Study on Effect of Nano-CaCO3 on Properties of Phosphorus Building Gypsum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Design
2.3. Experimental Methods
2.3.1. Fluidity and Setting Time
2.3.2. Absolute Dry Flexural Strength and Absolute Dry Compressive Strength
2.3.3. Water Absorption and Softening Coefficient
2.3.4. Microscopic Morphology of Gypsum Particles
3. Results
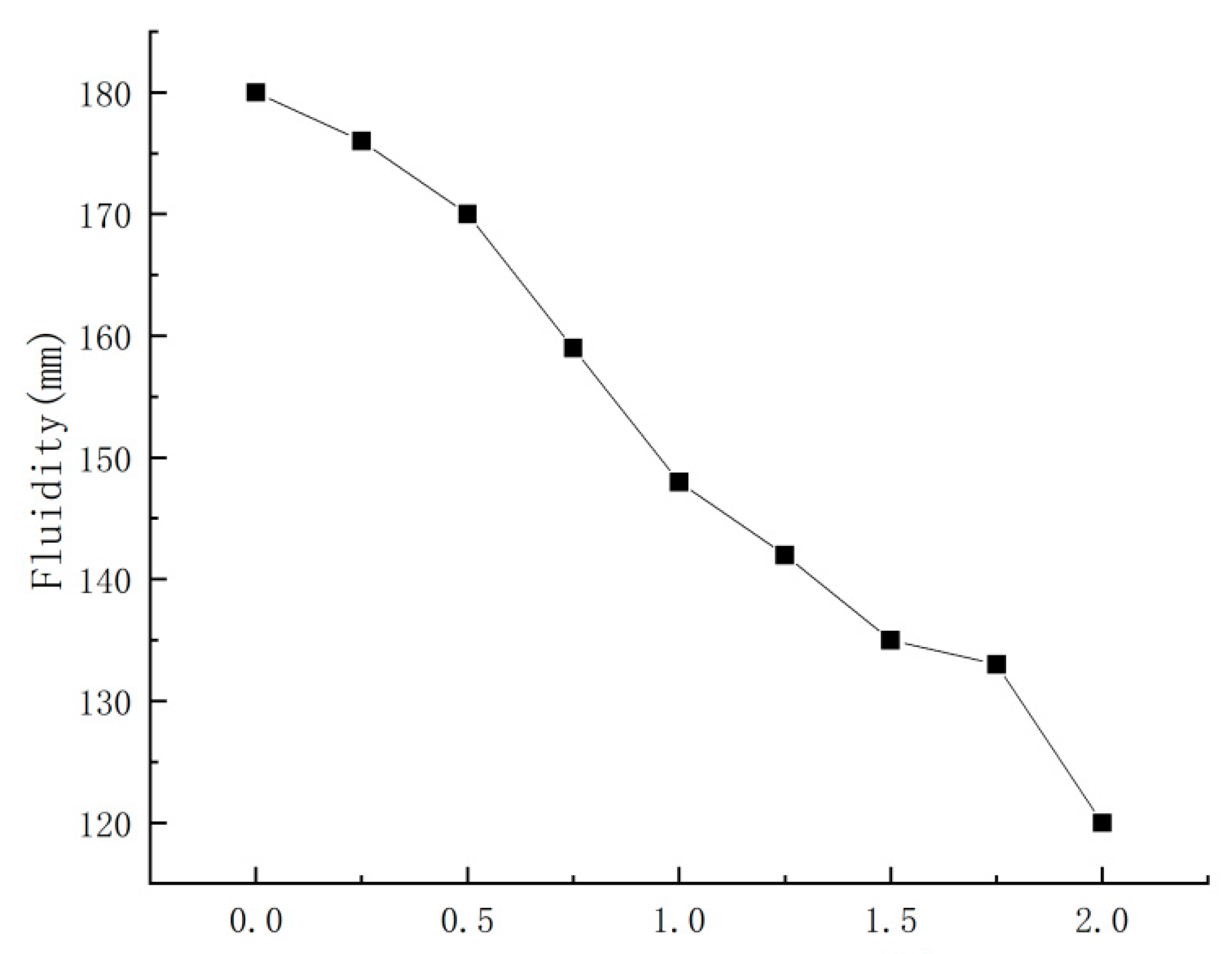
3.1. Effect of Nano-CaCO3 on PBG Fluidity
3.2. Effect of Nano-CaCO3 on Setting Time of PBG
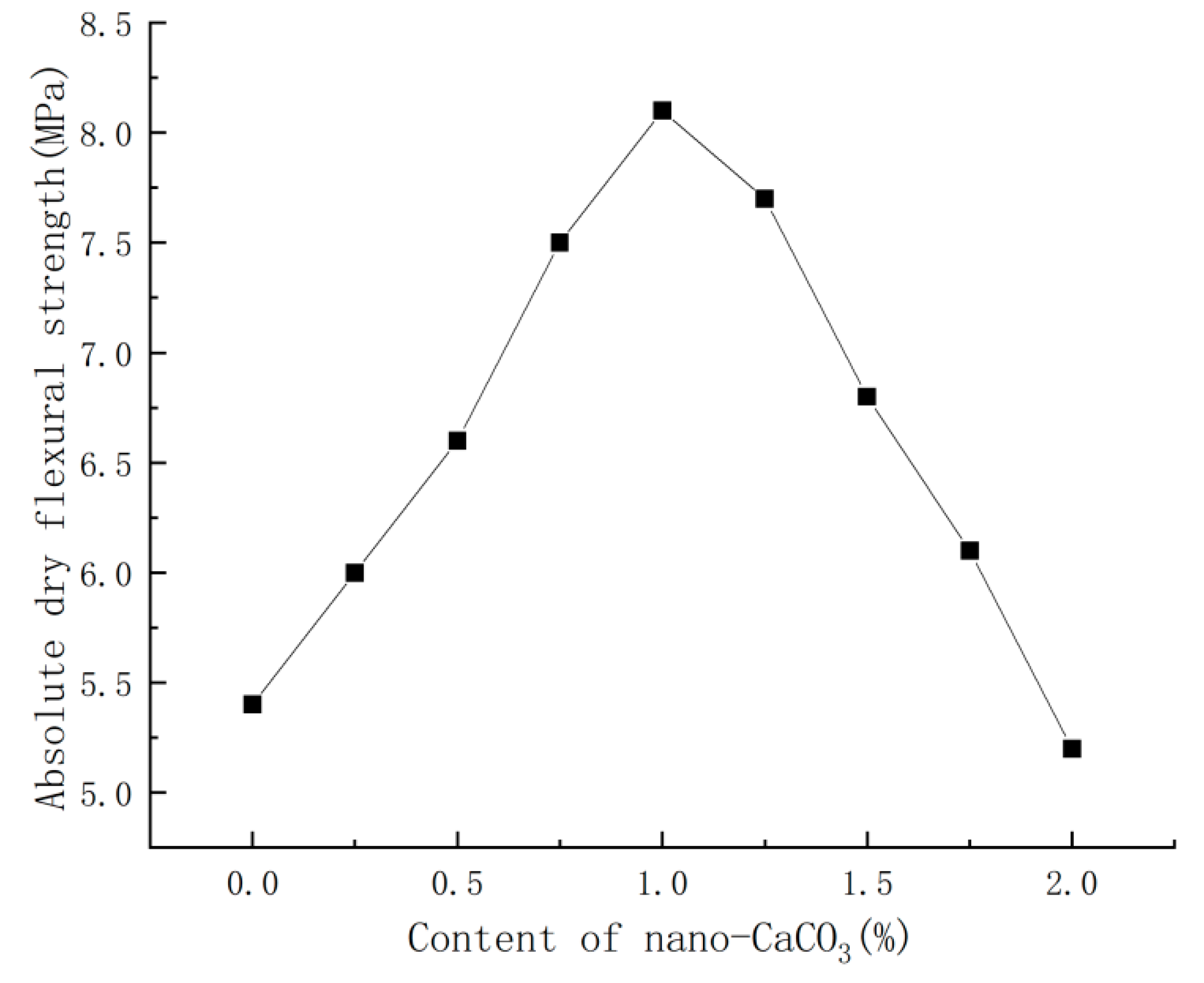
