MOF-Based Active Packaging Materials for Extending Post-Harvest Shelf-Life of Fruits and Vegetables
Abstract
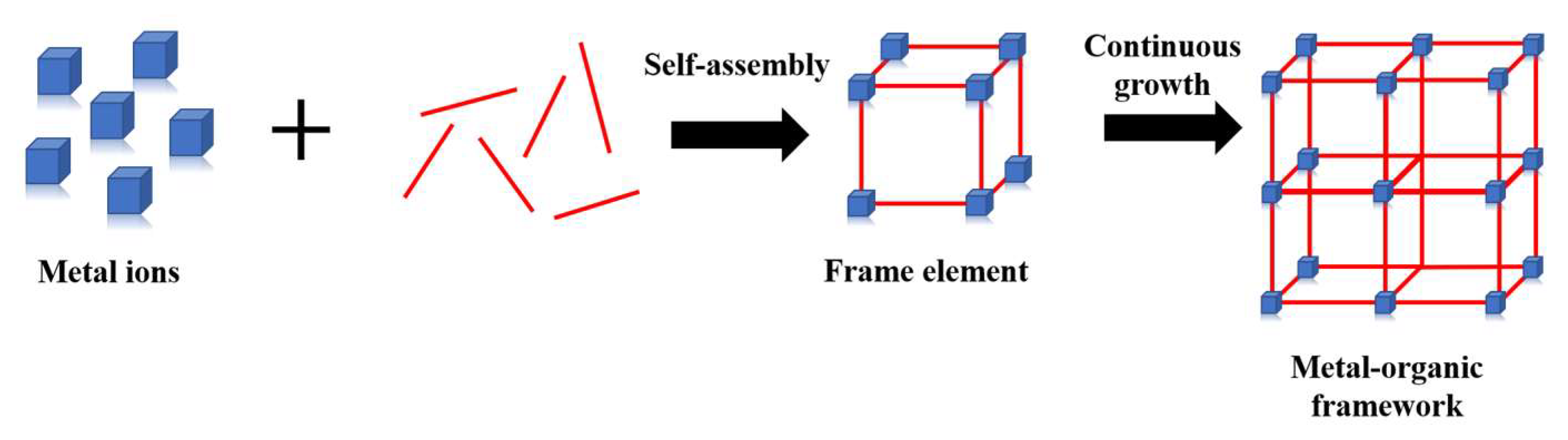
:1. Introduction
2. Fabrication of MOF Packaging Materials
2.1. Fabrication of Packaging Materials
2.1.1. Hydrothermal or Solvothermal Synthesis
2.1.2. Microwave-Assisted Synthesis
2.1.3. Sonochemical Synthesis
2.1.4. Electrochemical Synthesis
2.2. Forms Incorporated in Food Packaging
2.2.1. Impregnation in the Packaging Substrate
2.2.2. Sachets
2.2.3. Incorporation in Coatings

3. Gas Adsorption of MOF Packaging Materials
3.1. Application of MOF Packaging Materials in Fruit and Vegetable Preservation
3.1.1. Application of MOFs in Ethylene Adsorption/Desorption of Fruit and Vegetable Packaging
3.1.2. Combination of Different Materials in MOFs
3.2. Selective Adsorption/Desorption Mechanism of Ethylene in Packaging
Discussion of Selective Adsorption/Desorption Mechanism
- Adsorbent–absorbent interactions. The sorbent–adsorbent interaction is an affinity between the inner surface and the sorbent in the MOF. This affinity may be due to the interacting van der Waals forces. As a MOF has different adsorption sites, the distribution of the charge and electron cloud can change. Dutta et al. [43] showed computationally that chemical bonds form through the van der Waals force interaction of the aldehyde “tail” with the MOF junction. The different geometries of the metal sites and the pores of the MOFs they studied provided different contributions of the bonds to the adsorption energetics.
- Molecular sieve effect. Since MOFs are composed of nano-scale pores, there is a possibility of a molecular sieve effect, which means that MOFs can selectively adsorb specific gas molecules. The performance depends on the pore size or channel size. A study has shown that, due to their pore structure, MOFs can selectively adsorb nitrogen from mixtures containing ethylene [44].
