Synthesis, Characterization, and Polishing Properties of a Lanthanum Cerium Fluoride Abrasive
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
3. Results and Discussion
3.1. XRD Analysis
3.2. Sample Morphology
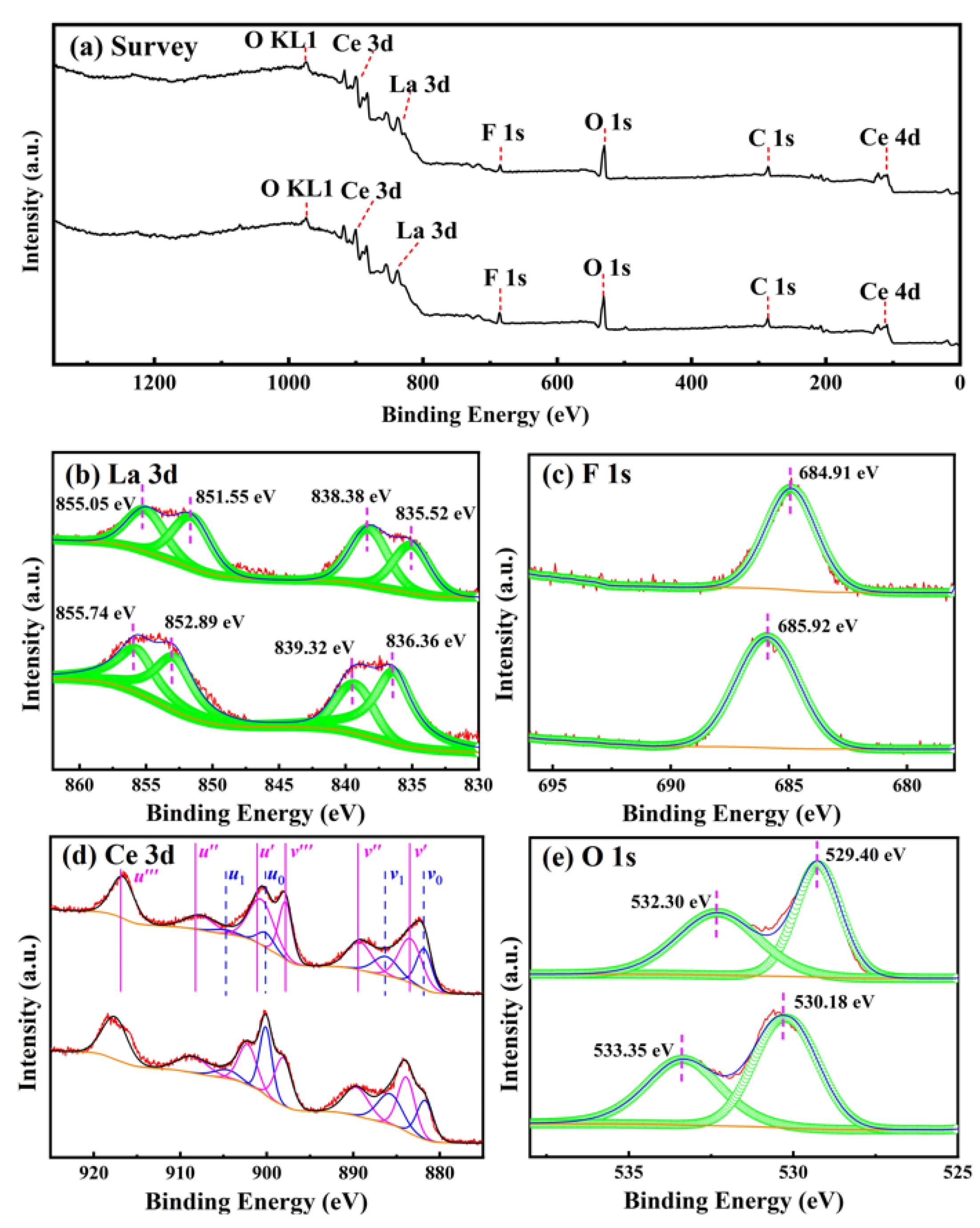
3.3. XPS Spectra
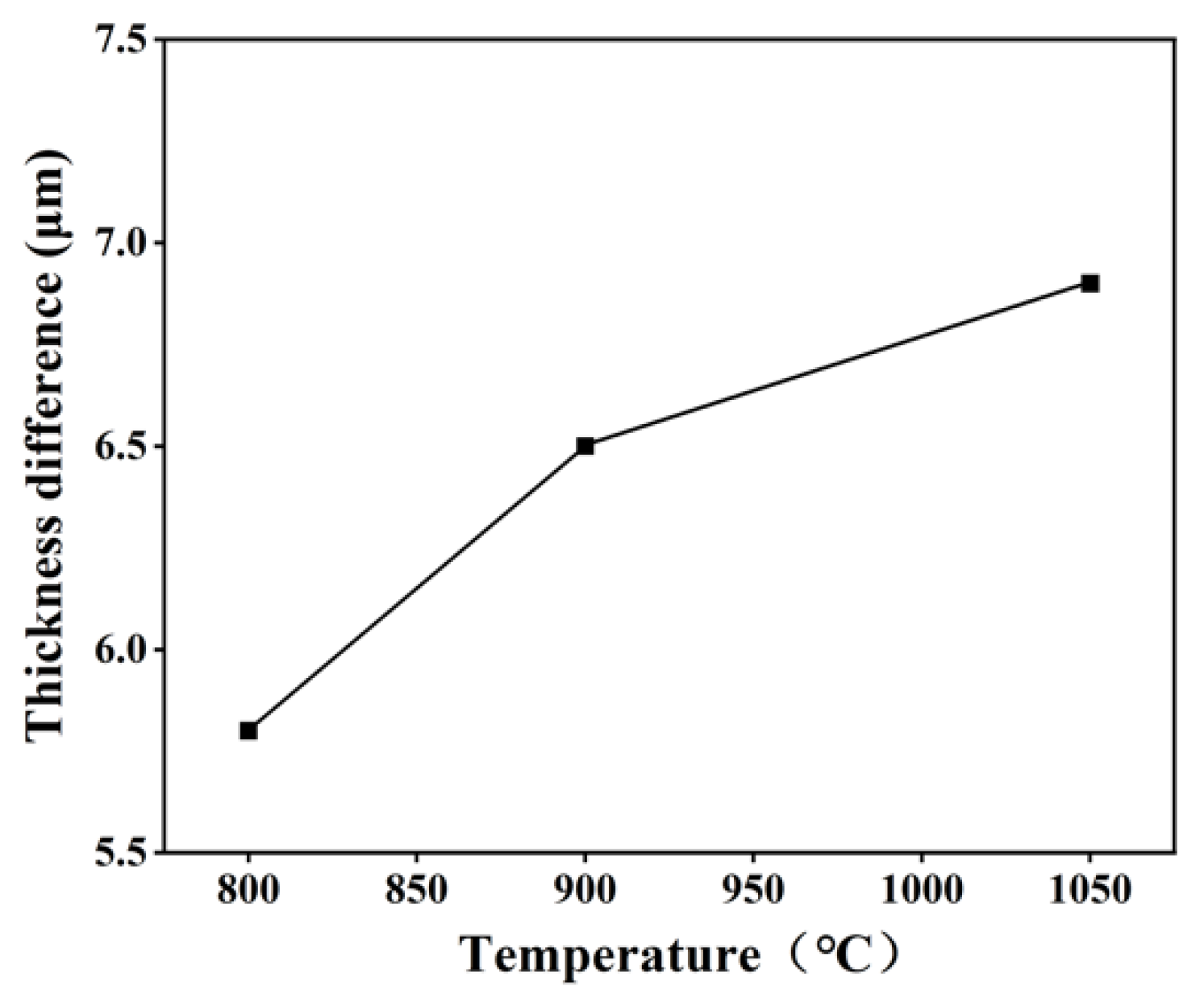
3.4. Assessment of Polishing Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ling, Y.; Wang, X.; Ma, Z.; Wei, K.; Wu, Y.; Khan, M.; Zheng, K.; Shen, S.; Wang, S. Review of experimental and modelling developments for ceria-based solid oxide fuel cells free from internal short circuits. J. Mater. Sci. 2020, 55, 1–23. [Google Scholar] [CrossRef]
- Jasinski, P.; Suzuki, T.; Anderson, H.U. Nanocrystalline undoped ceria oxygen sensor. Sens. Actuators B 2003, 95, 73–77. [Google Scholar] [CrossRef]
- Akil, J.; Siffert, S.; Pirault-Roy, L.; Royer, S.; Shen, F.; Chen, W.; Cousin, R.; Poupin, C. Investigation of catalysts M/CeO2 (M = Pt, Rh, or Pd) for purification of CO2 derived from oxycombustion in the absence or presence of water. Environ. Sci. Pollut. Res. 2021, 28, 12521–12532. [Google Scholar] [CrossRef]
- Alvarez-Asencio, R.; Corkery, R.W.; Ahniyaz, A. Solventless synthesis of cerium oxide nanoparticles and their application in UV protective clear coatings. RSC Adv. 2020, 10, 14818–14825. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.J.; Xie, P.B.; Su, Q.D. Size-dependent optical properties of nanocrystalline CeO2:Er obtained by combustion synthesis. Phys. Chem. Chem. Phys. 2001, 3, 5266–5269. [Google Scholar]