3.3. Effect of Nano-CaCO3 on Absolute Dry Flexural Strength of PBG
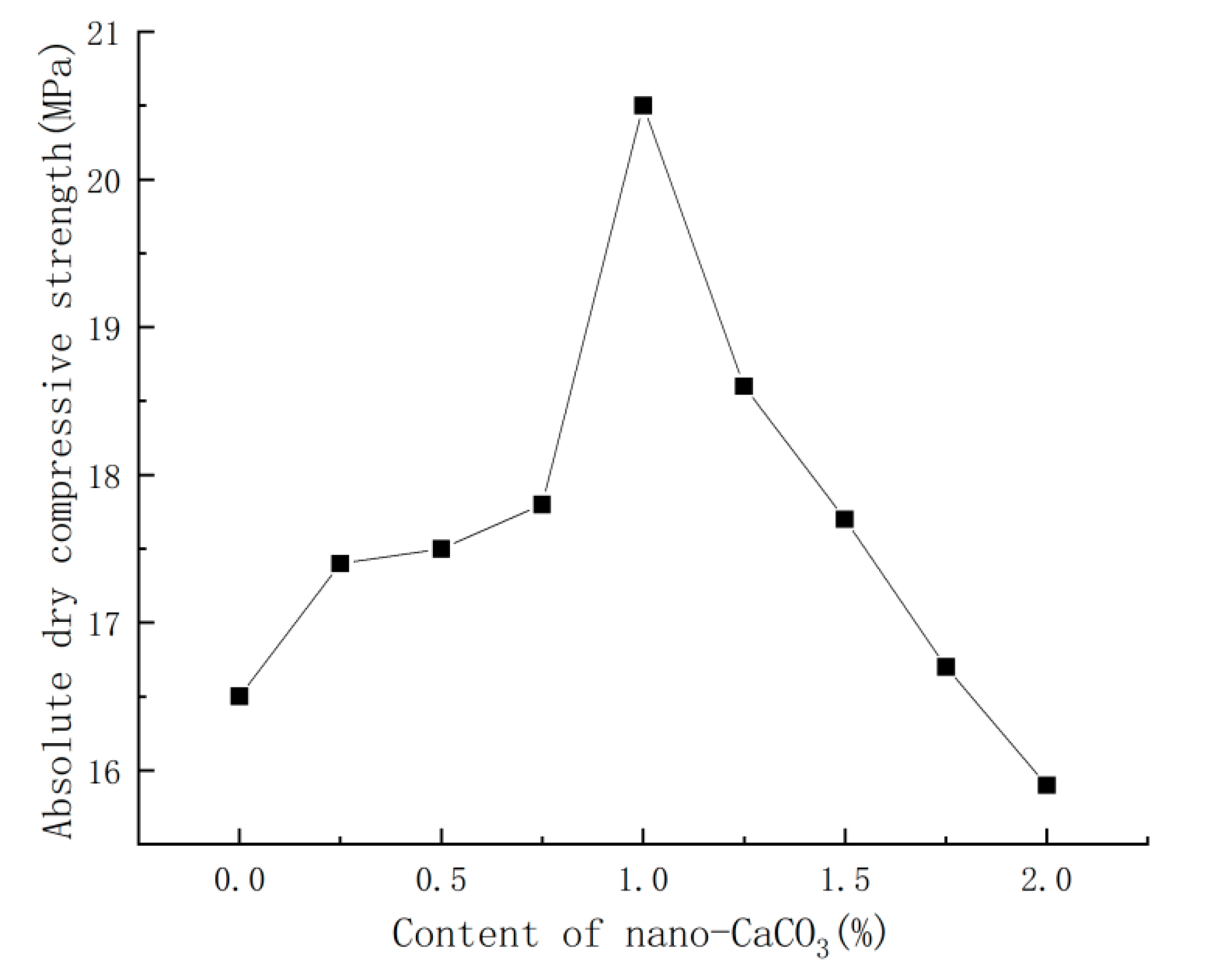
3.4. Effect of Nano-CaCO3 on Absolute Dry Compressive Strength of PBG
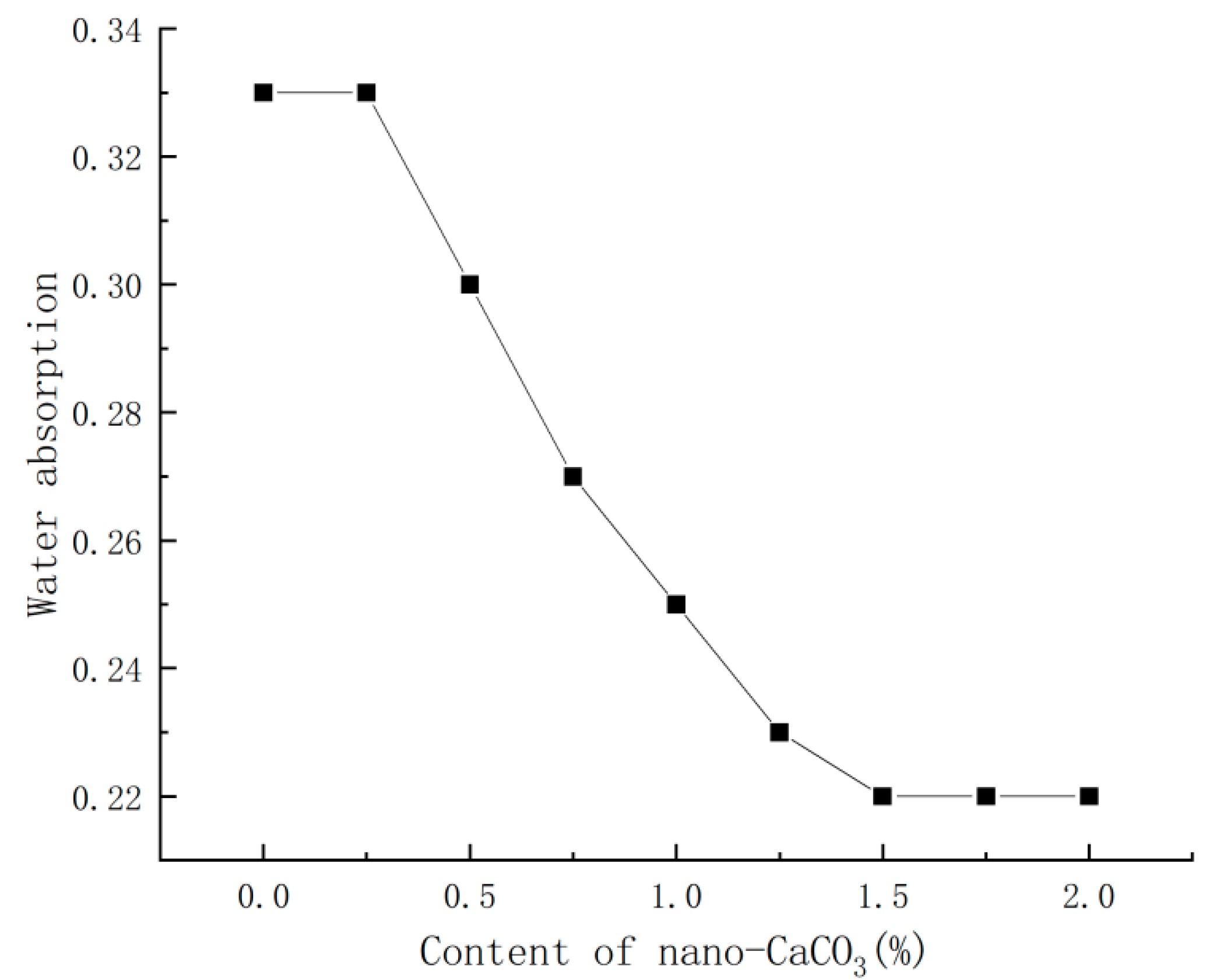
3.5. Effect of Nano-CaCO3 on Water Absorption of PBG
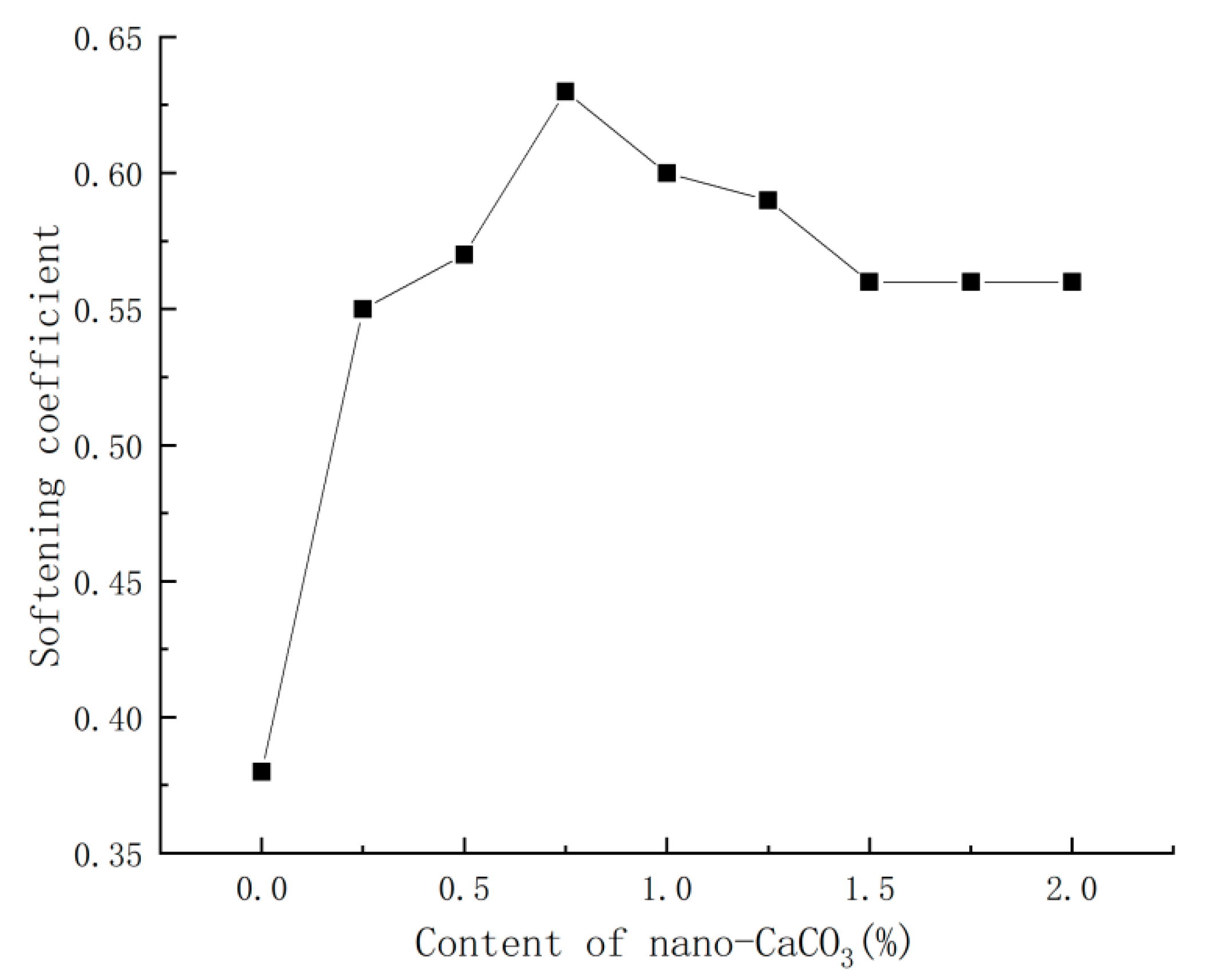
