Influence of PEO Electrolyzer Geometry on Current Density Distribution and Resultant Coating Properties on Zr-1Nb Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Electrolytic Oxidation Experimental Setup
2.2. Metal Sample Preparation
2.3. PEO Coating Formation
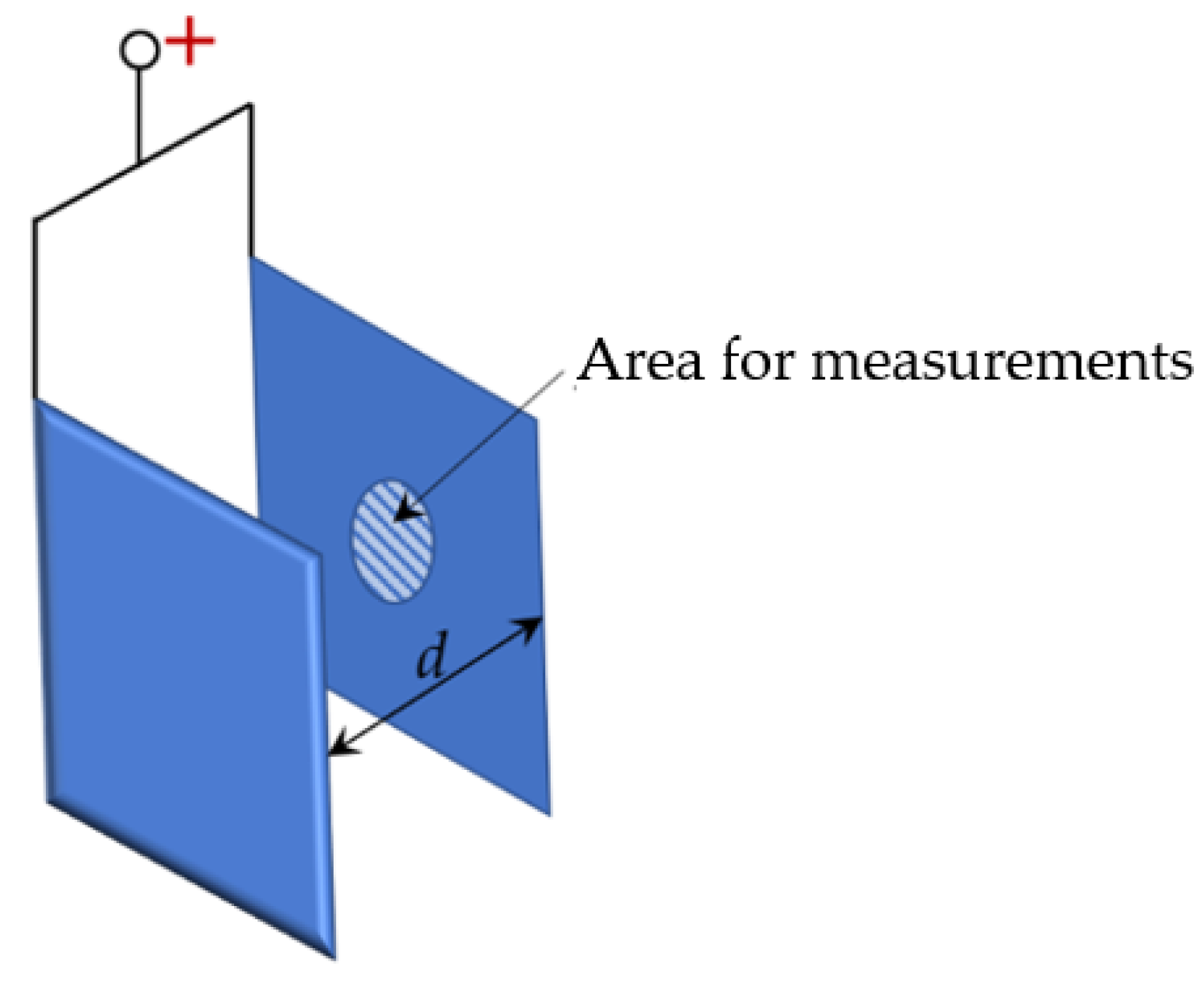
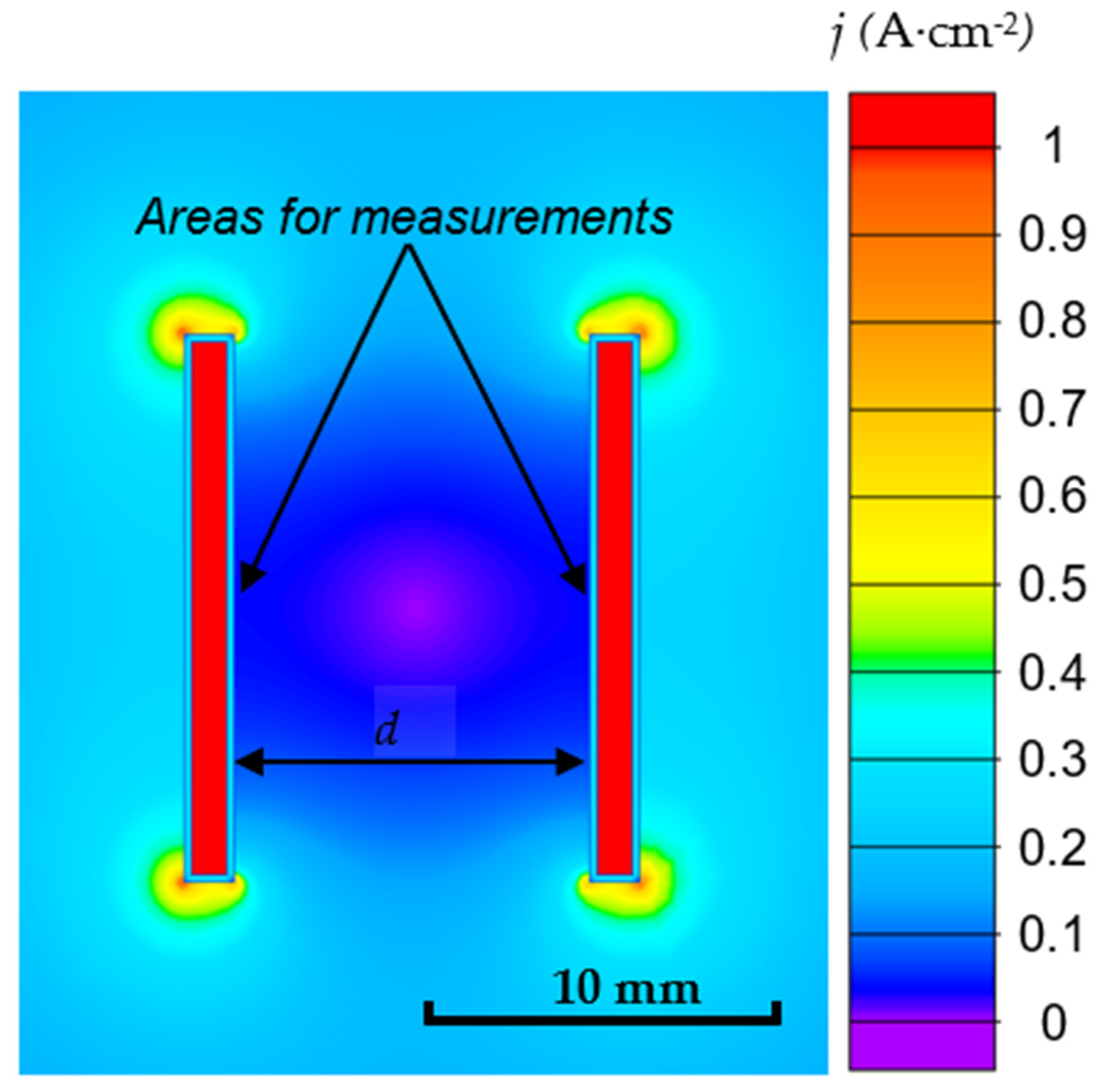
2.4. Electric Field Modeling
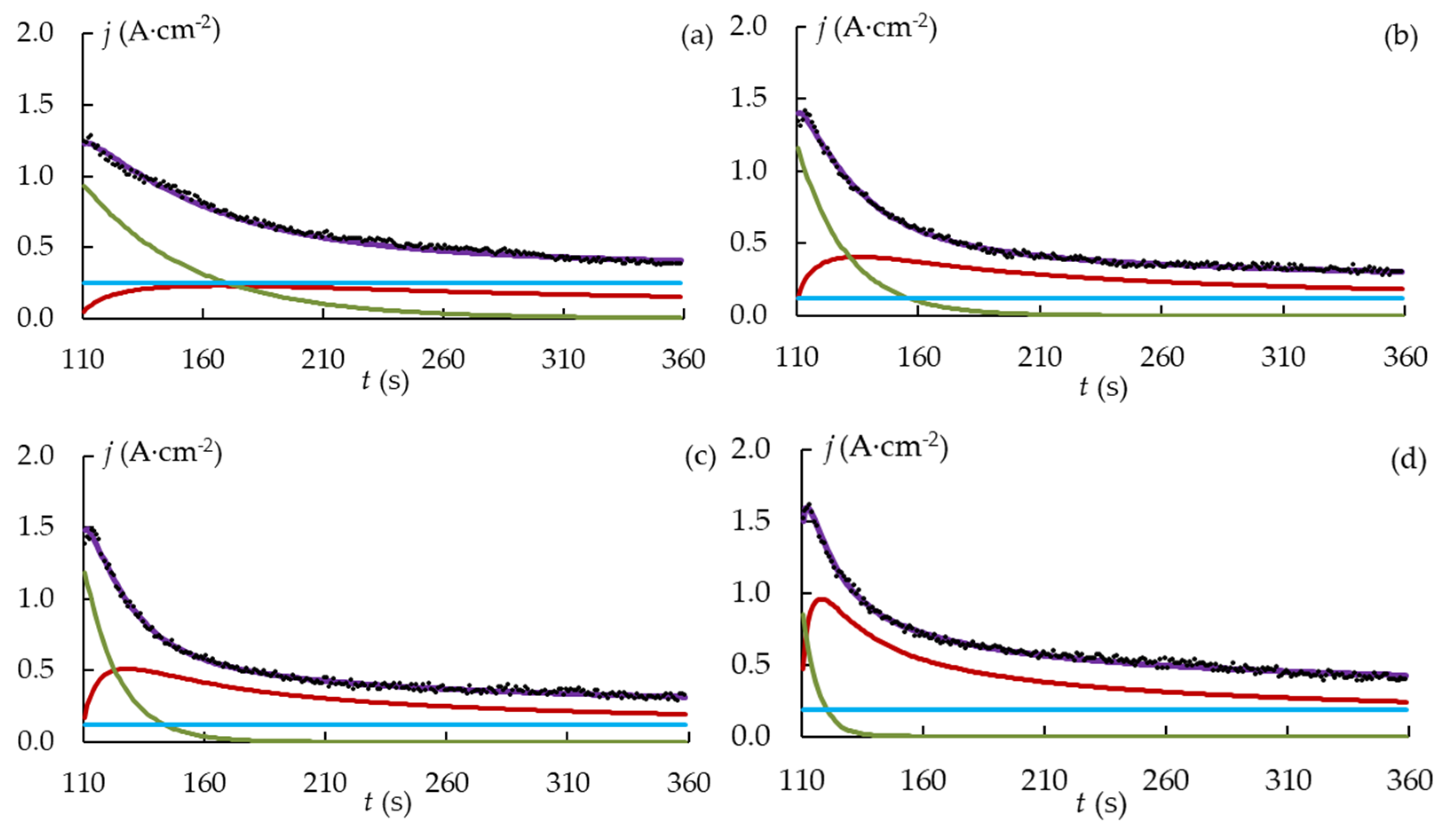
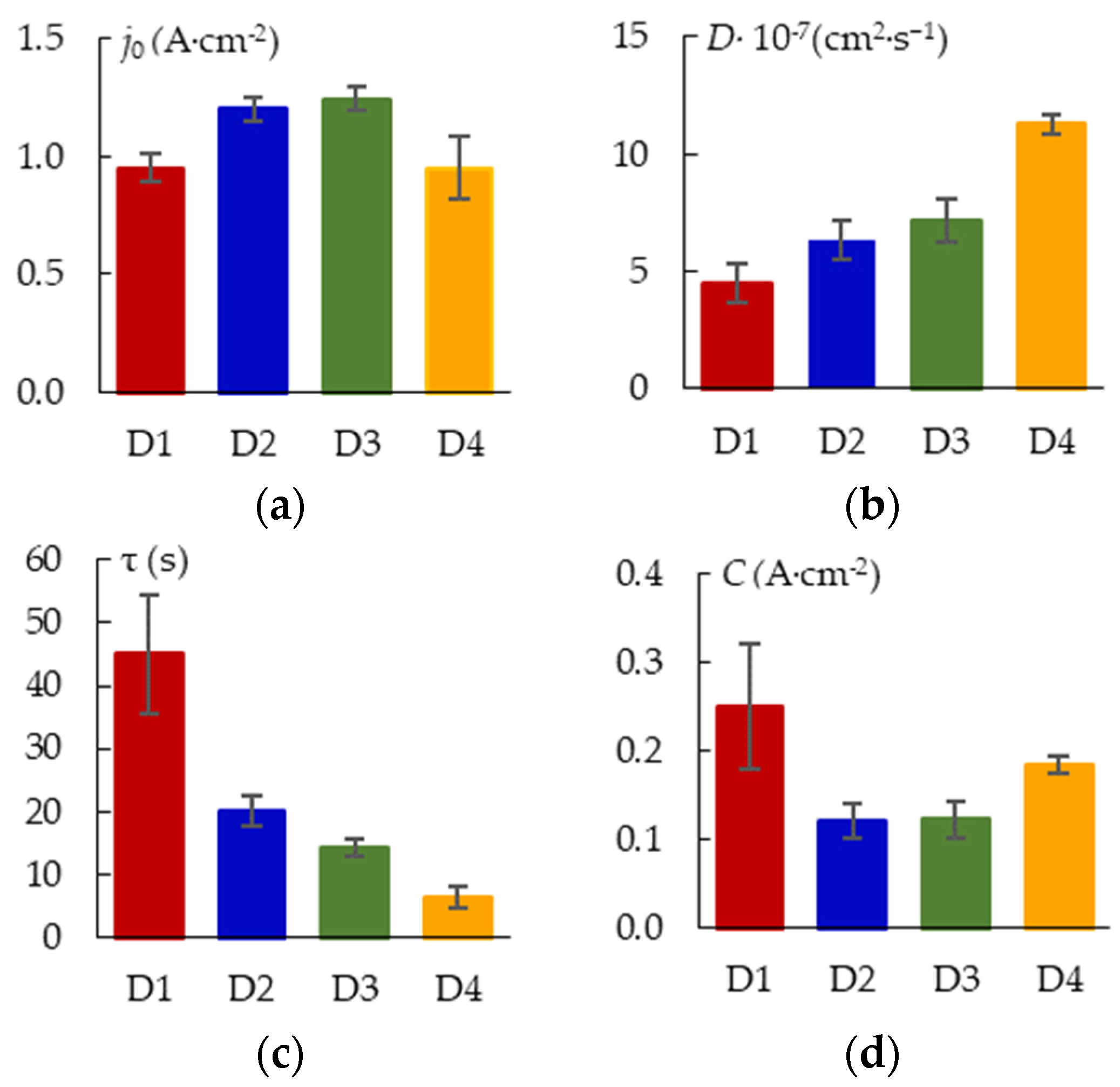
2.5. Estimation of Kinetic Coefficients of the PEO Process
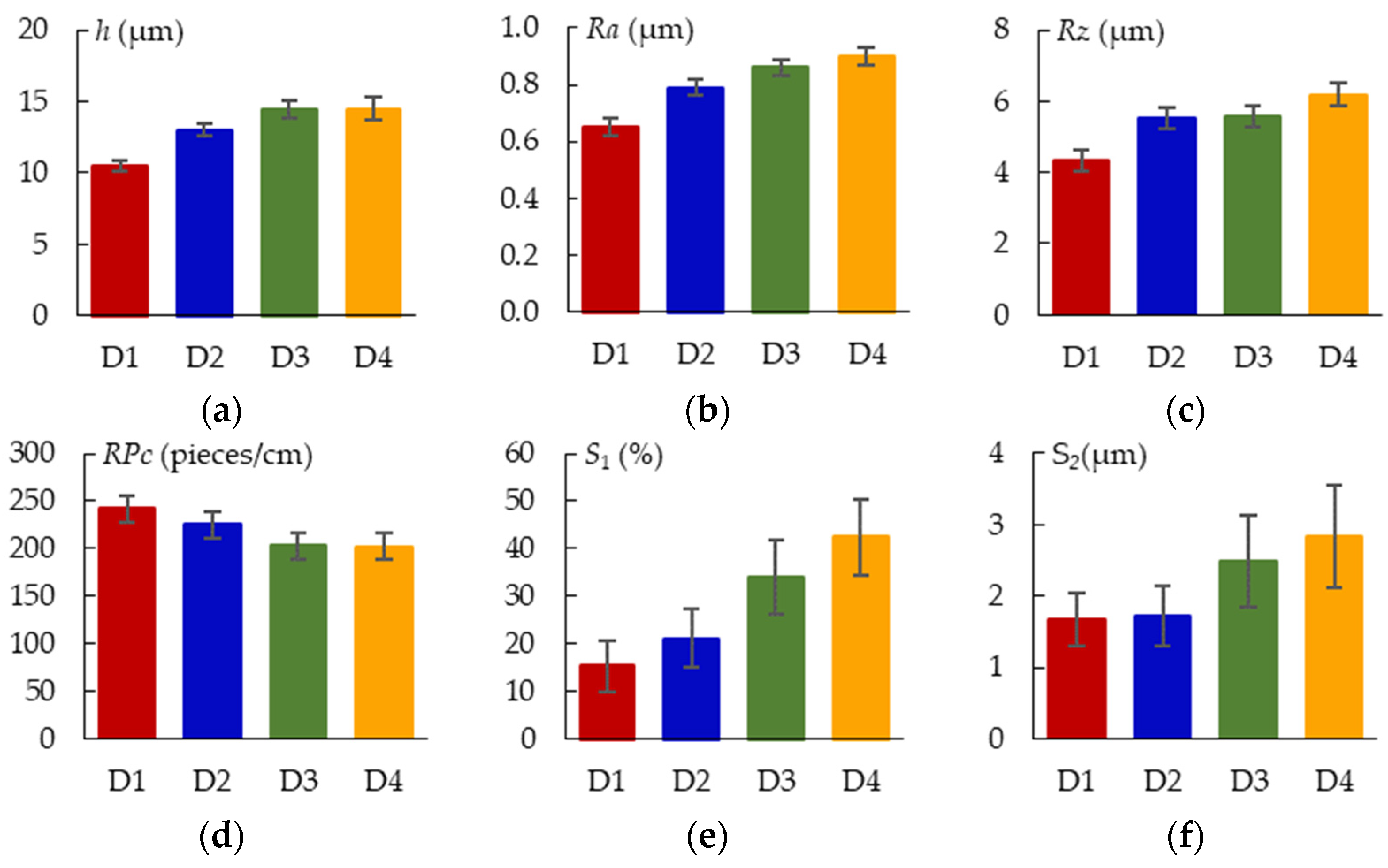
2.6. Surface Characterization
2.7. Evaluation of the Electrochemical Properties of the Samples
2.8. Wear Resistance Tests
2.9. Correlation Analysis
3. Results
3.1. Process Electrical Response of PEO of Zirconium Alloy
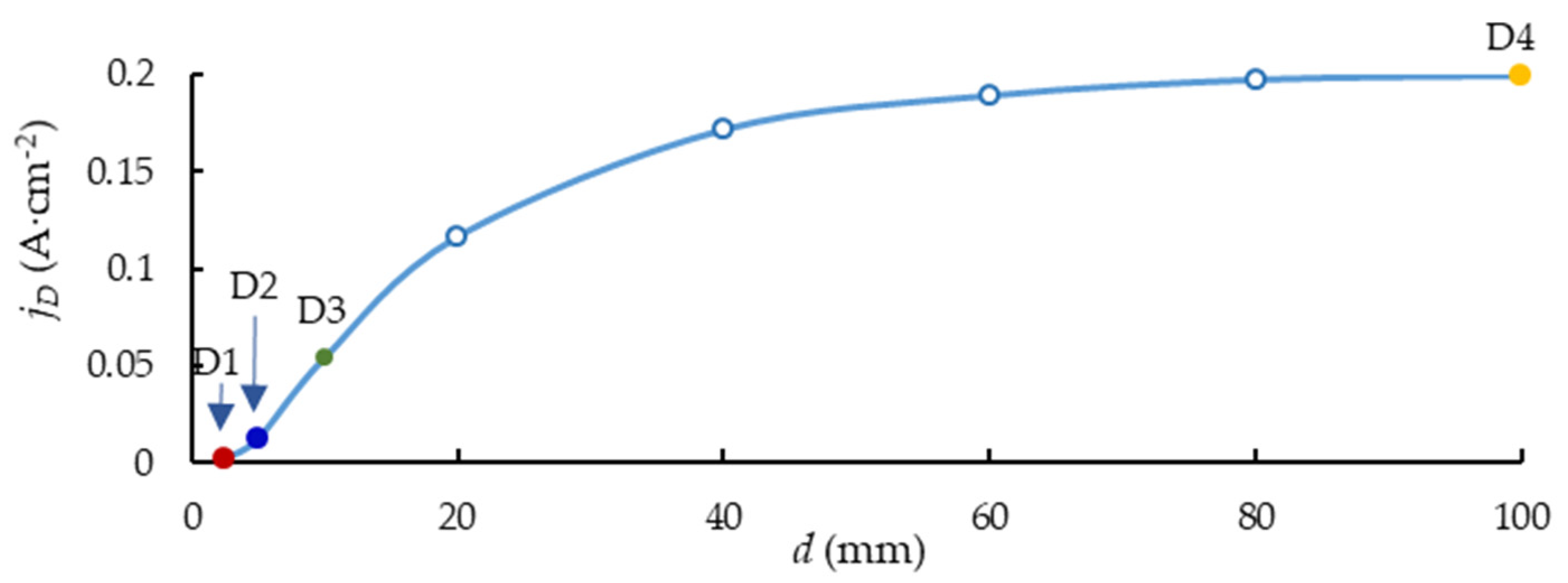
3.2. Electric Field Modelling Results
3.3. Quantitative Evaluation of the PEO Kinetics for the Zr-1Nb Alloy
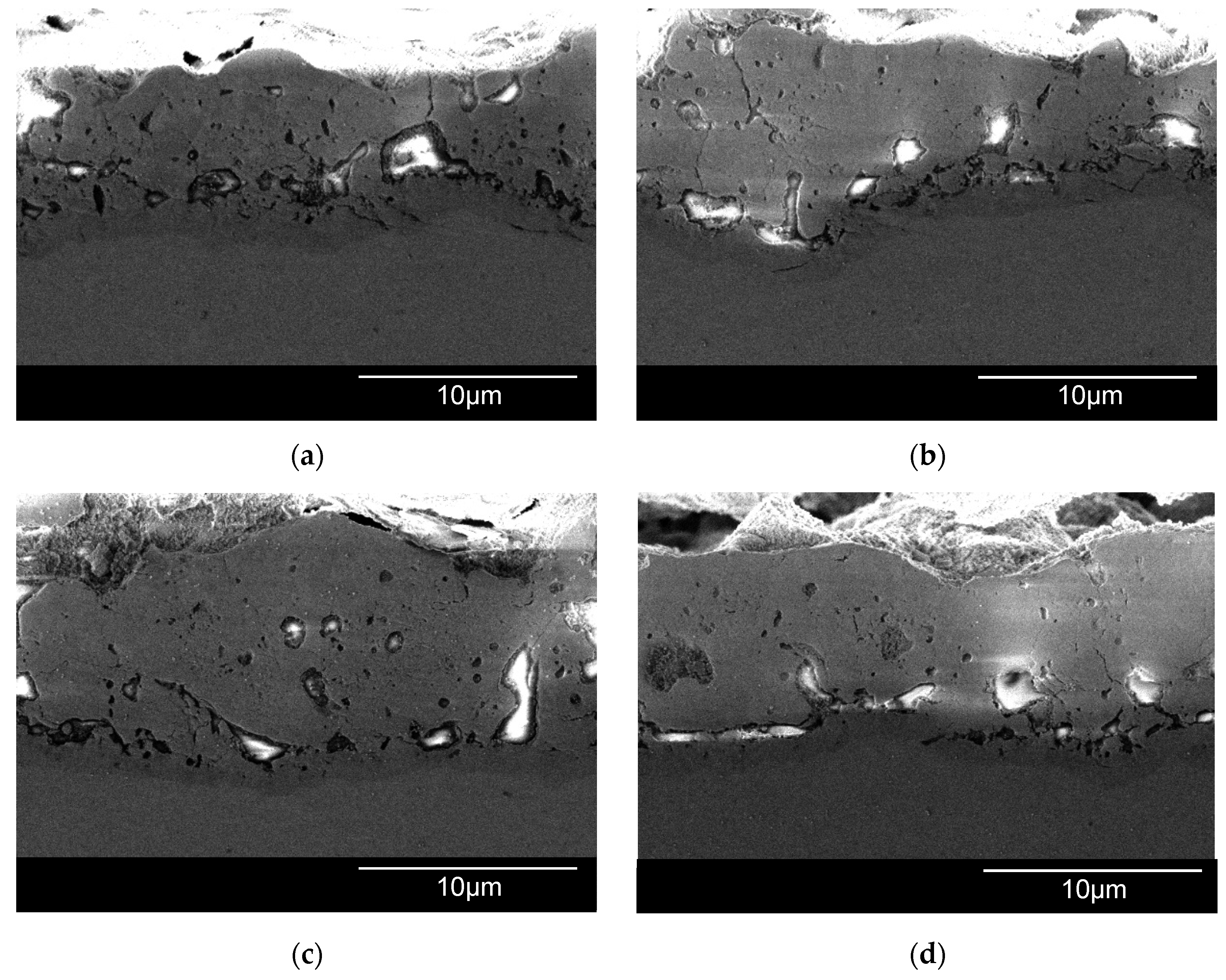
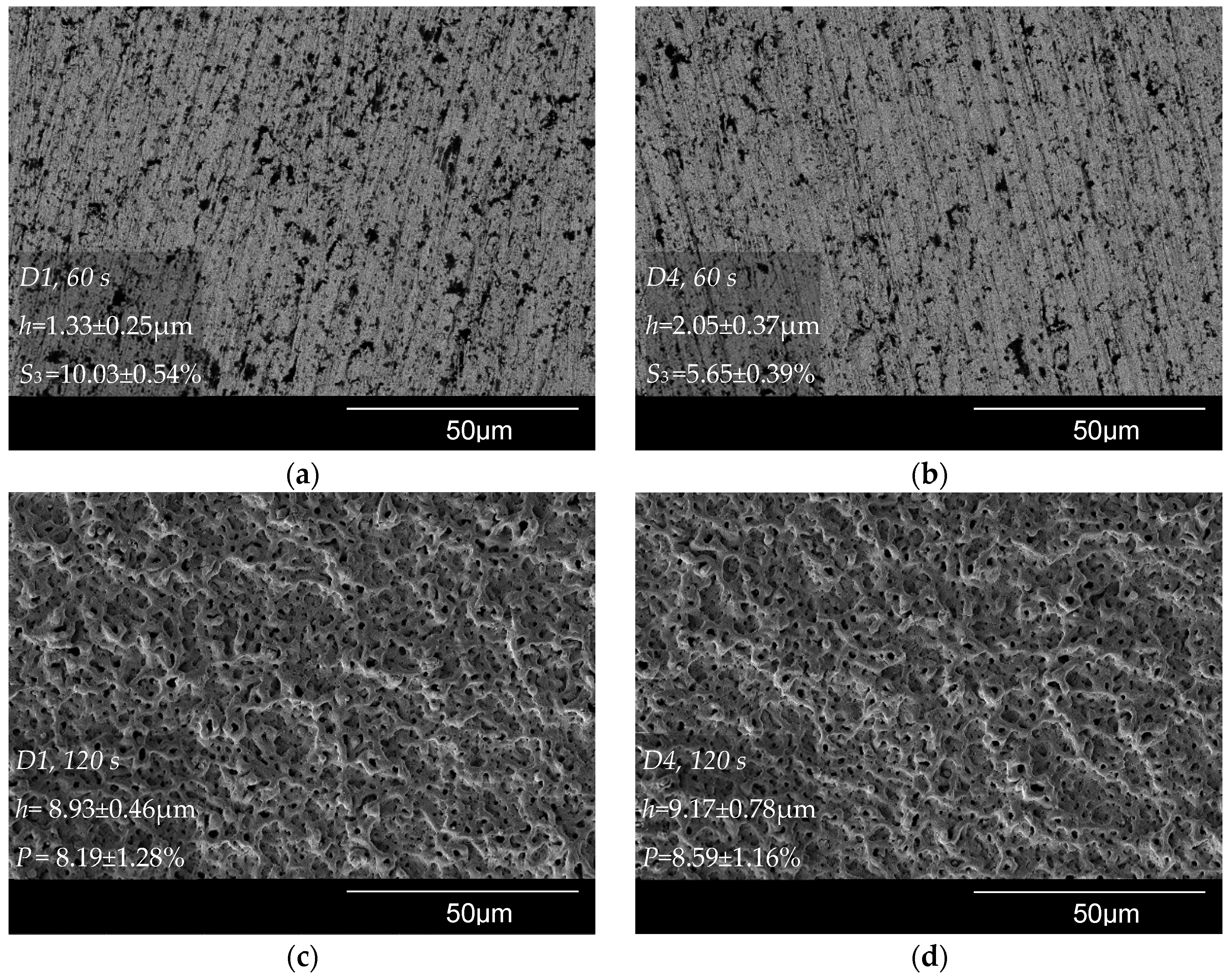
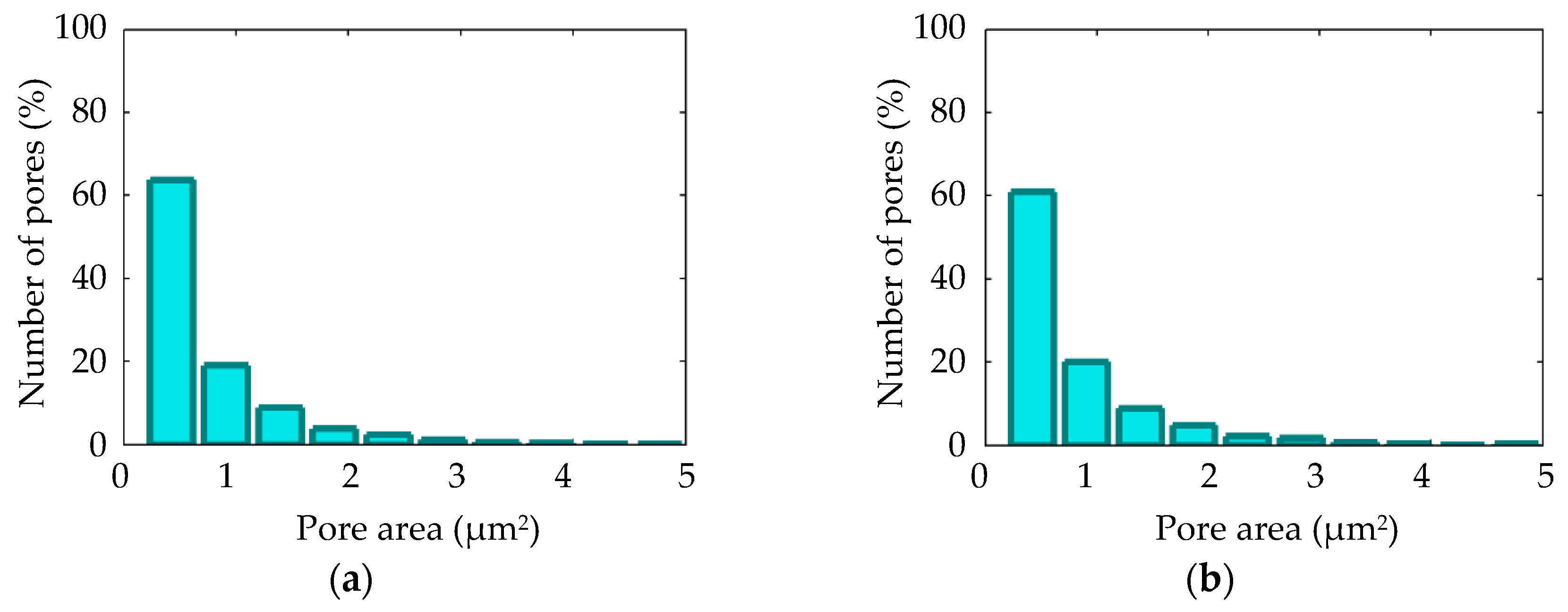
3.4. Thickness and Morphology of the PEO Coating as a Function of the Samples Arrangement
3.5. Thickness and Morphology of the PEO Coating as a Function of Time
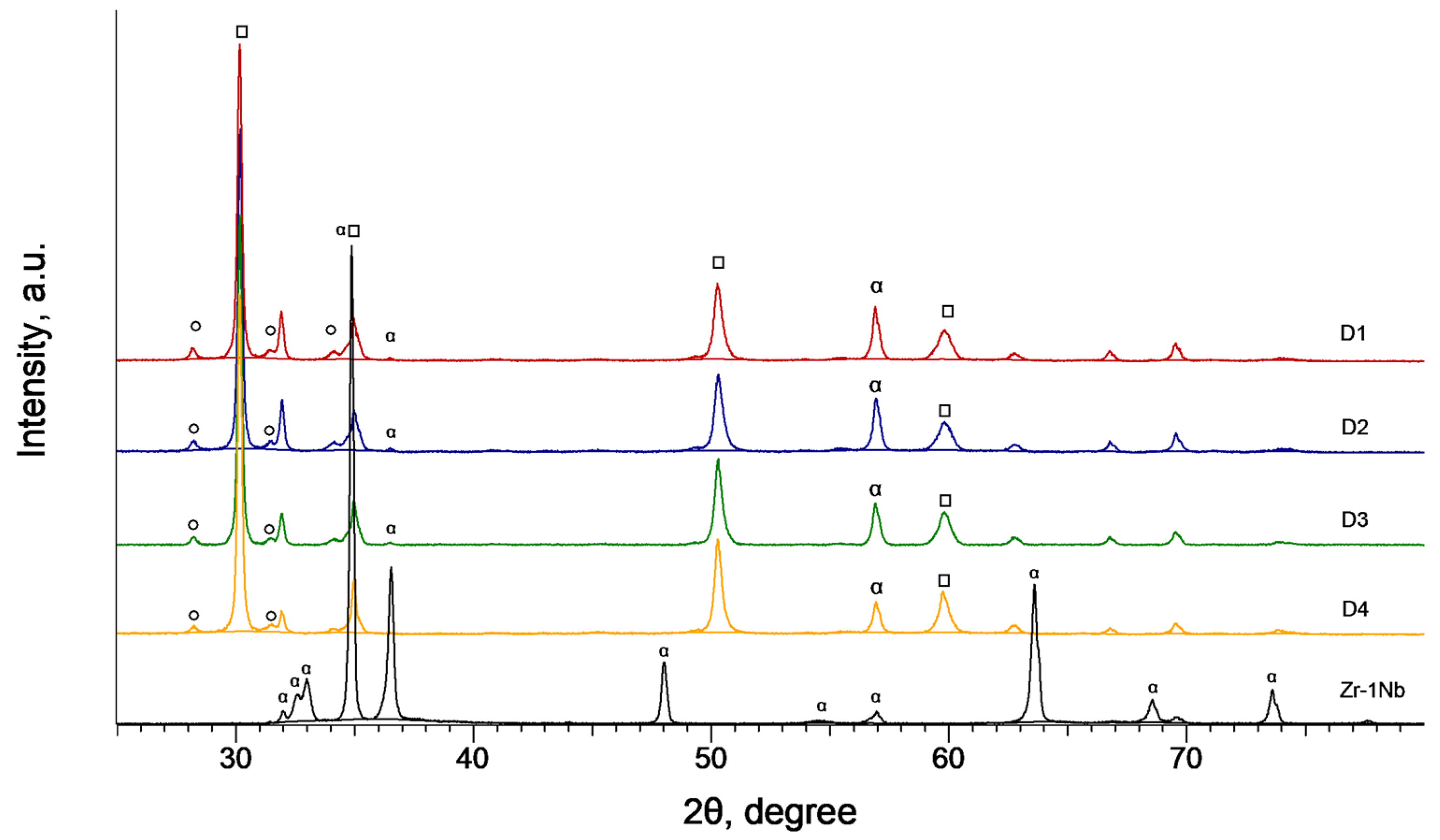
3.6. Phase Composition of the PEO Coatings as a Function of the Sample Arrangement
| Sample Code | Crystalline Phase Content in the PEO Coating (wt%) | |
|---|---|---|
| t-ZrO2 | m-ZrO2 | |
| D1 | 87 ± 2 | 13 ± 2 |
| D2 | 87 ± 2 | 13 ± 2 |
| D3 | 90 ± 2 | 10 ± 2 |
| D4 | 91 ± 2 | 9 ± 2 |
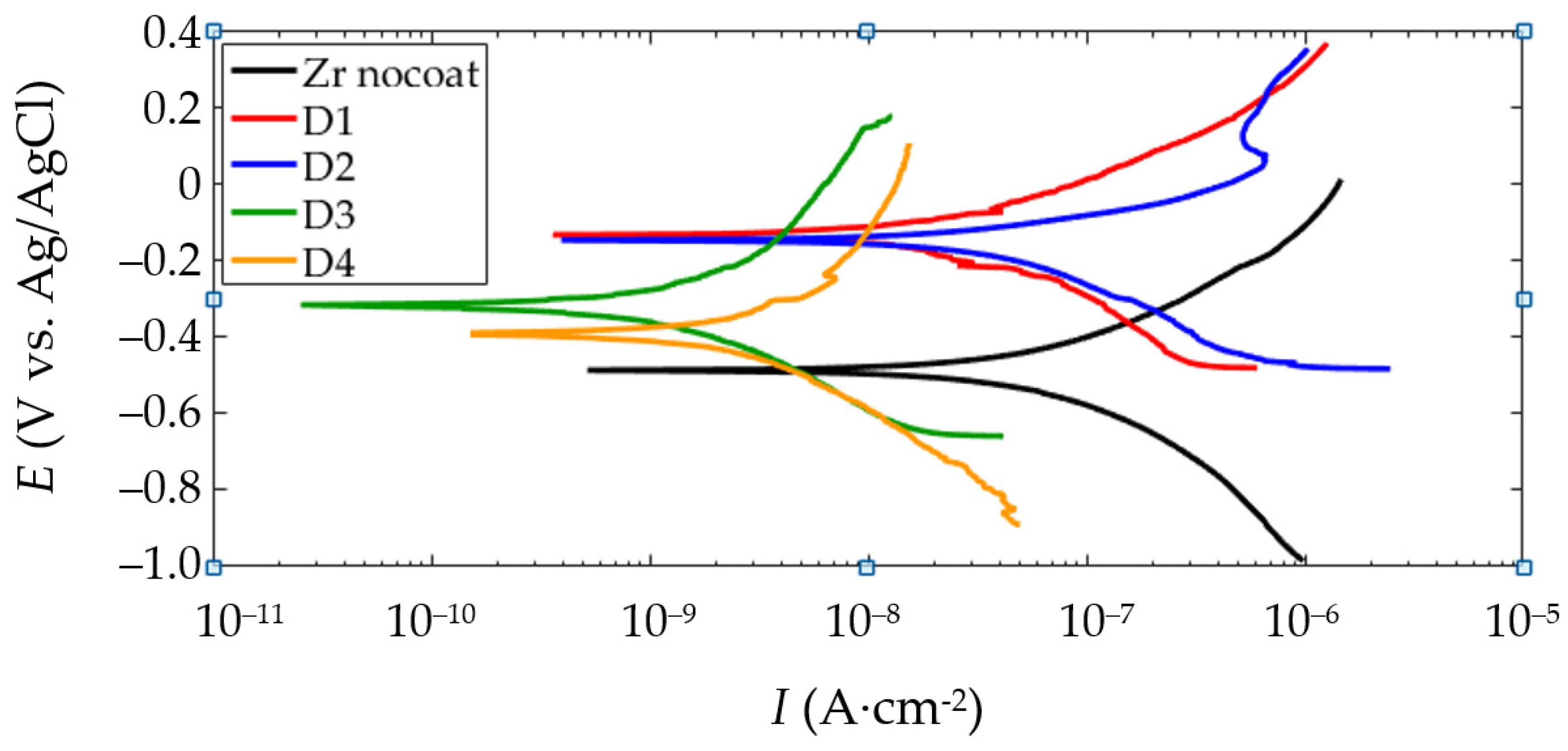
