Mineral Phase Reconstruction and Separation Behavior of Zinc and Iron from Zinc-Containing Dust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.3. Analytical Methods
3. Results and Discussion
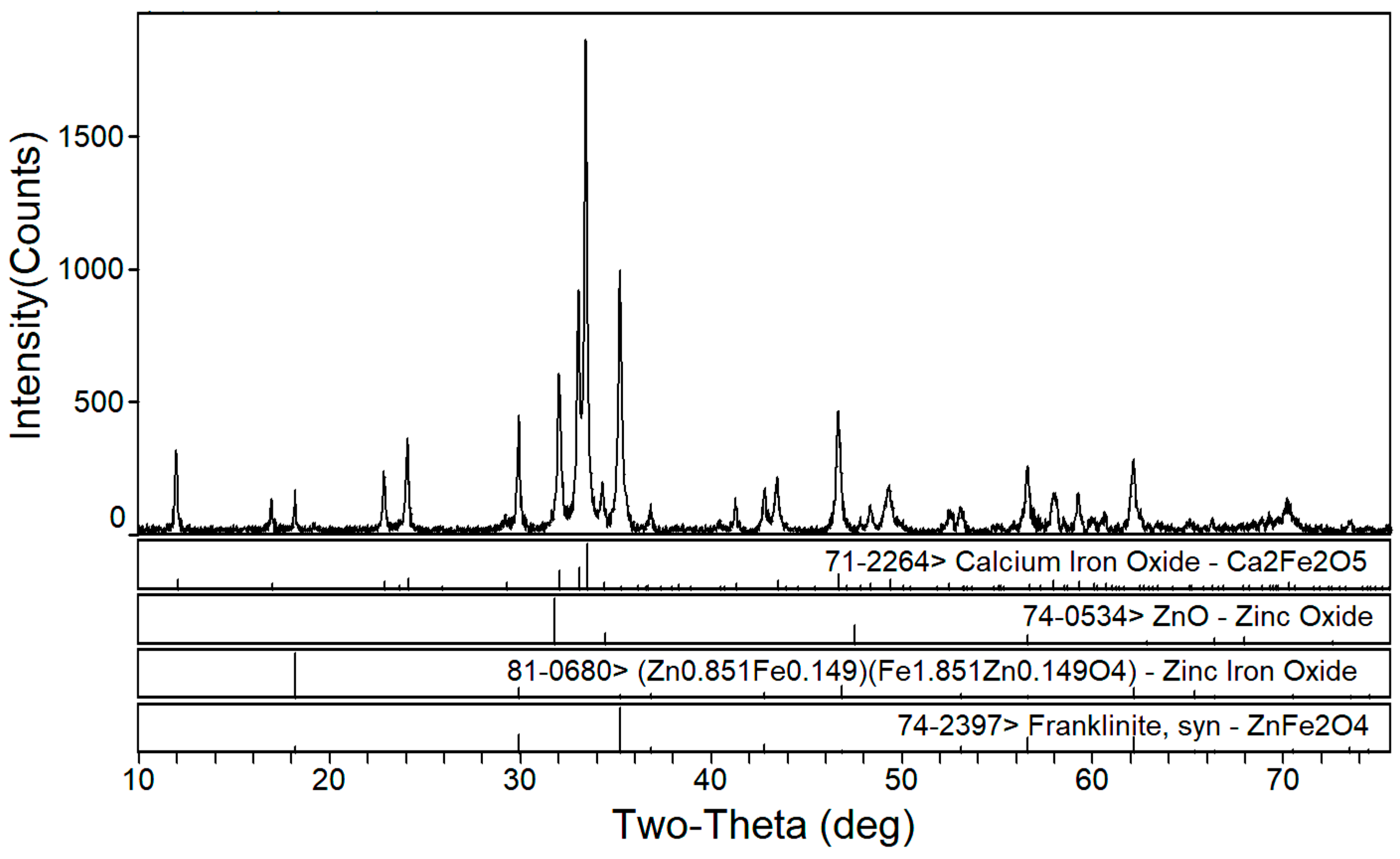
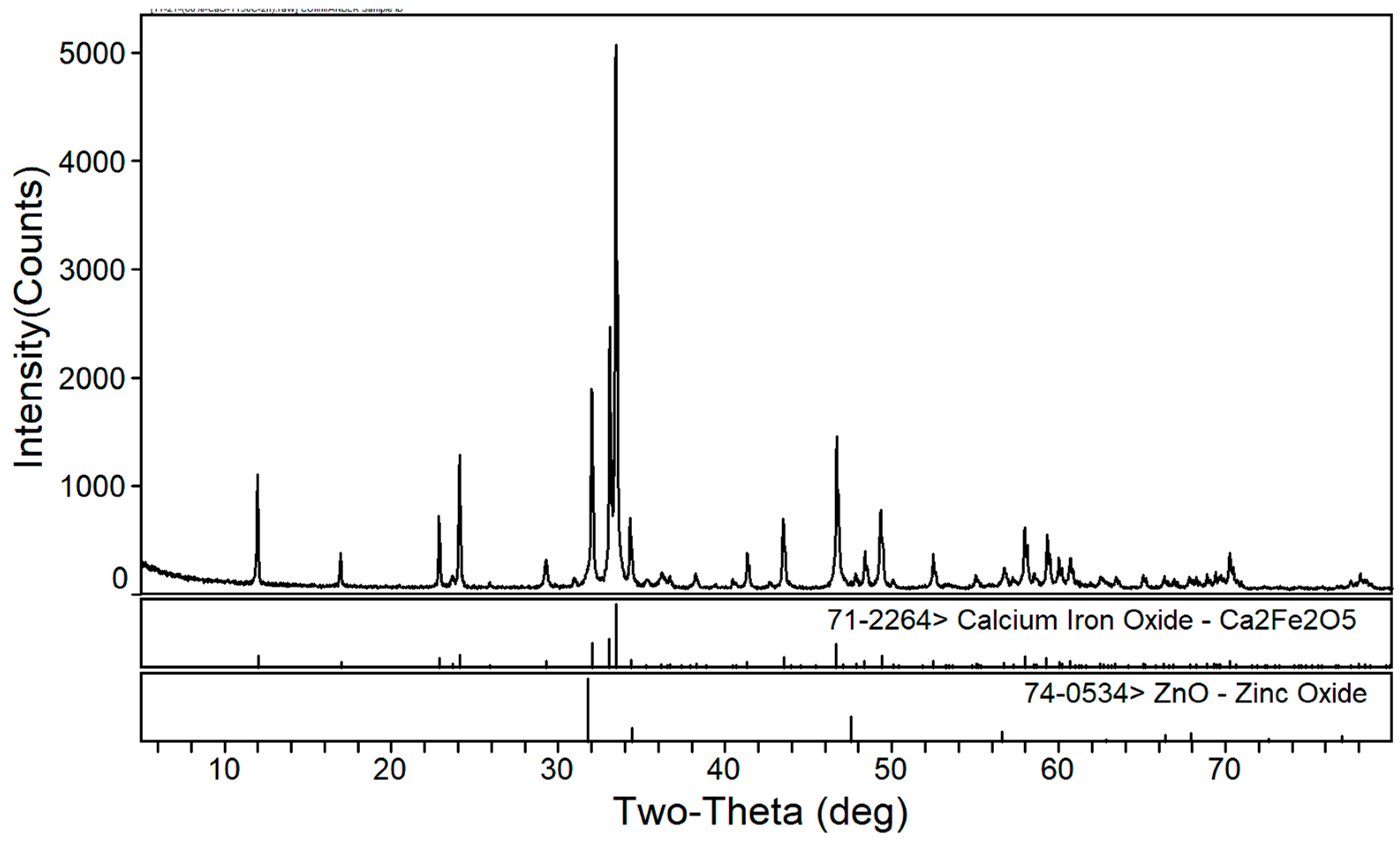
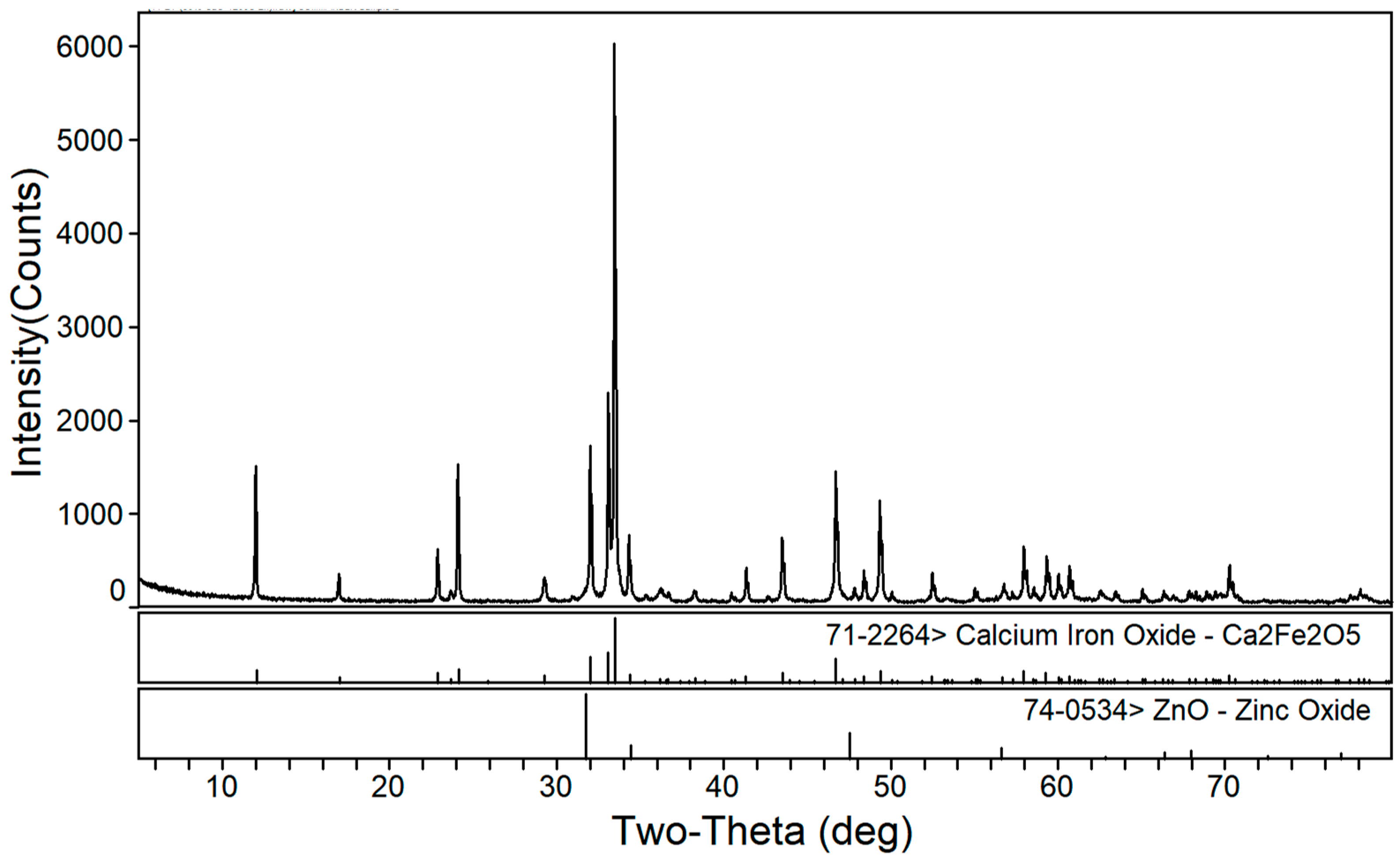
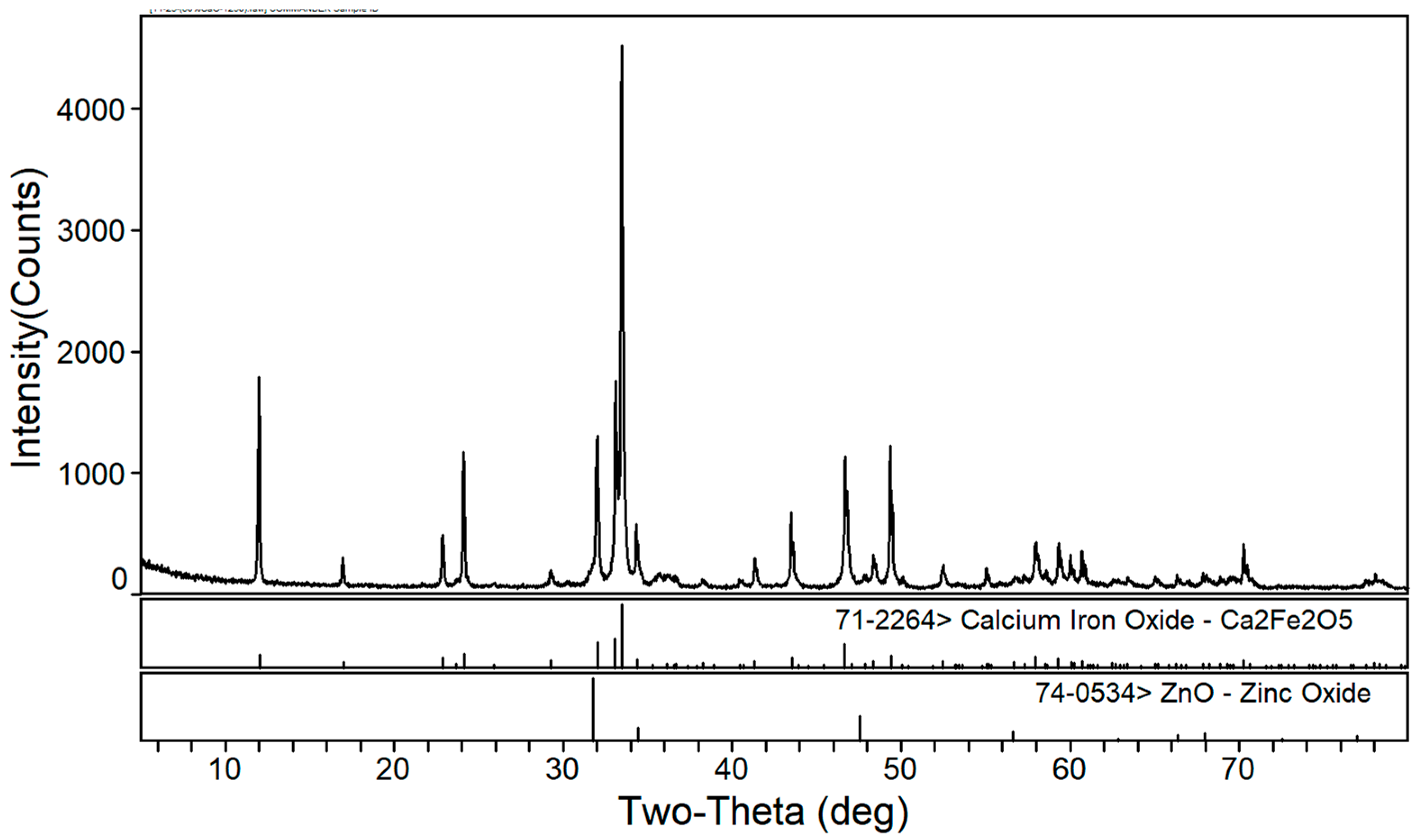
3.1. Phase Composition of the Calcium Roasting of EAF Dust
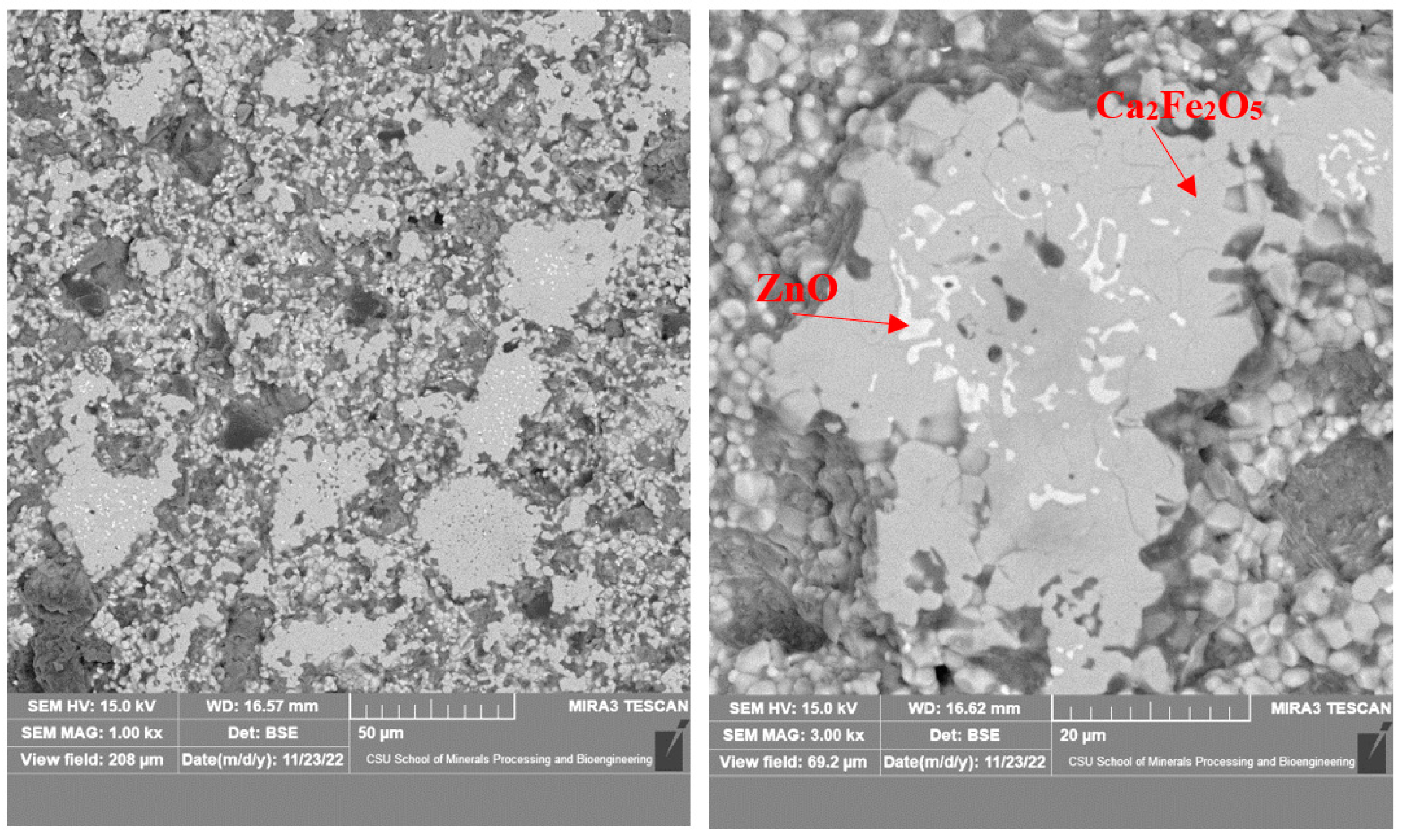
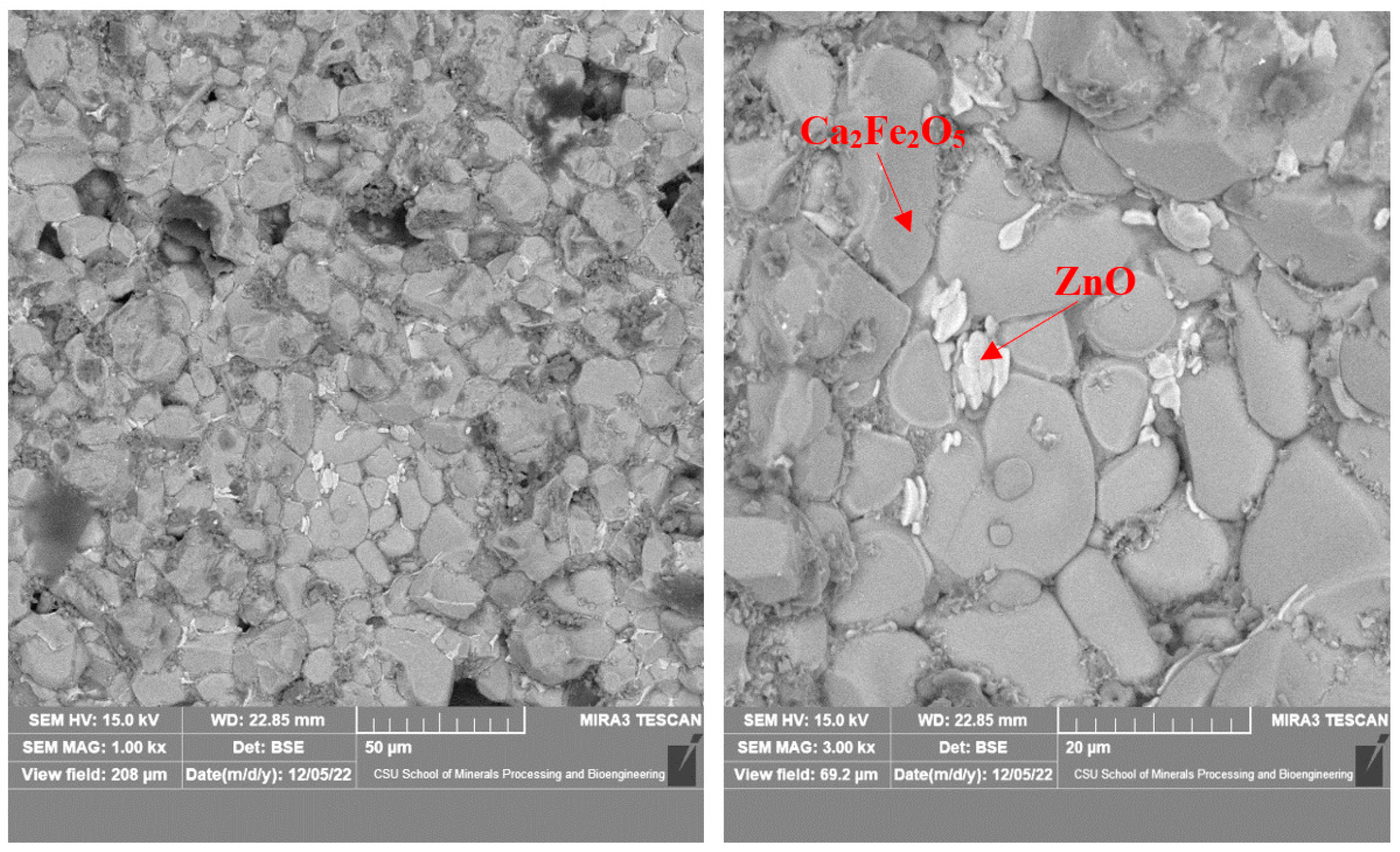
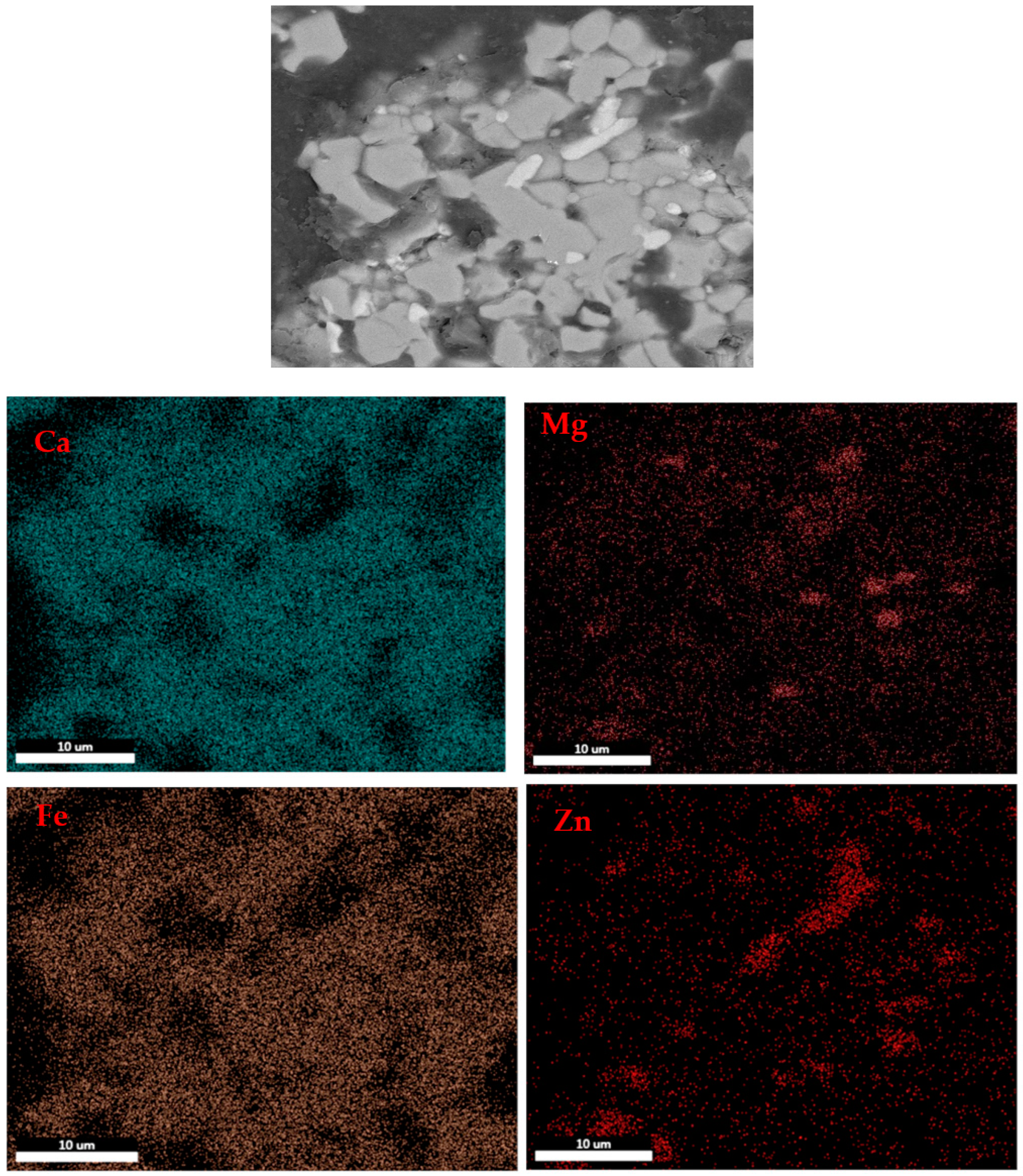
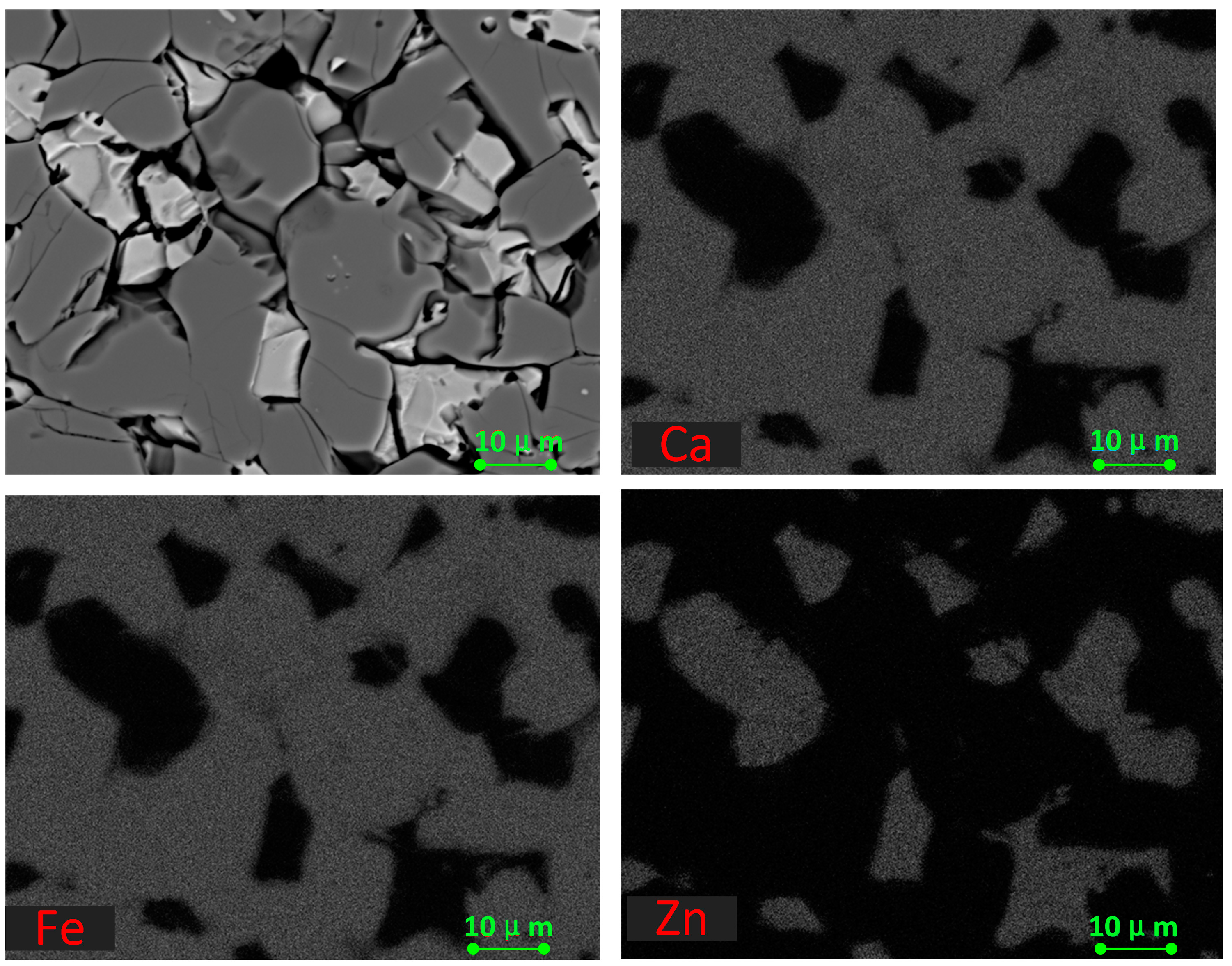
3.2. Microstructure of the Calcined Products of EAF Dust
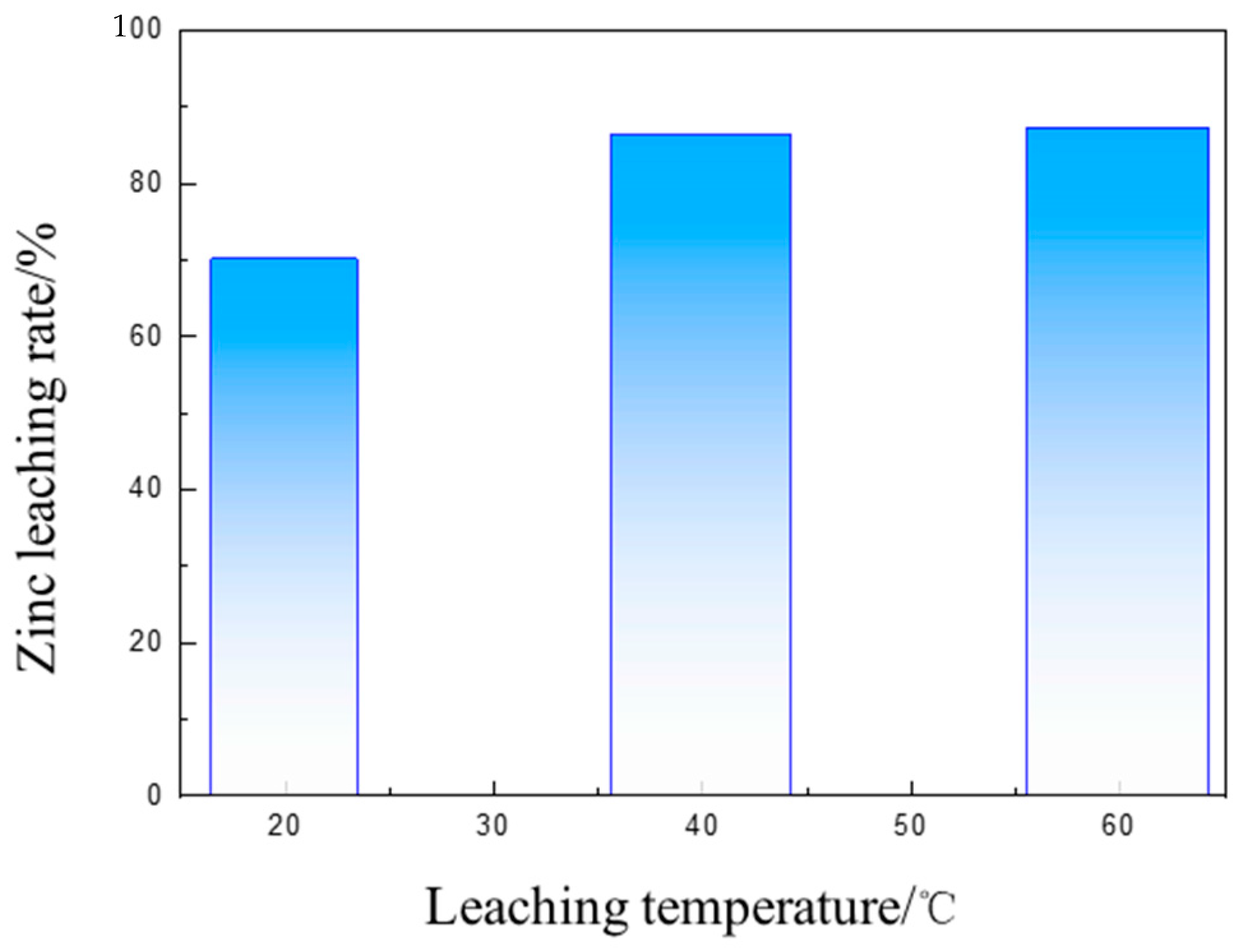
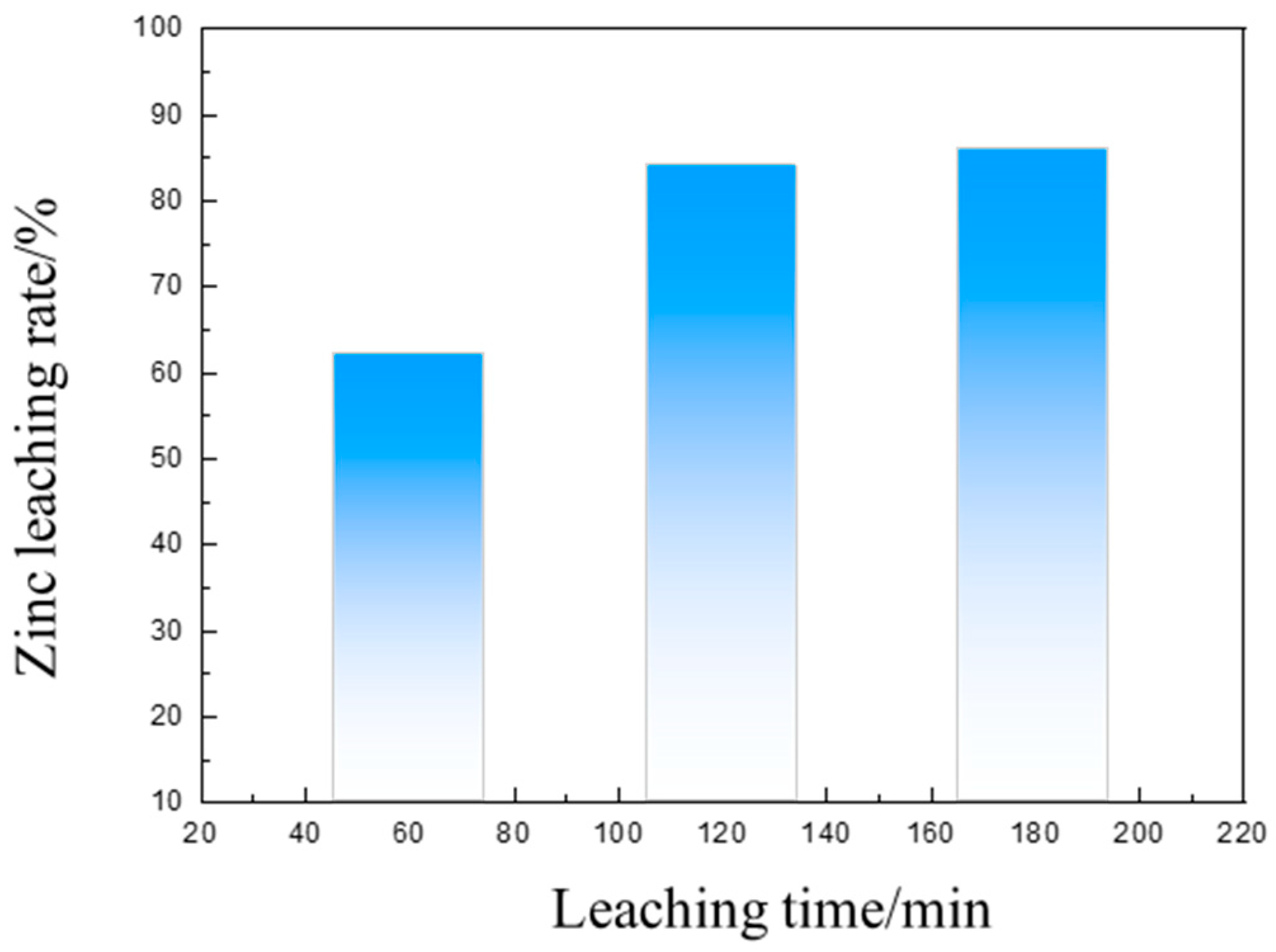
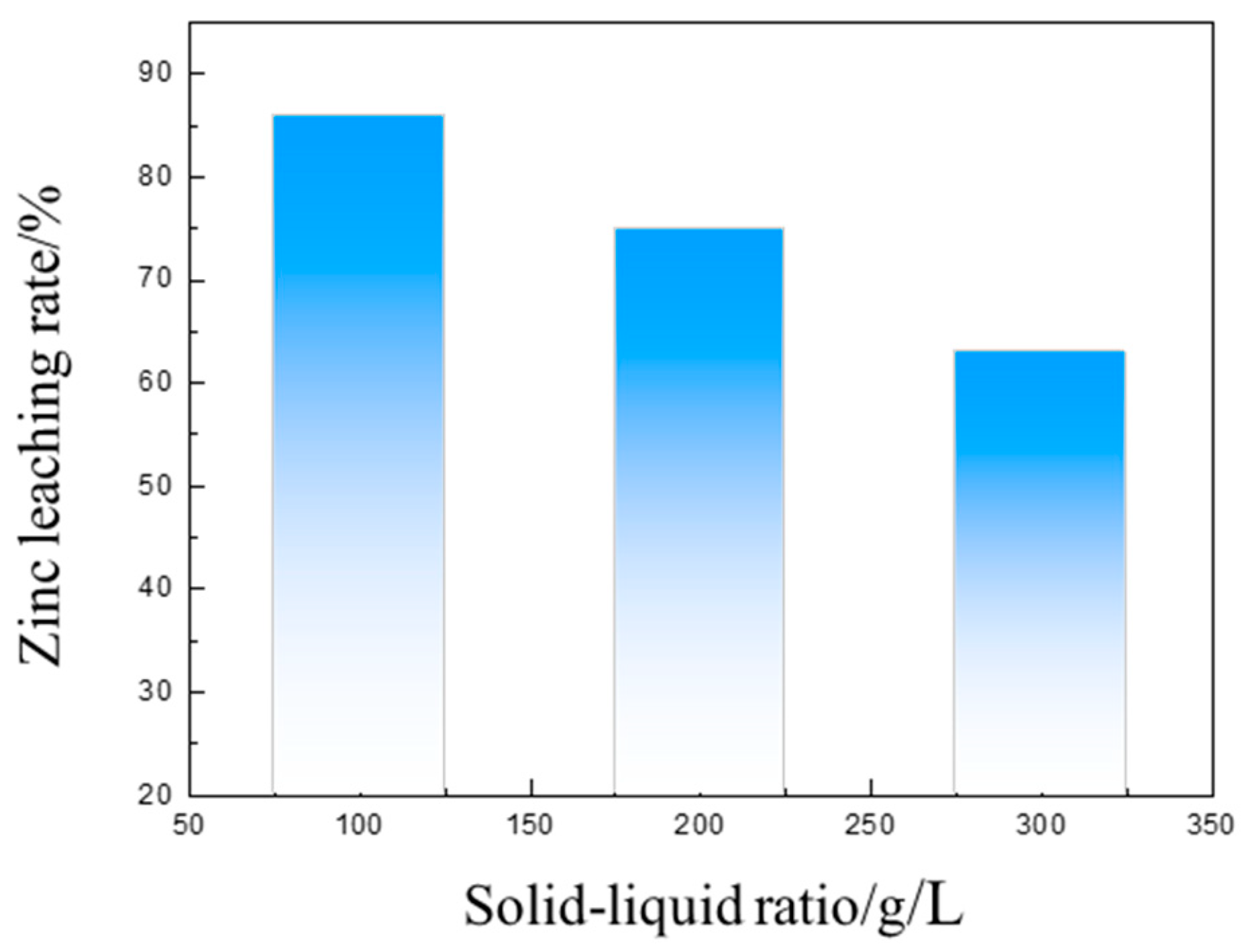
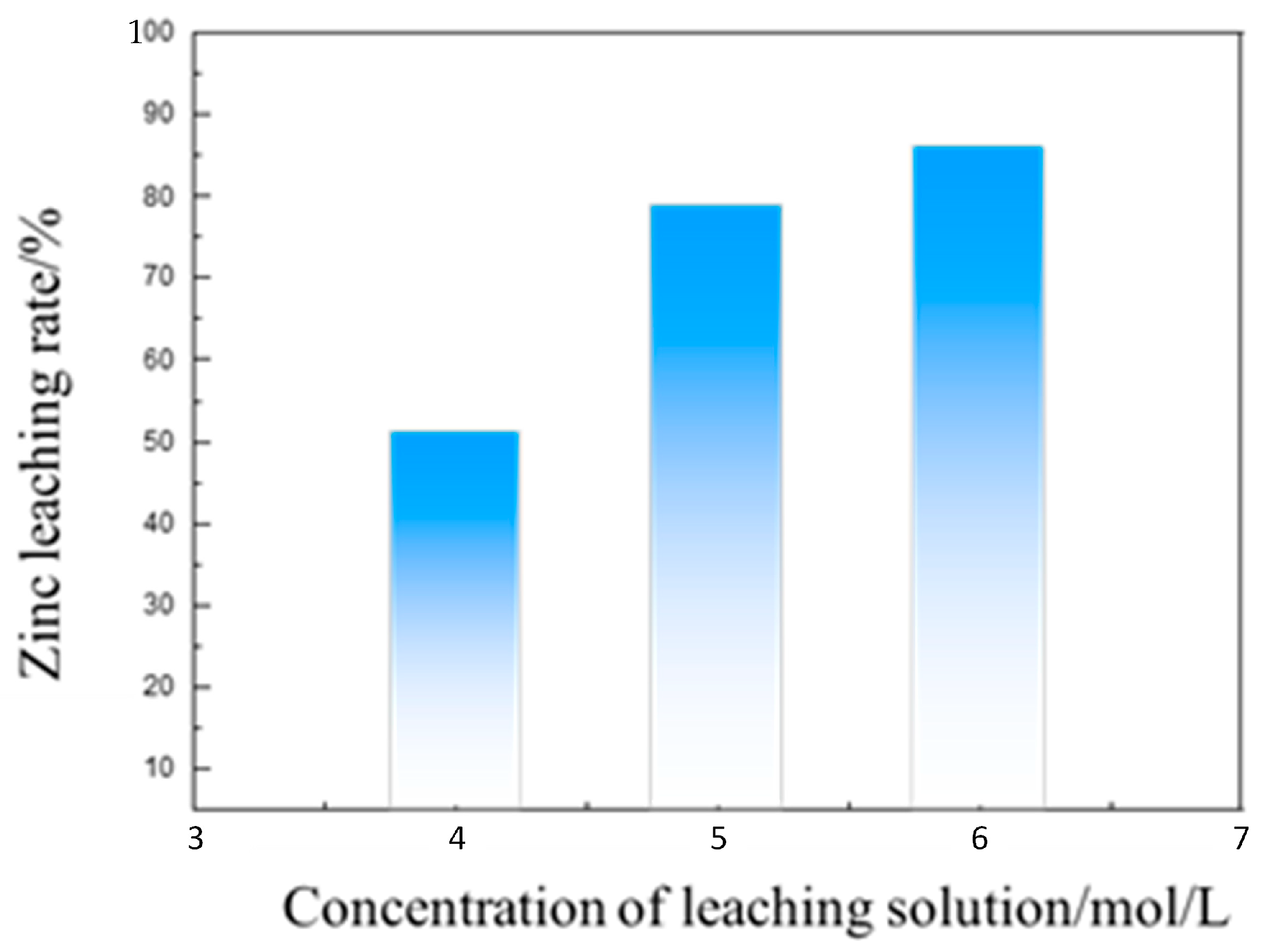
3.3. Separation of Iron and Zinc from the Calcined Products of EAF Dust by Ammonia Leaching
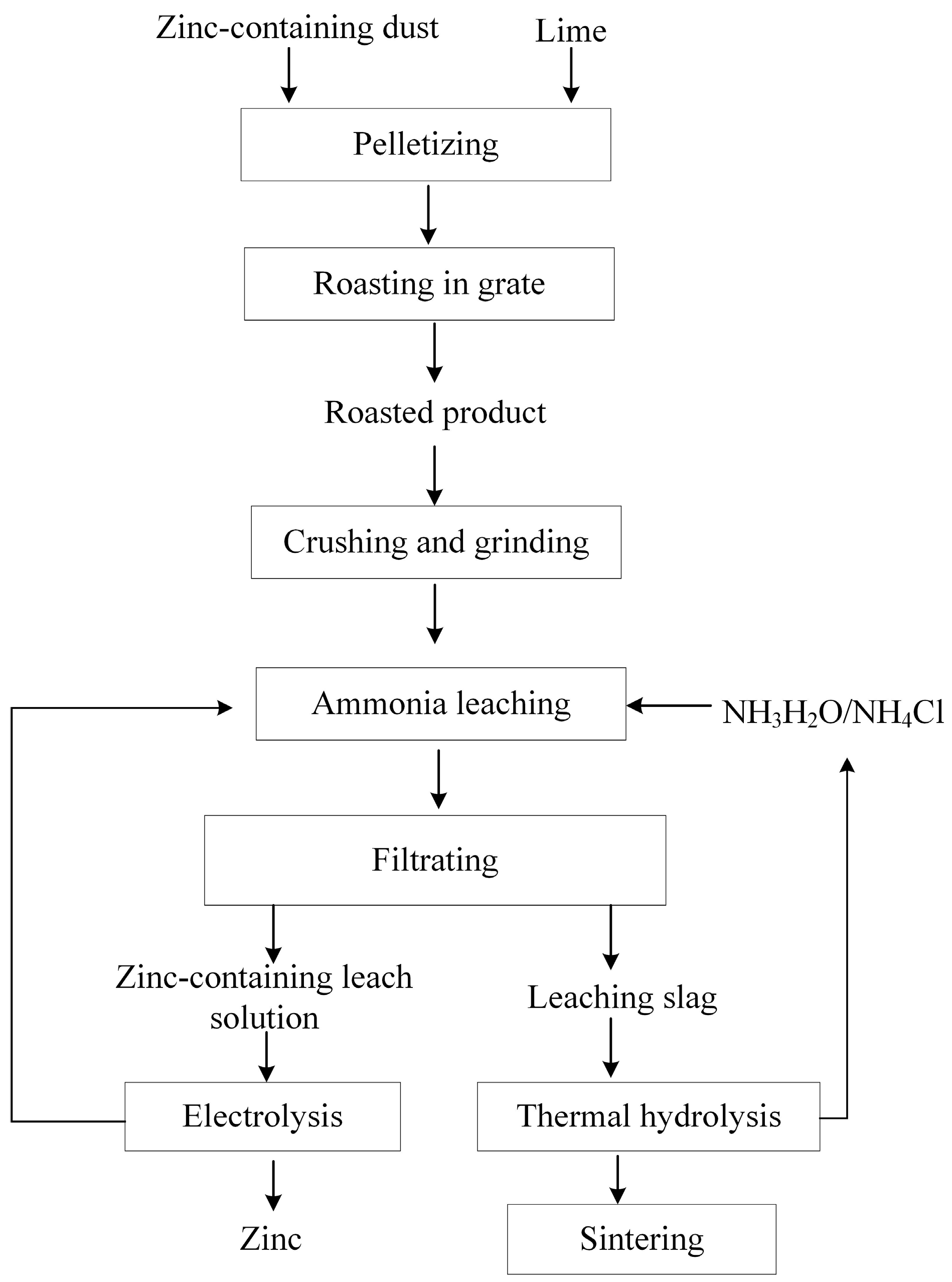
3.4. Proposed Process for the Comprehensive Utilization of Zinc and Iron in EAF Dust
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinc, J.; Kubasek, J.; Drahokoupil, J.; Capek, J.; Vojtech, D.; Skolakova, A. Microstructural and Mechanical Characterization of Newly Developed Zn-Mg-CaO Composite. Materials 2022, 15, 8703. [Google Scholar] [CrossRef]
