Investigation on Adsorption of Polar Molecules in Vegetable Insulating Oil by Functional Fossil Graphene
Abstract
1. Introduction
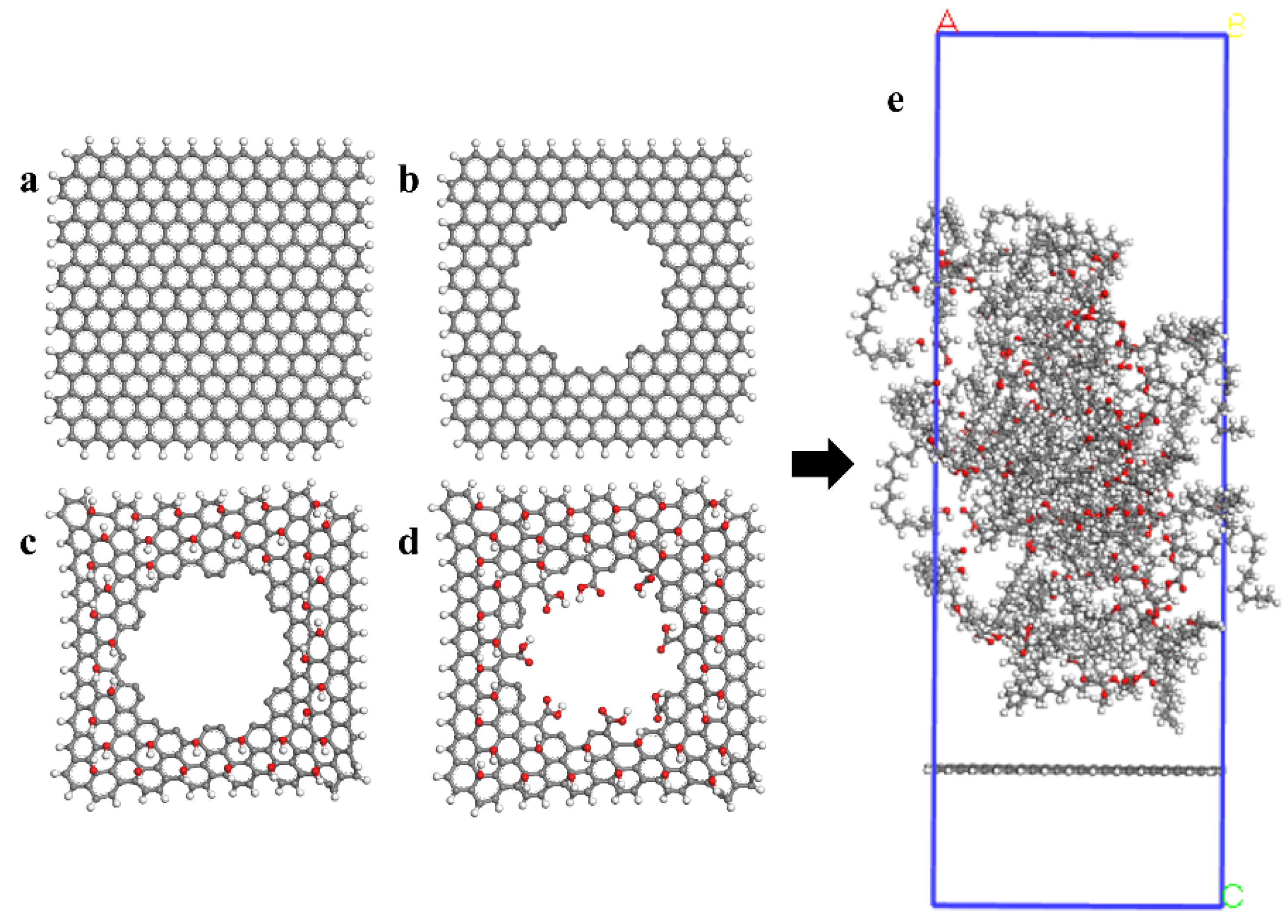
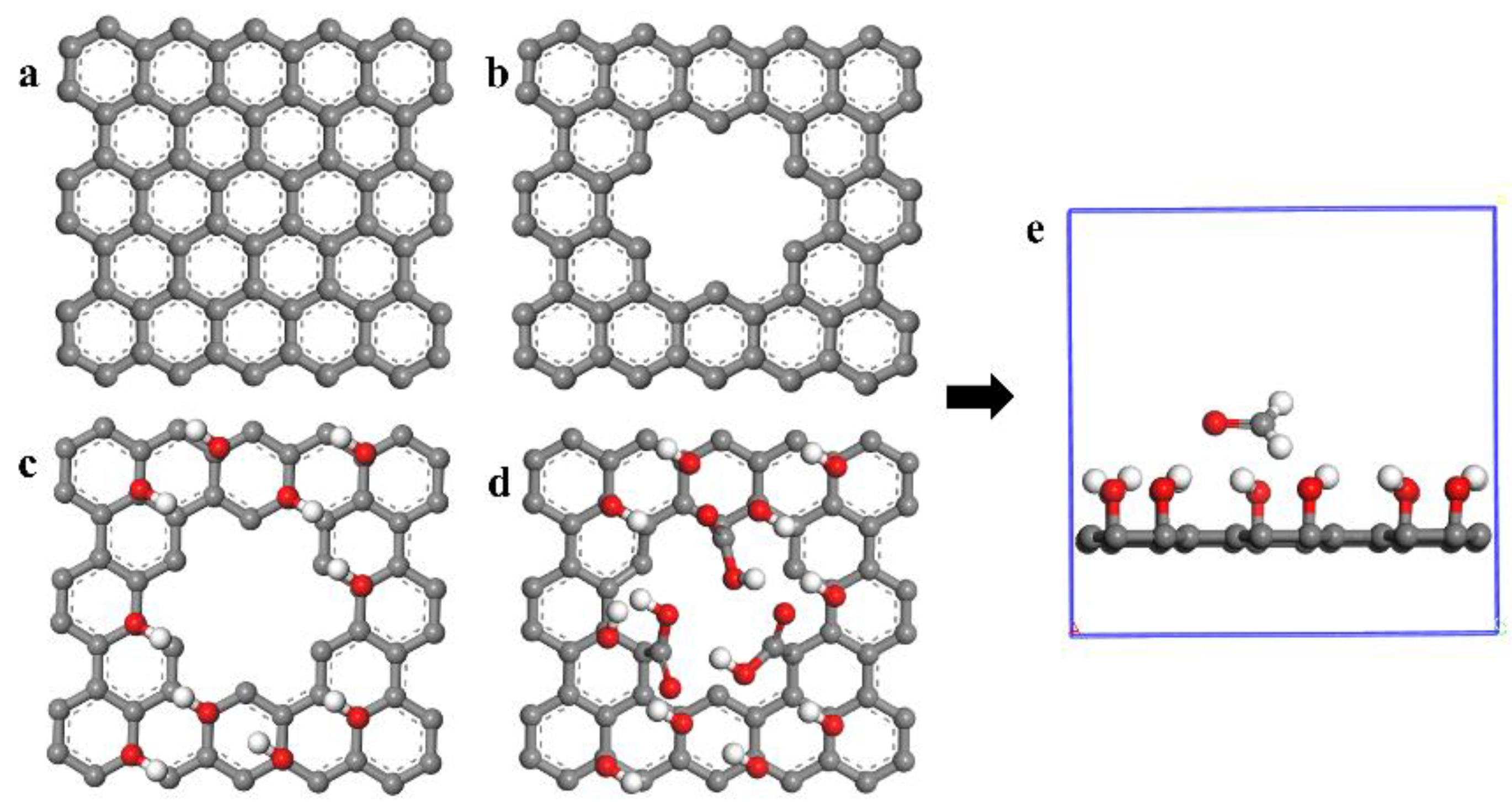
2. Computational Method and Physical Model
3. Results and Discussion
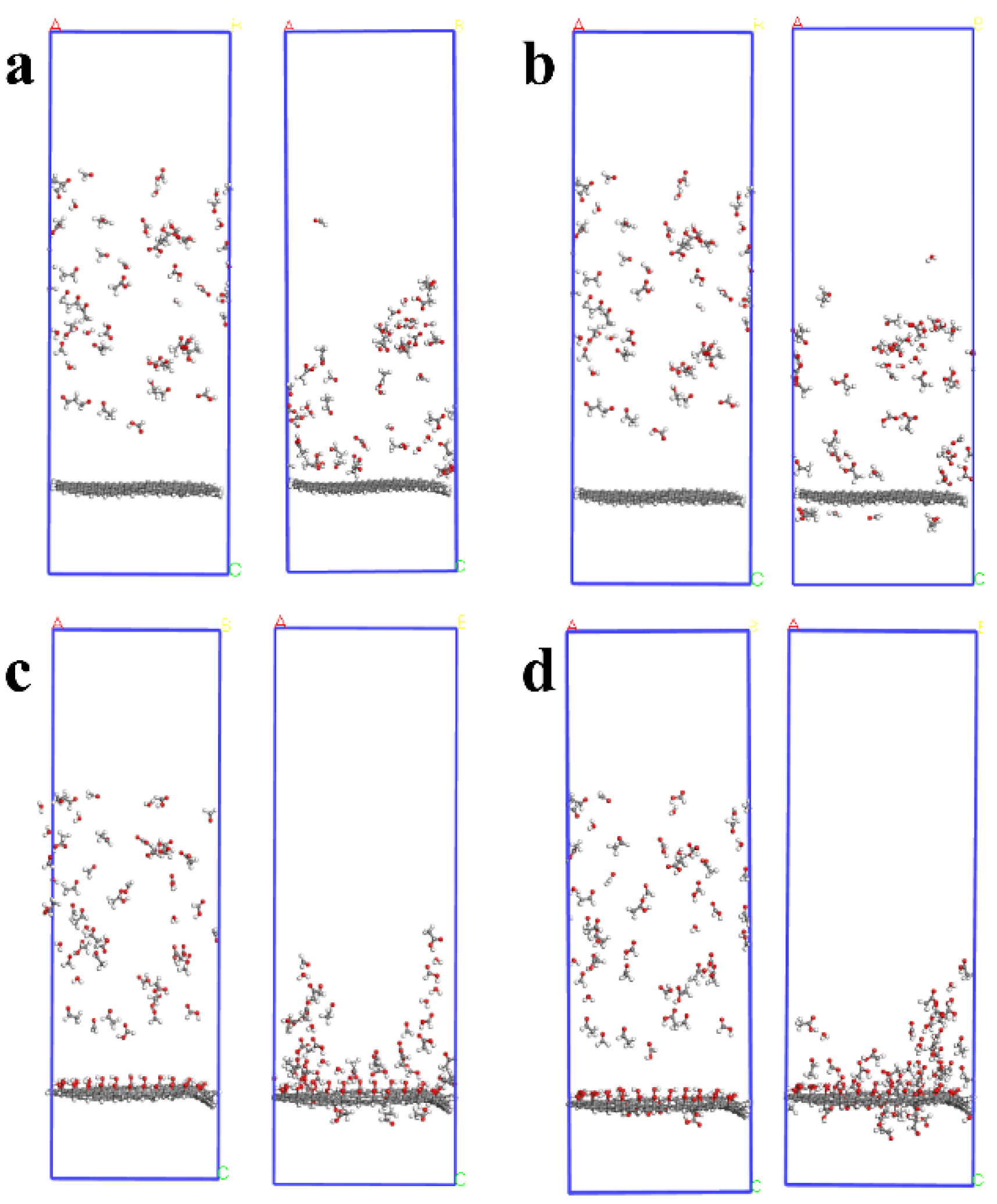
3.1. Diffusion Characteristic
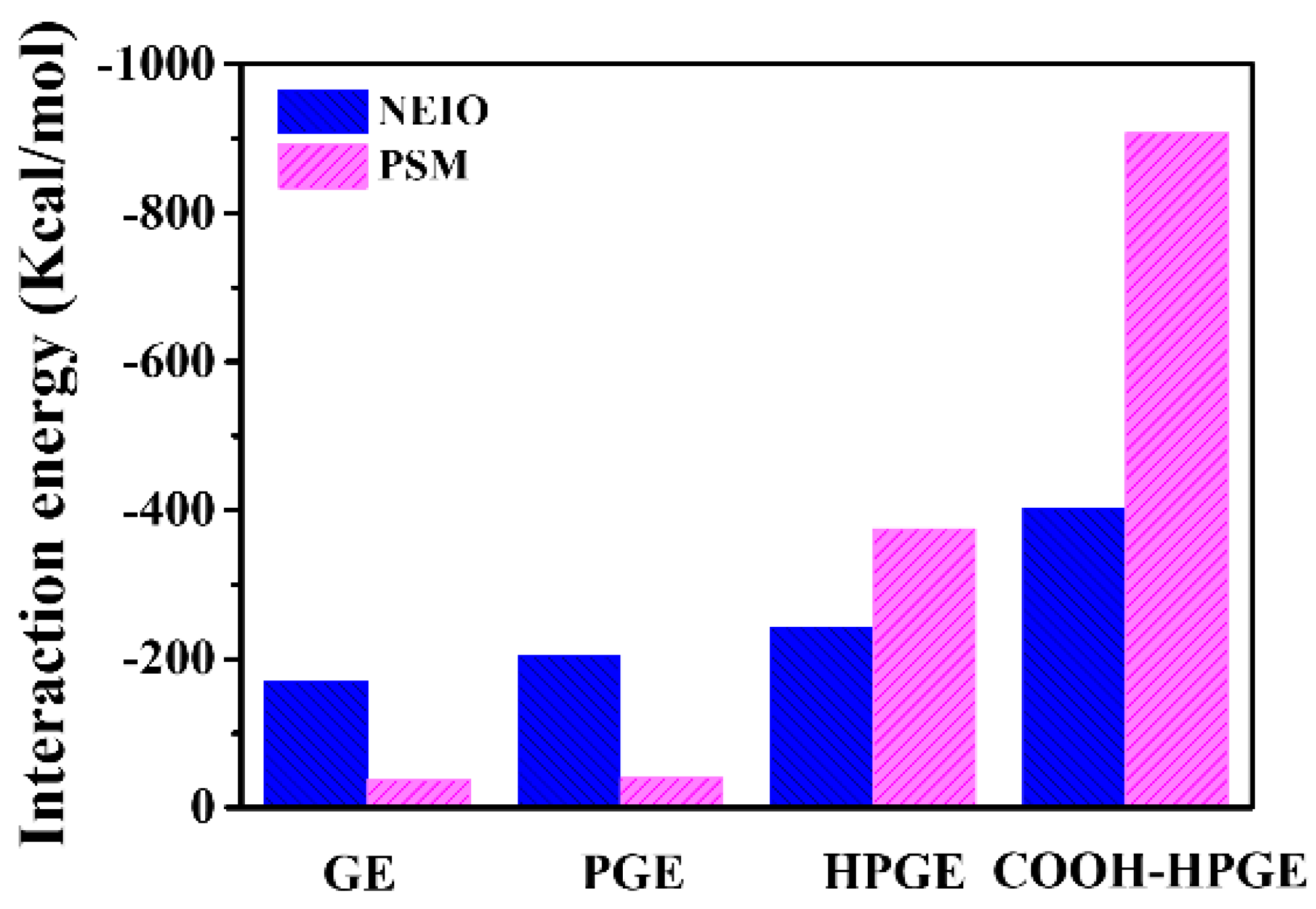
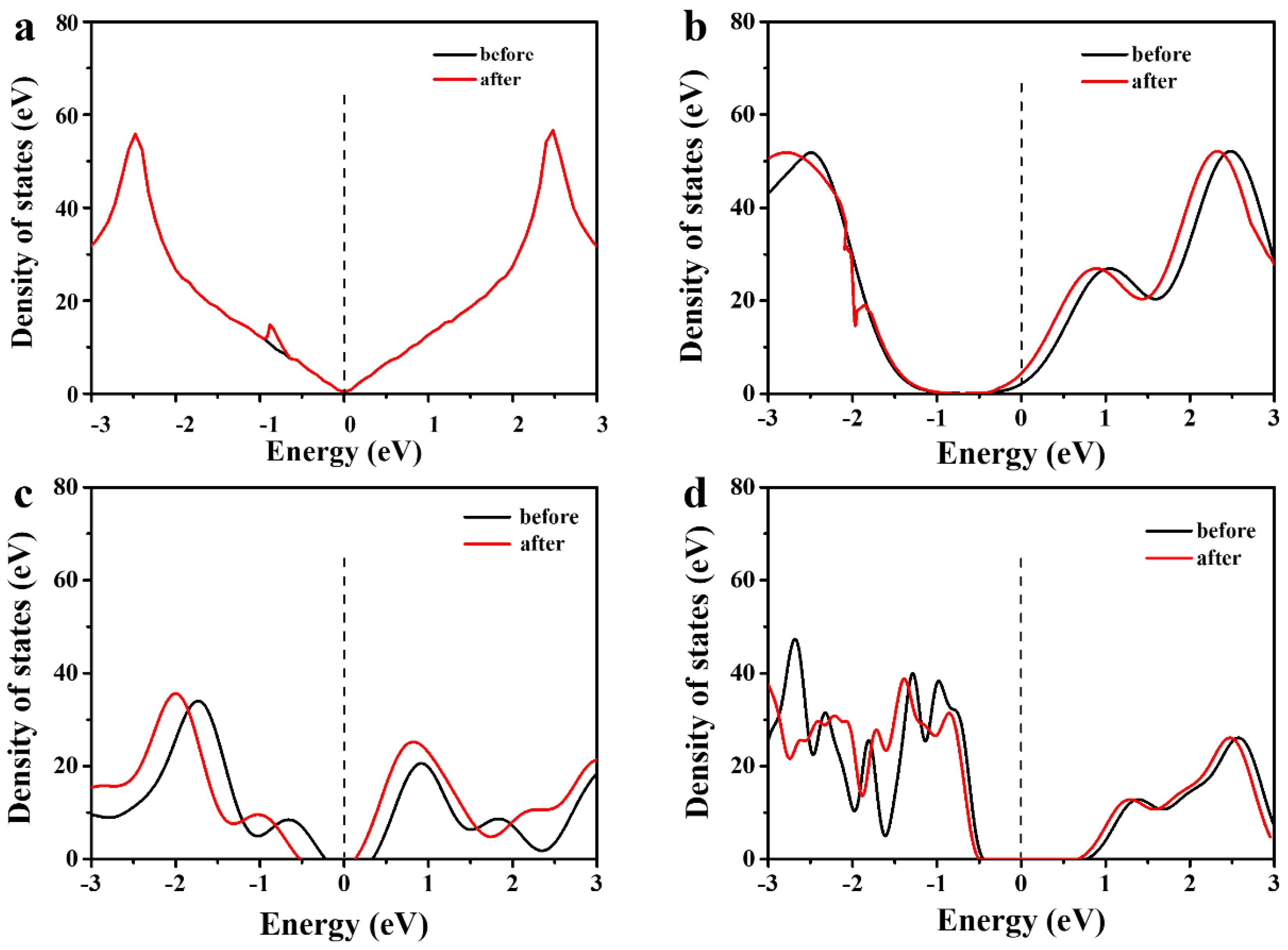
3.2. Interaction Energy
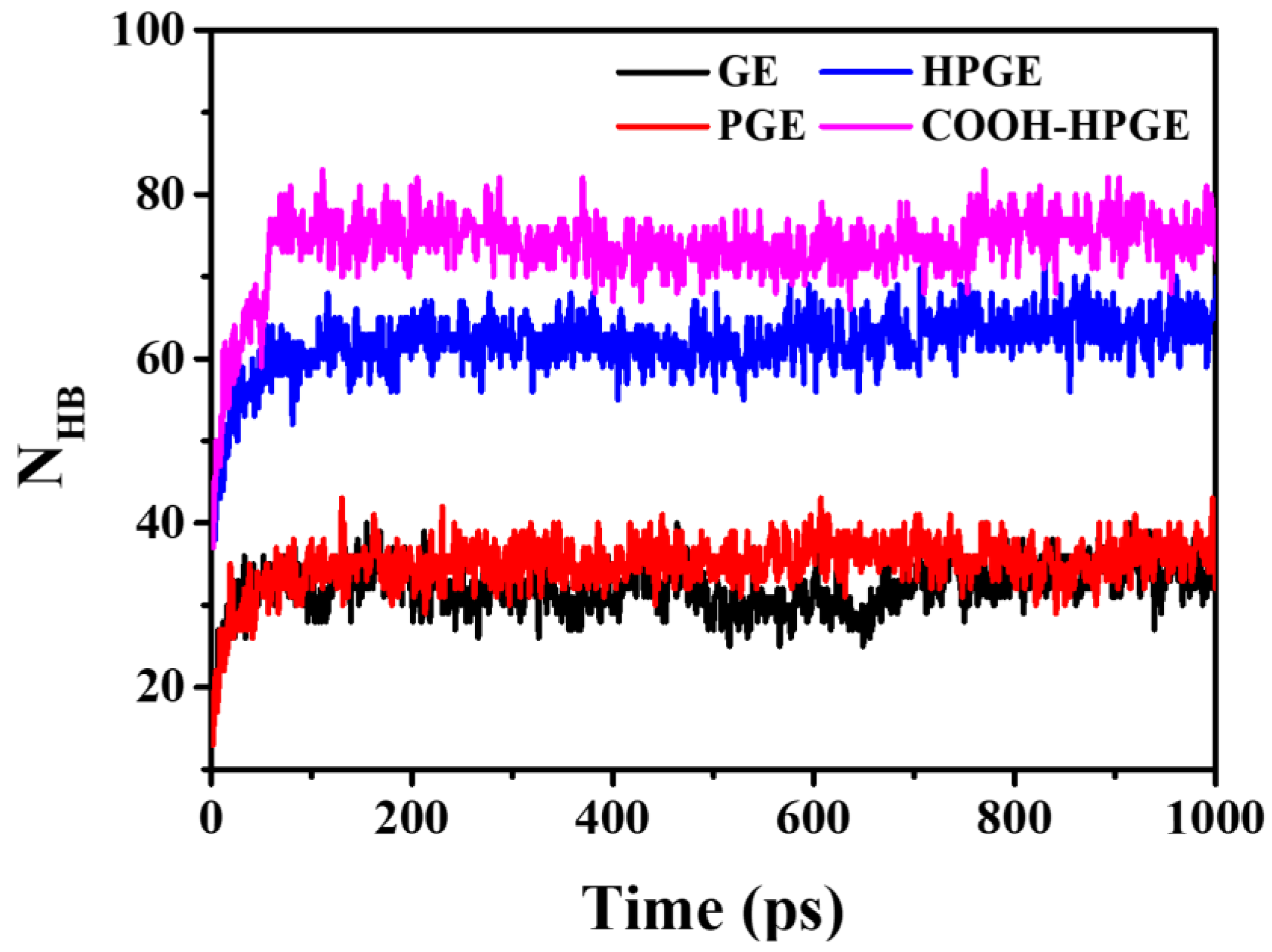
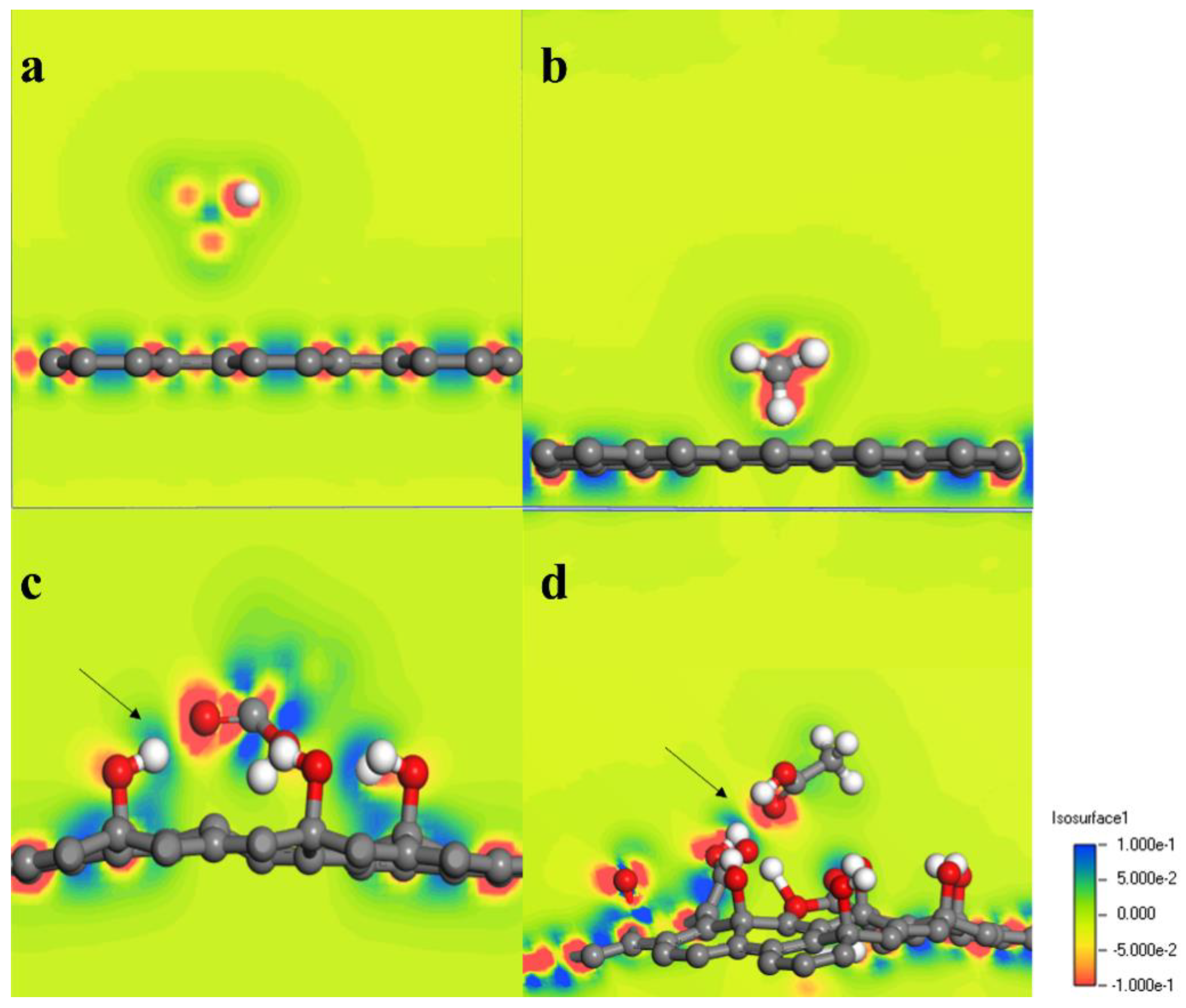
3.3. Adsorption Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Phan, D.-P.; Lee, E.Y. Catalytic Hydroisomerization Upgrading of Vegetable Oil-Based Insulating Oil. Catalysts 2018, 8, 131. [Google Scholar] [CrossRef]