3.6. Effect of Nano-CaCO3 on Softening Coefficient of PBG
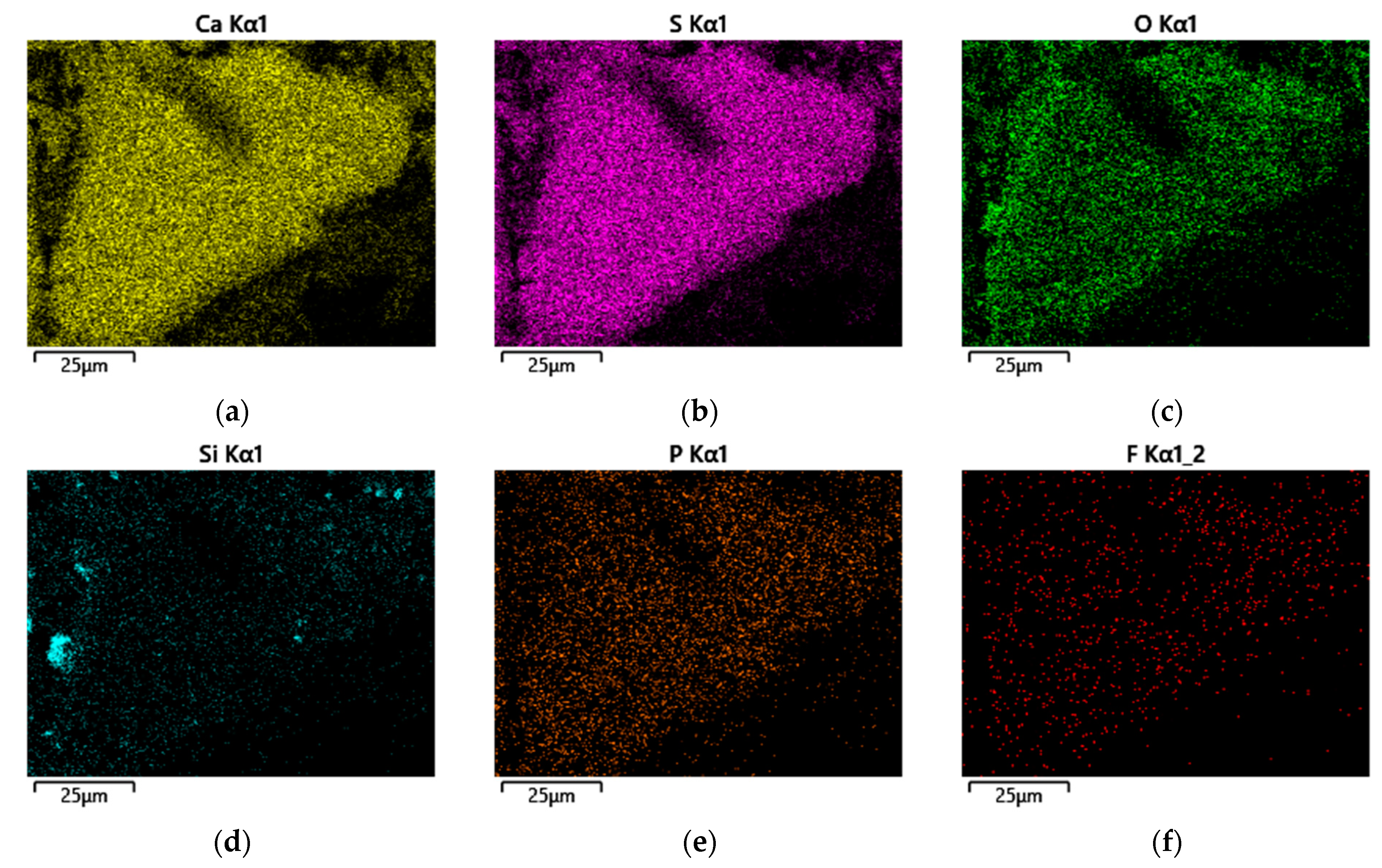
3.7. Microanalysis
3.8. Composition Analysis of Hydration Products
4. Discussion
5. Potential Applications and Prospects
6. Conclusions
- Nano-CaCO3 exerted a significant effect on the physical properties of PBG’s paste with the increase in nano-CaCO3 content: the fluidity and setting time of PBG were both decreased. Among them, the fluidity decreased by 33%, the initial setting time decreased by 38% and the final setting time decreased by 29%.