- Martynenko, N.; Anisimova, N.; Rybalchenko, O.; Kiselevskiy, M.; Rybalchenko, G.; Tabachkova, N.; Zheleznyi, M.; Temralieva, D.; Bazhenov, V.; Koltygin, A.; et al. Structure, Biodegradation, and In Vitro Bioactivity of Zn-1%Mg Alloy Strengthened by High-Pressure Torsion. Materials 2022, 15, 9073. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Cui, K.; Fu, T.; Gao, J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust—A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Tang, H.; Peng, Z.; Wang, L.; Shang, W.; Anzulevich, A.; Rao, M.; Li, G. Facile synthesis of zinc ferrite as adsorbent from high-zinc electric arc furnace dust. Powder Technol. 2022, 405, 117479. [Google Scholar] [CrossRef]
- Pickles, C.A. Thermodynamic modelling of the formation of zinc–manganese ferrite spinel in electric arc furnace dust. J. Hazard. Mater. 2010, 179, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Shawabkeh, R.A. Hydrometallurgical extraction of zinc from Jordanian electric arc furnace dust. Hydrometallurgy 2010, 104, 61–65. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Xu, A.; Yang, Q.; He, D.; Tian, N. Carbothermic Reduction of Zinc and Iron Oxides in Electric Arc Furnace Dust. J. Iron Steel Res. Int. 2014, 21, 427–432. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Jiao, F.; Yang, C.; Li, G.; Liu, S.; Qin, W. Separation and recovery of zinc, lead and iron from electric arc furnace dust by low temperature smelting. Sep. Purif. Technol. 2023, 312, 123355. [Google Scholar] [CrossRef]
