Influence of Plasma Treatment on Surface Characteristics of Aluminum Alloy Sheets and Bonding Performance of Glass Fiber-Reinforced Thermoplastic/Al Composites
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Surface Treatment
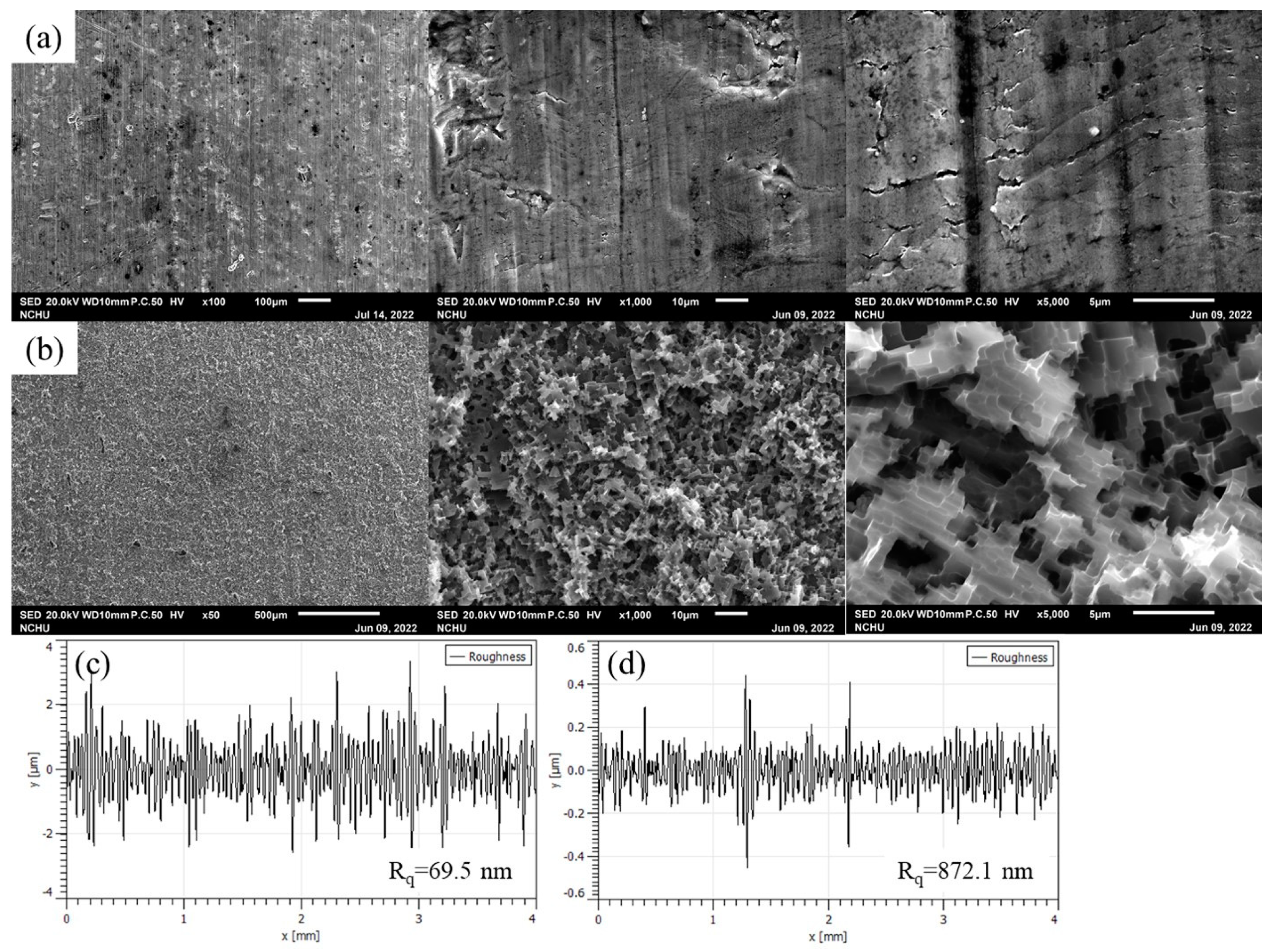
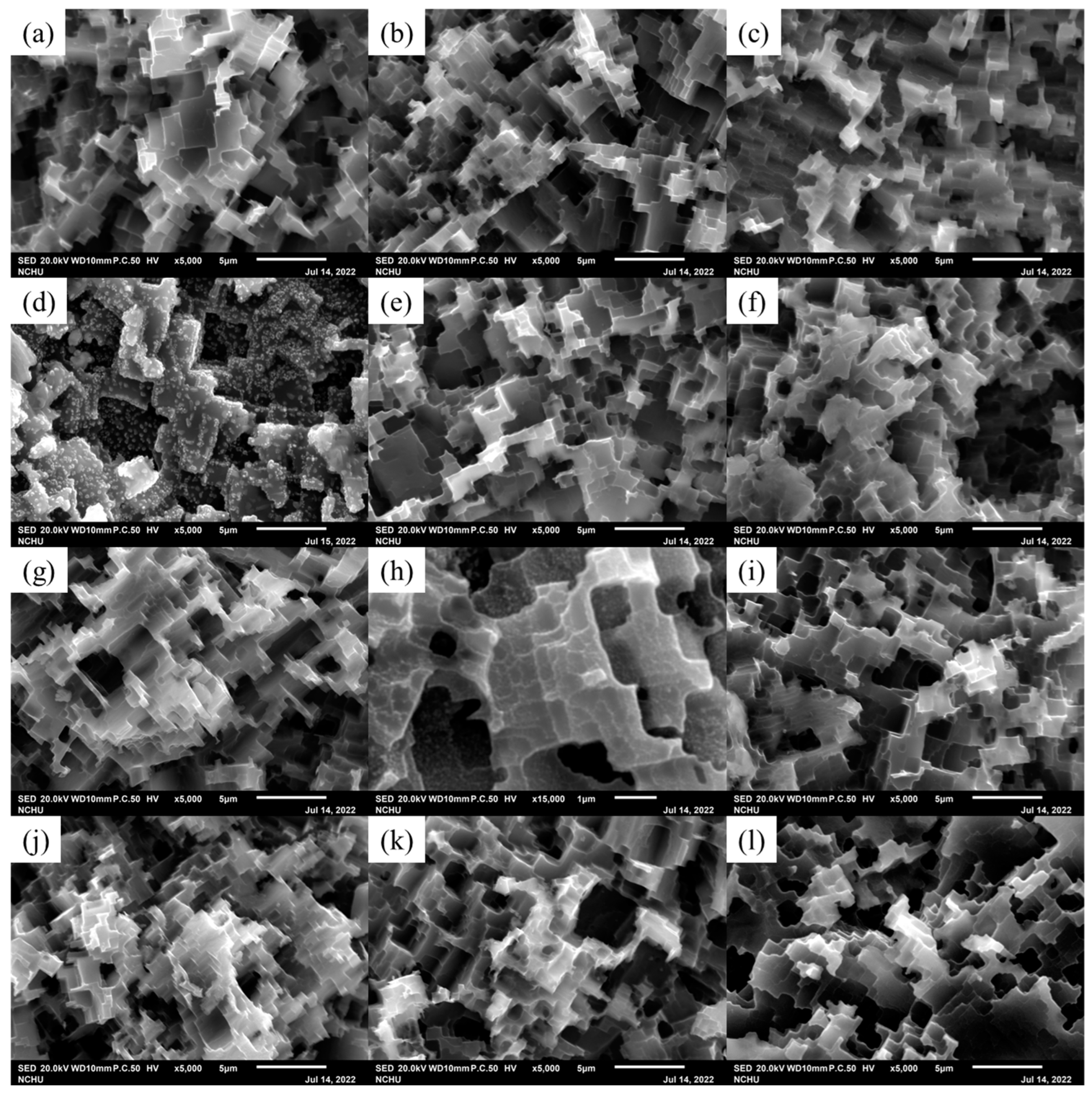
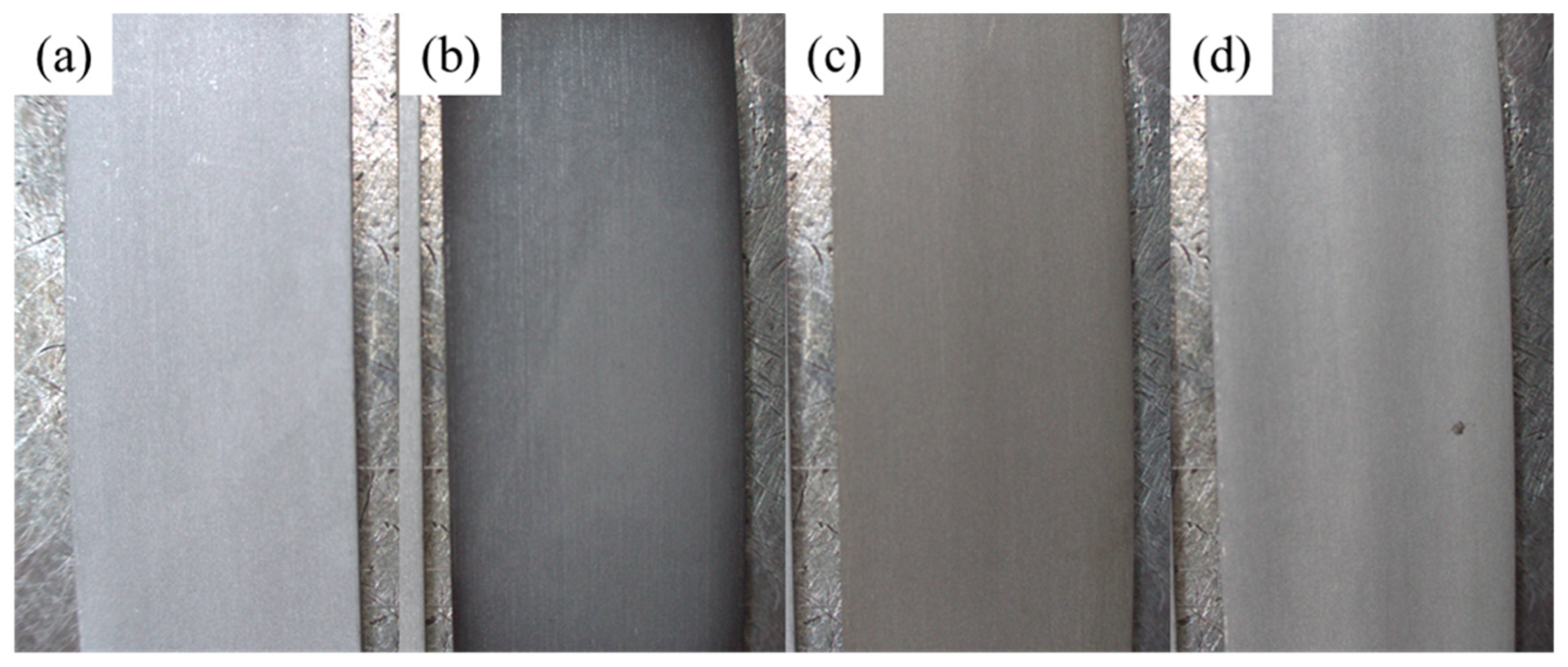
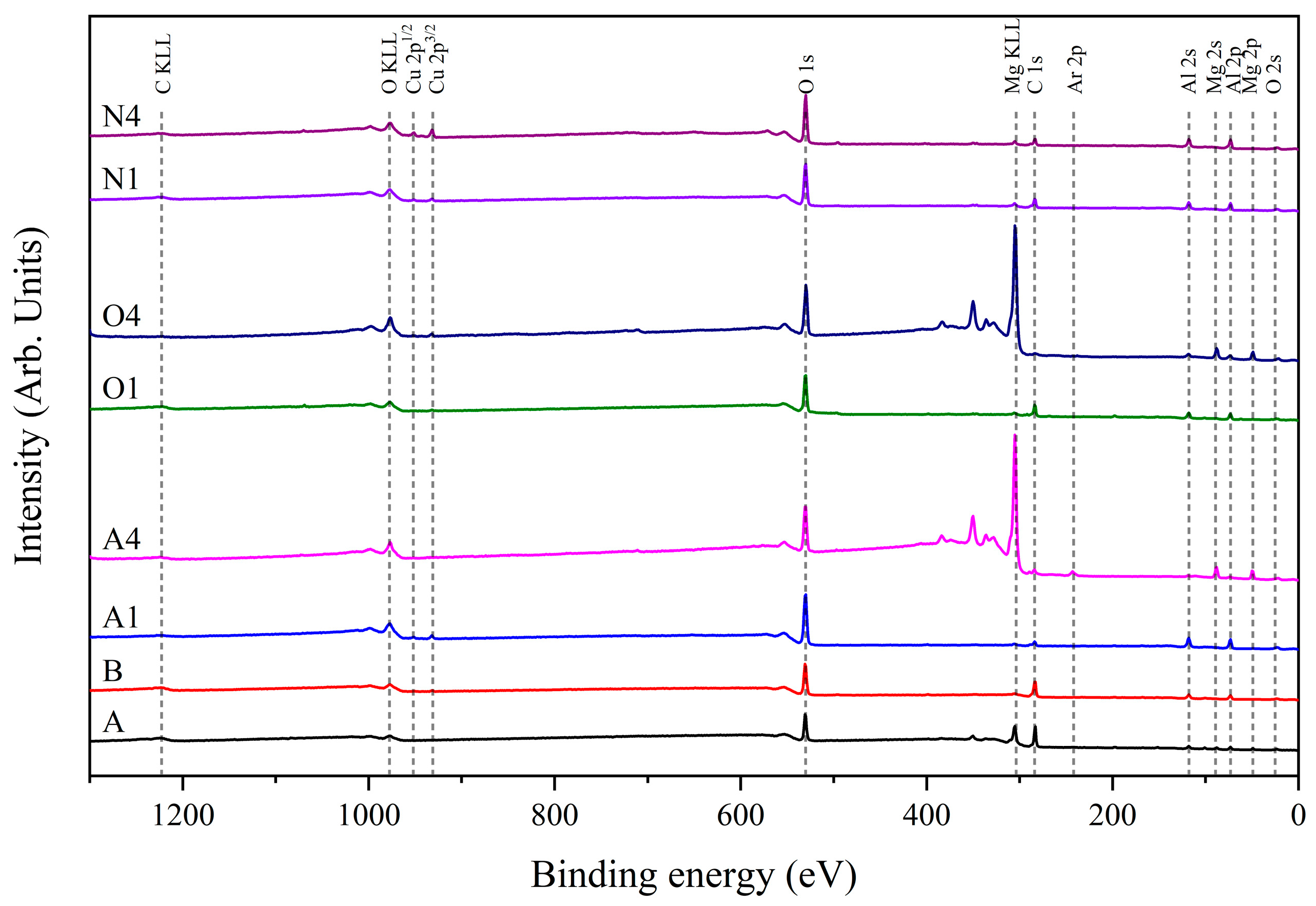
2.3. Structure Analysis
2.4. Lap-Shear Tensile Test
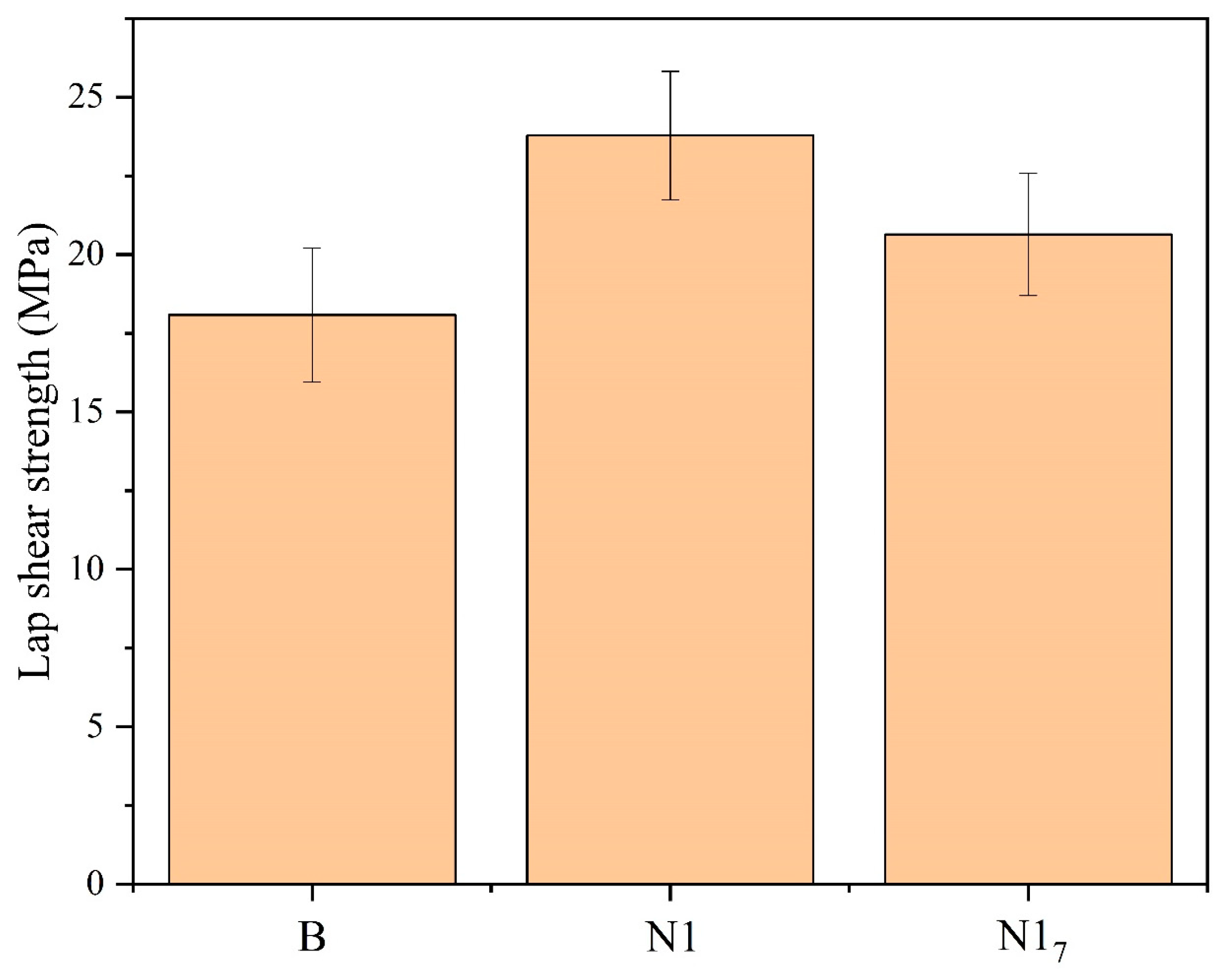
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinmazçelik, T.; Avcu, E.; Bora, M.Ö.; Çoban, O. A review: Fibre metal laminates, background, bonding types and applied test methods. Mater. Des. 2011, 32, 3671–3685. [Google Scholar] [CrossRef]
- Stoll, M.M.; Sessner, V.; Kramar, M.; Technau, J.; Weidenmann, K.A. The effect of an elastomer interlayer thickness variation on the mechanical properties of Fiber-Metal-Laminates. Compos. Struct. 2019, 219, 90–96. [Google Scholar] [CrossRef]
- Vogelesang, L.; Vlot, A. Development of fibre metal laminates for advanced aerospace structures. J. Mater. Process. Technol. 2000, 103, 1–5. [Google Scholar] [CrossRef]
- Mamalis, D.; Obande, W.; Koutsos, V.; Blackford, J.R.; Brádaigh, C.M.; Ray, D. Novel thermoplastic fibre-metal laminates manufactured by vacuum resin infusion: The effect of surface treatments on interfacial bonding. Mater. Des. 2019, 162, 331–344. [Google Scholar] [CrossRef]