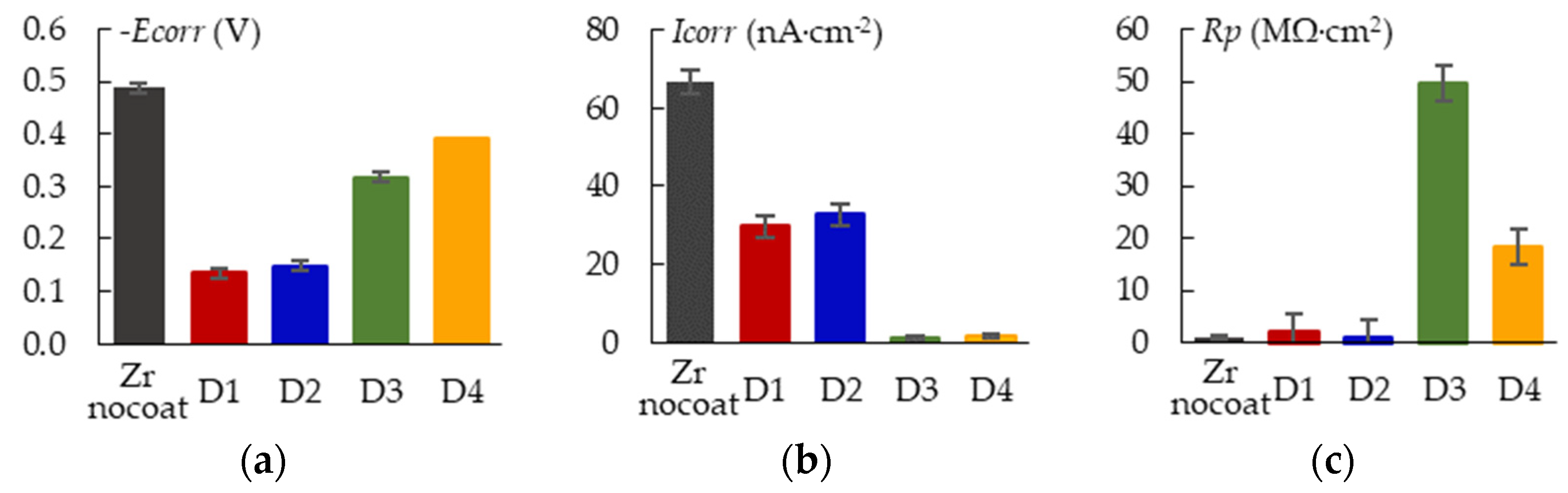
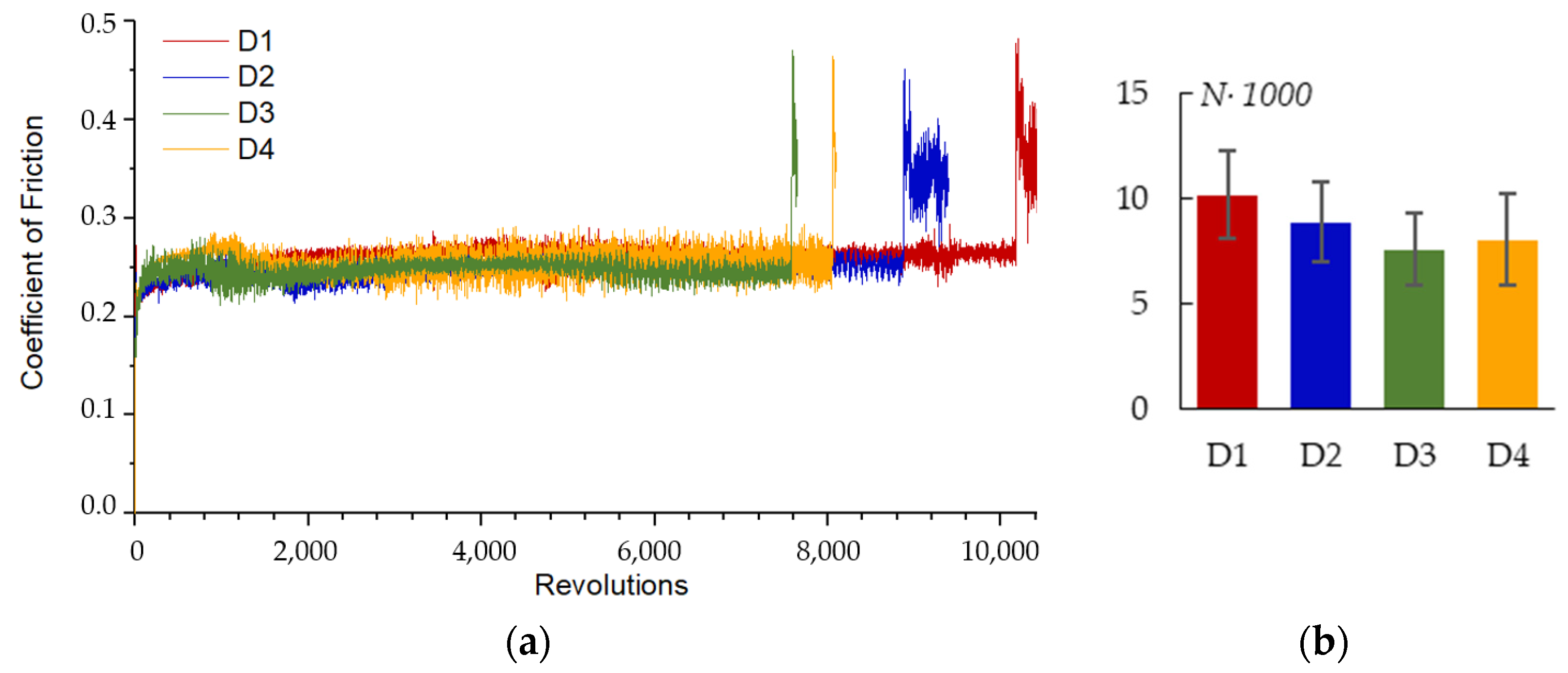
3.7. Corrosion and Wear Resistance of the PEO-Coating
4. Discussion
4.1. Influence of the Sample Spatial Arrangement on the Mechanism of the PEO Coating Growth on Zr-1Nb Alloy
4.2. Correlation Analysis of the PEO Process Parameters and the Resultant Coating Properties on Zr-1Nb Alloy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to plasma electrolytic oxidation-an overview of the process and applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Keshavarz, M.K.; Babaei, K. Surface characterization of bioceramic coatings on Zr and its alloys using plasma electrolytic oxidation (PEO): A review. Surf. Interfaces 2021, 25, 101283. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Effect of DC plasma electrolytic oxidation on surface characteristics and corrosion resistance of zirconium. Materials 2018, 11, 723. [Google Scholar] [CrossRef]
- Arun, S.; Hariprasad, S.; Saikiran, A.; Ravisankar, B.; Parfenov, E.V.; Mukaeva, V.R.; Rameshbabu, N. The effect of graphite particle size on the corrosion and wear behaviour of the PEO-EPD coating fabricated on commercially pure zirconium. Surf. Coat. Technol. 2019, 363, 301–313. [Google Scholar] [CrossRef]
- Savushkina, S.V.; Gerasimov, M.V.; Apelfeld, A.V.; Sivtsova, G.V.; Bogdashkina, N.L.; Ignatenko, V.E.; Suminov, I.V.; Vinogradov, A.V. Electrochemical study of Zr-1Nb alloy with oxide coatings formed by plasma electrolytic oxidation. J. Phys. Conf. Ser. 2020, 1713, 012039. [Google Scholar] [CrossRef]