- Stewart, D.J.C.; Barron, A.R. Pyrometallurgical removal of zinc from basic oxygen steelmaking dust—A review of best available technology. Resour. Conserv. Recycl. 2020, 157, 104746. [Google Scholar] [CrossRef]
- Ji, W.; Xie, K.; Yan, S. Separation and recovery of heavy metals zinc and lead from phosphorus flue dust by vacuum metallurgy. J. Environ. Manag. 2021, 294, 113001. [Google Scholar] [CrossRef]
- Gao, J.J.; Wang, H.; Wang, J.; Zhang, Y.Y.; Wang, F.; Yang, S.; Li, S.N. A New Process of Direct Zinc Oxide Production by Carbothermal Reduction of Zinc Ash. Materials 2022, 15, 5246. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Okumura, K. Recovery of Zinc by Reaction between Electric Arc Furnace Dust and Calcium Chloride. Tetsu Hagane 2020, 106, 82–90. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Heikkinen, E.P. Selective Zinc Removal from Electric Arc Furnace (EAF) Dust by Using Microwave Heating. J. Sustain. Metall. 2019, 5, 331–340. [Google Scholar] [CrossRef]
- Li, H.; Fu, Y.T.; Liang, J.L.; Wang, L.; Yan, H.Y.; Zhao, L.F. Preparation of Zinc Oxide and Zinc Ferrite from Zinc Hypoxide by Wet Process and Electrochemistry. Crystals 2021, 11, 1133. [Google Scholar] [CrossRef]
- Santos, F.; Brocchi, E.; Araujo, V.; Souza, R. Behavior of Zn and Fe Content in Electric Arc Furnace Dust as Submitted to Chlorination Methods. Metall. Mater. Trans. B 2015, 46, 1729–1741. [Google Scholar] [CrossRef]