- Yuan, X.Y.; Chen, C.D.; Lei, H.; Zhang, Z.F. Synthesis, characterization of CeO2@ZIF-8 composite abrasives and their chemical mechanical polishing behavior on glass substrate. Ceram. Int. 2023, 49, 5189–5198. [Google Scholar] [CrossRef]
- Kim, E.; Hong, J.; Hong, S.; Kanade, C.; Seok, H.; Kim, H.-U.; Kim, T. Improvement of oxide removal rate in chemical mechanical polishing by forming oxygen vacancy in ceria abrasives via ultraviolet irradiation. Mater. Chem. Phys. 2021, 273, 124967. [Google Scholar] [CrossRef]
- Krishnan, M.; Nalaskowski, J.W.; Cook, L.M. Chemical mechanical planarization: Slurry chemistry, materials, and mechanisms. Chem. Rev. 2010, 110, 178–204. [Google Scholar] [CrossRef]
- Kim, K.; Yi, D.K.; Paik, U. Increase in Ce3+ concentration of ceria nanoparticles for high removal rate of SiO2 in chemical mechanical planarization. ECS J. Solid State Sci. Technol. 2017, 6, P681–P685. [Google Scholar] [CrossRef]
- Oh, M.H.; Singh, R.K.; Gupta, S.; Cho, S.B. Polishing behaviors of single crystalline ceria abrasives on silicon dioxide and silicon nitride CMP. Microelectron. Eng. 2010, 87, 2633–2637. [Google Scholar] [CrossRef]
- Fu, M.; Wei, L.; Li, Y.; Zhou, X.; Hao, S.; Li, Y. Surface charge tuning of ceria particles by titanium doping: Towards significantly improved polishing performance. Solid State Sci. 2009, 11, 2133–2137. [Google Scholar] [CrossRef]
- Choi, J.; Shin, C.; Yang, J.; Chae, S.K.; Kim, T. Effect of ceria abrasive synthesized by supercritical hydrothermal method for chemical mechanical planarization. ECS J. Solid State Sci. Technol. 2019, 8, P3128–P3132. [Google Scholar] [CrossRef]