- Malingam, S.D.; Jumaat, F.A.; Ng, L.F.; Subramaniam, K.; Ab Ghani, A.F. Tensile and impact properties of cost-effective hybrid fiber metal laminate sandwich structures. Adv. Polym. Technol. 2018, 37, 2385–2393. [Google Scholar] [CrossRef]
- Mottaghian, F.; Taheri, F. Strength and failure mechanism of single-lap magnesium-basalt fiber metal laminate adhesively bonded joints: Experimental and numerical assessments. J. Compos. Mater. 2022, 56, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Zielecki, W.; Pawlus, P.; Perłowski, R.; Dzierwa, A. Surface topography effect on strength of lap adhesive joints after mechanical pre-treatment. Arch. Civ. Mech. Eng. 2013, 13, 175–185. [Google Scholar] [CrossRef]
- Mohamad, M.; Marzuki, H.F.A.; Ubaidillah, E.; Abidin, M.; Omar, S.; Rozi, I. Effect of Surface Roughness on Mechanical Properties of Aluminium-Carbon Laminates Composites. Adv. Mater. Res. 2014, 879, 51–57. [Google Scholar] [CrossRef]
- Yao, Y.; Shi, P.; Chen, M.; Chen, G.; Gao, C.; Boisse, P.; Zhu, Y. Experimental and numerical study on Mode I and Mode II interfacial fracture toughness of co-cured steel-CFRP hybrid composites. Int. J. Adhes. Adhes. 2022, 112, 103030. [Google Scholar] [CrossRef]