- Sarkar, A.; Chandanshive, S.A.; Thota, M.K.; Kapoor, R. High temperature deformation behavior of Zr-1Nb alloy. J. Alloys Compd. 2017, 703, 56–66. [Google Scholar] [CrossRef]
- Kapoor, R.; Bharat Reddy, G.; Sarkar, A. Dynamic Recrystallization in Zircaloy-2. Trans. Indian Inst. Met. 2022, 75, 975–982. [Google Scholar] [CrossRef]
- Farrakhov, R.G.; Mukaeva, V.R.; Fatkullin, A.R.; Gorbatkov, M.V.; Tarasov, P.V.; Lazarev, D.M.; Ramesh Babu, N.; Parfenov, E.V. Plasma electrolytic oxidation treatment mode influence on corrosion properties of coatings obtained on Zr-1Nb alloy in silicate-phosphate electrolyte. IOP Conf. Ser. Mater. Sci. Eng. 2018, 292, 012006. [Google Scholar] [CrossRef]
- Sukumaran, A.; Sampatirao, H.; Balasubramanian, R.; Parfenov, E.; Mukaeva, V.; Nagumothu, R. Formation of ZrO2–SiC Composite Coating on Zirconium by Plasma Electrolytic Oxidation in Different Electrolyte Systems Comprising of SiC Nanoparticles. Trans. Indian Inst. Met. 2018, 71, 1699–1713. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Ramana, C.V. Plasma electrolytic oxidation ceramic coatings on zirconium (Zr) and zralloys: Part i—Growth mechanisms, microstructure, and chemical composition. Coatings 2021, 11, 634. [Google Scholar] [CrossRef]
- Cengiz, S.; Uzunoglu, A.; Huang, S.M.; Stanciu, L.; Tarakci, M.; Gencer, Y. An in-vitro study: The effect of surface properties on bioactivity of the oxide layer fabricated on Zr substrate by PEO. Surf. Interfaces 2021, 22, 100884. [Google Scholar] [CrossRef]