- Lyutikova, M.; Korobeynikov, S.; Rao, U.M.; Fofana, I. Mixed Insulating Liquids with Mineral Oil for High Voltage Transformer Applications: A Review. IEEE Trans. Dielectr. Electr. Insul. 2022, 18, 454–461. [Google Scholar] [CrossRef]
- Zheng, H.; Li, X.; Feng, Y.; Yang, H.; Lv, W. Investigation on micro-mechanism of palm oil as natural ester insulating oil for overheating thermal fault analysis of transformers. High Volt. 2022, 7, 812–824. [Google Scholar] [CrossRef]
- Cong, H.; Shao, H.; Du, Y.; Hu, X.; Zhao, W.; Li, Q. Influence of Nanoparticles on Long-Term Thermal Stability of Vegetable Insulating Oil. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1642–1650. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Thermodynamic analysis of solar assisted steam distillation system for peppermint oil extraction. J. Food Process. Eng. 2023, 46, 12–16. [Google Scholar] [CrossRef]
- Nam, Y.; Ki, D.-K.; Koshino, M.; McCann, E.; Morpurgo, A.F. Interaction-induced insulating state in thick multilayer graphene. 2D Mater. 2016, 3, 045014. [Google Scholar] [CrossRef]
- Ariono, D.; Wardani, A.K.; Widodo, S.; Aryanti, P.T.P.; Wenten, I.G. Fouling mechanism in ultrafiltration of vegetable oil. Mater. Res. Express 2018, 5, 034009. [Google Scholar] [CrossRef]
- Ibarra-Rodríguez, M.; Sánchez, M. Lithium clusters on graphene surface and their ability to adsorb hydrogen molecules. Int. J. Hydrogen Energy 2021, 46, 21984–21993. [Google Scholar] [CrossRef]
- Huang, L.; Miao, S.; Wang, X.; Yang, X. DFT study of gas adsorbing and electronic properties of unsaturated nanoporous graphene. Mol. Simul. 2020, 46, 853–863. [Google Scholar] [CrossRef]
- Liu, T.; Aniagor, C.O.; Ejimofor, M.I.; Menkiti, M.C.; Wakawa, Y.M.; Li, J.; Akbour, R.A.; Yap, P.-S.; Lau, S.Y.; Jeevanandam, J. Recent developments in the utilization of modified graphene oxide to adsorb dyes from water: A review. J. Ind. Eng. Chem. 2023, 117, 21–37. [Google Scholar] [CrossRef]