- Janos, P.; Ederer, J.; Pilarova, V.; Henych, J.; Tolasz, J.; Milde, D.; Opletal, T. Chemical mechanical glass polishing with cerium oxide: Effect of selected physico-chemical characteristics on polishing efficiency. Wear 2016, 362–363, 114–120. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, X.L.; Ding, L.M.; Li, Y.; He, R.C.; Li, J. Changing the calcination temperature to tune the microstructure and polishing properties of ceria octahedrons. RSC Adv. 2022, 12, 16554–16560. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, S.; Li, Y.; Wang, T.Q.; Xie, L.L.; Lu, X.C. RE (La, Nd and Yb) doped CeO2 abrasive particles for chemical mechanical polishing of dielectric materials: Experimental and computational analysis. Appl. Surf. Sci. 2020, 506, 144668. [Google Scholar] [CrossRef]
- Ma, J.; Xu, N.; Luo, Y.; Lin, Y.; Pu, Y. Enhancing the polishing efficiency of CeO2 abrasives on the SiO2 substrates by improving the Ce3+ concentration on their surface. ACS Appl. Electron. Mater. 2023, 5, 526–536. [Google Scholar]
- Wang, J.; Jin, Y.Z.; Wang, Z.B. Effect of fluoride doping on properties of rare earth polishing powders. Inorg. Chem. Ind. 2019, 51, 37–41. (In Chinese) [Google Scholar]
- Pei, W.; Zhao, D.; Chen, X.; Wang, X.; Yang, X.; Wang, J.; Li, Z.; Zhou, L. Evolution of the phases and the polishing performance of ceria-based compounds synthesized by a facile calcination method. RSC Adv. 2019, 9, 26996–27001. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, N.; Feng, Z.; Tan, X.; Zhang, Z.; Han, H.; Huang, X. The effects of precursors on the morphology and chemical mechanical polishing performance of ceria-based abrasives. Materials 2022, 15, 7525. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Zhang, Z.Y.; Shi, C.J.; Feng, J.Y.; Zhuang, X.Y.; Li, L.; Meng, F.N.; Li, H.D.; Xue, Z.H.; Liu, D.D. Dispersion and polishing mechanism of a novel CeO2-LaOF-Based chemical mechanical polishing slurry for quartz glass. Materials 2023, 16, 1148. [Google Scholar] [CrossRef]
- El-Fayoumi, M.A.K.; Farouk, M. Structural properties of Li-borate glasses doped with Sm3+ and Eu3+ ions. J. Alloys Compd. 2009, 482, 356–360. [Google Scholar] [CrossRef]
- Ueda, J.; Tanabe, S. Review of luminescent properties of Ce3+-doped garnet phosphors: New insight into the effect of crystal and electronic structure. Opt. Mater. X 2019, 1, 100018. [Google Scholar] [CrossRef]
- Maensiri, S.; Labuayai, S.; Laokul, P.; Klinkaewnarong, J.; Swatsitang, E. Structure and optical properties of CeO2 nanoparticles prepared by using lemongrass plant extract solution. Jpn. J. Appl. Phys. 2014, 53, 06JG14. [Google Scholar] [CrossRef]
- Leoni, M.; Maggio, R.D.; Polizzi, S.; Scardi, P. X-ray diffraction methodology for the microstructural analysis of nanocrystalline powders: Application to cerium oxide. J. Am. Ceram. Soc. 2004, 87, 1133–1140. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Cryst. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Roosen, A.R.; McCormack, R.P.; Carter, W.C. Wulffman: A tool for the calculation and display of crystal shapes. Comp. Mater. Sci. 1998, 11, 16–26. [Google Scholar] [CrossRef]
- Sorensen, O.T. Thermodynamic studies of the phase relationships of nonstoichiometric cerium oxides at higher temperatures. J. Solid State Chem. 1976, 18, 217–233. [Google Scholar] [CrossRef]
