Effect of Annealing Temperature on Electrochemical Properties of Zr56Cu19Ni11Al9Nb5 in PBS Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Tests
3. Results and Discussion
3.1. Electrochemical Tests
3.2. Optical Microscopy Observation
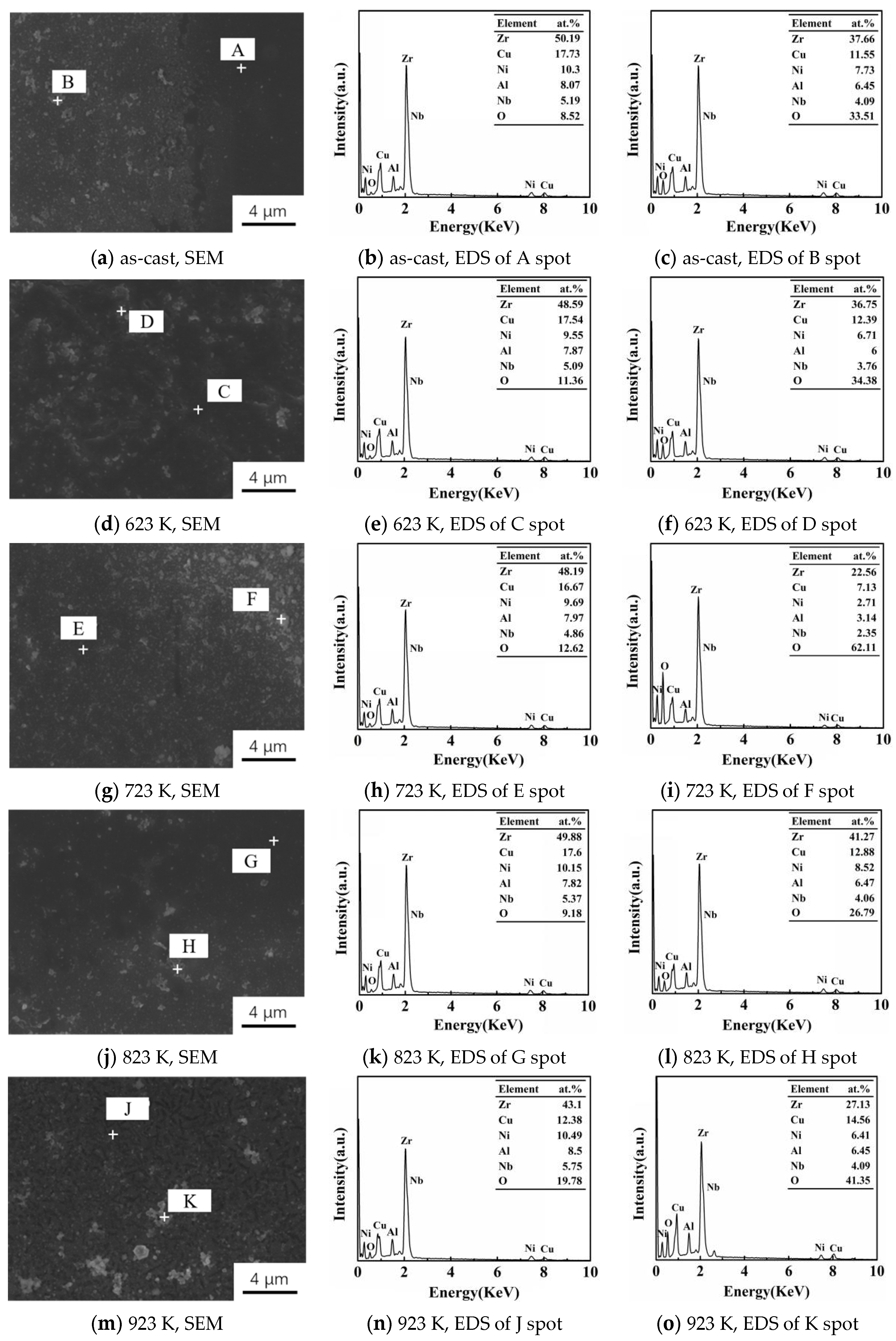
3.3. SEM and EDS Analysis
3.4. XPS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, K.; Kirk, D.; Singh, C.; Thorpe, S. Optimizing electrochemical micromachining parameters for Zr-based bulk metallic glass. J. Manuf. Process. 2017, 25, 227–234. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, H. Micro machining of bulk metallic glasses: A review. Int. J. Adv. Manuf. Technol. 2019, 100, 637–661. [Google Scholar] [CrossRef]
- Pan, J.; Ivanov, Y.P.; Zhou, W.H.; Li, Y.; Greer, A.L. Strain-hardening and suppression of shear-banding in rejuvenated bulk metallic glass. Nature 2020, 578, 559–562. [Google Scholar] [CrossRef]
- Geng, C.; Huang, B.; Zhang, N.; Yi, J.; Wang, Q.; Jia, Y.; Li, F.; Luan, J.; Hou, X.; Huang, W.; et al. Evolution of local densities during shear banding in Zr-based metallic glass micropillars. Acta Mater. 2022, 235, 118068. [Google Scholar] [CrossRef]
- Dhale, K.; Banerjee, N.; Outeiro, J.; Singh, R.K. Investigation of the softening behavior in severely deformed microm-achined sub-surface of Zr-based bulk metallic glass via nanoindentation. J. Non-Cryst. Solids 2022, 576, 121280. [Google Scholar] [CrossRef]
- Cai, Z.; Kang, S.; Lv, J.; Zhang, S.; Shi, Z.; Yang, Y.; Ma, M. Effect of adding remelting materials on the properties of die-cast Zr-based amorphous alloy gear castings. J. Non-Cryst. Solids 2022, 581, 121428. [Google Scholar] [CrossRef]
- Cao, Q.; Peng, S.; Zhao, X.; Wang, X.; Zhang, D.; Jiang, J. Effect of Nb substitution for Cu on glass formation and corrosion behavior of Zr Cu Ag Al Be bulk metallic glass. J. Alloys Compd. 2016, 683, 22–31. [Google Scholar] [CrossRef]