- Wang, H.G.; Gao, J.M.; Liu, W.W.; Zhang, M.; Guo, M. Recovery of metal-doped zinc ferrite from zinc-containing electric arc furnace dust: Process development and examination of elemental migration. Hydrometallurgy 2016, 166, 1–8. [Google Scholar] [CrossRef]
- Maiorova, A.V.; Kulikova, T.V.; Shubin, A.B. Extraction of Zinc and Arsenic from Metallurgical Furnace Dust. JOM 2021, 73, 3588–3596. [Google Scholar] [CrossRef]
- Li, Y.; Feng, H.X.; Wang, J.S.; She, X.F.; Wang, G.; Zuo, H.B.; Xue, Q.G. Current status of the technology for utilizing difficult-to-treat dust and sludge produced from the steel industry. J. Clean. Prod. 2022, 367, 132909. [Google Scholar] [CrossRef]
- Lv, W.; Gan, M.; Fan, X.H.; Sun, Z.Q.; Zhang, R.C.; Ji, Z.Y.; Chen, X.L. Reaction Behavior and Transformation Path of Zinc in the Heating-Up Zone during Sintering Process. Sustainability 2022, 14, 10147. [Google Scholar] [CrossRef]
- Wang, Y.N.; Gan, M.; Fan, X.H.; Lv, W.; Ji, Z.Y. Phase Transformation and Enhanced Zn Removal Technology During the Iron Ore Sintering Process. JOM 2022, 75, 310–320. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, D.; Pan, J.; Tian, H.; Xue, Y. A study on the zinc removal kinetics and mechanism of zinc-bearing dust pellets in direct reduction. Powder Technol. 2021, 380, 273–281. [Google Scholar] [CrossRef]