- Brach, I.; Schulz, H. Determination of the diffusion path in the ionic conductor LaF3. Solid State Ion. 1985, 15, 135–138. [Google Scholar] [CrossRef]
- Zachariasen, W.H. Crystal chemical studies of the 5f-series of elements. XIV. Oxyfluorides, XOF. Acta Crystallogr. 1951, 4, 231–236. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Jiang, Y.F.; Gao, Q.; Chu, X.F.; Dong, Y.P.; Liang, S.M.; Chakraborty, A.K. Highly responsive and selective formaldehyde sensor based on La3+-doped barium stannate microtubes prepared by electrospinning. J. Mater. Res. 2019, 34, 2067–2077. [Google Scholar] [CrossRef]
- Sunding, M.F.; Hadidi, K.; Diplas, S.; Løvvik, O.M.; Norby, T.E.; Gunnæs, A.E. XPS characterization of in situ treated lanthanum oxide and hydroxide using tailored charge referencing and peak fitting procedures. J. Electron Spectrosc. Relat. Phenom. 2011, 184, 399–409. [Google Scholar] [CrossRef]
- Makarowicz, A.; Bailey, C.L.; Weiher, N.; Kemnitz, E.; Schroeder, S.L.M.; Mukhopadhyay, S.; Wander, A.; Searle, B.G.; Harrison, N.M. Electronic structure of lewis acid sites on high surface area aluminium fluorides: A combined XPS and ab initio investigation. Phys. Chem. Chem. Phys. 2009, 11, 5664–5673. [Google Scholar] [CrossRef]
- Ahmad, S.; Kharkwal, M.; Govind; Nagarajan, R. Application of KZnF3 as a single source precursor for the synthesis of nanocrystals of ZnO2:F and ZnO:F; synthesis, characterization, optical, and photocatalytic properties. J. Phys. Chem. C 2011, 115, 10131–10139. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, F.; Zhao, L.; Ma, Y.; Yao, J. Comparative XPS study of surface reduction for nanocrystalline and microcrystalline ceria powder. Appl. Surf. Sci. 2006, 252, 4931–4935. [Google Scholar] [CrossRef]
