Structural and Scintillation Properties of Ce3+:Gd3Al3Ga2O12 Translucent Ceramics Prepared by One-Step Sintering
Abstract
:1. Introduction
2. Materials and Methods
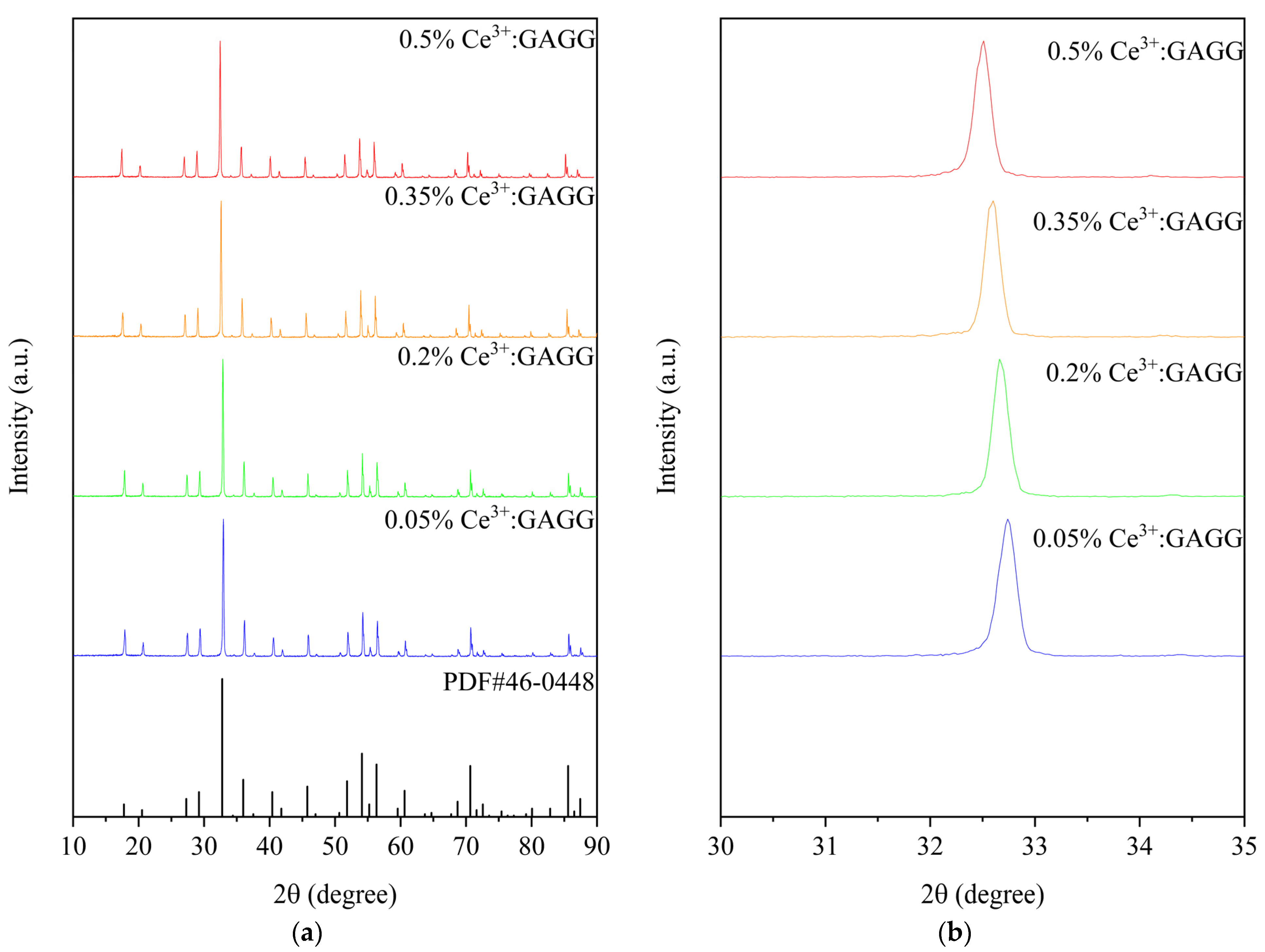
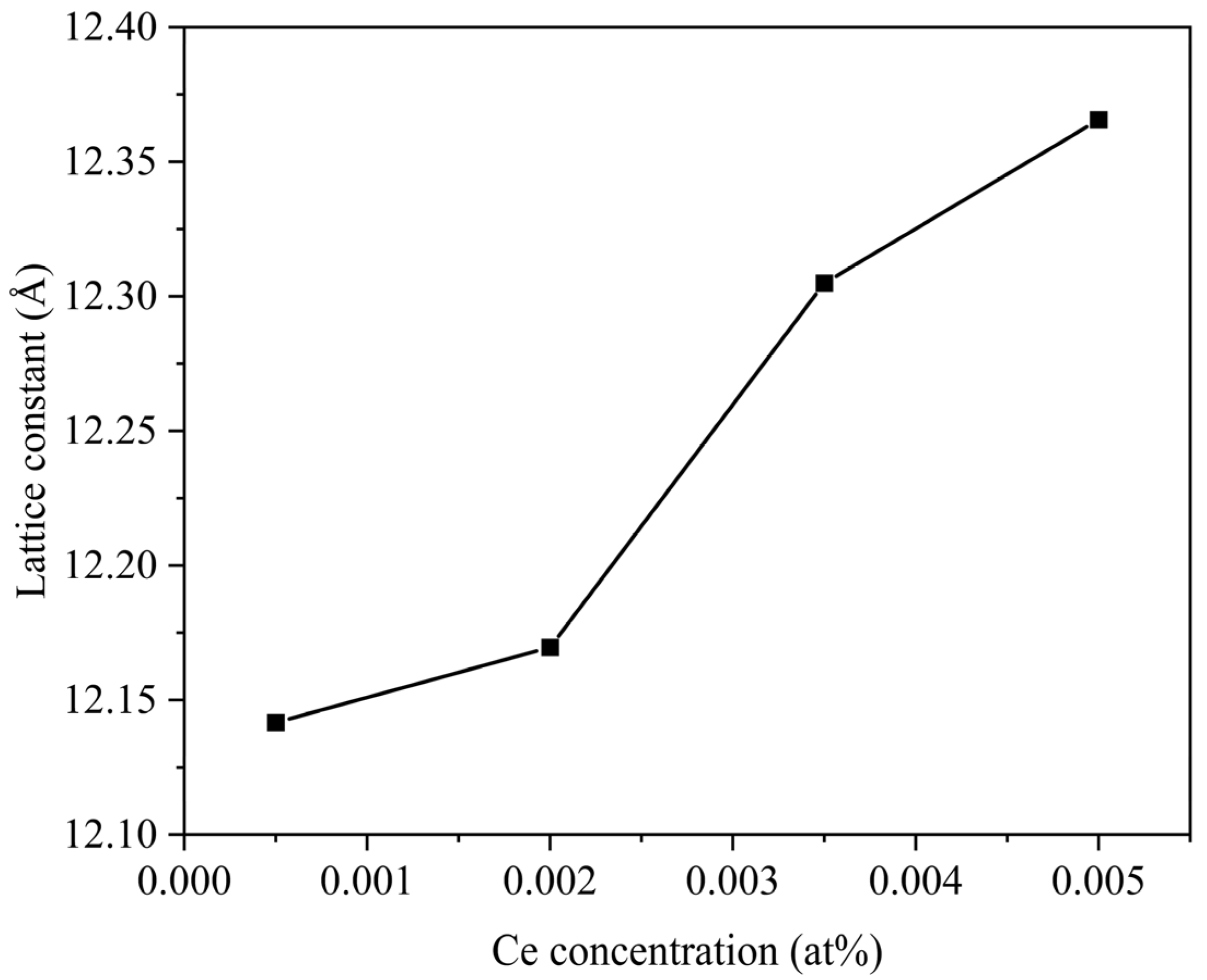
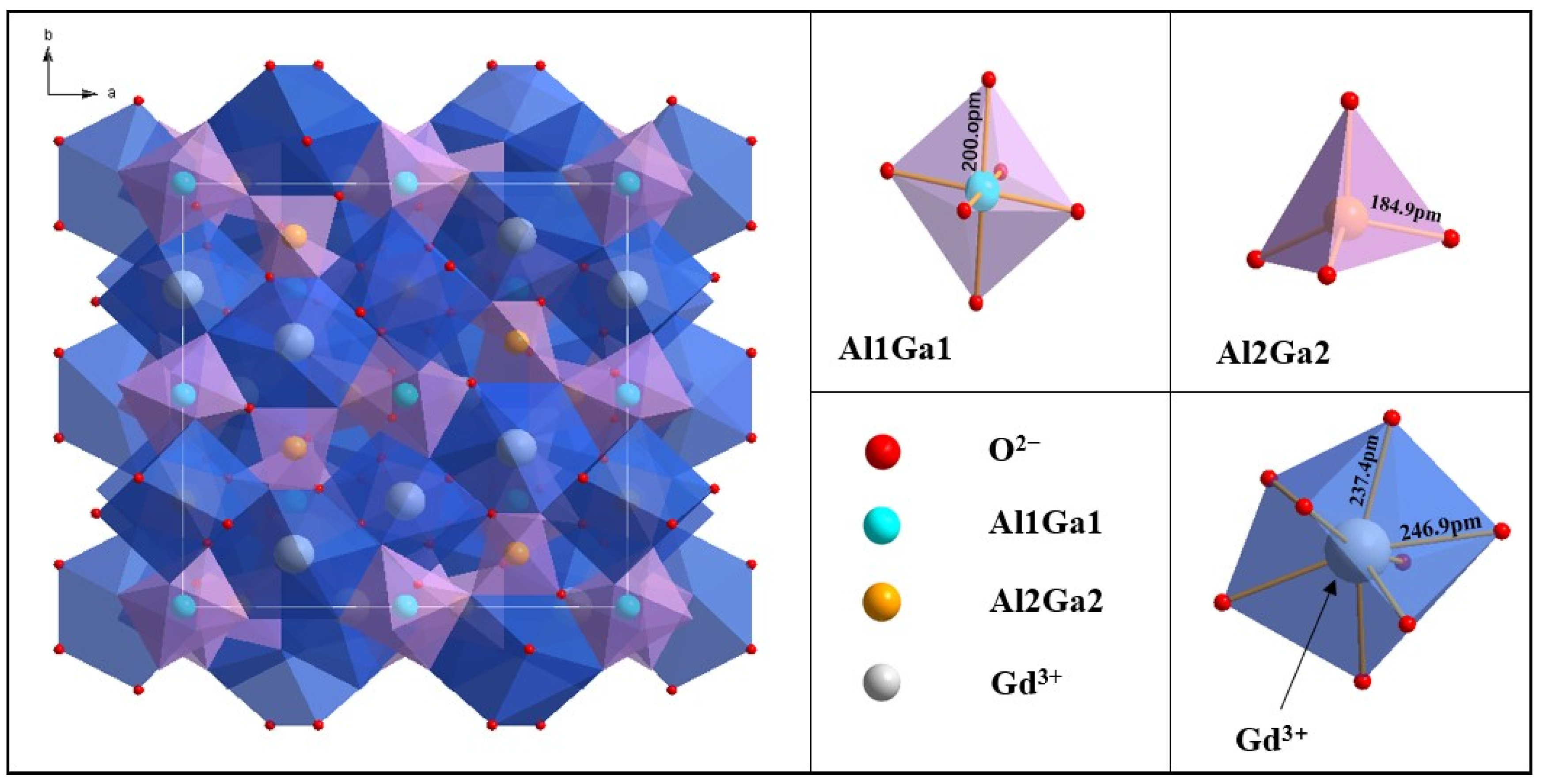
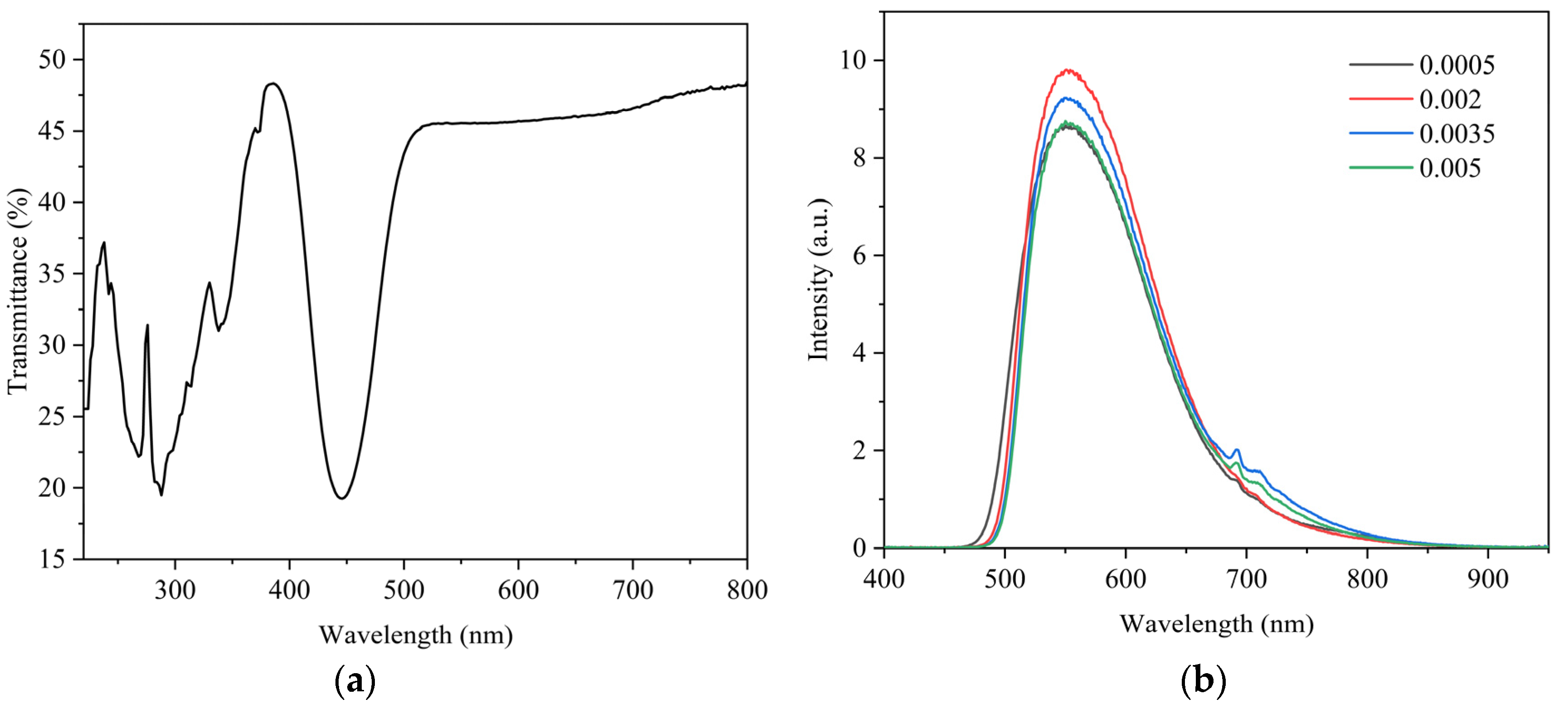
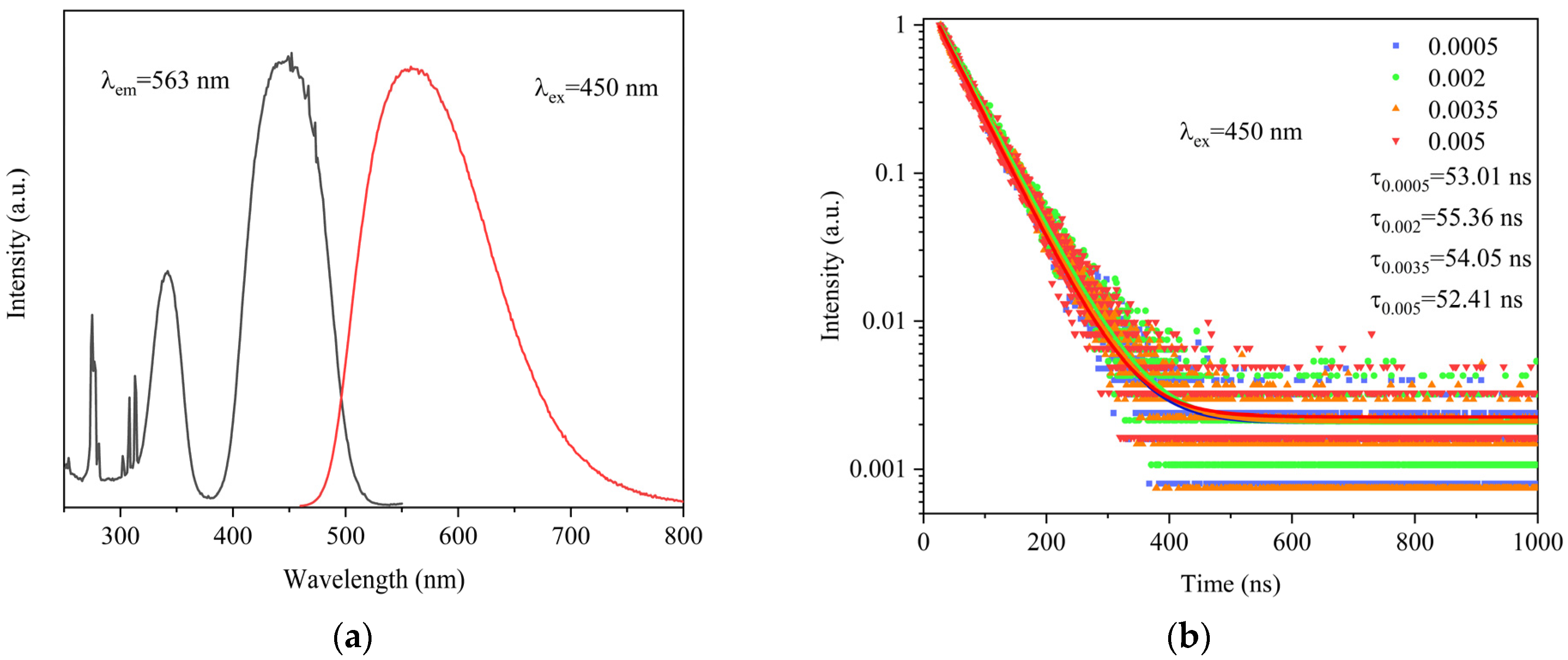
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yanagida, T. Inorganic scintillating materials and scintillation detectors. Proc. Jpn. Acad. Ser. B 2018, 94, 75–97. [Google Scholar] [CrossRef]
- Sibczynski, P.; Iwanowska-Hanke, J.; Moszyński, M.; Swiderski, L.; Szawłowski, M.; Grodzicka, M.; Szczęśniak, T.; Kamada, K.; Yoshikawa, A. Characterization of GAGG:Ce scintillators with various Al-to-Ga ratio. Nucl. Instrum. Methods Phys. Res. A 2015, 772, 112–117. [Google Scholar] [CrossRef]