- Stimulus Response Gate. When a stimulus such as temperature [45], pressure [46,47], or light triggers the MOF response gate, the gate is opened and gas molecules enter the pores of the MOF [48,49]. In a study of the photosensitive properties of an MOF, the diaryl ethylene-azobenzene metal–organic backbone showed different adsorption properties at different sites [50].
| Metal–Organic Frameworks | Active Compound | Synthesis Method | Food | Outcomes | Reference |
|---|---|---|---|---|---|
| Cyclodextrin-based MOF | Hexanal | Vapor diffusion method | Mango | Shelf-life was extended to 15 days | Moussa et al. [54] |
| Single-walled nickel–organic framework | Hexanal | — | Banana | Banana placed in a 1-L MOF jar showed no sign of spoilage until day 30; in the control a dark spot was observed on day 9 of storage | Li et al. [55] |
| MIL-101@CMFP and UIO-66@CMFP | Curcumin | — | Pitaya | Curcumin-loaded nano-metal–organic framework extended the shelf-life of pitaya to 6 days; the control showed signs of spoilage on day 2 of storage | Huang et al. [56] |
| Electrospun pullulan/polyvinyl alcohol nanofibers incorporated in a porphyrin MOF | Thymol | — | Fresh grapes and strawberries | Grapes wrapped in the MOF showed no spoilage for 7 days; control showed rot on day 7 Strawberries remained fresh for 7 days when wrapped in the MOF; control showed mold growths | Min et al. [57] |
| Silver-based MOF | Chitosan | One-pot synthesis method | Pitaya | Spraying metal–organic framework solution on pitaya maintained its freshness for 14 days; control showed mold growth on day 7 | Zhang et al. [58] |
| Copper terephthalate MOF | Chitosan | Solvothermal | Bananas and avocados | The shelf-life of bananas and avocados was extended | Zhang et al. [17] |
| Fresh Food Type | Ethylene Sensitivity | Effect of Ethylene |
|---|---|---|
| Apples | Very high (>100 mL kg−1h−1) | Brown, soften |
| Passion fruit | Very high (>100 mL kg−1 h−1) | Decay |
| Peaches | High (10–100 mL kg−1 h−1) | Soften |
| Pears | High (10–100 mL kg−1 h−1) | Fermentation, mildew |
| Banana | Medium (1–10 mL kg−1 h−1) | Decay |
| Mango | Medium (1–10 mL kg−1 h−1) | Spot |
| Cherries | Low (<1.0 mL kg−1 h−1) | Soften |
| Grapes | Low (<1.0 mL kg−1 h−1) | Decay |
| Broccoli | Low (<1.0 mL kg−1 h−1) | Mildew |
| Cauliflowers | Low (<1.0 mL kg−1 h−1) | Mildew |
| Ethylene Effect | Affected Organ | Fresh Food Type | Reference |
|---|---|---|---|
| Abscission | Bunch Stalk Calyx | Cherry Tomato Muskmelon | Beno-Moualem et al. [59] Dong et al. [60] |
| Sprouting | Tubercle, bulb | Persimmon Potato | Salvador et al. [61] Brasil and Siddiqui [4] |
| Color | Yellowing Stem browning | Onion Broccoli Sweet cherry | Bufler [62] Suzuki et al. [63] Zhao et al. [64] |
| Off-flavors | Volatiles | Banana | Cecchini et al. [65] |
| Toughness | Lignification | Asparagus | Toscano et al. [66] |
| Bitterness | Isocoumarin | Carrot, lettuce | Fan et al. [67] |
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahajan, P.V.; Caleb, O.J.; Gil, M.I.; Izumi, H.; Colelli, G.; Watkins, C.B.; Zude, M. Quality and Safety of Fresh Horticultural Commodities: Recent Advances and Future Perspectives. Food Packag. Shelf Life 2017, 14, 2–11. [Google Scholar] [CrossRef]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and Metal Nanoparticles and Their Antimicrobial Activity in Food Packaging Applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites Materials for Food Packaging Applications: Concepts and Future Outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Brasil, I.M.; Siddiqui, M.W. Chapter 1—Postharvest Quality of Fruits and Vegetables: An Overview. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–40. ISBN 978-0-12-809807-3. [Google Scholar]