- Guo, D.L.; Hu, C.G.; Xi, Y. UV-irradiation-enhanced ferromagnetism of barium vanadate (Ba3V2O8) nanoflowers. J. Alloys Compd. 2013, 550, 389–394. [Google Scholar] [CrossRef]
- Yang, S.; Han, D.L.; Wang, Z.; Liu, Y.; Chen, G.; Luan, H.M.; Bayanheshig; Yang, L.L. Synthesis and magnetic properties of ZnFe1.97RE0.03O4 (RE = Eu and Nd) nanoparticles. Mater. Sci. Semicon. Proc. 2014, 27, 854–859. [Google Scholar] [CrossRef]
- Zhou, G.T.; Wang, X.C.; Yu, J.C. Selected-control synthesis of NaV6O15 and Na2V6O16·3H2O single-crystalline nanowires. Cryst. Growth Des. 2005, 5, 969–974. [Google Scholar] [CrossRef]
- Abi-aad, E.; Bechara, R.; Grimblot, J.; Aboukais, A. Preparation and characterization of CeO2 under an oxidizing atmosphere. Thermal analysis, XPS, and EPR study. Chem. Mater. 1993, 5, 793–797. [Google Scholar] [CrossRef]
- Holgado, J.P.; Munuera, G.; Espinos, J.P.; Gonzalez-Elipe, A.R. XPS study of oxidation processes of CeOx defective layers. Appl. Surf. Sci. 2000, 158, 164–171. [Google Scholar] [CrossRef]
- Seo, J.; Moon, J.; Kim, J.H.; Lee, K.; Hwang, J.; Yoon, H.; Yi, D.K.; Paik, U. Role of the oxidation state of cerium on the ceria surfaces for silicate adsorption. Appl. Surf. Sci. 2016, 389, 311–315. [Google Scholar] [CrossRef]
- Cook, L.M. Chemical processes in glass polishing. J. Non-Cryst. Solids 1990, 120, 152–171. [Google Scholar] [CrossRef]
- Srinivasan, R.; VR Dandu, P.; Babu, S.V. Shallow trench isolation chemical mechanical planarization: A review. ECS J. Solid State Sci. Technol. 2015, 4, P5029–P5039. [Google Scholar] [CrossRef]
