Research on Salt Corrosion Resistance of Lithium-Based Protective Coating on Mortar Substrate
Abstract
:1. Introduction
2. Materials and Method
2.1. Raw Materials
2.2. Experimental Programme
2.2.1. Penetration Depth of Free Chloride Ion in a Coated Mortar Matrix
2.2.2. Effect of Sulfate Attack on Coating Treated Mortar Matrix
2.2.3. Study on the Effect of Coating Treatment on Chloride Ion Penetration Resistance of Concrete
2.3. Microscopic Test
SEM Characterization & Testing
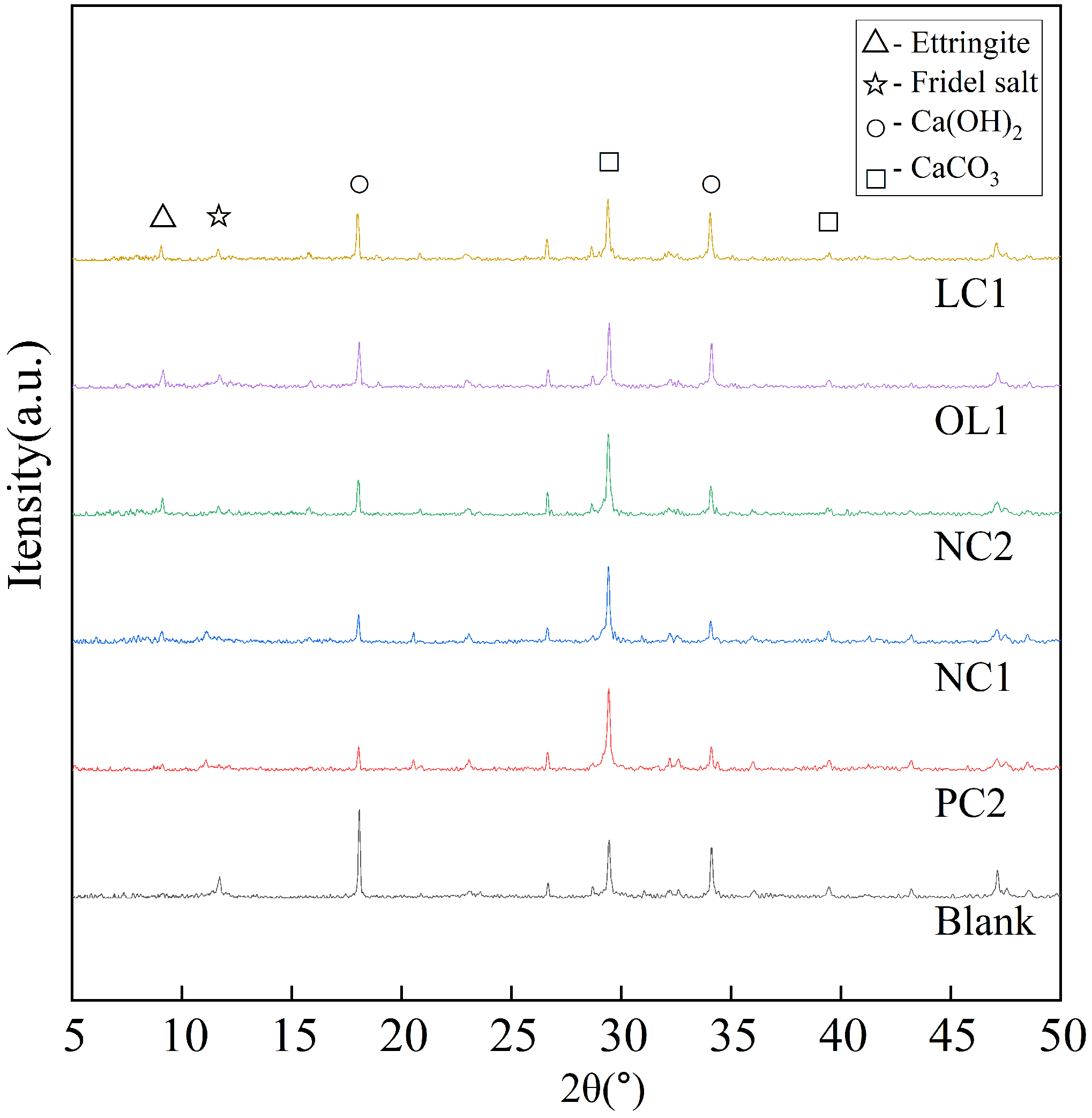
2.4. XRD Characterization & Testing
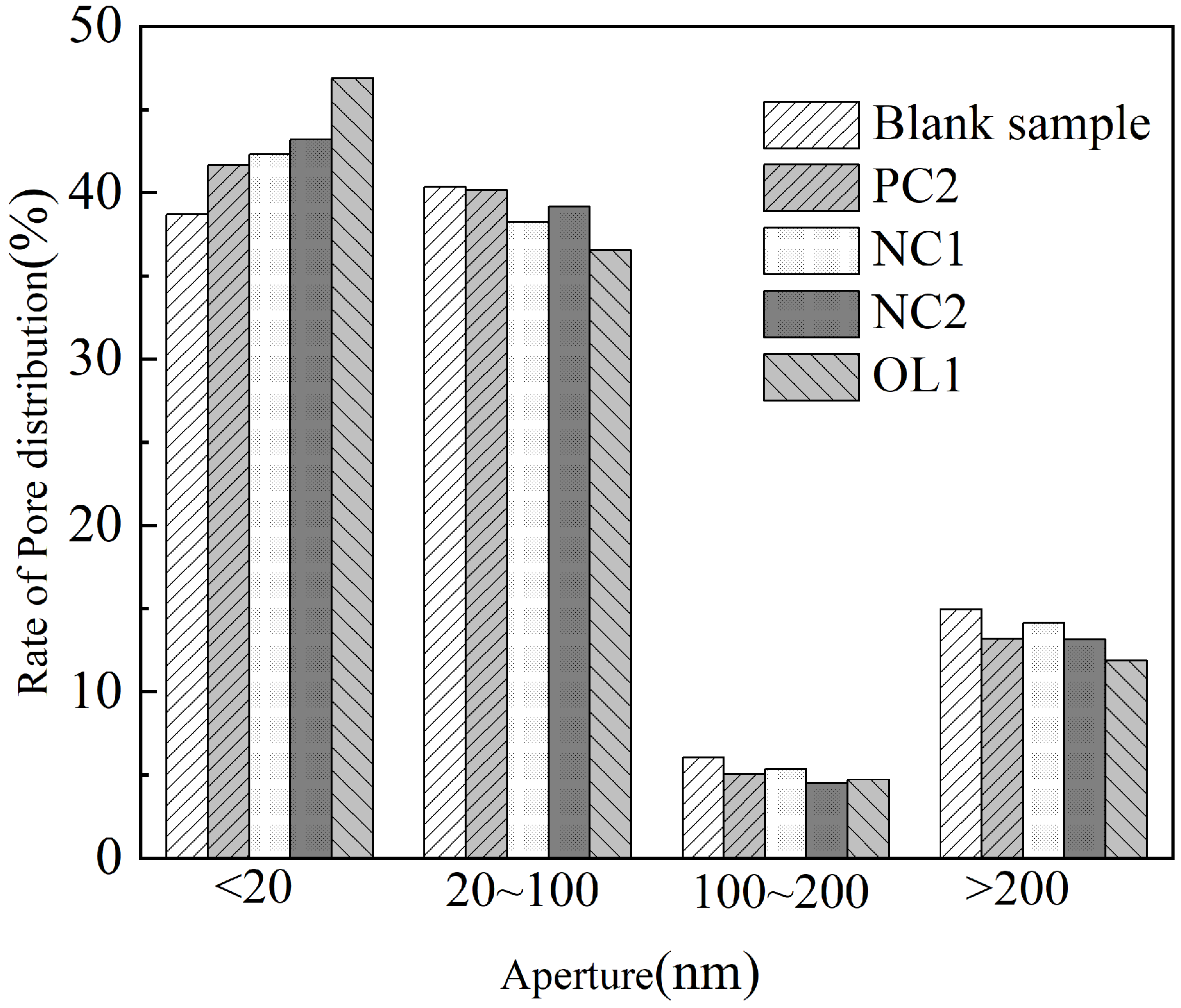
2.5. Mercury Injection Test
3. Results and Discussion
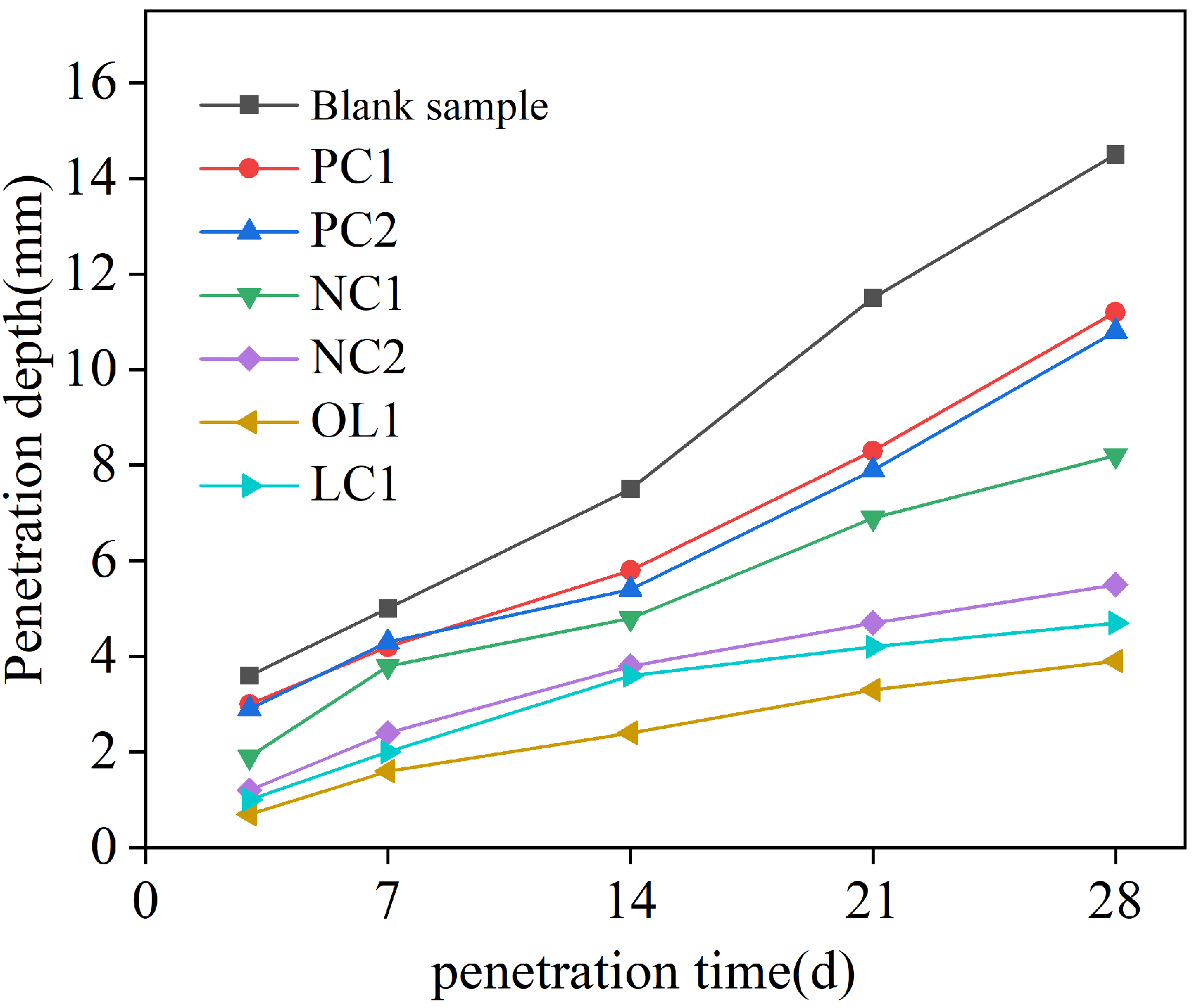
3.1. Penetration Depth of Free Chloride Ion in a Coated Mortar Matrix
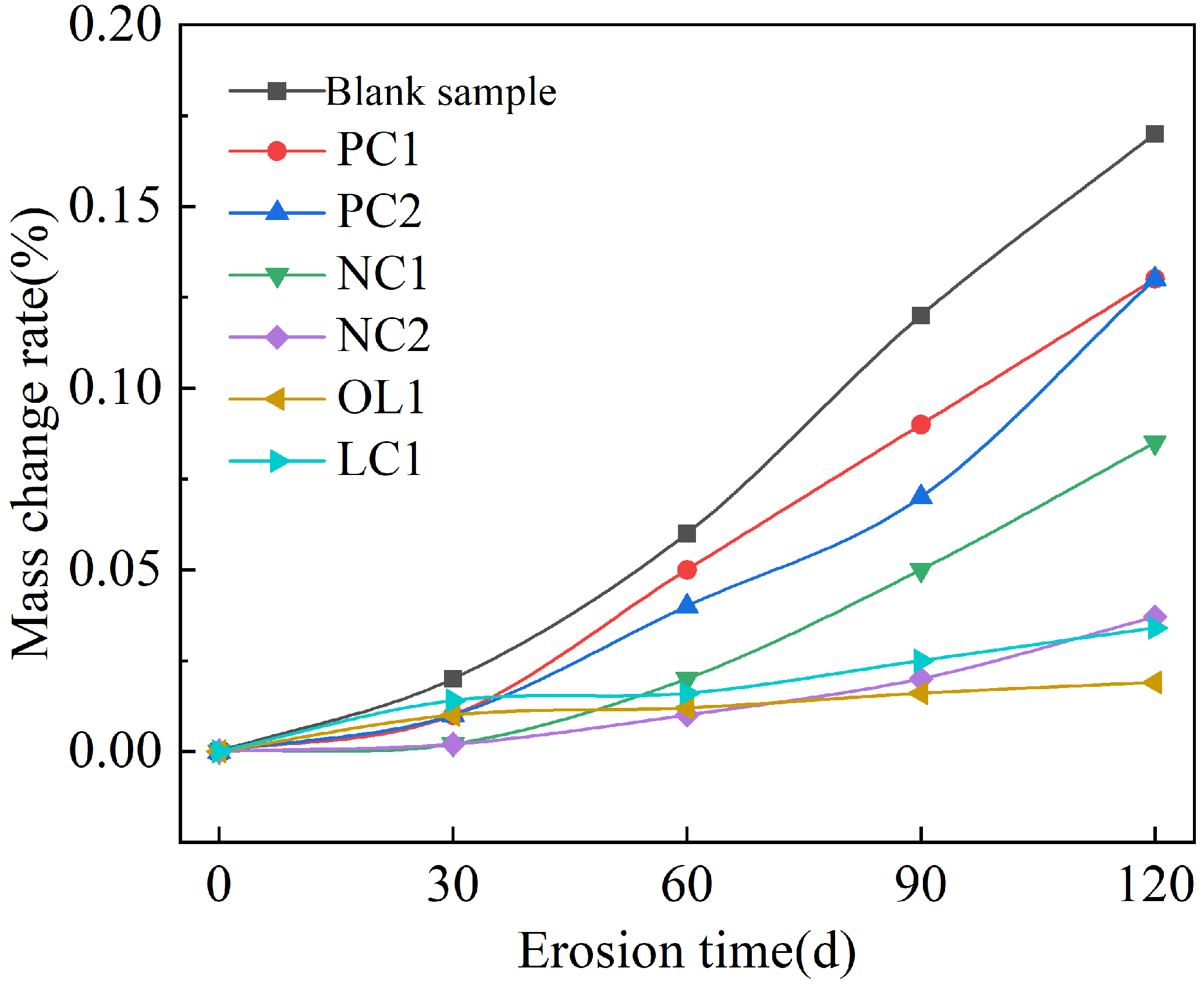
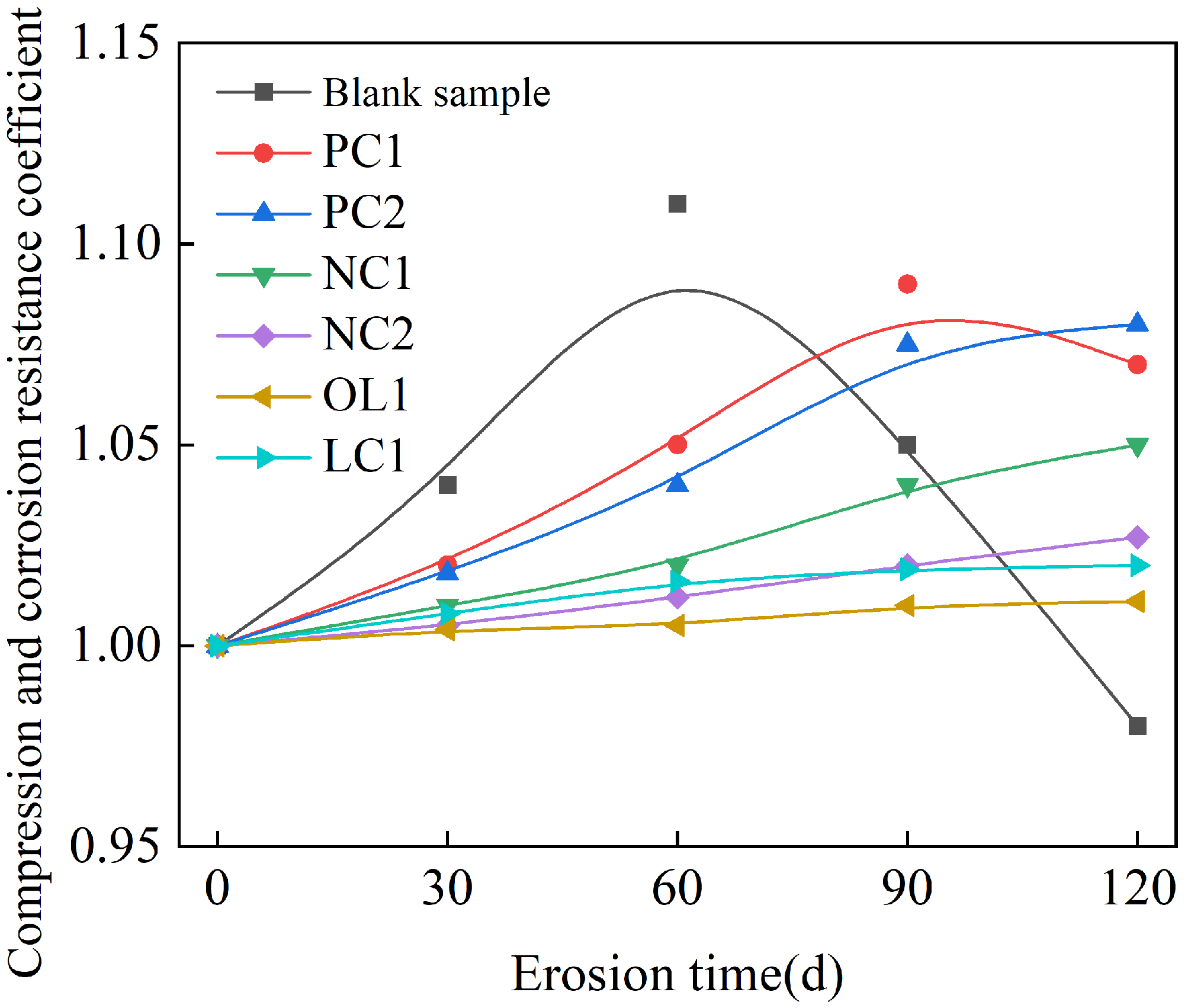
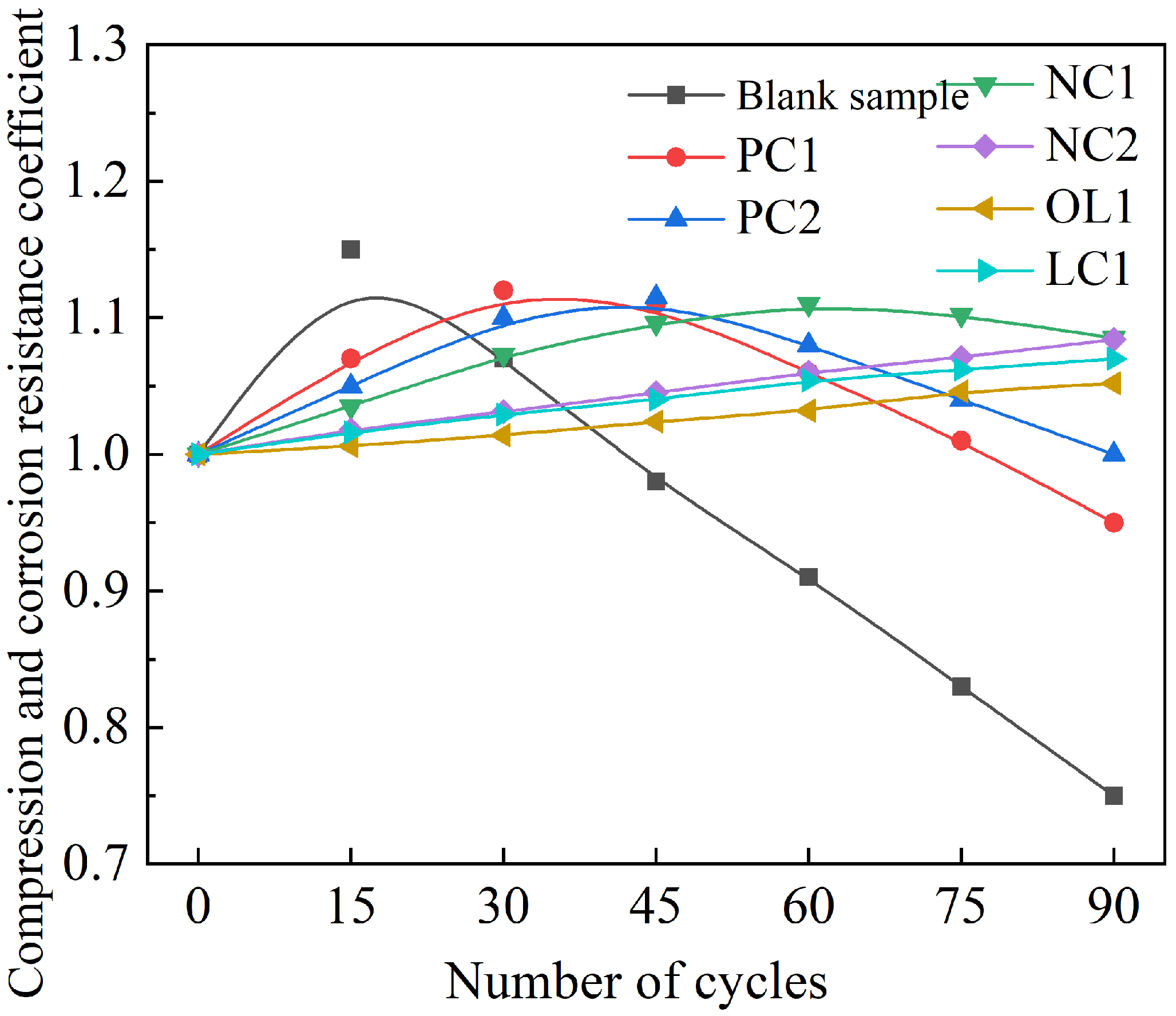
3.2. Effect of Sulfate Attack on Coating-Treated Mortar Matrix
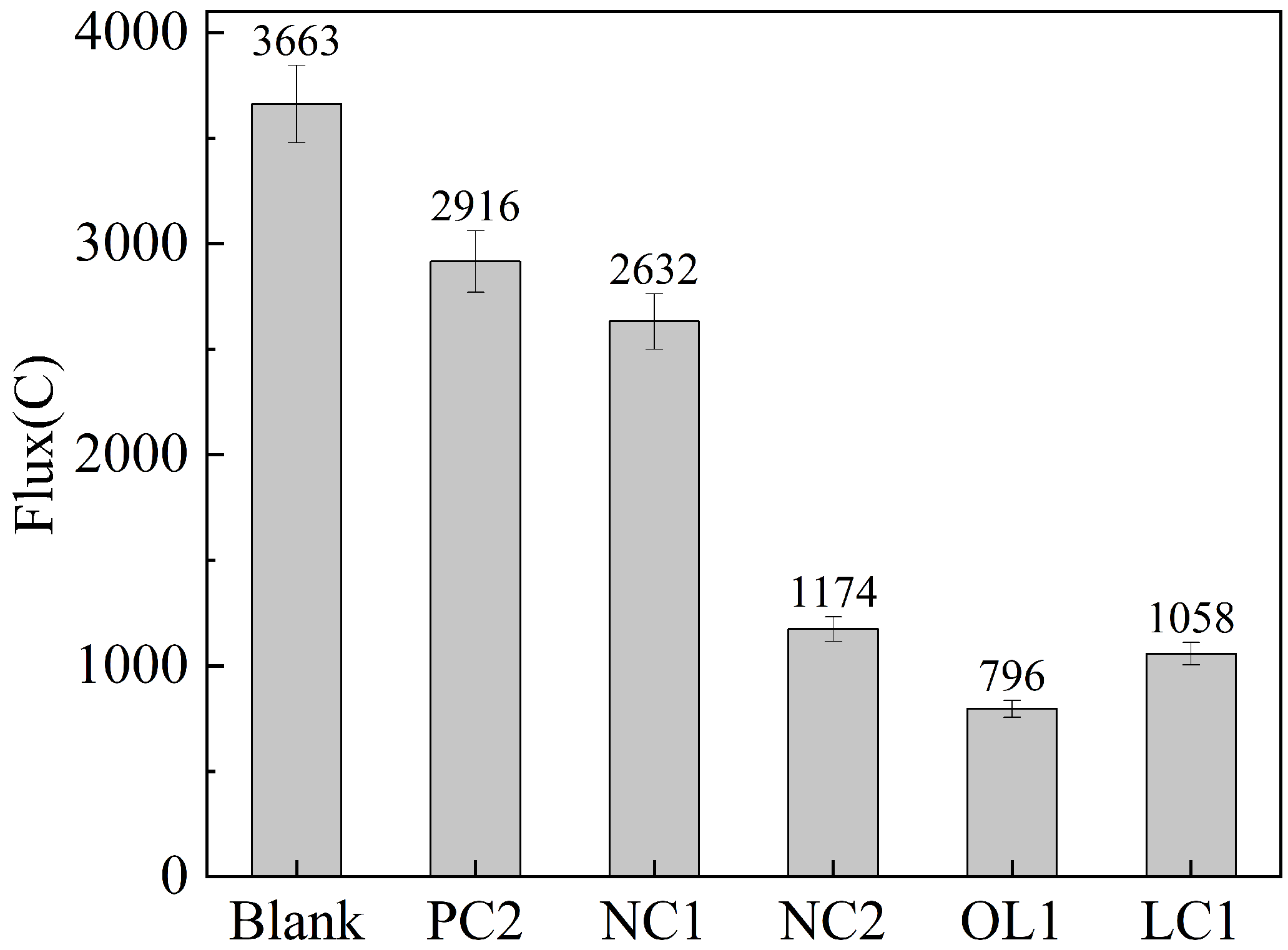
3.3. Study on the Effect of Coating Treatment on Chloride Ion Penetration Resistance of Concrete