- Nishiyama, K.; Guis, M.; Rose, J.K.C.; Kubo, Y.; Bennett, K.A.; Wangjin, L.; Kato, K.; Ushijima, K.; Nakano, R.; Inaba, A.; et al. Ethylene Regulation of Fruit Softening and Cell Wall Disassembly in Charentais Melon. J. Exp. Bot. 2007, 58, 1281–1290. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Bailén, G.; Serrano, M.; Guillén, F.; Valverde, J.M.; Zapata, P.; Castillo, S.; Valero, D. Tools to Maintain Postharvest Fruit and Vegetable Quality through the Inhibition of Ethylene Action: A Review. Crit. Rev. Food Sci. Nutr. 2007, 47, 543–560. [Google Scholar] [CrossRef]
- Zhang, H.; Han, M.; Xie, Y.; Wang, M.; Cao, C. Application of Ethylene-Regulating Packaging in Post-Harvest Fruits and Vegetables Storage: A Review. Packag. Technol. Sci. 2022, 35, 461–471. [Google Scholar] [CrossRef]
- Shorter, A.J.; Scott, K.J.; Ward, G.; Best, D.J. Effect of Ethylene Absorption on the Storage of Granny Smith Apples Held in Polyethylene Bags. Postharvest Biol. Technol. 1992, 1, 189–194. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Khalil, H.P.S.A. Biodegradable Green Packaging with Antimicrobial Functions Based on the Bioactive Compounds from Tropical Plants and Their By-Products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Sultana, A.; Luo, H.; Ramakrishna, S. Harvesting of Antimicrobial Peptides from Insect (Hermetia Illucens) and Its Applications in the Food Packaging. Appl. Sci. 2021, 11, 6991. [Google Scholar] [CrossRef]
- Öhrström, L.; Amombo Noa, F.M. Metal-Organic Frameworks; ACS In Focus; American Chemical Society: Washington, DC, USA, 2020. [Google Scholar]
- Sharanyakanth, P.S.; Radhakrishnan, M. Synthesis of Metal-Organic Frameworks (MOFs) and Its Application in Food Packaging: A Critical Review. Trends Food Sci. Technol. 2020, 104, 102–116. [Google Scholar] [CrossRef]
- Lashkari, E.; Wang, H.; Liu, L.; Li, J.; Yam, K. Innovative Application of Metal-Organic Frameworks for Encapsulation and Controlled Release of Allyl Isothiocyanate. Food Chem. 2017, 221, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Qazvini, O.T.; Babarao, R.; Telfer, S.G. Selective Capture of Carbon Dioxide from Hydrocarbons Using a Metal-Organic Framework. Nat. Commun. 2021, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- D’Arras, L.; Sassoye, C.; Rozes, L.; Sanchez, C.; Marrot, J.; Marre, S.; Aymonier, C. Fast and Continuous Processing of a New Sub-Micronic Lanthanide-Based Metal–Organic Framework. New J. Chem. 2014, 38, 1477–1483. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Bao, Z.; Xing, H.; Zhang, Z.; Su, B.; Yang, Q.; Yang, Y.; Ren, Q. Adsorption Separation of Acetylene and Ethylene in a Highly Thermostable Microporous Metal-Organic Framework. Sep. Purif. Technol. 2018, 195, 238–243. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Kanyuck, K.; Bauchan, G.; Mowery, J.; Zavalij, P. Development of Metal-Organic Framework for Gaseous Plant Hormone Encapsulation To Manage Ripening of Climacteric Produce. J. Agric. Food Chem. 2016, 64, 5164–5170. [Google Scholar] [CrossRef] [PubMed]