- Ayyagari, A.; Hasannaeimi, V.; Arora, H.; Mukherjee, S. Electrochemical and Friction Characteristics of Metallic Glass Composites at the Microstructural Length-scales. Sci. Rep. 2018, 8, 906. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Fan, Y.; Zhang, W.; Wei, R.; Guan, S.; Wang, T.; Kong, B.; Zhang, T.; Li, F. Crystallization and corrosion resistance of Zr-Ti-Y-Al-Cu-Ni-Co-Fe complex multi-component bulk metallic glasses. Intermetallics 2020, 118, 106688. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, Z.; Fu, H.; Zhang, H.; Zhu, Z.; Wang, A.; Li, H.; Zhang, L.; Zhang, H. Corrosion mechanisms of Zr-based bulk metallic glass in NaF and NaCl solutions. J. Mater. Sci. Technol. 2020, 46, 33–43. [Google Scholar] [CrossRef]
- Luo, Y.; Xing, L.; Jiang, Y.; Li, R.; Lu, C.; Zeng, R.; Luo, J.; Zhang, P.; Liu, W. Additive Manufactured Large Zr-Based Bulk Metallic Glass Composites with Desired Deformation Ability and Corrosion Resistance. Materials 2020, 13, 597. [Google Scholar] [CrossRef]
- Imai, K.; Zhou, X.; Liu, X. Application of Zr and Ti-Based Bulk Metallic Glasses for Orthopaedic and Dental Device Materials. Metals 2020, 10, 203. [Google Scholar] [CrossRef]
- Han, K.-M.; Qiang, J.-B.; Wang, Y.-M.; Zhao, B.-B.; Häussler, P. Zr55.8Al19.4(Co1−xCux)24.8 (x = 0–0.8 at.%) bulk metallic glasses for surgical devices applications. J. Iron Steel Res. Int. 2018, 25, 644–649. [Google Scholar] [CrossRef]
- Han, K.; Wang, Y.; Qiang, J.; Jiang, H.; Gu, L. Low-cost Zr-based bulk metallic glasses for biomedical devices applications. J. Non-Cryst. Solids 2019, 520, 119442. [Google Scholar] [CrossRef]