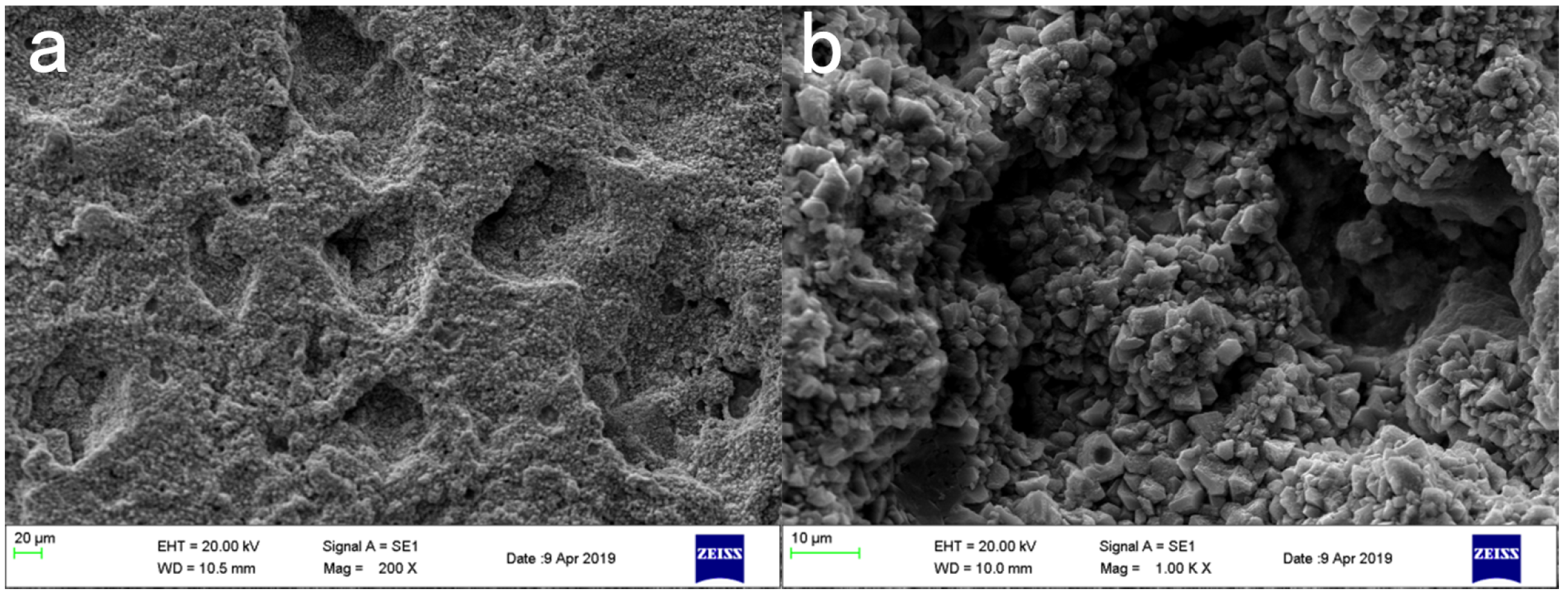
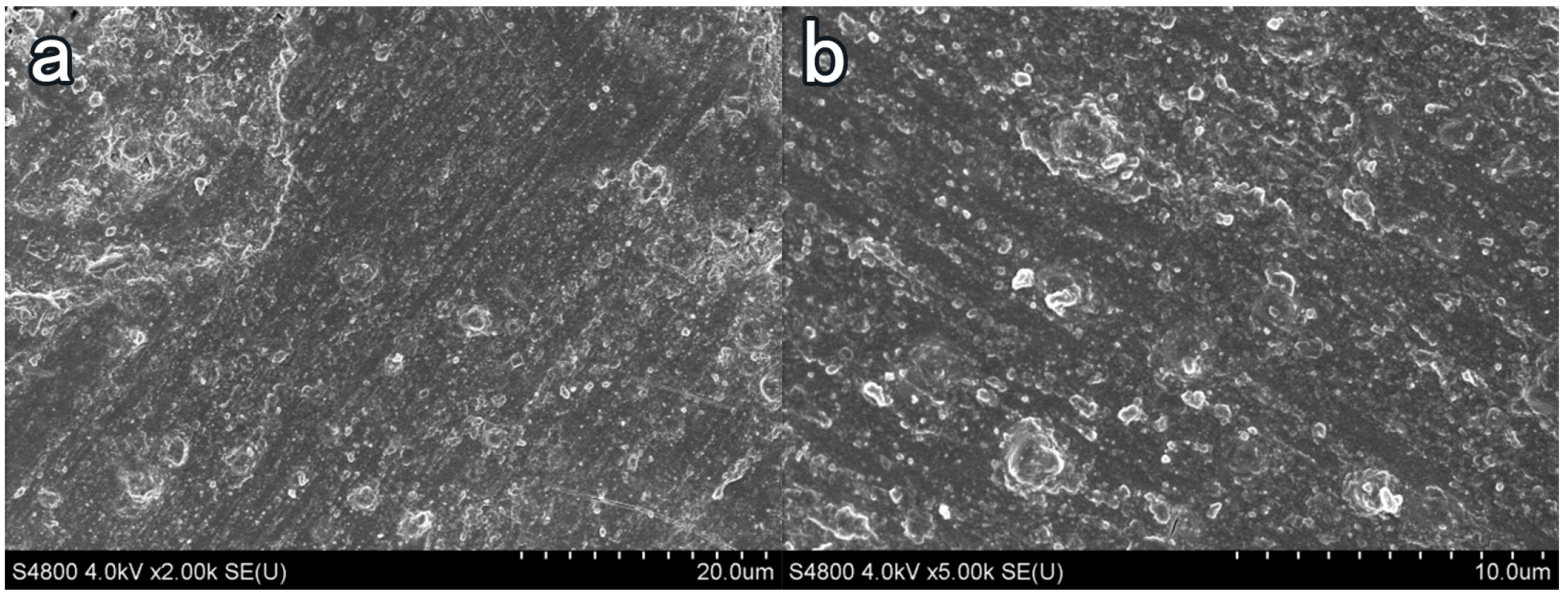
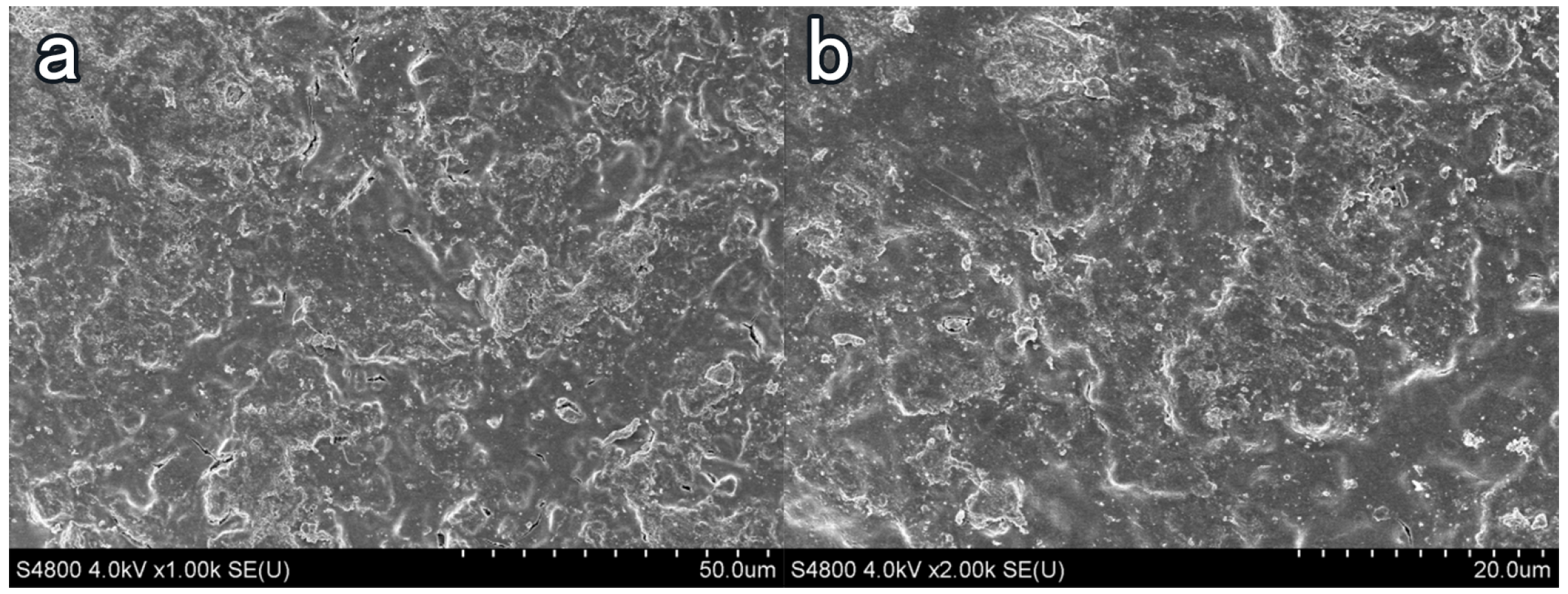
3.4. Influence of Coating Treatment on Surface Morphology of Cement-Based Materials
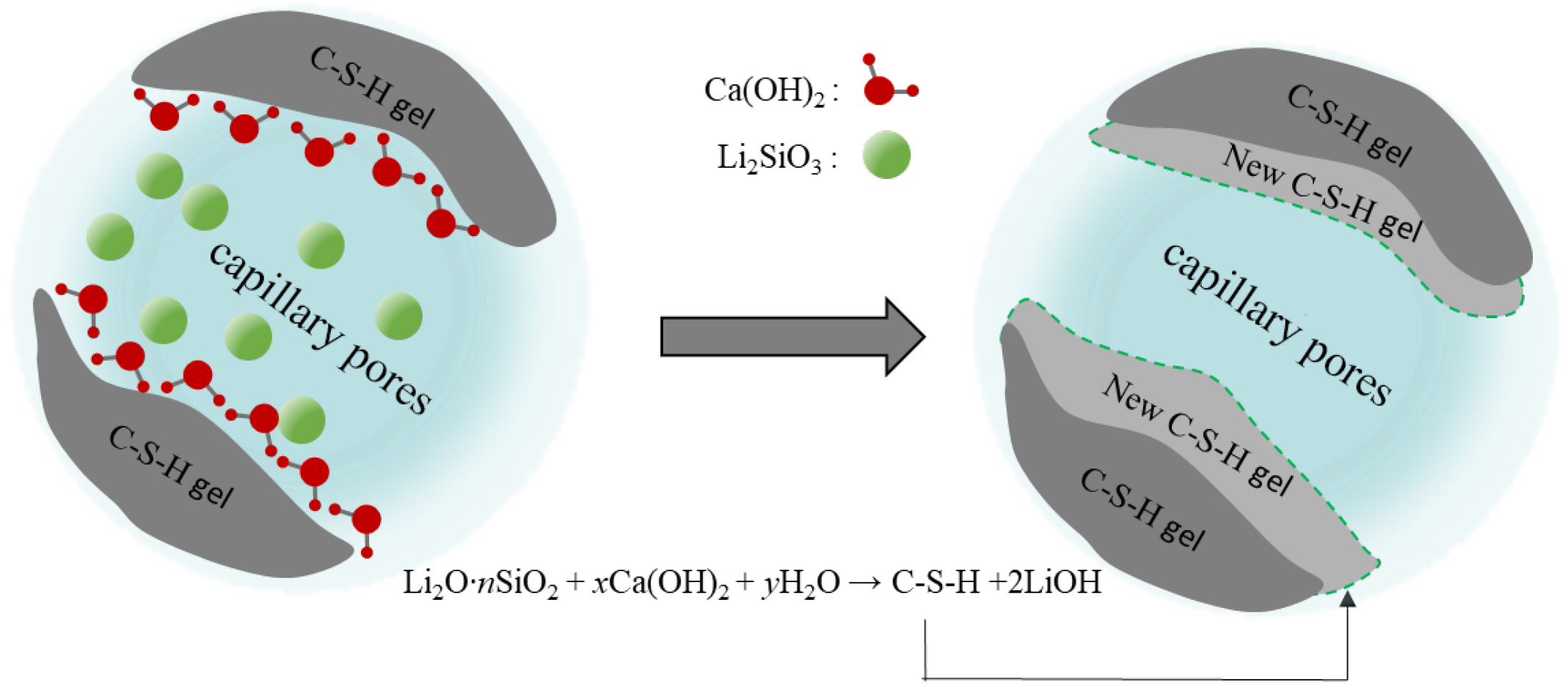
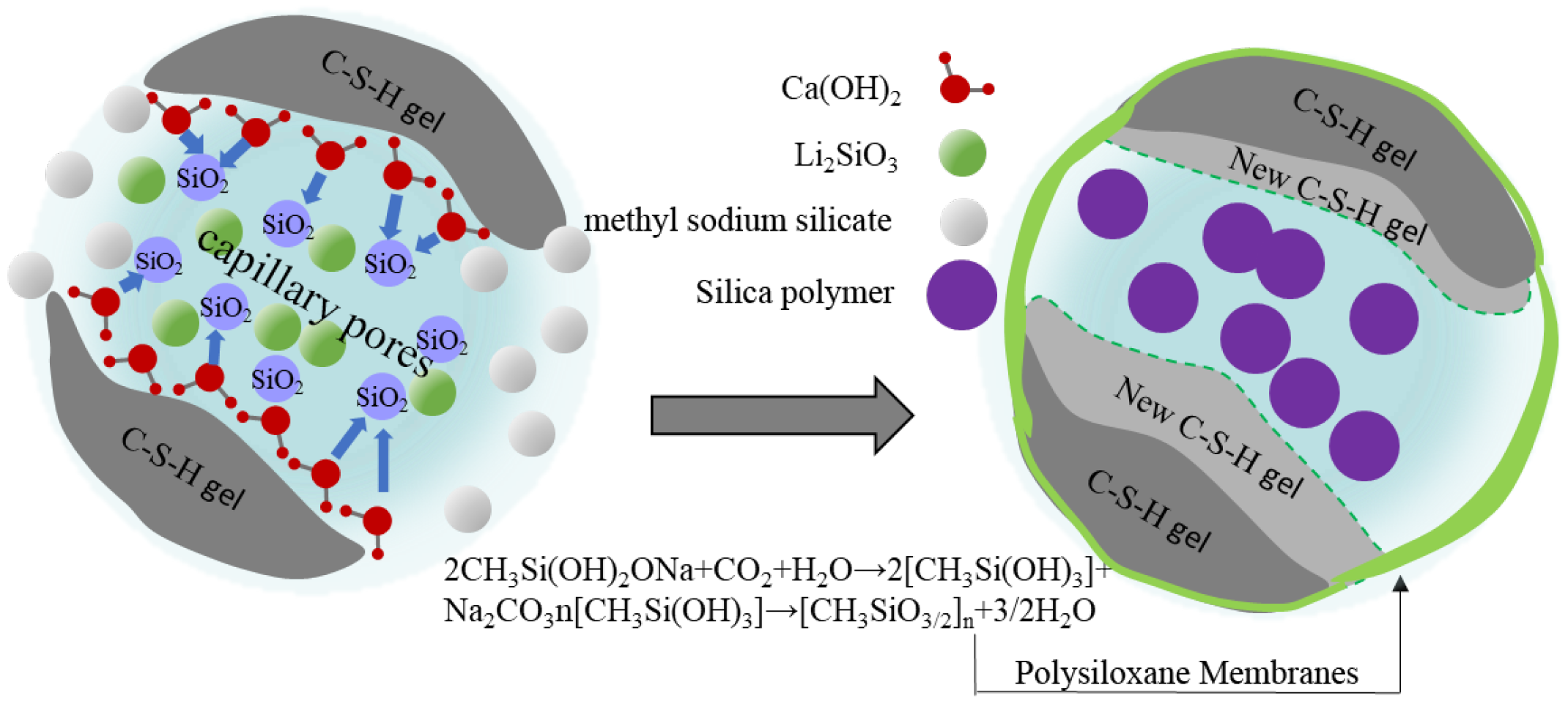
3.5. Analysis of Protective Mechanism of Coating on Cement-Based Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gong, Y.; Liu, C. Durability of Concrete and Its Protection and Repair; China Architecture & Building Press: Beijing, China, 1999; pp. 74–79. [Google Scholar]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- He, Z.; Liu, Y.; Bai, K.; Liu, J. Progress in research on carbonation of concrete. Mater. Rep. 2008, 22, 353–357. [Google Scholar]
- Pan, C.; Li, Y.; Shu, D.; Wang, Z.; Zhu, S.; Mao, J.; Jin, W. Development of migrating corrosion inhibitorin reinforced concrete. Mater. Rep. 2016, 30, 145–151. [Google Scholar]