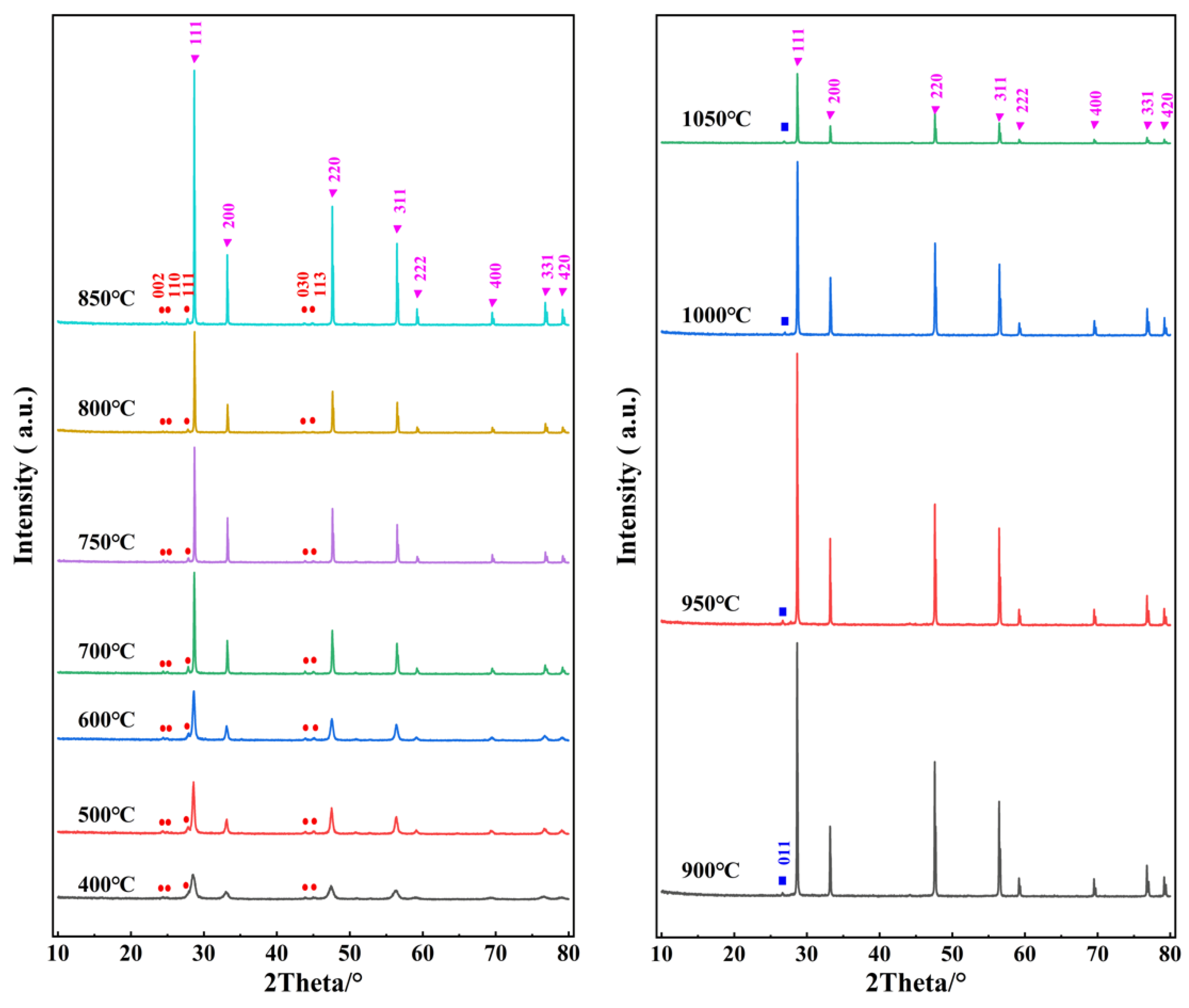
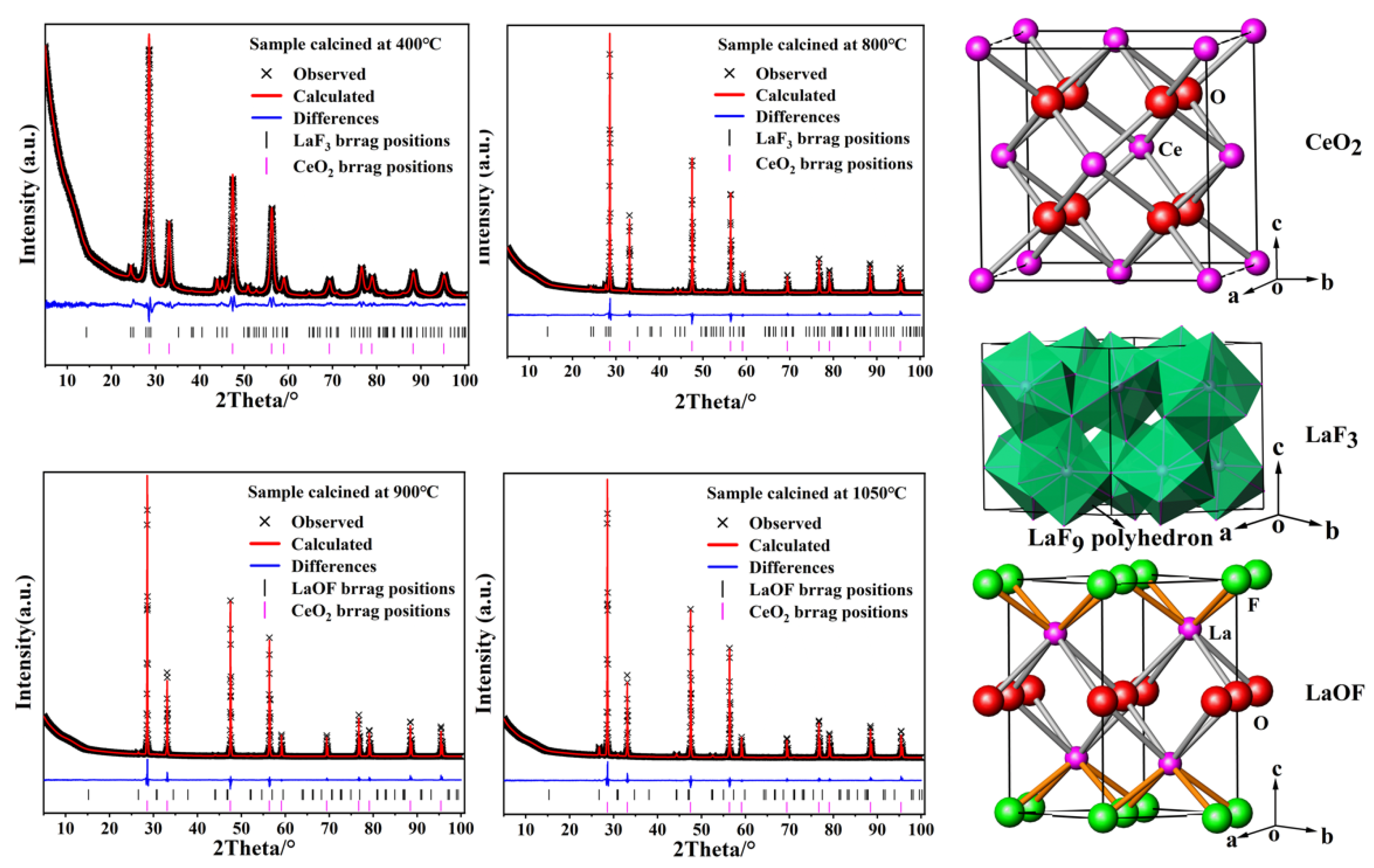


| T/°C | CeO2 (111) 2θ/° | CeO2 (111) FWHM/° | LaF3 (111) 2θ/° | LaOF (011) 2θ/° | a/Å | Scherrer Grain Size/nm | LaF3 (111)/CeO2 (111) % | LaOF (011)/CeO2 (111) % |
|---|---|---|---|---|---|---|---|---|
| 400 | 28.511 | 0.591 | 27.918 | 5.412(2) | 13.71 | 14.2 | ||
| 500 | 28.572 | 0.308 | 27.817 | 5.413(1) | 26.32 | 12.6 | ||
| 600 | 28.614 | 0.322 | 27.898 | 5.409(1) | 25.18 | 11.4 | ||
| 700 | 28.675 | 0.158 | 27.838 | 5.404(1) | 51.32 | 8.6 | ||
| 750 | 28.714 | 0.112 | 27.893 | 5.401(2) | 72.40 | 5.3 | ||
| 800 | 28.714 | 0.119 | 27.796 | 5.401(2) | 68.14 | 4.1 | ||
| 850 | 28.676 | 0.101 | 27.738 | 5.403(1) | 80.28 | 1.9 | ||
| 900 | 28.656 | 0.093 | 26.671 | 5.405(1) | 87.18 | 1.5 | ||
| 950 | 28.660 | 0.104 | 26.657 | 5.404(1) | 77.96 | 2.2 | ||
| 1000 | 28.701 | 0.111 | 26.975 | 5.402(2) | 73.05 | 1.8 | ||
| 1050 | 28.682 | 0.110 | 26.892 | 5.403(2) | 73.71 | 4.5 |
| Sample | Phase | Space Group | Lattice Parameters (Å) | Phase | |
|---|---|---|---|---|---|
| a | c | Fraction | |||
| Calcined at 400 °C | CeO2 | Fm-3m | 5.4216(2) | 90.24(18)% | |
| Rp = 2.47% | LaF3 | P-3c1 | 7.1408(6) | 7.297(1) | 9.76(18)% |
| Rwp = 3.69% | |||||
| GOF = 2.04 | |||||
| Calcined at 800 °C | CeO2 | Fm-3m | 5.4112(1) | 93.05(19)% | |
| Rp = 3.09% | LaF3 | P-3c1 | 7.1804(7) | 7.346(1) | 6.95(19)% |
| Rwp = 5.28% | |||||
| GOF = 2.88 | |||||
| Calcined at 900 °C | CeO2 | Fm-3m | 5.4112 (1) | 98.61(18)% | |
| Rp = 3.22% | LaOF | P4/nmm | 4.104(3) | 5.829(8) | 1.39(18)% |
| Rwp = 5.92% | |||||
| GOF = 3.28 | |||||