- Klinowski, J.; Paz, F.A.A.; Silva, P.; Rocha, J. Microwave-Assisted Synthesis of Metal-Organic Frameworks. Dalton Trans. 2011, 40, 321–330. [Google Scholar] [CrossRef]
- Kim, S.-H.; Babu, R.; Kim, D.-W.; Lee, W.; Park, D.-W. Cycloaddition of CO2 and Propylene Oxide by Using M(HBTC)(4,4′-Bipy)·3DMF (M = Ni, Co, Zn) Metal-Organic Frameworks. Chin. J. Catal. 2018, 39, 1311–1319. [Google Scholar] [CrossRef]
- Yu, K.; Lee, Y.-R.; Seo, J.Y.; Baek, K.-Y.; Chung, Y.-M.; Ahn, W.-S. Sonochemical Synthesis of Zr-Based Porphyrinic MOF-525 and MOF-545: Enhancement in Catalytic and Adsorption Properties. Microporous Mesoporous Mat. 2021, 316, 110985. [Google Scholar] [CrossRef]
- Peng, G.; Gao, F.; Zou, J.; Wang, X.; Gao, Y.; Zhou, H.; Liu, S.; Li, M.; Lu, L. One-Step Electrochemical Synthesis of Tremella-like Co-MOFs/Carbon Nanohorns Films for Enhanced Electrochemical Sensing of Carbendazim in Vegetable and Fruit Samples. J. Electroanal. Chem. 2022, 918, 116462. [Google Scholar] [CrossRef]
- Datta, S.J.; Mayoral, A.; Murthy Srivatsa Bettahalli, N.; Bhatt, P.M.; Karunakaran, M.; Carja, I.D.; Fan, D.; Graziane, M.; Mileo, P.; Semino, R.; et al. Rational Design of Mixed-Matrix Metal-Organic Framework Membranes for Molecular Separations. Science 2022, 376, 1080–1087. [Google Scholar] [CrossRef]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X.; Gascon, J. Metal–Organic Framework Nanosheets in Polymer Composite Materials for Gas Separation. Nat. Mater. 2015, 14, 48–55. [Google Scholar] [CrossRef]
- Chopra, S.; Dhumal, S.; Abeli, P.; Beaudry, R.; Almenar, E. Metal-Organic Frameworks Have Utility in Adsorption and Release of Ethylene and 1-Methylcyclopropene in Fresh Produce Packaging. Postharvest Biol. Technol. 2017, 130, 48–55. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Han, Y.; Luo, X.; Tang, W.; Yue, T.; Li, Z. Robust MOF Film of Self-Rearranged UiO-66-NO2 Anchored on Gelatin Hydrogel via Simple Thermal-Treatment for Efficient Pb(II) Removal in Water and Apple Juice. Food Control 2021, 130, 108409. [Google Scholar] [CrossRef]
- Sultana, A.; Kathuria, A.; Gaikwad, K.K. Metal–Organic Frameworks for Active Food Packaging. A Review. Env. Chem. Lett. 2022, 20, 1479–1495. [Google Scholar] [CrossRef]
- Hu, Z.; Sun, Y.; Zeng, K.; Zhao, D. Structural-Failure Resistance of Metal-Organic Frameworks toward Multiple-Cycle CO2 Sorption. Chem. Commun. 2017, 53, 8653–8656. [Google Scholar] [CrossRef] [PubMed]
- Kapelewski, M.T.; Runčevski, T.; Tarver, J.D.; Jiang, H.Z.H.; Hurst, K.E.; Parilla, P.A.; Ayala, A.; Gennett, T.; Fitz Gerald, S.A.; Brown, C.M.; et al. Record High Hydrogen Storage Capacity in the Metal-Organic Framework Ni2(m-Dobdc) at Near-Ambient Temperatures. Chem. Mater. 2018, 30, 8179–8189. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–Organic Frameworks: Structures and Functional Applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, V.; Singh, S.; Saini, S.; Gaikwad, K.K. Pine Needles Lignocellulosic Ethylene Scavenging Paper Impregnated with Nanozeolite for Active Packaging Applications. Ind. Crops Prod. 2021, 170, 113752. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, F.; Wang, F.; Zhang, H.; Kang, M. Development and Characterization of Zein-Based Active Packaging Films Containing Catechin Loaded β-Cyclodextrin Metal-Organic Frameworks. Food Packag. Shelf Life 2022, 31, 100810. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, T.-J.; Liu, Y.-Y.; Chen, L. Porphyrinic Metal–Organic Framework-Loaded Polycaprolactone Composite Films with a High Photodynamic Antibacterial Activity for the Preservation of Fresh-Cut Apples. ACS Appl. Polym. Mater. 2022, 5, 560–566. [Google Scholar] [CrossRef]