- Yan, L.; Deng, W.; Wang, N.; Xue, X.; Hua, J.; Chen, Z. Anti-Corrosion Reinforcements Using Coating Technologies—A Review. Polymers 2022, 14, 4782. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Zhang, S.S.; Chen, E.; Li, W.G. A review on corrosion detection and protection of existing reinforced concrete (RC) structures. Constr. Build. Mater. 2022, 325, 126718. [Google Scholar] [CrossRef]
- Baltazar, L.; Santana, J.; Lopes, B.; Paula Rodrigues, M.; Correia, J.R. Surface skin protection of concrete with silicate-based impregnations: Influence of the substrate roughness and moisture. Constr. Build. Mater. 2014, 70, 191–200. [Google Scholar] [CrossRef]
- Zheng, B.; Yuan, C.; Sun, Y.; Li, W. Research progress of modified SiO2 composite coating on concrete surface. J. Funct. Mater. 2021, 52. [Google Scholar]
- Liao, G.; Yang, H.; He, Z.; Zhao, P. Research of permeable multi-function treatment agent on concrete surface. Concrete 2015, 11, 61–62. [Google Scholar]
- Ibrahim, M.; Al-Gahtani, A.S.; Maslehuddin, M.; Almusallam, A.A. Effectiveness of concrete surface treatmentmaterials in reducing chloride-induced reinforcement corrosion. Concr. Build. Mater. 1997, 11, 443–451. [Google Scholar] [CrossRef]