- Luo, X.; Wang, C.; Shi, X.; Li, X.; Wei, C.; Li, M.; Deng, Z. Selective separation of zinc and iron/carbon from blast furnace dust via a hydrometallurgical cooperative leaching method. Waste Manag. 2022, 139, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, X.; Zhang, N.; Guo, S.; Xie, Z.; Xu, C. A novel process for the treatment of steelmaking converter dust: Selective leaching and recovery of zinc sulfate and synthesis of iron oxides@HTCC photocatalysts by carbonizing carbohydrates. Hydrometallurgy 2023, 217, 106039. [Google Scholar] [CrossRef]
- Bruckard, W.J.; Davey, K.J.; Rodopoulos, T.; Woodcock, J.T.; Italiano, J. Water leaching and magnetic separation for decreasing the chloride level and upgrading the zinc content of EAF steelmaking baghouse dusts. Int. J. Miner. Process. 2005, 75, 1–20. [Google Scholar] [CrossRef]
- Kelebek, S.; Yörük, S.; Davis, B. Characterization of basic oxygen furnace dust and zinc removal by acid leaching. Miner. Eng. 2004, 17, 285–291. [Google Scholar] [CrossRef]
- Oustadakis, P.; Tsakiridis, P.E.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD): Part I: Characterization and leaching by diluted sulphuric acid. J. Hazard. Mater. 2010, 179, 1–7. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, Y.; Kang, Y.; Gao, J.; Guo, Z. Promoted acid leaching of Zn from hazardous zinc-containing metallurgical dusts: Focusing on transformation of Zn phases in selective reduction roasting. Process Saf. Environ. 2022, 163, 353–361. [Google Scholar] [CrossRef]