- Wang, H.; Lashkari, E.; Lim, H.; Zheng, C.; Emge, T.J.; Gong, Q.; Yam, K.; Li, J. The Moisture-Triggered Controlled Release of a Natural Food Preservative from a Microporous Metal-Organic Framework. Chem. Commun. 2016, 52, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Banerjee, D.; Liu, J.; Schaef, H.T.; Crum, J.V.; Fernandez, C.A.; Kukkadapu, R.K.; Nie, Z.; Nune, S.K.; Motkuri, R.K.; et al. Redox-Active Metal-Organic Composites for Highly Selective Oxygen Separation Applications. Adv. Mater. 2016, 28, 3572. [Google Scholar] [CrossRef]
- Guan, Y.; Teng, Z.; Mei, L.; Zhang, J.; Wang, Q.; Luo, Y. An Entrapped Metal-Organic Framework System for Controlled Release of Ethylene. J. Colloid Interface Sci. 2019, 533, 207–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Chen, X.; Chen, M.; Sun, B.; Kim, D.; Yam, K. Potential of Metal-Organic Frameworks to Adsorb Ethylene for Fresh Produce Active Packaging Applications. Food Packaging Shelf Life 2023, 35, 101034. [Google Scholar] [CrossRef]
- Awalgaonkar, G.; Beaudry, R.; Almenar, E. Ethylene-Removing Packaging: Basis for Development and Latest Advances. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3980–4007. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, L.; Li, C.; Fu, X.; Huang, Q.; Zhang, B. Metal-Organic Framework Based on α-Cyclodextrin Gives High Ethylene Gas Adsorption Capacity and Storage Stability. ACS Appl. Mater. Interfaces 2020, 12, 34095–34104. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.-W. Metal-Organic Frameworks for Biomedical Applications. Small 2020, 16, e1906846. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Sheikh-Assadi, M.; Sharafi, Y.; Farmani, B.; Fard, J.R.; Razavi, F. Employing Exogenous Melatonin Applying Confers Chilling Tolerance in Tomato Fruits by Upregulating ZAT2/6/12 Giving Rise to Promoting Endogenous Polyamines, Proline, and Nitric Oxide Accumulation by Triggering Arginine Pathway Activity. Food Chem. 2019, 275, 549–556. [Google Scholar] [CrossRef]
- Golden, K.D.; Williams, O.J.; Dunkley, H.M. Ethylene in Postharvest Technology: A Review. Asian J. Biol. Sci. 2014, 7, 135–143. [Google Scholar] [CrossRef]
- He, J.; Li, X. Metal–Organic Framework for Selective Gas Scavenging. J. Mol. Eng. Mater. 2016, 4, 1640014. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Brown, R.J.C.; Kim, Y.-H.; Boukhvalov, D. Metal-Organic Framework and Tenax-TA as Optimal Sorbent Mixture for Concurrent GC-MS Analysis of C1 to C5 Carbonyl Compounds. Sci. Rep. 2018, 8, 5033. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, D.E.; Reed, D.A.; Jiang, H.Z.H.; Oktawiec, J.; Mara, M.W.; Forse, A.C.; Lussier, D.J.; Murphy, R.A.; Cunningham, M.; Colombo, V.; et al. Selective Nitrogen Adsorption via Backbonding in a Metal-Organic Framework with Exposed Vanadium Sites. Nat. Mater. 2020, 19, 517–521. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bureekaew, S.; Kitagawa, S. A Dynamic, Isocyanurate-Functionalized Porous Coordination Polymer. Angew. Chem. Int. Ed. Engl. 2008, 47, 3403–3406. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. Engl. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Luo, F.; Fan, C.B.; Luo, M.B.; Wu, X.L.; Zhu, Y.; Pu, S.Z.; Xu, W.-Y.; Guo, G.-C. Photoswitching CO2 Capture and Release in a Photochromic Diarylethene Metal-Organic Framework. Angew. Chem. Int. Ed. Engl. 2014, 53, 9298–9301. [Google Scholar] [CrossRef] [PubMed]