- Nano-CaCO3 also presented a significant impact on the mechanical properties of PBG: with the increase in nano-CaCO3 content, both the absolute dry flexural strength and absolute dry compressive strength of PBG first increased then decreased. When the content of nano-CaCO3 was 1%, the absolute dry flexural strength of PBG increased by 50% and the absolute dry compressive strength increased by 24%. When the content of nano-CaCO3 reached a certain level, it imposed a negative impact on the mechanical properties of PBG.
- Nano-CaCO3 also had a significant influence on other properties of PBG: with the increase in nano-CaCO3 content, the water absorption of PBG first decreased then stabilized gradually, decreasing by 33%, while the softening coefficient first increased, then decreased and finally tended to stabilize, with a maximum increase of 66%.
- After nano-CaCO3 was added into PBG, it could fill the voids within the hardened body and improve PBG’s pore structure. Meanwhile, based on the original structure, it could form a new one around nano-CaCO3 as the crystal nucleus, which reduced both the internal surface area and porosity of PBG, increased PBG’s compactness and improved PBG’s various properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, Y.; Wang, Q.; Xue, J. Novel Foam Insulation Material Produced by Calcined Phosphogypsum and H2O2. J. Mater. Civ. Eng. 2020, 32, 04020379. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Y.; Xue, J. Study on the improvement of the waterproof and mechanical properties of hemihydrate phosphogypsum-based foam insulation materials. Constr. Build. Mater. 2019, 230, 117014. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Yan, Y. Utilization of original phosphogypsum as raw material for the preparation of self-leveling mortar. J. Clean. Prod. 2016, 127, 204–213. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Kang, X.; Yu, J.; Fan, Y.; Dang, Y.; Zhang, W.; Wang, S. Belite-calcium sulfoaluminate cement prepared with phosphogypsum: Influence of P2O5 and F on the clinker formation and cement performances. Constr. Build. Mater. 2019, 203, 432–442. [Google Scholar] [CrossRef]
- Singh, M. Role of phosphogypsum impurities on strength and microstructure of selenite plaster. Constr. Build. Mater. 2005, 19, 480–486. [Google Scholar] [CrossRef]
- Mei, L.I.; Jiahui, P.; Jianxin, Z.; Huan, Z. Research on Characteristics of Phosphorus Building Gypsum and Its Modification. Bull. Chin. Ceram. Soc. 2012, 31, 553–558. [Google Scholar] [CrossRef]
- He, S.; Shi, Y.; Li, Q. Effect of Particle Gradation on Properties of Phosphogypsum–based Cement Paste Backfill. DEStech Trans. Environ. Energy Earth Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.U.; Zhiman, Z.; Rui, T.; Sichen, Q.; Yang, L.; Jiyuan, H.; Bo, W.; Haijian, D. Orthogonal Test of Modified Phosphogypsum by Polypropylene Fibers and Polyvinyl Alcohol. Bull. Chin. Ceram. Soc. 2018, 4022–4026. [Google Scholar] [CrossRef]
- Lei, W.; Zhiman, Z.; Rui, T.; Bo, W.; Jiyuan, H.; Yang, L.; Haijian, D. Study on Strength of Short-cut Polypropylene Fiber Reinforced Phosphate Building Gypsum. Non-Met. Mines. 2018, 41, 4–6. [Google Scholar]
- Wu, L.; Zhao, Z.-m.; Sichen, Q.; Cun, W.; Zhuo, L. Influence of Chopped Fiber on the Working Performance of Phosphorus Building Gypsum. Bull. Chin. Ceram. Soc. 2019, 38, 7. [Google Scholar] [CrossRef]
- Liang, X.; Fang, L.; Yaowen, X.; Zhennan, Y. Study on Modification of Hemihydrate Phosphogypsum with Ordinary Portland Cement and Its Hydration Mechanism. Non-Met. Mines 2018, 41, 45–47. [Google Scholar]
