. Technol. 2020, 47, 223–230. [Google Scholar] [CrossRef]
- Ismail, A.A.; Ibrahim, I.A.; Ahmed, M.S.; Mohamed, R.M.; El-Shall, H. Sol–gel synthesis of titania–silica photocatalyst for cyanide photodegradation. J. Photochem. Photobiol. A-Chem. 2004, 163, 445–451. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Wang, W.; Zou, L.; Lian, Y.; Zhang, Z. Silica–titania composite aerogel photocatalysts by chemical liquid deposition of titania onto nanoporous silica scaffolds. ACS Appl. Mater. Interfaces 2015, 7, 5400–5409. [Google Scholar] [CrossRef]
- Chastain, J.; King, R.C., Jr. Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corpor. 1992, 40, 221. [Google Scholar]
- Luo, L.; Cooper, A.T.; Fan, M. Preparation and application of nanoglued binary titania–silica aerogel. J. Hazard. Mater. 2009, 161, 175–182. [Google Scholar] [CrossRef]
- Chinh, V.D.; Broggi, A.; Di Palma, L.; Scarsella, M.; Speranza, G.; Vilardi, G.; Thang, P.N. XPS spectra analysis of Ti2+, Ti3+ ions and dye photodegradation evaluation of titania-silica mixed oxide nanoparticles. J. Electron. Mater. 2018, 47, 2215–2224. [Google Scholar] [CrossRef]
- Rasalingam, S.; Kibombo, H.S.; Wu, C.M.; Budhi, S.; Peng, R.; Baltrusaitis, J.; Koodali, R.T. Influence of Ti–O–Si hetero-linkages in the photocatalytic degradation of Rhodamine B. Catal. Commun. 2013, 31, 66–70. [Google Scholar] [CrossRef]
- Zou, W.; Zhang, S.; Ali, Z.; Miao, Z.; Abbas, Y.; Liu, W.; Qiu, M.; Wu, Z. Structure control of heteroatoms’ self-doped porous carbon materials derived from cyclomatrix polyphosphazene for high-performance supercapacitor application. Ionics 2022, 28, 3985–3999. [Google Scholar] [CrossRef]
- Barzetti, T.; Selli, E.; Moscotti, D.; Forni, L. Pyridine and ammonia as probes for FTIR analysis of solid acid catalysts. J. Chem. Soc. Faraday Trans. 1996, 92, 1401–1407. [Google Scholar] [CrossRef]
- Ryczkowski, J. IR spectroscopy in catalysis. Catal. Today 2001, 68, 263–381. [Google Scholar]
- Song, Y.; Zhang, J.; Zhang, Y.; Zhou, X.; Wang, J.-A.; Xu, L. Effect of crystallization mode of hydrous zirconia support on the isomerization activity of Pt/WO3–ZrO2. Catal. Today 2011, 166, 79–83. [Google Scholar] [CrossRef]
- Rafler, G.; Faserforschu, U.S.W. Kinetics of polycondensation of BHET. Textiltechn 1973, 24, 235–239. [Google Scholar]


| Samples | SBET (m2/g) | Sext (m2/g) | Vtot (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|
| TiO2-AG | 332.86 | 275.01 | 0.39 | 7.56 |
| TS91 | 524.59 | 350.29 | 0.85 | 8.08 |
| TS82 | 536.81 | 495.99 | 0.86 | 8.12 |
| TS73 | 524.04 | 484.16 | 0.84 | 7.51 |
| TS64 | 441.37 | 411.81 | 0.66 | 7.24 |
| TS55 | 402.87 | 377.93 | 0.46 | 7.02 |
| Samples | Element Content (atom.%) | |||
|---|---|---|---|---|
| Ti | Si | C | O | |
| TS91 | 13.70 | 2.66 | 44.99 | 38.65 |