- Sun, S.; Wu, G.; Sun, L.; Shan, X.; Li, M.; Ji, S. Effects of different surface treatments of aluminum alloy 5083 on interlaminar strength and anticorrosion properties of FMLs. Mater. Res. Express 2018, 5, 116506. [Google Scholar] [CrossRef]
- Critchlow, G.W.; Yendall, K.A.; Bahrani, D.; Quinn, A.; Andrews, F. Strategies for the replacement of chromic acid anodising for the structural bonding of aluminium alloys. Int. J. Adhes. Adhes. 2006, 26, 419–453. [Google Scholar] [CrossRef]
- Lawcock, G.; Ye, L.; Mai, Y.-W.; Sun, C.-T. The effect of adhesive bonding between aluminum and composite prepreg on the mechanical properties of carbon-fiber-reinforced metal laminates. Compos. Sci. Technol. 1997, 57, 35–45. [Google Scholar] [CrossRef]
- Prolongo, S.G.; del Rosario, G.; Ureña, A. Comparative study on the adhesive properties of different epoxy resins. Int. J. Adhes. Adhes. 2006, 26, 125–132. [Google Scholar] [CrossRef]
- Aghamohammadi, H.; Abbandanak, S.N.; Eslami-Farsani, R.; Siadati, S.H. Effects of various aluminum surface treatments on the basalt fiber metal laminates interlaminar adhesion. Int. J. Adhes. Adhes. 2018, 84, 184–193. [Google Scholar] [CrossRef]