- Kamada, K.; Yanagida, T.; Endo, T.; Tsutumi, K.; Usuki, Y.; Nikl, M.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A. 2 inch diameter single crystal growth and scintillation properties of Ce:Gd3Al2Ga3O12. J. Cryst. Growth 2012, 352, 88–90. [Google Scholar] [CrossRef]
- Dormenev, V.; Amelina, A.; Auffray, E.; Brinkmann, K.-T.; Dosovitskiy, G.; Cova, F.; Fedorov, A.; Gundacker, S.; Kazlou, D.; Korjik, M.; et al. Multipurpose Ce-doped Ba-Gd silica glass scintillator for radiation measurements. Nucl. Inst. Methods Phys. Res. A 2021, 1015, 10. [Google Scholar] [CrossRef]
- Chen, X.; Qin, H.; Zhang, Y.; Luo, Z.; Jiang, J.; Jiang, H. Preparation and Optical Properties of Transparent (Ce,Gd)3Al3Ga2O12 Ceramics. J. Am. Ceram. Soc. 2015, 98, 2352–2356. [Google Scholar] [CrossRef]
- Cester, D.; Nebbia, G.; Stevanato, L.; Pino, F.; Viesti, G. Experimental tests of the new plastic scintillator with pulse shape discrimination capabilities EJ-299-33. Nucl. Instrum. Methods Phys. Res. A 2014, 735, 202–206. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, H.J.; Hwang, Y.S.; Kim, D.H.; Park, H.W. Scintillation properties of quantum-dot doped styrene based plastic scintillators. J. Lumin. 2014, 146, 157–161. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, P.; Yan, D.; Xu, X.; Zhang, J. Fabrication of Ce3+ doped Gd3Ga3Al2O12 ceramics by reactive sintering method. Opt. Mater. 2017, 71, 23–26. [Google Scholar] [CrossRef]
- Chen, X.; Qin, H.; Zhang, Y.; Jiang, J.; Jiang, H. Highly transparent ZrO2-doped (Ce,Gd)3Al3Ga2O12 ceramics prepared via oxygen sintering. J. Eur. Ceram. Soc. 2015, 35, 3879–3883. [Google Scholar] [CrossRef]
- Babin, V.; Bohacek, P.; Grigorjeva, L.; Kucera, M.; Nikl, M.; Zazubovich, S.; Zolotarjovs, A. Effect of Mg2+ ions co-doping on luminescence and defects formation processes in Gd3(Ga,Al)5O12:Ce single crystals. Opt. Mater. 2017, 66, 48–58. [Google Scholar] [CrossRef]
- McDonald, K.; Schweitzer, G. Synthesis of GAGG:Ce3+ powder for ceramics using mechanochemical and solution combustion methods. J. Am. Ceram. Soc. 2018, 101, 3837–3849. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Qian, S.; Zhu, Y.; Hu, P.; Wu, Q.; Chen, P.; Ma, L.; Peng, S.; Zhang, L.; et al. Performance study of GAGG:Ce scintillator for gamma and neutron detection. J. Instrum. 2020, 15, C06031. [Google Scholar] [CrossRef]
- Bok, J.; Lalinský, O.; Hanuš, M.; Onderišinová, Z.; Kelar, J.; Kučera, M. GAGG: Ce single crystalline films: New perspective scintillators for electron detection in SEM. Ultramicroscopy 2016, 163, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Park, K.; Kim, H.; So, J.; Kim, J. Potential of GAGG:Ce scintillation crystals for synchrotron X-Ray micro-imaging. Current Appl. Phys. 2019, 19, 303–307. [Google Scholar] [CrossRef]
- Gerasimov, I.; Nepokupnaya, T.; Boyarintsev, A.; Sidletskiy, O.; Kurtsev, D.; Voloshyna, O.; Trubaieva, O.; Boyarintseva, Y.; Sibilieva, T.; Shaposhnyk, A.; et al. GAGG: Ce composite scintillator for X-ray imaging. Opt. Mater. 2020, 109, 110305. [Google Scholar] [CrossRef]
- Mori, M.; Xu, J.; Okada, G.; Yanagida, T.; Ueda, J.; Tanabe, S. Comparative study of optical and scintillation properties of Ce:YAGG, Ce:GAGG and Ce:LuAGG transparent ceramics. J. Ceram. Soc. Jpn. 2016, 124, 569–573. [Google Scholar] [CrossRef]
- Chen, X.; Qin, H.; Zhang, Y.; Jiang, J.; Wu, Y.; Jiang, H. Effects of Ga substitution for Al on the fabrication and optical properties of transparent Ce:GAGG-based ceramics. J. Eur. Ceram. Soc. 2017, 37, 4109–4114. [Google Scholar] [CrossRef]
- Mohammadi, F.; Mirzaee, O.; Tajally, M. Influence of TEOS and MgO addition on slurry rheological, optical, and microstructure properties of YAG transparent ceramic. Opt. Mater. 2018, 85, 174–182. [Google Scholar] [CrossRef]
- Kamada, K.; Kurosawa, S.; Prusa, P.; Nikl, M.; Kochurikhin, V.; Endo, T.; Tsutumi, K.; Sato, H.; Yokota, Y.; Sugiyama, K.; et al. Cz grown 2-in. size Ce:Gd3(Al,Ga)5O12 single crystal; relationship between Al, Ga site occupancy and scintillation properties. Opt. Mater. 2014, 36, 1942–1945. [Google Scholar] [CrossRef]