| TS82 | 12.55 | 4.10 | 43.67 | 39.68 |
| TS73 | 12.45 | 6.40 | 31.30 | 49.85 |
| TS64 | 11.30 | 10.20 | 25.42 | 53.08 |
| TS55 | 11.09 | 12.40 | 23.31 | 53.19 |
| Samples | L Acid Content (μmol/g) | B Acid Content (μmol/g) | L Acid Density (μmol/m2) |
|---|---|---|---|
| TiO2-AG | 221.29 | 6.04 | 0.665 |
| TS91 | 370.29 | 20.05 | 0.705 |
| TS82 | 301.33 | 16.67 | 0.564 |
| TS73 | 299.51 | 8.74 | 0.571 |
| TS64 | 208.83 | 9.85 | 0.473 |
| TS55 | 164.51 | 13.27 | 0.407 |
| Catalyst | Introduction Stage of Catalyst | [η] (dL/g) | Mn (g/mol) | L | a | b | Terminal Carboxyl Content (mol/t) |
|---|---|---|---|---|---|---|---|
| TiO2-AG | After esterification | 1.14 | 4.03 × 104 | 90.69 | −0.03 | 2.08 | 32.05 |
| TS91 | After esterification | 1.74 | 7.72 × 104 | 90.73 | −0.17 | 1.97 | 25.45 |
| TS82 | After esterification | 1.64 | 7.05 × 104 | 90.70 | −0.31 | 2.01 | 24.32 |
| TS73 | After esterification | 1.61 | 6.85 × 104 | 90.21 | −0.27 | 2.67 | 25.31 |
| TS64 | After esterification | 1.35 | 5.22 × 104 | 89.15 | −0.16 | 3.43 | 23.32 |
| TS55 | After esterification | 0.93 | 2.94 × 104 | 88.02 | −0.20 | 4.51 | 26.44 |
| TBT | After esterification | 1.89 | 8.74 × 104 | 88.14 | −0.29 | 4.61 | 51.68 |
| No catalyst | - | 0.63 | 1.62 × 104 | 82.21 | −0.18 | 9.81 | 82.75 |
| TS91 | Before esterification | 1.78 | 7.99 × 104 | 91.03 | −0.21 | 1.93 | 24.43 |
| TBT | Before esterification | 1.26 | 4.70 × 104 | 88.26 | −0.32 | 4.79 | 62.37 |
| Polycondensation Temperature | Linear Regression Equations (TS91) | Linear Regression Equations (TBT) |
|---|---|---|
| 220 °C | Mt = 2923 + 481t; K = 241 | Mt = 2923 + 716t; K = 358 |
| 225 °C | Mt = 2923 + 656t; K = 328 | Mt = 2923 + 933t; K = 467 |
| 230 °C | Mt = 2923 + 779t; K = 390 | Mt = 2923 + 1042t; K = 521 |
| 235 °C | Mt = 2923 + 946t; K = 473 | Mt = 2923 + 1053t; K = 527 |
| 240 °C | Mt = 2923 + 972t; K = 486 | Mt = 2923 + 1180t; K = 590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Bian, H.; Guo, J.; Xu, J.; Guo, B. Preparation of Titania–Silica Composite Aerogel at Atmospheric Pressure and Its Catalytic Performance in the Synthesis of Poly (Butylene Succinate). Materials 2023, 16, 3296. https://doi.org/10.3390/ma16093296
Zou W, Bian H, Guo J, Xu J, Guo B. Preparation of Titania–Silica Composite Aerogel at Atmospheric Pressure and Its Catalytic Performance in the Synthesis of Poly (Butylene Succinate). Materials. 2023; 16(9):3296. https://doi.org/10.3390/ma16093296
Chicago/Turabian StyleZou, Wenqi, Hongli Bian, Jinjing Guo, Jun Xu, and Baohua Guo. 2023. "Preparation of Titania–Silica Composite Aerogel at Atmospheric Pressure and Its Catalytic Performance in the Synthesis of Poly (Butylene Succinate)" Materials 16, no. 9: 3296. https://doi.org/10.3390/ma16093296