- Gonzalez-Canche, N.G.; Flores-Johnson, E.A.; Cortes, P.; Carrillo, J.G. Evaluation of surface treatments on 5052-H32 aluminum alloy for enhancing the interfacial adhesion of thermoplastic-based fiber metal laminates. Int. J. Adhes. Adhes. 2018, 82, 90–99. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Shen, Y.; Liu, S.; Wang, W.; Tao, J. Improvement of adhesion performance between aluminum alloy sheet and epoxy based on anodizing technique. Int. J. Adhes. Adhes. 2016, 70, 74–80. [Google Scholar] [CrossRef]
- Santos, A.L.; Nakazato, R.; Schmeer, S.; Botelho, E.C. Influence of anodization of aluminum 2024 T3 for application in aluminum/Cf/ epoxy laminate. Compos. Part B Eng. 2020, 184, 107718. [Google Scholar] [CrossRef]
- He, P.; Chen, K.; Yang, J. Surface modifications of Ti alloy with tunable hierarchical structures and chemistry for improved metal–polymer interface used in deepwater composite riser. Appl. Surf. Sci. 2015, 328, 614–622. [Google Scholar] [CrossRef]
- Klébert, S.; Mohai, M.; Csiszár, E. Can Plasma Surface Treatment Replace Traditional Wood Modification Methods? Coatings 2022, 12, 487. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, G.; Li, J.J. Advances in implant surface modifications to improve osseointegration. Mater. Adv. 2021, 2, 6901–6927. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, X.; Zhao, Z.; Liu, J.; Chen, Q.; Wang, X. Rapid and Continuous Atmospheric Plasma Surface Modification of PAN-Based Carbon Fibers. ACS Omega 2022, 7, 10963–10969. [Google Scholar] [CrossRef]