- Heinke, L.; Cakici, M.; Dommaschk, M.; Grosjean, S.; Herges, R.; Bräse, S.; Wöll, C. Photoswitching in Two-Component Surface-Mounted Metal-Organic Frameworks: Optically Triggered Release from a Molecular Container. ACS Nano 2014, 8, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Lyndon, R.; Konstas, K.; Ladewig, B.P.; Southon, P.D.; Kepert, P.C.J.; Hill, M.R. Dynamic Photo-Switching in Metal-Organic Frameworks as a Route to Low-Energy Carbon Dioxide Capture and Release. Angew. Chem. Int. Ed. Engl. 2013, 52, 3695–3698. [Google Scholar] [CrossRef]
- Fan, C.B.; Liu, Z.Q.; Gong, L.L.; Zheng, A.M.; Zhang, L.; Yan, C.S.; Wu, H.Q.; Feng, X.F.; Luo, F. Photoswitching Adsorption Selectivity in a Diarylethene-Azobenzene MOF. Chem. Commun. 2017, 53, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, R.-B.; Krishna, R.; Li, H.; Xiang, S.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Ethane/Ethylene Separation in a Metal-Organic Framework with Iron-Peroxo Sites. Science 2018, 362, 443–446. [Google Scholar] [CrossRef]
- Lin, R.-B.; Li, L.; Zhou, H.-L.; Wu, H.; He, C.; Li, S.; Krishna, R.; Li, J.; Zhou, W.; Chen, B. Molecular Sieving of Ethylene from Ethane Using a Rigid Metal–Organic Framework. Nat. Mater. 2018, 17, 1128–1133. [Google Scholar] [CrossRef]
- Yang, J.; Tong, M.; Han, G.; Chang, M.; Yan, T.; Ying, Y.; Yang, Q.; Liu, D. Solubility-Boosted Molecular Sieving-Based Separation for Purification of Acetylene in Core–Shell IL@MOF Composites. Adv. Funct. Mater. 2023, 33, 2213743. [Google Scholar] [CrossRef]
- Moussa, Z.; Hmadeh, M.; Abiad, M.G.; Dib, O.H.; Patra, D. Encapsulation of Curcumin in Cyclodextrin-Metal Organic Frameworks: Dissociation of Loaded CD-MOFs Enhances Stability of Curcumin. Food Chem. 2016, 212, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, T.; Pan, X. Metal-Organic-Framework-Based Materials for Antimicrobial Applications. ACS Nano 2021, 15, 3808–3848. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yan, Y.; Xu, D.; Wu, J.; Xu, C.; Fu, L.; Lin, B. Curcumin-Loaded NanoMOFs@CMFP: A Biological Preserving Paste with Antibacterial Properties and Long-Acting, Controllable Release. Food Chem. 2021, 337, 127987. [Google Scholar] [CrossRef] [PubMed]
- Min, T.; Sun, X.; Zhou, L.; Du, H.; Zhu, Z.; Wen, Y. Electrospun Pullulan/PVA Nanofibers Integrated with Thymol-Loaded Porphyrin Metal-Organic Framework for Antibacterial Food Packaging. Carbohydr. Polym. 2021, 270, 118391. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Z.; He, Q.; Deng, Y.; Wei, F.; Xu, C.; Fu, L.; Lin, B. Enhanced Aqueous Stability and Long-Acting Antibacterial of Silver-Based MOFs via Chitosan-Crosslinked for Fruit Fresh-Keeping. Appl. Surf. Sci. 2022, 571, 151351. [Google Scholar] [CrossRef]