- Zhang, X. Preparation of Silane Protective Material for Concrete and Its Influence on Durability of Concrete. Master’s Thesis, Qingdao University of Technology, Qingdao, China, 2014. [Google Scholar]
- Zhang, G.; Shi, D.; Jiang, T.; Zhang, Q. Research progress of siloxane protection and repair materials for concrete. China Concr. Cem. Prod. 2018, 10, 18–23. [Google Scholar]
- Liu, J. Preparation and Properties of Anticorrosive Epoxy Resin Coating Material for Concrete. Master’s Thesis, Chang’an University, Chang’an, China, 2015. [Google Scholar]
- Fang, S. The Preparation of Graphene Oxide/Acrylic Resin Coating and Its Protective Effect on Concrete. Master’s Thesis, South China University of Technology, Guangzhou, China, 2019. [Google Scholar]
- Zhu, Y.; Kou, S.; Poon, C.; Dai, J.; Li, Q. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.C.; Li, N. A review on surface treatment for concrete - Part 2: Performance. Constr. Build. Mater. 2017, 133, 81–90. [Google Scholar] [CrossRef]
- Li, T.; Wu, Y.; Wu, H. A Study on Impact of Different Surface Treatment Agents on the Durability of Airport Pavement Concrete. Coatings 2022, 12, 162. [Google Scholar] [CrossRef]
- Sudbrink, B.; Moradllo, M.K.; Hu, Q.; Ley, M.T.; Davis, J.M.; Materer, N.; Apblett, A. Imaging the presence of silane coatings in concrete with micro X-ray fluorescence. Cem. Concr. Res. 2017, 92, 121–127. [Google Scholar] [CrossRef]
- Jones, M.; Dhir, R.; Gill, J. Concrete surface treatment: Effect of exposure temperature on chloride diffusion resistance. Cem. Concr. Res. 1995, 25, 197–208. [Google Scholar] [CrossRef]
- Shen, L.; Jiang, H.; Wang, T.; Chen, K.; Zhang, H. Performance of silane -based surface treatments for protecting degraded historic concrete. Prog. Org. Coat. 2019, 129, 209–216. [Google Scholar] [CrossRef]
- Batis, G.; Pantazopoulou, P.; Routoulas, A. Corrosion protection investigation of reinforce- ent by inorganic coating in the presence of alkanolamine-based inhibitor. Cem. Concr. Compos. 2003, 25, 371–377. [Google Scholar] [CrossRef]
- McGettigan, E. Silicon-based weatherproofing materials. Concr. Int. 1992, 14, 52. [Google Scholar]