- Zhang, E.; Zhou, K.; Zhang, X.; Wu, Y.; Liu, J.; Chen, W.; Peng, C. Selective separation of copper and zinc from high acid leaching solution of copper dust using a sulfide precipitation-pickling approach. Process Saf. Environ. 2021, 156, 100–108. [Google Scholar] [CrossRef]
- Chairaksa-Fujimoto, R.; Maruyama, K.; Miki, T.; Nagasaka, T. The selective alkaline leaching of zinc oxide from Electric Arc Furnace dust pre-treated with calcium oxide. Hydrometallurgy 2016, 159, 120–125. [Google Scholar] [CrossRef]
- Yakornov, S.A.; Panshin, A.M.; Grudinsky, P.I.; Dyubanov, V.G.; Leontiev, L.I.; Kozlov, P.A.; Ivakin, D.A. Method of Electric Arc Furnace Dust Processing by Calcination with Lime with Following Alkaline Leaching. Russ. Metall. 2018, 2018, 1282–1287. [Google Scholar] [CrossRef]
- Jaafar, I.; Griffiths, A.J.; Hopkins, A.C.; Steer, J.M.; Griffiths, M.H.; Sapsford, D.J. An evaluation of chlorination for the removal of zinc from steelmaking dusts. Miner. Eng. 2011, 24, 1028–1030. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T. Utilization of blast furnace sludge for the removal of zinc from steelmaking dusts using microwave heating. Sep. Purif. Technol. 2019, 210, 867–884. [Google Scholar] [CrossRef]
- Gargul, K.; Boryczko, B. Removal of zinc from dusts and sludges from basic oxygen furnaces in the process of ammoniacal leaching. Arch. Civ. Mech. Eng. 2015, 15, 179–187. [Google Scholar] [CrossRef]