- Aubakirova, V.; Farrakhov, R.; Astanin, V.; Parfenov, E.; Sharipov, A.; Gorbatkov, M. Plasma Electrolytic Oxidation of Zr-1%Nb Alloy: Effect of Sodium Silicate and Boric Acid Addition to Calcium Acetate-Based Electrolyte. Materials 2022, 15, 2003. [Google Scholar] [CrossRef] [PubMed]
- Apelfeld, A.; Grigoriev, S.; Krit, B.; Ludin, V.; Suminov, I.; Chudinov, D. Improving the stability of the coating properties for group plasma electrolytic oxidation. Manuf. Lett. 2022, 33, 54–59. [Google Scholar] [CrossRef]
- Ma, X.; Blawert, C.; Höche, D.; Kainer, K.U.; Zheludkevich, M.L. Simulation assisted investigation of substrate geometry impact on PEO coating formation. Surf. Coat. Technol. 2018, 350, 281–297. [Google Scholar] [CrossRef]
- Ma, X.; Blawert, C.; Höche, D.; Zheludkevich, M.L.; Kainer, K.U. Investigation of electrode distance impact on PEO coating formation assisted by simulation. Appl. Surf. Sci. 2016, 388, 304–312. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Farrakhov, R.G.; Mukaeva, V.R.; Gusarov, A.V.; Nevyantseva, R.R.; Yerokhin, A. Electric field effect on surface layer removal during electrolytic plasma polishing. Surf. Coat. Technol. 2016, 307, 1329–1340. [Google Scholar] [CrossRef]
- Khan, R.H.U.; Yerokhin, A.L.; Li, X.; Dong, H.; Matthews, A. Influence of current density and electrolyte concentration on DC PEO titania coatings. Surf. Eng. 2014, 30, 102–108. [Google Scholar] [CrossRef]
- Shin, K.R.; Kim, Y.S.; Yang, H.W.; Ko, Y.G.; Shin, D.H. In vitro biological response to the oxide layer in pure titanium formed at different current densities by plasma electrolytic oxidation. Appl. Surf. Sci. 2014, 314, 221–227. [Google Scholar] [CrossRef]