- Beno-Moualem, D.; Gusev, L.; Dvir, O.; Pesis, E.; Meir, S.; Lichter, A. The Effects of Ethylene, Methyl Jasmonate and 1-MCP on Abscission of Cherry Tomatoes from the Bunch and Expression of Endo-1,4-β-Glucanases. Plant Sci. 2004, 167, 499–507. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Einhorn, T.C. Postharvest Physiology, Storage Quality and Physiological Disorders of ‘Gem’ Pear (Pyrus Communis L.) Treated with 1-Methylcyclopropene. Sci. Hortic. 2018, 240, 631–637. [Google Scholar] [CrossRef]
- Salvador, A.; Arnal, L.; Monterde, A.; Cuquerella, J. Reduction of Chilling Injury Symptoms in Persimmon Fruit Cv. ‘Rojo Brillante’ by 1-MCP. Postharvest Biol. Technol. 2004, 33, 285–291. [Google Scholar] [CrossRef]
- Bufler, G. Exogenous Ethylene Inhibits Sprout Growth in Onion Bulbs. Ann. Bot. 2009, 103, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kimura, T.; Takahashi, D.; Terai, H. Ultrastructural Evidence for the Inhibition of Chloroplast-to-Chromoplast Conversion in Broccoli Floret Sepals by Ethanol Vapor. Postharvest Biol. Technol. 2005, 35, 237–243. [Google Scholar] [CrossRef]
- Zhao, Y.; Collins, H.P.; Knowles, N.R.; Oraguzie, N. Respiratory Activity of ‘Chelan’, ‘Bing’ and ‘Selah’ Sweet Cherries in Relation to Fruit Traits at Green, White-Pink, Red and Mahogany Ripening Stages. Sci. Hortic. 2013, 161, 239–248. [Google Scholar] [CrossRef]
- Cecchini, M.; Contini, M.; Massantini, R.; Monarca, D.; Moscetti, R. Effects of Controlled Atmospheres and Low Temperature on Storability of Chestnuts Manually and Mechanically Harvested. Postharvest Biol. Technol. 2011, 61, 131–136. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Leonardi, C.; Romano, D. PAL Activities in Asparagus Spears during Storage after Ammonium Sulfate Treatments. Postharvest Biol. Technol. 2018, 140, 34–41. [Google Scholar] [CrossRef]
- Fan, X.; Argenta, L.; Mattheis, J.P. Inhibition of Ethylene Action by 1-Methylcyclopropene Prolongs Storage Life of Apricots. Postharvest Biol. Technol. 2000, 20, 135–142. [Google Scholar] [CrossRef]
- Hendon, C.H.; Rieth, A.J.; Korzyński, M.D.; Dincă, M. Grand Challenges and Future Opportunities for Metal-Organic Frameworks. ACS Cent. Sci. 2017, 3, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Chen, N.; Chang, G.; Zhao, X.; Sun, Y.; Chen, S.; Zhang, H.; Yang, D. Selective Capture of Toxic Selenite Anions by Bismuth-Based Metal-Organic Frameworks. Angew. Chem. Int. Ed. Engl. 2018, 57, 13197–13201. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Yang, D.; Chen, Y.; Shi, J.; Zhang, X.; Hao, Y.; Zhang, Z.; Sun, Y.; Zhang, J. MOF-Based Active Packaging Materials for Extending Post-Harvest Shelf-Life of Fruits and Vegetables. Materials 2023, 16, 3406. https://doi.org/10.3390/ma16093406
Fu Y, Yang D, Chen Y, Shi J, Zhang X, Hao Y, Zhang Z, Sun Y, Zhang J. MOF-Based Active Packaging Materials for Extending Post-Harvest Shelf-Life of Fruits and Vegetables. Materials. 2023; 16(9):3406. https://doi.org/10.3390/ma16093406
Chicago/Turabian StyleFu, Yabo, Dan Yang, Yiyang Chen, Jiazi Shi, Xinlin Zhang, Yuwei Hao, Zhipeng Zhang, Yunjin Sun, and Jingyi Zhang. 2023. "MOF-Based Active Packaging Materials for Extending Post-Harvest Shelf-Life of Fruits and Vegetables" Materials 16, no. 9: 3406. https://doi.org/10.3390/ma16093406
APA StyleFu, Y., Yang, D., Chen, Y., Shi, J., Zhang, X., Hao, Y., Zhang, Z., Sun, Y., & Zhang, J. (2023). MOF-Based Active Packaging Materials for Extending Post-Harvest Shelf-Life of Fruits and Vegetables. Materials, 16(9), 3406. https://doi.org/10.3390/ma16093406