- Lv, W.; Gan, M.; Fan, X.; Ji, Z.; Chen, X. Mechanism of calcium oxide promoting the separation of zinc and iron in metallurgical dust under reducing atmosphere. J. Mater. Res. Technol. 2019, 8, 5745–5752. [Google Scholar] [CrossRef]
- Yakornov, S.A.; Pan’Shin, A.M.; Grudinsky, P.I.; Dyubanov, V.G.; Leont’Ev, L.I.; Kozlov, P.A.; Ivakin, D.A. Thermodynamic Analysis of Zinc Ferrite Decomposition in Electric Arc Furnace Dust by Lime. Russ. J. Non-Ferr. Met. 2017, 58, 586–590. [Google Scholar] [CrossRef]
- Chairaksa-Fujimoto, R.; Inoue, Y.; Umeda, N.; Itoh, S.; Nagasaka, T. New pyrometallurgical process of EAF dust treatment with CaO addition. Int. J. Min. Met. Mater. 2015, 22, 788–797. [Google Scholar] [CrossRef]
- Wang, W.A.; Li, X.M.; He, Y.; Xi, H.D.; Wang, J.L.; Qiu, G.X. Effect of CaCO3 on volatilization of self-reduced zinc from blast furnace dust. J. Iron Steel Res. Int. 2022, 29, 1404–1411. [Google Scholar] [CrossRef]
- Xie, Z.Q.; Jiang, T.; Chen, F.; Guo, Y.F.; Wang, S.; Yang, L.Z. Phase Transformation and Zinc Extraction from Zinc Ferrite by Calcium Roasting and Ammonia Leaching Process. Crystals 2022, 12, 641. [Google Scholar] [CrossRef]
- GB/T 8151.1-2012; Methods for Chemical Analysis of Zinc Concentrates—Part 1:Determination of Zinc Content—Precipitate Separation-Na2EDTA Titrimetric Method and Extractive Separation-Na2EDTA Titrimetric Method. State Standardization Administration of China: Beijing, China, 2012.