- Nadaraia, K.V.; Suchkov, S.N.; Imshinetskiy, I.M.; Mashtalyar, D.V.; Sinebrykhov, S.L.; Gnedenkov, S.V. Some new aspects of the study of dependence of properties of PEO coatings on the parameters of current in potentiodynamic mode. Surf. Coat. Technol. 2021, 426, 127744. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Lu, X.; Lv, Y.; Jiang, D.; Yu, Y.; Peng, Z.; Dong, Z. Characterization and property of dual-functional Zn-incorporated TiO2 micro-arc oxidation coatings: The influence of current density. J. Alloys Compd. 2019, 810, 151893. [Google Scholar] [CrossRef]
- Mukaeva, V.R.; Gorbatkov, M.V.; Farrakhov, R.G.; Lazarev, D.M.; Stotskiy, A.G.; Parfenov, E.V. Advanced plasma electrolysis research equipment with in-situ process diagnostics. In Proceedings of the 2020 International Conference on Electrotechnical Complexes and Systems (ICOECS), Ufa, Russia, 27–30 October 2020; p. 9278498. [Google Scholar]
- Snizhko, L.O.; Yerokhin, A.; Gurevina, N.L.; Misnyankin, D.O.; Ciba, A.V.; Matthews, A. Voltastatic studies of magnesium anodising in alkaline solutions. Surf. Coat. Technol. 2010, 205, 1527–1531. [Google Scholar] [CrossRef]
- Lazarev, D.M.; Farrakhov, R.G.; Mukaeva, V.R.; Kulyasova, O.B.; Parfenov, E.V.; Yerokhin, A.L. Growth Kinetics and Corrosion Protection Properties of Plasma Electrolytic Oxidation Coatings on Biodegradable Mg−2% Sr Alloy. Surf. Eng. Appl. Electrochem. 2020, 56, 83–92. [Google Scholar] [CrossRef]