- Qiao, Y.; Shin, Y.; Pallaka, M.R.; Nickerson, E.K.; Merkel, D.R.; Seffens, R.J.; Ortiz, A.; Ramos, J.L.; Simmons, K.L. Plasma surface modification coupled with thermal and step-over distance effects on significant fracture improvement of adhesively-bonded metal-CFRTP dissimilar materials. Compos. Sci. Technol. 2023, 232, 109833. [Google Scholar] [CrossRef]
- Mui, T.S.M.; Silva, L.L.G.; Prysiazhnyi, V.; Kostov, K.G. Surface modification of aluminium alloys by atmospheric pressure plasma treatments for enhancement of their adhesion properties. Surf. Coat. Technol. 2017, 312, 32–36. [Google Scholar] [CrossRef]
- Laban, O.; Mahdi, E. Enhancing mode I inter-laminar fracture toughness of aluminum/fiberglass fiber-metal laminates by combining surface pre-treatments. Int. J. Adhes. Adhes. 2017, 78, 234–239. [Google Scholar] [CrossRef]
- Lin, Y.; Li, H.; Wang, Q.; Gong, Z.; Tao, J. Effect of plasma surface treatment of aluminum alloy sheet on the properties of Al/Gf/PP laminates. Appl. Surf. Sci. 2020, 507, 145062. [Google Scholar] [CrossRef]