- Jasso-Terán, R.A.; Cortés-Hernández, D.A.; Sánchez-Fuentes, H.J.; Reyes-Rodríguez, P.Y.; De-León-Prado, L.E.; Escobedo-Bocardo, J.C.; Almanza-Robles, J.M. Synthesis, characterization and hemolysis studies of Zn1−x CaxFe2O4 ferrites synthesized by sol-gel for hyperthermia treatment applications. J. Magn. Magn. Mater. 2017, 427, 241–244. [Google Scholar] [CrossRef]





| Fe | O | Zn | Ca | Mn | Na | Cl | Si |
|---|---|---|---|---|---|---|---|
| 50 | 29 | 8 | 3 | 2 | 2 | 2 | 1 |
| Tfe | SiO2 | ZnO |
|---|---|---|
| 52.34 | 2.27 | 9.41 |
| Element | 1 (ZnO) | 2 (ZnO) | 3 (Ca2Fe2O5) |
|---|---|---|---|
| O | 16.11 | 16.83 | 16.57 |
| Mg | 3.98 | 5.96 | 0.95 |
| Ca | 3.06 | 2.48 | 34.35 |
| Fe | 6.07 | 5.45 | 41.68 |
| Zn | 70.79 | 69.28 | 6.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Li, G.; Guo, Y.; Wang, S.; Chen, F.; Yang, L.; Fu, G.; Jiang, T. Mineral Phase Reconstruction and Separation Behavior of Zinc and Iron from Zinc-Containing Dust. Materials 2023, 16, 3481. https://doi.org/10.3390/ma16093481
Xie Z, Li G, Guo Y, Wang S, Chen F, Yang L, Fu G, Jiang T. Mineral Phase Reconstruction and Separation Behavior of Zinc and Iron from Zinc-Containing Dust. Materials. 2023; 16(9):3481. https://doi.org/10.3390/ma16093481
Chicago/Turabian StyleXie, Zeqiang, Guang Li, Yufeng Guo, Shuai Wang, Feng Chen, Lingzhi Yang, Ganghua Fu, and Tao Jiang. 2023. "Mineral Phase Reconstruction and Separation Behavior of Zinc and Iron from Zinc-Containing Dust" Materials 16, no. 9: 3481. https://doi.org/10.3390/ma16093481