- Farrakhov, R.; Melnichuk, O.; Parfenov, E.; Mukaeva, V.; Raab, A.; Sheremetyev, V.; Zhukova, Y.; Prokoshkin, S. Comparison of Biocompatible Coatings Produced by Plasma Electrolytic Oxidation on cp-Ti and Ti-Zr-Nb Superelastic Alloy. Coatings 2021, 11, 401. [Google Scholar] [CrossRef]
- Kirch, W. Descriptive Studies. In Encyclopedia of Public Health; Kirch, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; p. 261. [Google Scholar] [CrossRef]
- Gorbatkov, M.V.; Parfenov, E.V.; Tarasov, P.V.; Mukaeva, V.R.; Farrakhov, R.G. Method for Measuring Coating Thickness during the Process of Plasma Electrolytic Oxidation. RU Patent 2 668 344, 28 September 2018. [Google Scholar]
- Gao, Y.; Yerokhin, A.; Parfenov, E.; Matthews, A. Application of Voltage Pulse Transient Analysis during Plasma Electrolytic Oxidation for Assessment of Characteristics and Corrosion Behaviour of Ca- and P-containing Coatings on Magnesium. Electrochim. Acta 2014, 149, 218–230. [Google Scholar] [CrossRef]
- Apelfeld, A.V.; Betsofen, S.Y.; Borisov, A.M.; Vladimirov, B.V.; Savushkina, S.V.; Knyazev, E.V. Stabilization of the high-temperature phases in ceramic coatings on zirconium alloy produced by plasma electrolytic oxidation. J. Phys. Conf. Ser. 2016, 748, 012019. [Google Scholar] [CrossRef]
- Fabris, S.; Paxton, A.T.; Finnis, M.W. A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater. 2002, 50, 5171–5178. [Google Scholar] [CrossRef]
- Liu, J.; Saw, R.E.; Kiang, Y.H. Calculation of Effective Penetration Depth in X-Ray Diffraction for Pharmaceutical Solids. J. Pharm. Sci. 2010, 99, 3807–3814. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2018, 64, 127–162. [Google Scholar] [CrossRef]