- Dighton, C.; Rezai, A.; Ogin, S.L.; Watts, J.F. Atmospheric plasma treatment of CFRP composites to enhance structural bonding investigated using surface analytical techniques. Int. J. Adhes. Adhes. 2019, 91, 142–149. [Google Scholar] [CrossRef]
- Schafer, J.; Hofmann, T.; Holtmannspötter, J.; Frauenhofer, M.; Von Czarnecki, J.; Gudladt, H.-J. Atmospheric-pressure plasma treatment of polyamide 6 composites for bonding with polyurethane. J. Adhes. Sci. Technol. 2015, 29, 1807–1819. [Google Scholar] [CrossRef]
- Zaldivar, R.J.; Kim, H.I.; Steckel, G.L.; Nokes, J.P.; Morgan, B.A. Effect of Processing Parameter Changes on the Adhesion of Plasma-treated Carbon Fiber Reinforced Epoxy Composites. J. Compos. Mater. 2010, 44, 1435–1453. [Google Scholar] [CrossRef]
- Dyer, C.K.; Alwitt, R.S. Surface Changes during A.C. Etching of Aluminum. J. Electrochem. Soc. 1981, 128, 300. [Google Scholar] [CrossRef]
- Lin, C.S.; Fu, S.M. Etch Film and Pit Structure of AA1050 Aluminum Plates Electrograined in Nitric and Hydrochloric Acids. J. Electrochem. Soc. 2001, 148, C240. [Google Scholar] [CrossRef]
- Yi, C.X.; Wang, S.K.; Xu, X.B.; Tian, Y.F.; Bao, M.D. Study on plasma cleaning of surface contaminants on pure copper. Mater. Res. Express 2023, 10, 016506. [Google Scholar] [CrossRef]