- Ma, Q.; Yuxiang, L.; Hongbin, T.; Meihui, T. Effect of Recycled Brick Powder on the Properties of Phosphorus Building Gypsum. Non-Met. Mines 2022, 45, 103–106. [Google Scholar]
- Li, L.; Li, B.; Yang, Y. Study on Inorganic Cementitious Material Composite Modified Phosphorus Building Gypsum. Non-Met. Mines 2022, 45, 102–106. [Google Scholar]
- Ji, M.; Jiang, B.; Huangfu, S. Study on the Effect of Carbon Nanotubes Modified Phosphorous Building Gypsum. Non-Met. Mines 2021, 4, 53–55. [Google Scholar]
- Zhao, B.; Zhiman, Z.; Sichen, Q.; Yi, Z.; Dan, L.; Sihua, W. Effect of Mineral Admixture on the Strength of Phosphorus Building Gypsum Mortar. Non-Met. Mines 2019, 42, 45–48. [Google Scholar]
- Nasir, M.; Aziz, M.A.; Zubair, M.; Manzar, M.S.; Ashraf, N.; Mu′Azu, N.D.; Al-Harthi, M.A. Recent review on synthesis, evaluation, and SWOT analysis of nanostructured cellulose in construction applications. J. Build. Eng. 2021, 46, 103747. [Google Scholar] [CrossRef]
- Ibrahim, M.; Nasir, M.; Hussaini, S. R; Najamuddin, S.K; Ewebajo, A.O. Performance of structurally viable green concrete derived from natural Pozzolan and Nanosilica. Mag. Civ. Eng. 2022, 107, 10710. [Google Scholar] [CrossRef]
- Lu, C.; Li, L.; Wang, W.; Feng, L. Bending strength properties of cement modified with nano-materials. Electron. J. Geotech. Eng. 2015, 20, 3647–3654. [Google Scholar]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Nanotechnology: Advantages and drawbacks in the field of construction and building materials. Constr. Build. Mater. 2011, 25, 582–590. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide–cement composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Siddique, R.; Mehta, A. Effect of carbon nanotubes on properties of cement mortars. Constr. Build. Mater. 2014, 50, 116–129. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L. Advances in the applied research of nano-material in concrete. Concrete 2004, 11, 4. [Google Scholar]
- Xiaoyan, G.; Bing, Z.; Yupeng, G. Synthesis and characterization of well-dispersed polyurethane/CaCO3 nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2010, 371, 1–7. [Google Scholar]
- Kawashima, S.; Pengkun, H.; David, J. Modification of cement-based materials with nanoparticles. Cem. Concr. Compos. 2016, 36, 8–15. [Google Scholar] [CrossRef]
- Camiletti, J.; Soliman, A.M.; Nehdi, M.L. Effects of nano- and micro-limestone addition on early-age properties of ultra-high-performance concrete. Mater. Struct. 2012, 46, 881–898. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Liu, A.; Wang, X. Effect of nano-CaCO3 on properties of cement paste. Energy Procedia 2012, 16, 991–996. [Google Scholar] [CrossRef]
- Hawkins, P.; Tennis, P.D.; Detwiler, R.J. The Use of Limestone in Portland Cement: A State-of-the-Art Review; Portland Cement Association: Skokie, IL, USA, 1996; p. 36. [Google Scholar]
- Qian, K.L.; Meng, T.; Qian, X.Q.; Zhan, S.L. Research on Some Properties of Fly Ash Concrete with Nano-CaCO3 Middle Slurry. Key Eng. Mater. 2009, 405–406, 186–190. [Google Scholar] [CrossRef]
- GB/T 17669.4-1999.1999-02-08; Gypsum Plasters—Determination of Physical Properties of Pure Paste. State Bureau of Building Materials Industry: Beijing, China, 1999.
- GB/T 17669.3-1999.1999-02-08; Gypsum Plasters—Determination of Mechanical Properties. State Bureau of Building Materials Industry: Beijing, China, 1999.