- Han, K.; Jiang, H.; Wang, Y.; Qiang, J.; Yu, C. Antimicrobial Zr-based bulk metallic glasses for surgical devices applications. J. Non-Cryst. Solids 2021, 564, 120827. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, W.; Wei, X.; Ding, Y.; Shen, X.; Liu, W. Effect of Ti addition on mechanical properties and corrosion resistance of Ni-free Zr-based bulk metallic glasses for potential biomedical applications. J. Alloys Compd. 2020, 815, 152636. [Google Scholar] [CrossRef]
- Hua, N.; Liao, Z.; Chen, W.; Huang, Y.; Zhang, T. Effects of noble elements on the glass-forming ability, mechanical property, electrochemical behavior and tribocorrosion resistance of Ni- and Cu-free Zr-Al-Co bulk metallic glass. J. Alloys Compd. 2017, 725, 403–414. [Google Scholar] [CrossRef]
- Khan, M.M.; Shabib, I.; Haider, W. A combinatorially developed Zr-Ti-Fe-Al metallic glass with outstanding corrosion resistance for implantable medical devices. Scr. Mater. 2018, 162, 223–229. [Google Scholar] [CrossRef]
- Tabeshian, A.; Persson, D.; Arnberg, L.; Aune, R. Comparison of the electrochemical behavior of amorphous Zr55Cu30Ni5Al10, stainless steel (316LVM), and CoCrMo(F75) in simulated body fluid with and without addition of protein. Mater. Corros. 2019, 70, 652–660. [Google Scholar] [CrossRef]
- Jabed, A.; Rahman, Z.U.; Khan, M.M.; Haider, W.; Shabib, I. Combinatorial development and in-vitro characterization of the quaternary Zr-Ti-X-Y (X-Y = Cu-Ag/Co-Ni) metallic glass for prospective bio-implants. Adv. Eng. Mater. 2019, 21, 1900726. [Google Scholar] [CrossRef]
- Jabed, A.; Khan, M.M.; Camiller, J.; Greenlee-Wacker, M.; Haider, W.; Shabib, I. Property optimization of Zr-Ti-X (X = Ag, Al) metallic glass via combinatorial development aimed at prospective biodemidcal application. Surf. Coat. Technol. 2019, 372, 278–287. [Google Scholar] [CrossRef]
- Zhou, M.; Hagos, K.; Huang, H.; Yang, M.; Ma, L. Improved mechanical properties and pitting corrosion resistance of Zr65Cu17.5Fe10Al7.5 bulk metallic glass by isothermal annealing. J. Non-Cryst. Solids 2016, 452, 50–56. [Google Scholar] [CrossRef]