- Chen, X.; Qin, H.; Zhang, Y.; Liu, Y.; Jiang, J.; Jiang, H. Microstructure and optical properties of transparent Nd:GAGG ceramics prepared via solid-state reactive sintering. Opt. Mater. Express 2016, 6, 610–619. [Google Scholar] [CrossRef]
- Lee, S.H.; Kochawattana, S.; Messing, G.L.; Dumm, J.Q.; Quarles, G.; Castillo, V. Solid-State Reactive Sintering of Transparent Polycrystalline Nd: YAG Ceramics. J. Am. Ceram. Soc. 2006, 89, 1945–1950. [Google Scholar] [CrossRef]
- Xu, J.; Shi, Y.; Xie, J.; Lei, F. Fabrication, Microstructure, and Luminescent Properties of Ce3+—Doped Lu3Al5O12 (Ce:LuAG) Transparent Ceramics by Low-Temperature Vacuum Sintering. J. Am. Ceram. Soc. 2013, 96, 1930–1936. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Xie, R.; Hirosaki, N.; Xu, X.; Huang, L. Cerium-doped lutetium aluminum garnet optically transparent ceramics fabricated by a sol-gel combustion process. J. Mater. Res. 2006, 21, 1519–1525. [Google Scholar] [CrossRef]
- Nikl, M.; Kamada, K.; Babin, V.; Pejchal, J.; Pilarova, K.; Mihokova, E.; Yoshikawa, A. Defect engineering in Ce-doped aluminum garnet single crystal scintillators. Cryst. Growth Des. 2014, 14, 4827–4833. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Z.; Feng, Y.; Liu, X.; Chen, H.; Shi, Y.; Kucerkova, R.; Beitlerova, A.; Nikl, M.; Li, J. Luminescence and scintillation characteristics of cerium doped Gd2YGa3Al2O12 ceramics. Opt. Mater. 2019, 90, 20–25. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, S.; Wang, Z.; Guo, H.; Ma, L.; Wang, Z.; Wu, Q. Scintillation properties of GAGG:Ce ceramic and single crystal. Opt. Mater. 2020, 105, 109964. [Google Scholar] [CrossRef]
- Taggart, M.; Nakhostin, M.; Sellin, P. Investigation into the potential of GAGG:Ce as a neutron detector. Nucl. Inst. Methods Phys. Res. A 2019, 931, 121–126. [Google Scholar] [CrossRef]
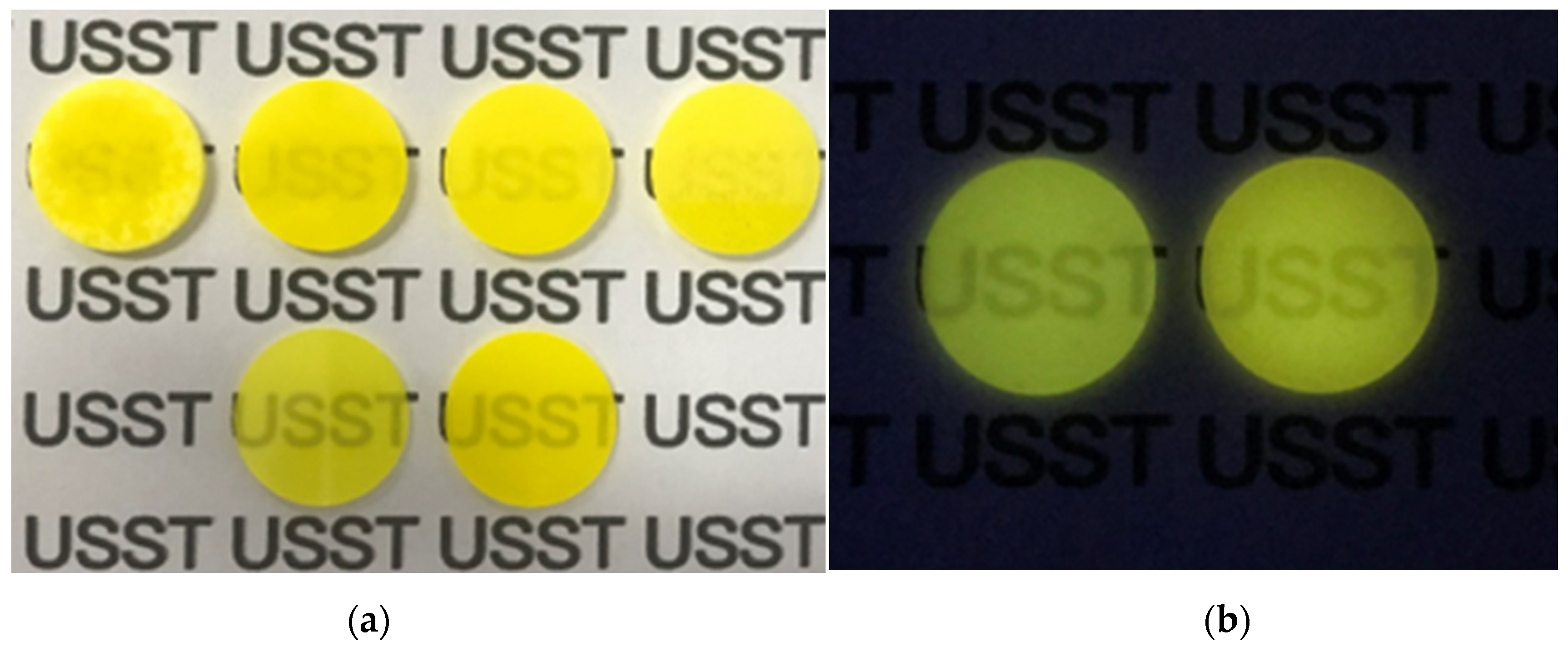


| Sample Location | Sintering Holding Temperature |
|---|---|
| First in the first row | 1550 °C |
| Second in the first row | 1575 °C |
| Third in the first row | 1625 °C |
| Fourth in the first row | 1650 °C |
| Second row | 1600 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Q.; Lin, H.; Hong, R.; Han, Z.; Zhang, D.; Ding, Y. Structural and Scintillation Properties of Ce3+:Gd3Al3Ga2O12 Translucent Ceramics Prepared by One-Step Sintering. Materials 2023, 16, 3373. https://doi.org/10.3390/ma16093373
You Q, Lin H, Hong R, Han Z, Zhang D, Ding Y. Structural and Scintillation Properties of Ce3+:Gd3Al3Ga2O12 Translucent Ceramics Prepared by One-Step Sintering. Materials. 2023; 16(9):3373. https://doi.org/10.3390/ma16093373
Chicago/Turabian StyleYou, Qi, Hui Lin, Ruijin Hong, Zhaoxia Han, Dawei Zhang, and Yuchong Ding. 2023. "Structural and Scintillation Properties of Ce3+:Gd3Al3Ga2O12 Translucent Ceramics Prepared by One-Step Sintering" Materials 16, no. 9: 3373. https://doi.org/10.3390/ma16093373