| Calcined at 1050 °C | CeO2 | Fm-3m | 5.4114(1) | 95.47(15)% | |
| Rp = 3.14% | LaOF | P4/nmm | 4.0784(5) | 5.802(1) | 4.53(15)% |
| Rwp = 5.60% | |||||
| GOF = 3.15 | |||||
| Ce 3d5/2 | Ce 3d3/2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak Assignment | ||||||||||||
| Ce3+ | Ce4+ | Ce3+ | Ce4+ | Ce4+ | Ce3+ | Ce4+ | Ce3+ | Ce4+ | Ce4+ | |||
| Temperature 800 °C | Binding energy (eV) | 881.8 | 884.1 | 886.6 | 889.7 | 898.7 | 900.3 | 902.6 | 905.5 | 908.9 | 917.7 | [Ce3+] (%) |
| Peak area (%) | 5.58 | 8.96 | 5.74 | 11.34 | 17.24 | 11.12 | 12.45 | 2.69 | 8.57 | 16.30 | 25.13 | |
| 900 °C | Binding energy (eV) | 881.3 | 883.6 | 886.4 | 889.3 | 897.9 | 900.0 | 901.3 | 904.4 | 907.9 | 916.9 | [Ce3+] (%) |
| Peak area (%) | 12.17 | 14.01 | 5.89 | 11.08 | 16.67 | 3.54 | 15.79 | 1.13 | 4.92 | 14.79 | 22.73 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, Y.; Chen, W.; Chen, X. Synthesis, Characterization, and Polishing Properties of a Lanthanum Cerium Fluoride Abrasive. Materials 2023, 16, 3393. https://doi.org/10.3390/ma16093393
Mei Y, Chen W, Chen X. Synthesis, Characterization, and Polishing Properties of a Lanthanum Cerium Fluoride Abrasive. Materials. 2023; 16(9):3393. https://doi.org/10.3390/ma16093393
Chicago/Turabian StyleMei, Yan, Wenjuan Chen, and Xuean Chen. 2023. "Synthesis, Characterization, and Polishing Properties of a Lanthanum Cerium Fluoride Abrasive" Materials 16, no. 9: 3393. https://doi.org/10.3390/ma16093393
APA StyleMei, Y., Chen, W., & Chen, X. (2023). Synthesis, Characterization, and Polishing Properties of a Lanthanum Cerium Fluoride Abrasive. Materials, 16(9), 3393. https://doi.org/10.3390/ma16093393