- Speight, J. Lange’s Handbook of Chemistry, 70th Anniversary Edition; McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Li, B.; Li, J.; Liang, C.; Li, H.; Guo, L.; Liu, S.; Wang, H. Surface Roughness and Hydrophilicity of Titanium after Anodic Oxidation. Rare Met. Mater. Eng. 2016, 45, 858–862. [Google Scholar] [CrossRef]
- Yamasaki, S.; Das, U.K.; Ishikawa, K. Direct observation of surface dangling bonds during plasma process: Chemical reactions during H2 and Ar plasma treatments. Thin Solid Film. 2002, 407, 139–143. [Google Scholar] [CrossRef]
- Shun’ko, E.V.; Belkin, V.S. Cleaning properties of atomic oxygen excited to metastable state 2s22p4(S10). J. Appl. Phys. 2007, 102, 083304. [Google Scholar] [CrossRef]
- Mathioudaki, S.; Barthélémy, B.; Detriche, S.; Vandenabeele, C.; Delhalle, J.; Mekhalif, Z.; Lucas, S. Plasma Treatment of Metal Oxide Nanoparticles: Development of Core–Shell Structures for a Better and Similar Dispersibility. ACS Appl. Nano Mater. 2018, 1, 3464–3473. [Google Scholar] [CrossRef]
- Bartnik, A.; Skrzeczanowski, W.; Fiedorowicz, H.; Wachulak, P.; Fok, T. Low-temperature plasmas induced in nitrogen by extreme ultraviolet (EUV) pulses. Laser Part. Beams 2018, 36, 76–83. [Google Scholar] [CrossRef]
- AZoM. Improved Remote Plasma Cleaning for Hydrocarbon Mitigation. 2021. Available online: https://www.azom.com/article.aspx?ArticleID=14789 (accessed on 24 February 2023).