- Thompson, J.; Silsbee, M.; Gill, P.; Scheetz, B. Characterization of silicate sealers on concrete. Cem. Concr. Res. J. Mater. Sci. 1997, 17, 1561–1567. [Google Scholar] [CrossRef]
- Li, X.; Pan, C.; Li, D.; Geng, J.; Chen, N.; He, J.; Liu, S. Design of CNS-Li2SiO3 Permeable Protective Coatings and Effects on Mortar Matrix. Materials 2020, 13, 1733. [Google Scholar] [CrossRef]
- Liu, F.; Liu, A.; Tao, W.; Yang, Y. Preparation of UV curable organic/inorganic hybrid coatings—A review. Prog. Org. Coat. 2020, 145, 105685. [Google Scholar] [CrossRef]
- Wang, H.; Feng, P.; Lv, Y.; Geng, Z.; Liu, Q.; Liu, X. A comparative study on UV degradation of organic coatings for concrete: Structure, adhesion, and protection performance. Prog. Org. Coat. 2020, 149, 105892. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, G.; Zhuo, M.; Fan, Z.; Miao, L.; Chen, L.; Zeng, A.; Yin, R.; Ou, Y.; Shi, Z.; et al. Next-generation sequencing based mutation profiling reveals heterogeneity of clinical response and resistance to osimertinib. Lung Cancer 2020, 141, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, F.; Qi, X.; Lin, Z. Mechanical, electrochemical, and durability behavior of graphene nano-platelet loaded epoxy-resin composite coatings. Compos. Part B Eng. 2019, 176, 107103. [Google Scholar] [CrossRef]
- Wei, H.; Xia, J.; Zhou, W.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K.K. Adhesion and cohesion of epoxy-based industrial composite coatings. Compos. Part B Eng. 2020, 193, 108035. [Google Scholar] [CrossRef]
- Habib, S.; Hassanein, A.; Kahraman, R.; Mahdi Ahmed, E.; Shakoor, R. Self-healing behavior of epoxy-based double-layer nanocomposite coatings modified with Zirconia nanoparticles. Mater. Des. 2021, 207, 109839. [Google Scholar] [CrossRef]
- Li, G.; Hu, W.; Cui, H.; Zhou, J. Long-term effectiveness of carbonation resistance of concrete treated with nano-SiO2 modified polymer coatings. Constr. Build. Mater. 2019, 201, 623–630. [Google Scholar] [CrossRef]
- ISO 679:2009; Methods of Testing Cements—Determination of Strength. ISO: Geneva, Switzerland, 2009.