- Tang, J.; Yu, L.; Qiao, J.; Wang, Y.; Wang, H.; Duan, M.; Chamas, M. Effect of atomic mobility on the electrochemical properties of a Zr58Nb3Cu16Ni13Al10 bulk metallic glass. Electrochim. Acta 2018, 267, 222–233. [Google Scholar] [CrossRef]
- Tang, J.; Wang, Y.; Zhu, Q.; Chamas, M.; Wang, H.; Qiao, J.; Zhu, Y.; Normand, B. Passivation behavior of a Zr60Cu20Ni8Al7Hf3Ti2 bulk metallic glass in sulfuric acid solutions. Int. J. Electrochem. Sci. 2018, 13, 6913–6929. [Google Scholar] [CrossRef]
- Ge, W.; Li, B.; Axinte, E.; Zhang, Z.; Shang, C.; Wang, Y. Crystallization and Corrosion Resistance in Different Aqueous Solutions of Zr50.7Ni28Cu9Al12.3 Amorphous Alloy and Its Crystallization Counterparts. JOM 2017, 69, 776–783. [Google Scholar] [CrossRef]
- Aditya, A.; Wu, H.F.; Arora, H.; Mukherjee, S. Amorphous Metallic Alloys: Pathways for Enhanced Wear and Corrosion Resistance. JOM 2017, 69, 2150–2155. [Google Scholar] [CrossRef]
- Hua, N.; Liao, Z.; Wang, Q.; Zhang, L.; Ye, Y.; Brechtl, J.; Liaw, P.K. Effects of crystallization on mechanical behavior and corrosion performance of a ductile Zr68Al8Ni8Cu16 bulk metallic glass. J. Non-Cryst. Solids 2020, 529, 119782. [Google Scholar] [CrossRef]
- Shi, H.; Tang, C.; Zhao, X.; Ding, Y.; Ma, L.; Shen, X. Effect of isothermal annealing on mechanical performance and corrosion resistance of Ni-free Zr59Ti6Cu17.5Fe10Al7.5 bulk metallic glass. J. Non-Cryst. Solids 2020, 537, 120013. [Google Scholar] [CrossRef]
- Emran, K.M.; Al-Refai, H. Electrochemical and surface investigation of Ni-Cr glassy alloys in nitric acid solution. Int. J. Electrochem. Sci. 2017, 12, 6404–6416. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, L.; Zhao, X.; Ma, L. Enhanced chloride ion corrosion resistance of Zr-based bulk metallic glasses with cobalt substitution. J. Non-Cryst. Solids 2018, 496, 18–23. [Google Scholar] [CrossRef]