- Mikailzade, F.; Önal, F.; Maksutoglu, M.; Zarbali, M.; Göktaş, A. Structure and magnetization of polycrystalline La0.66Ca0.33MnO3 and La0.66Ba0.33MnO3 films prepared using sol-gel technique. J. Supercond. Nov. Magn. 2018, 31, 4141–4145. [Google Scholar] [CrossRef]
- Altiner, M.; Top, S.; Kaymakoğlu, B.; Seçkin, İ.Y.; Vapur, H. Production of precipitated calcium carbonate particles from gypsum waste using venturi tubes as a carbonation zone. J. CO2 Util. 2019, 29, 117–125. [Google Scholar] [CrossRef]
- Mikailzade, F.; Türkan, H.; Önal, F.; Zarbali, M.; Göktaş, A.; Tumbul, A. Structural and magnetic properties of polycrystalline Zn1−xMnxO films synthesized on glass and p-type Si substrates using Sol–Gel technique. Appl. Phys. A 2021, 127, 408. [Google Scholar] [CrossRef]
- Huang, Z.; Cao, F. Effects of Nano-materials on the Performance of UHPC. Mater. Rev. 2012, 26, 136–141. [Google Scholar]
- An, Y.X. Effect of nano materials on durability of high performance concrete. In Advanced Materials Research; Trans Tech Publications Ltd.: Shanghai, China, 2013; Volume 742, pp. 220–223. [Google Scholar]
- Zhang, H.; Wu, Y. Influence of nano SiO2 on mechanical property of the high performance concrete. New Build. Mater. 2012, 39, 78–80. [Google Scholar]
- Liu, Z.; Yang, J.; Bing, C. Experimental Study on Properties of Modified Raw Soil Material adding Phosphogypsum and Fly Ash. Fly Ash Compr. Util. 2016, 1, 3–6. [Google Scholar]




| Component | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | K2O | P2O5 | SO3 | Organism |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 14.52 | 1.66 | 0.15 | 0.005 | 0.17 | 31.94 | 0.22 | 0.94 | 45.38 | 0.25 |
| Appearance | CaCO3 Content (%) | Specific Surface Area (m2/g) | Particle Size Distribution | pH | ||
|---|---|---|---|---|---|---|
| D10 (nm) | D50 (nm) | D90 (nm) | ||||
| White powder | 98 | 22 | 54.65 | 74.66 | 100.73 | 9.05 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Contents of Nano-CaCO3(%) | 0 | 0.25 | 0.5 | 0.75 | 1 | 1.25 | 1.5 | 1.75 | 2 |
| No. | Fluidity (mm) | Initial Setting Time (s) | Final Setting Time (s) | Absolute Dry Flexural Strength (MPa) | Absolute Dry Compressive Strength (MPa) | Water Absorption (%) | Softening Coefficient (%) |
|---|---|---|---|---|---|---|---|
| 1 | 180 | 786 | 1861 | 5.4 | 16.5 | 0.33 | 0.38 |
| 2 | 176 | 781 | 1803 | 6 | 17.4 | 0.33 | 0.55 |
| 3 | 170 | 722 | 1742 | 6.6 | 17.5 | 0.3 | 0.57 |
| 4 | 159 | 669 | 1622 | 7.5 | 17.8 | 0.27 | 0.63 |
| 5 | 148 | 604 | 1562 | 8.1 | 20.5 | 0.25 | 0.6 |
| 6 | 142 | 545 | 1445 | 7.7 | 18.6 | 0.23 | 0.59 |
| 7 | 135 | 542 | 1384 | 6.8 | 17.7 | 0.22 | 0.56 |
| 8 | 133 | 540 | 1329 | 6.1 | 16.7 | 0.22 | 0.56 |
| 9 | 120 | 485 | 1321 | 5.2 | 15.9 | 0.22 | 0.56 |
| Performance Index | Here | 9 | 10 | 11 | 14 |
|---|---|---|---|---|---|
| Materials | Nano-CaCO3 | Short-cut polypropylene fiber | Chopped basalt fiber | Ordinary Portland cement | Multi-walled carbon nanotubes |
| Content (%) | 1 | 1.5 | 1.6 | 10 | 0.03 |
| Fluidity (mm) | 148 | 170 | 60 | - | - |
| Initial setting time (s) | 604 | - | 610 | 480 | - |
| Final setting time (s) | 1562 | - | 1530 | 1200 | - |
| Absolute dry flexural strength (MPa) | 8.1 | 6.46 | - | 5.1 | 4.94 |
| Absolute dry compressive strength (MPa) | 20.5 | 17.63 | - | 16.05 | 17.46 |
| Softening coefficient | 0.6 | - | - | 0.42 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tao, Z.; Wu, L.; Zhang, Z.; Zhao, Z. Study on Effect of Nano-CaCO3 on Properties of Phosphorus Building Gypsum. Materials 2023, 16, 3354. https://doi.org/10.3390/ma16093354
Zhang Y, Tao Z, Wu L, Zhang Z, Zhao Z. Study on Effect of Nano-CaCO3 on Properties of Phosphorus Building Gypsum. Materials. 2023; 16(9):3354. https://doi.org/10.3390/ma16093354
Chicago/Turabian StyleZhang, Yi, Zhong Tao, Lei Wu, Zhiqi Zhang, and Zhiman Zhao. 2023. "Study on Effect of Nano-CaCO3 on Properties of Phosphorus Building Gypsum" Materials 16, no. 9: 3354. https://doi.org/10.3390/ma16093354