- GB/T 17671-1999; Method of Testing Cements—Determination of Strength. China Association for Standardization: Beijing, China, 2022.
- Wang, C.; Gao, X.; Zhao, Y.; Jin, W. Peak value distribution of surface chloride concentration and convection depth of Concrete. Bull. Chin. Ceram. Soc. 2010, 29, 262–267. [Google Scholar]
- Wang, M. Study on the Influence of Lithium Silicate Based Epoxy Coating on Concrete Performance. Master’s Thesis, Changsha University of Science & Technology, Changsha, China, 2017. [Google Scholar]
- Zhang, H.; Zhan, S.; Wu, H.; Wang, J.; Xu, Y. Research on the influence of lithium silicate composite sol on concrete surface properties. Rare Met. Mater. Eng. 2016, 45, 85–88. [Google Scholar]
- Yang, K. An experimental study on concrete under the long-term of immersion attacked by sulfate. J. Huaiyin Inst. Technol. 2011, 20, 48–52. [Google Scholar]
- Gao, R.; Zhao, S.; Li, Q.; Chen, J. Experimental study of the deterioration mechanism of concrete under sulfate attack in wet-dry cycle. China Civ. Eng. J. 2010, 43, 48–52. [Google Scholar]
- ASTMC876-15; Standard Test Method for Corrosion Potentials of Uncoated ReinforcingSteel in Concrete. ASTM International: West Conshohocken, PA, USA, 2015.
- Yang, J.; Li, J.; Lei, Z.; Zhang, P.; Wang, N.; Wang, Y. Analysis on affecting factors of concrete electric flux. China Harb. Eng. 2012, 4, 57–61. [Google Scholar]
- Chen, J.; Fan, Q.; Zhang, L. Research and development of building waterproofing agent in silicone. Zhejiang Chem. Ind. 2004, 4, 25–26. [Google Scholar]
- Wu, Z.; Lian, H. High Performance Concrete; China Railway Publishing House: Beijing, China, 1999; pp. 20–50. [Google Scholar]
- Miao, J. Experimental Investigation on the Evolution of Pore Structure and Chloride Diffusion for Cement Mortars. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2021. [Google Scholar]
- Wang, C.; Jiang, J.; Ren, C.; Sun, W.; Han, J. Progress of Preparation and Applications of Metal Oxide Nanocrystallines. China Concr. Cem. Prod. 2011, 25, 41–44.67. [Google Scholar]
- Jo, B.W.; Kim, C.H.; Tae, G.H.; Park, J.B. Characteristics of cement mortar with nano-SiO2 particles. Constr. Build. Mater. 2005, 21, 1351–1355. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Zhu, B.; Li, X. Effect of silica sol on microstructure and properties of corundum castables bonded with aluminate cement. J. Chin. Ceram. Soc. 2018, 46, 427–433. [Google Scholar]
- Lu, F.; Nie, J. Study on the application of sodium methyl silicate (silicone waterproofing agent). Jiangxi Chem. Ind. 1995, 2, 31–34. [Google Scholar]
- He, T.; Kang, Z.; Chen, C. Influence of Sodium Methyl Silicateon Waterpro of Property of Desulfurized Gypsum Block. J. Build. Mater. 2020, 2454, 012025. [Google Scholar]




| Test Items | Technical Standard | Test Result |
|---|---|---|
| Flexural strength at 3 d/MPa | ≥3.5 | 4.6 |
| Flexural strength at 28 d/MPa | ≥6.5 | 7.3 |
| Flexural strength at 3 d/MPa | ≥17.0 | 19.8 |
| Flexural strength at 28 d/MPa | ≥42.5 | 42.9 |
| Initial setting time | ≥45 | 110 |
| Final setting time | ≤390 | 350 |
| Stability | Must be qualified | qualified |
| Fineness (80) µm sieve residue (%) | ≤10.00 | 3.79 |
| Specific surface area/m2/kg | >300 | 347 |
| Chemical Composition | SiO2 | CaO | Fe2O3 | Al2O3 | MgO | Alkali Content | Loss on Ignition |
|---|---|---|---|---|---|---|---|
| content/% | 20.56 | 60.96 | 4.89 | 5.56 | 2.93 | 0.5 | 1.64 |
| ISO 679:2009 [32] | China ISO Standard Sand [33] | ||
|---|---|---|---|
| Grain Size Specification (µm) | Cumulative Sieving Amount (%) | Grain Size Specification (µm) | Cumulative Sieving Amount (%) |
| 2000 | 0 | 2000 | 0 |
| 1600 | 7 ± 5 | 1600 | 7 ± 4 |
| 1000 | 33 ± 5 | 1000 | 33 ± 4 |
| 500 | 67 ± 5 | 500 | 67 ± 4 |
| 160 | 87 ± 5 | 160 | 87 ± 4 |
| 80 | 99 ± 1 | 80 | 99 ± 1 |
| Number | Lithium Silicate | Sodium Silicate | Silica Sol | Sodium Methylsilicate | Silane Coupling Agent | Methyl Silicone Oil | Helper Component |
|---|---|---|---|---|---|---|---|
| PC1 | 40% | — | — | — | — | — | Surfactants, pH regulators, dispersants, defoamers, film-forming AIDS, etc. |
| PC2 | 40% | 10% | — | — | — | — | |
| NC1 | 40% | 10% | 30% × PC2 | — | — | — | |
| NC2 | 40% | 10% | 30% × PC2 | 5% × PC2 | — | — | |
| OL1 | 40% | 10% | — | — | 100% × PC2 | 2% × PC2 |
| Quantity of Charge q (in Coulombs) | Chloride Ion Permeability |
|---|---|
| >4000 | High |
| 2000–4000 | medium |
| 1000–2000 | low |
| 100–1000 | very low |
| <100 | could be ignored |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, J.; Pan, C.; Li, X.; Chen, K.; Chen, D. Research on Salt Corrosion Resistance of Lithium-Based Protective Coating on Mortar Substrate. Materials 2023, 16, 3463. https://doi.org/10.3390/ma16093463
Zang J, Pan C, Li X, Chen K, Chen D. Research on Salt Corrosion Resistance of Lithium-Based Protective Coating on Mortar Substrate. Materials. 2023; 16(9):3463. https://doi.org/10.3390/ma16093463
Chicago/Turabian StyleZang, Jiawei, Chonggen Pan, Xu Li, Keyu Chen, and Danting Chen. 2023. "Research on Salt Corrosion Resistance of Lithium-Based Protective Coating on Mortar Substrate" Materials 16, no. 9: 3463. https://doi.org/10.3390/ma16093463
APA StyleZang, J., Pan, C., Li, X., Chen, K., & Chen, D. (2023). Research on Salt Corrosion Resistance of Lithium-Based Protective Coating on Mortar Substrate. Materials, 16(9), 3463. https://doi.org/10.3390/ma16093463