- Sikora, A.; Czylkowski, D.; Hrycak, B.; Moczała-Dusanowska, M.; Łapiński, M.; Dors, M.; Jasiński, M. Surface modification of PMMA polymer and its composites with PC61BM fullerene derivative using an atmospheric pressure microwave argon plasma sheet. Sci. Rep. 2021, 11, 9270. [Google Scholar] [CrossRef]
- Butcher, K.S.; Georgiev, V.; Georgieva, D.; Gergova, R.; Terziyska, P.; Binsted, P.W. Downstream Electric Field Effects during Film Deposition with a Radio Frequency Plasma and Observations of Carbon Reduction. Coatings 2022, 12, 1581. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Berger, M.B.; Bosh, K.B.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Benchtop Plasma Treatment of Titanium Surfaces Enhances Cell Response. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2021, 37, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Song, G.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Bordes, J.M.; Bordes, C.; Ehret, E.; Gschwind, R.; Bauer, P. Theoretical sputtering yields of Al and Mg targets in physical vapor deposition processes. J. Vac. Sci. Technol. A 2001, 19, 805–811. [Google Scholar] [CrossRef]
- Duchoslav, J.; Kehrer, M.; Truglas, T.; Groiß, H.; Nadlinger, M.; Hader-Kregl, L.; Riener, C.K.; Arndt, M.; Stellnberger, K.H.; Luckeneder, G.; et al. The effect of plasma treatment on the surface chemistry and structure of ZnMgAl coatings. Appl. Surf. Sci. 2020, 504, 144457. [Google Scholar] [CrossRef]
- Kim, J.W.; Yoo, S.H.; Kong, Y.B.; Cho, S.O.; Lee, E.J. Wetting Property Modification of Al2O3 by Helium Ion Irradiation: Effects of Beam Energy and Fluence on Contact Angle. Langmuir 2021, 37, 11301–11308. [Google Scholar] [CrossRef]
- Saini, C.P.; Barman, A.; Das, D.; Satpati, B.; Bhattacharyya, S.R.; Kanjilal, D.; Ponomaryov, A.; Zvyagin, S.; Kanjilal, A. Role of Oxygen Vacancy on the Hydrophobic Behavior of TiO2 Nanorods on Chemically Etched Si Pyramids. J. Phys. Chem. C 2017, 121, 278–283. [Google Scholar] [CrossRef]
- Islam, M.S.; Tong, L.; Falzon, P.J. Influence of metal surface preparation on its surface profile, contact angle, surface energy and adhesion with glass fibre prepreg. Int. J. Adhes. Adhes. 2014, 51, 32–41. [Google Scholar] [CrossRef]


| Sample | Plasma Gas | Discharge Power (W) | Exposure Time (s) |
|---|---|---|---|
| A1 | Ar | 50 | 30 |
| A2 | 300 | 30 | |
| A3 | 300 | 120 | |
| A4 | 300 | 300 | |
| O1 | O2 | 50 | 30 |
| O2 | 300 | 30 | |
| O3 | 300 | 120 | |
| O4 | 300 | 300 | |
| N1 | N2 | 50 | 30 |
| N2 | 300 | 30 | |
| N3 | 300 | 120 | |
| N4 | 300 | 300 |
| A | B | A1 | A4 | O1 | O4 | N1 | N4 | |
|---|---|---|---|---|---|---|---|---|
| [Al]/([Al]+[Mg]) | 57.3 | 96.4 | 97.6 | 9.1 | 95.4 | 24.7 | 96.8 | 97.0 |
| [Mg]/([Al]+[Mg]) | 42.7 | 3.6 | 2.4 | 90.9 | 4.6 | 75.3 | 3.2 | 3.0 |
| [C]/([C]+[O]) | 61.8 | 53.3 | 23.7 | 21.8 | 40.1 | 27.7 | 32.4 | 25.6 |
| Element | Reaction | Oxidation-Reduction Potential (V) |
|---|---|---|
| Magnesium | Mg2+ + 2e− → Mg | −2.37 |
| Aluminum | Al3+ + 3e− → Al | −1.66 |
| Manganese | Mn2+ + 2e− → Mn | −1.18 |
| Iron | Fe2+ + 2e−→ Fe | −0.44 |
| Copper | Cu2+ + 2e− → Cu | +0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, D.-C.; Chang, Z.-C.; Chen, E.-C.; Huang, Y.-L.; Jiang, Y.-C.; Shieu, F.-S. Influence of Plasma Treatment on Surface Characteristics of Aluminum Alloy Sheets and Bonding Performance of Glass Fiber-Reinforced Thermoplastic/Al Composites. Materials 2023, 16, 3317. https://doi.org/10.3390/ma16093317
Tsai D-C, Chang Z-C, Chen E-C, Huang Y-L, Jiang Y-C, Shieu F-S. Influence of Plasma Treatment on Surface Characteristics of Aluminum Alloy Sheets and Bonding Performance of Glass Fiber-Reinforced Thermoplastic/Al Composites. Materials. 2023; 16(9):3317. https://doi.org/10.3390/ma16093317
Chicago/Turabian StyleTsai, Du-Cheng, Zue-Chin Chang, Erh-Chiang Chen, Yen-Lin Huang, Yun-Chen Jiang, and Fuh-Sheng Shieu. 2023. "Influence of Plasma Treatment on Surface Characteristics of Aluminum Alloy Sheets and Bonding Performance of Glass Fiber-Reinforced Thermoplastic/Al Composites" Materials 16, no. 9: 3317. https://doi.org/10.3390/ma16093317