- Fu, H.; Huang, J.; Gray, K. Crumpled graphene balls adsorb micropollutants from water selectively and rapidly. Carbon 2021, 183, 958–969. [Google Scholar] [CrossRef]
- Wilhelm, H.M.; Franch, V.; Tulio, L.; Franch, A.F. Compatibility of transformer construction materials with natural ester-based insulating fluids. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2703–2708. [Google Scholar] [CrossRef]
- Zuo, H.; Wang, F.; Huang, Z.; Wang, Q.; Li, J.; Rozga, P. Synergistic effect of electric field and temperature on POSS modified natural ester insulating oil: A molecular dynamics study. J. Mol. Liq. 2022, 355, 118923. [Google Scholar] [CrossRef]
- Prathab, B.; Subramanian, V.; Aminabhavi, T. Molecular dynamics simulations to investigate polymer–polymer and polymer–metal oxide interactions. Polymer 2007, 48, 409–416. [Google Scholar] [CrossRef]
- Qiu, Q.; Tian, W.; Yang, L.; Ding, Z.; Zhang, J.; Tang, C. Simulation effect of SiO2 nanoparticles on the water molecules diffusion inside insulating oil at different temperatures. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 610, 125738. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, W.; Yao, W.; Jiang, Y.; Xu, Z.; Gao, C. Ion Diffusion-Directed Assembly Approach to Ultrafast Coating of Graphene Oxide Thick Multilayers. ACS Nano. 2017, 10, 9663–9670. [Google Scholar] [CrossRef]
- Shi, M.; Tang, C.; Yang, X.; Zhou, J.; Jia, F.; Han, Y.; Li, Z. Superhydrophobic silica aerogels reinforced with polyacrylonitrile fibers for adsorbing oil from water and oil mixtures. RSC Adv. 2017, 7, 4039–4045. [Google Scholar] [CrossRef]