| D1 | D2 | D3 | D4 |
|---|---|---|---|
 |  |  |  |
| Nb | O | Hf | Fe | Ca | C | Ni | Cr | Si | Zr |
|---|---|---|---|---|---|---|---|---|---|
| 1.10 | 0.10 | 0.05 | 0.05 | 0.03 | 0.02 | 0.02 | 0.02 | 0.02 | Balance |
| j | h | Ra | Rz | RPc | RSm | S1 | S2 | t-ZrO2 | Ecorr | Icorr | Rp | N | j0 | D | τ | C | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d | 98 | 55 | 65 | 70 | −61 | 68 | 81 | 80 | 77 | −80 | −61 | 8 | −43 | −53 | 94 | −65 | 11 |
| j | 100 | 67 | 76 | 77 | −74 | 80 | 90 | 89 | 87 | −89 | −74 | 26 | −57 | −40 | 97 | −74 | 0 |
| h | 100 | 99 | 95 | −98 | 97 | 90 | 84 | 82 | −84 | −79 | 67 | −98 | 40 | 80 | −98 | −71 | |
| Ra | 100 | 97 | −98 | 98 | 93 | 87 | 85 | −87 | −80 | 60 | −95 | 29 | 87 | −99 | −64 | ||
| Rz | 100 | −89 | 91 | 88 | 78 | 75 | −79 | −66 | 40 | −86 | 23 | 89 | −99 | −63 | |||
| RPc | 100 | −100 | −95 | −92 | −92 | 92 | 90 | −75 | 98 | −29 | −82 | 94 | 58 | ||||
| RSm | 100 | 98 | 95 | 94 | −95 | −91 | 71 | −95 | 21 | 87 | −94 | −52 | |||||
| S1 | 100 | 99 | 98 | −99 | −92 | 61 | −86 | −1 | 94 | −90 | −34 | ||||||
| S2 | 100 | 100 | −100 | −96 | 67 | −82 | −8 | 89 | −81 | −22 | |||||||
| t-ZrO2 | 100 | −100 | −98 | 70 | −82 | −7 | 87 | −79 | −22 | ||||||||
| Ecorr | 100 | 96 | −66 | 82 | 8 | −89 | 82 | 23 | |||||||||
| Icorr | 100 | −83 | 83 | −4 | −74 | 72 | 25 | ||||||||||
| Rp | 100 | −80 | 48 | 30 | −51 | −47 | |||||||||||
| N | 100 | −50 | −69 | 92 | 73 | ||||||||||||
| j0 | 100 | −22 | −31 | −89 | |||||||||||||
| D | 100 | −86 | −21 | ||||||||||||||
| τ | 100 | 68 | |||||||||||||||
 | -well-known correlations | ||||||||||||||||