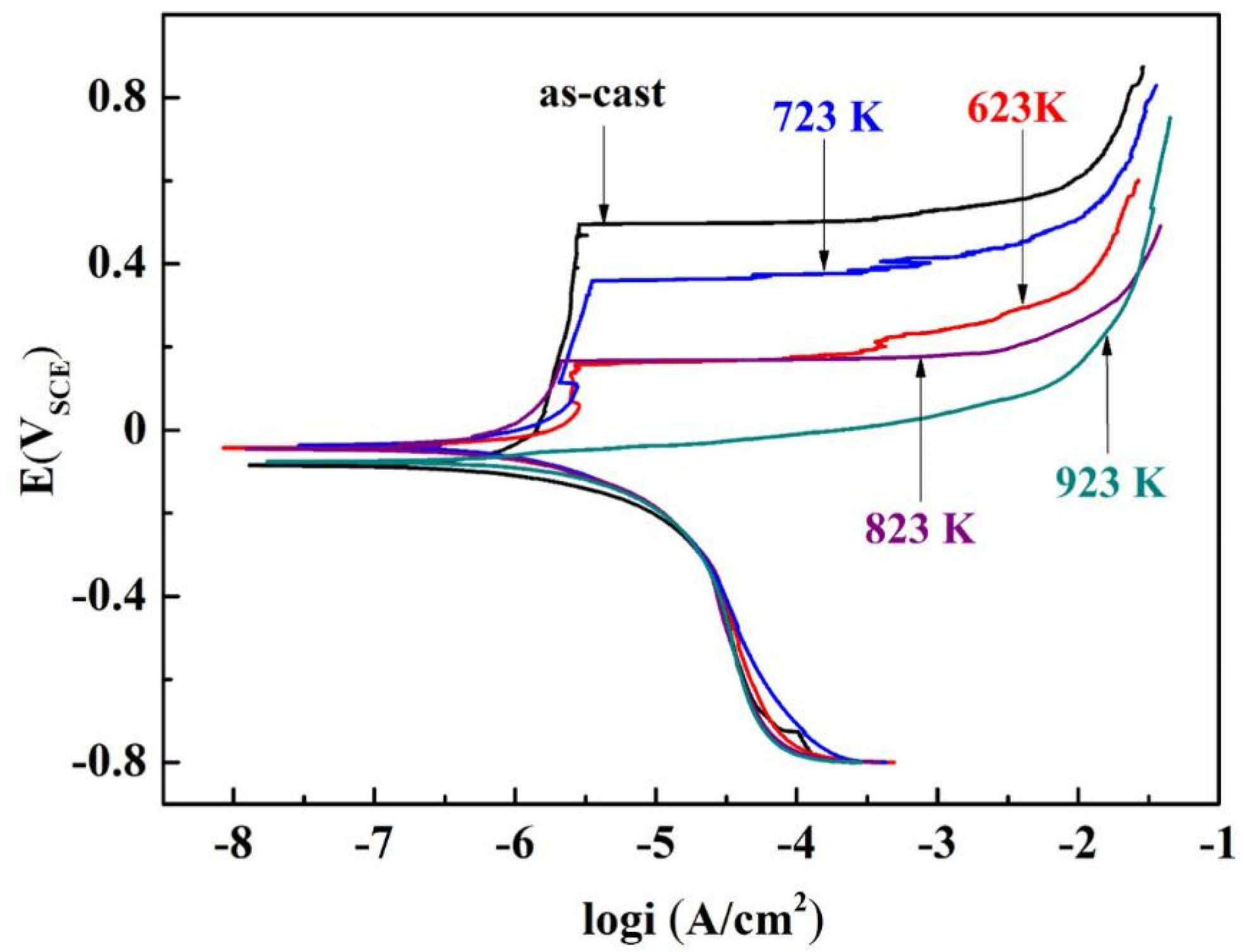




| Annealing Temperature (K) | Ecorr (VSCE) | icorr (A/cm2) | Epit (VSCE) | Epit–Ecorr (V) |
|---|---|---|---|---|
| As-cast | −0.083 | 1.866 × 10−5 | 0.496 | 0.579 |
| 623 | −0.042 | 1.466 × 10−5 | 0.157 | 0.199 |
| 723 | −0.036 | 9.977 × 10−6 | 0.359 | 0.395 |
| 823 | −0.045 | 1.549 × 10−5 | 0.165 | 0.210 |
| 923 | −0.075 | 1.879 × 10−5 | / | / |
| Annealing Temperature (K) | EIS Fitting Results | |||||||
|---|---|---|---|---|---|---|---|---|
| Rs (Ω·cm2) | Y01 (Ω−1·cm−2·sn) | n1 | Rf (Ω·cm2) | Y02 (Ω−1·cm−2·sn) | n2 | Rct (Ω·cm2) | Rct + Rf (Ω·cm2) | |
| As-cast | 18.1 | 1.25 × 10−4 | 0.792 | 3647 | 1.88 × 10−4 | 0.696 | 204 | 3851 |
| 623 | 11.6 | 1.56 × 10−4 | 0.846 | 5224 | 2.71 × 10−4 | 0.675 | 92 | 5316 |
| 723 | 13.0 | 1.64 × 10−3 | 0.793 | 5303 | 1.50 × 10−4 | 0.775 | 57 | 5360 |
| 823 | 11.5 | 4.50 × 10−4 | 0.562 | 225 | 1.30 × 10−4 | 0.913 | 5684 | 5909 |
| 923 | 12.0 | 1.50 × 10−4 | 0.914 | 2010 | 2.77 × 10−4 | 0.659 | 163 | 2173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhong, X.; Teng, X.; Huang, Y.; Han, H.; Chen, T.; Zhang, Q.; Yang, X.; Gong, Y. Effect of Annealing Temperature on Electrochemical Properties of Zr56Cu19Ni11Al9Nb5 in PBS Solution. Materials 2023, 16, 3389. https://doi.org/10.3390/ma16093389
Zhang Z, Zhong X, Teng X, Huang Y, Han H, Chen T, Zhang Q, Yang X, Gong Y. Effect of Annealing Temperature on Electrochemical Properties of Zr56Cu19Ni11Al9Nb5 in PBS Solution. Materials. 2023; 16(9):3389. https://doi.org/10.3390/ma16093389
Chicago/Turabian StyleZhang, Zhiying, Xinwei Zhong, Xiujin Teng, Yanshu Huang, Han Han, Tao Chen, Qinyi Zhang, Xiao Yang, and Yanlong Gong. 2023. "Effect of Annealing Temperature on Electrochemical Properties of Zr56Cu19Ni11Al9Nb5 in PBS Solution" Materials 16, no. 9: 3389. https://doi.org/10.3390/ma16093389