- Miao, S.; Xu, Z.; Yang, X. DFT study of non-covalent interaction mechanisms of solvents with GO surfaces and the solvent-mediated GO interaction. Appl. Surf. Sci. 2017, 499, 143926. [Google Scholar] [CrossRef]
- Wei, D.; Zhao, C.; Khan, A.; Sun, L.; Ji, Y.; Ai, Y.; Wang, X. Sorption mechanism and dynamic behavior of graphene oxide as an effective adsorbent for the removal of chlorophenol based environmental-hormones: A DFT and MD simulation study. Chem. Eng. J. 2019, 375, 121964. [Google Scholar] [CrossRef]
- Zhao, C.; Fu, H.; Yang, X.; Xiong, S.; Han, D.; An, X. Adsorption and photocatalytic performance of Au nanoparticles decorated porous Cu2O nanospheres under simulated solar light irradiation. Appl. Surf. Sci. 2021, 545, 149014. [Google Scholar] [CrossRef]
- Sirohi, A.; SanthiBhushan, B.; Srivastava, A. Charge transport in polythiophene molecular device: DFT analysis. J. Mol. Model. 2021, 27, 77. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Ren, X.; Xu, J.; Li, H.; Zheng, Y.; Hong, Y.; Lin, Y.; Zhou, Y.; Chen, Y.; Zhang, W. Excellent adsorption property and mechanism of oxygen vacancies-assisted hexagonal MoO3 nanosheets for methylene blue and rhodamine b dyes. Appl. Surf. Sci. 2022, 597, 153699. [Google Scholar] [CrossRef]

| Graphene Based | GE | PGE | HPGE | COOH-HPGE | |
|---|---|---|---|---|---|
| Small Molecules | |||||
| H2O | −0.121 | −0.157 | −1.219 | −1.757 | |
| HCOOH | −0.126 | −0.172 | −1.030 | −1.660 | |
| CH3CHO | −0.186 | −0.235 | −0.923 | −1.055 | |
| HCHO | −0.128 | −0.175 | −1.006 | −1.634 | |
| CH3COOH | −0.172 | −0.202 | −0.998 | −1.557 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Yang, Z.; Shao, X.; Zheng, Y.; Wang, Q.; Huang, Z. Investigation on Adsorption of Polar Molecules in Vegetable Insulating Oil by Functional Fossil Graphene. Materials 2023, 16, 3434. https://doi.org/10.3390/ma16093434
Liang S, Yang Z, Shao X, Zheng Y, Wang Q, Huang Z. Investigation on Adsorption of Polar Molecules in Vegetable Insulating Oil by Functional Fossil Graphene. Materials. 2023; 16(9):3434. https://doi.org/10.3390/ma16093434
Chicago/Turabian StyleLiang, Suning, Zhi Yang, Xianjun Shao, Yiming Zheng, Qiang Wang, and Zhengyong Huang. 2023. "Investigation on Adsorption of Polar Molecules in Vegetable Insulating Oil by Functional Fossil Graphene" Materials 16, no. 9: 3434. https://doi.org/10.3390/ma16093434
APA StyleLiang, S., Yang, Z., Shao, X., Zheng, Y., Wang, Q., & Huang, Z. (2023). Investigation on Adsorption of Polar Molecules in Vegetable Insulating Oil by Functional Fossil Graphene. Materials, 16(9), 3434. https://doi.org/10.3390/ma16093434