| -new uncovered correlations | |||||||||||||||||
| -new correlations that statistically require additional studies | |||||||||||||||||
| -indirect correlations, due to the mutual influence of parameters on each other | |||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubakirova, V.; Gunderov, D.; Farrakhov, R.; Astanin, V.; Stotskiy, A.; Sharipov, A.; Demin, A.; Khalilov, L.; Parfenov, E. Influence of PEO Electrolyzer Geometry on Current Density Distribution and Resultant Coating Properties on Zr-1Nb Alloy. Materials 2023, 16, 3377. https://doi.org/10.3390/ma16093377
Aubakirova V, Gunderov D, Farrakhov R, Astanin V, Stotskiy A, Sharipov A, Demin A, Khalilov L, Parfenov E. Influence of PEO Electrolyzer Geometry on Current Density Distribution and Resultant Coating Properties on Zr-1Nb Alloy. Materials. 2023; 16(9):3377. https://doi.org/10.3390/ma16093377
Chicago/Turabian StyleAubakirova, Veta, Dmitry Gunderov, Ruzil Farrakhov, Vasily Astanin, Andrey Stotskiy, Arseny Sharipov, Alexey Demin, Leonard Khalilov, and Evgeny Parfenov. 2023. "Influence of PEO Electrolyzer Geometry on Current Density Distribution and Resultant Coating Properties on Zr-1Nb Alloy" Materials 16, no. 9: 3377. https://doi.org/10.3390/ma16093377
APA StyleAubakirova, V., Gunderov, D., Farrakhov, R., Astanin, V., Stotskiy, A., Sharipov, A., Demin, A., Khalilov, L., & Parfenov, E. (2023). Influence of PEO Electrolyzer Geometry on Current Density Distribution and Resultant Coating Properties on Zr-1Nb Alloy. Materials, 16(9), 3377. https://doi.org/10.3390/ma16093377







