Cysteine as an Alternative Eco-Friendly Corrosion Inhibitor for Absorption-Based Carbon Capture Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tested Specimens and Absorption Solutions
2.2. Electrochemical Measurements
2.3. Weight Loss Corrosion Measurements
3. Results and Discussion
3.1. Electrochemical Inhibition Performance
3.1.1. Effect of Cysteine Concentration
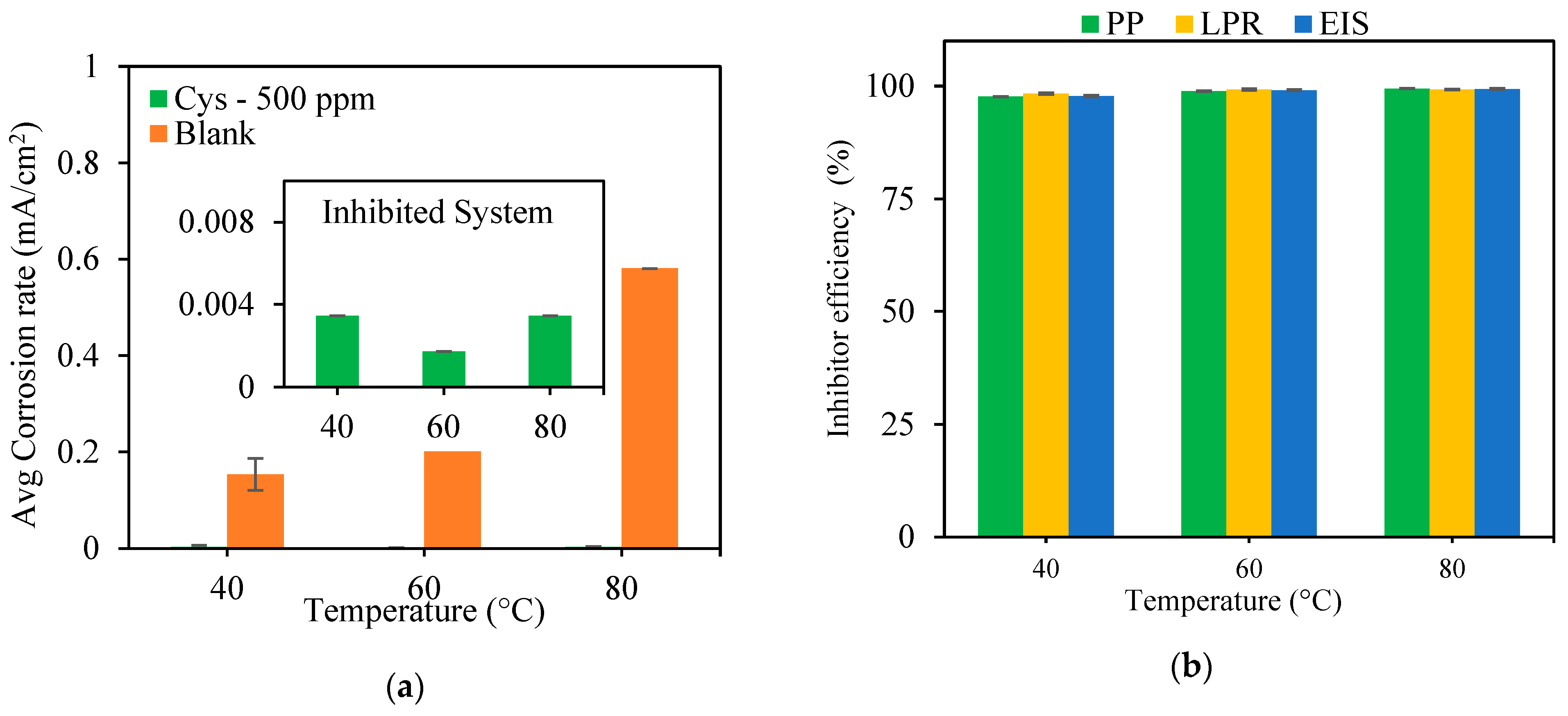
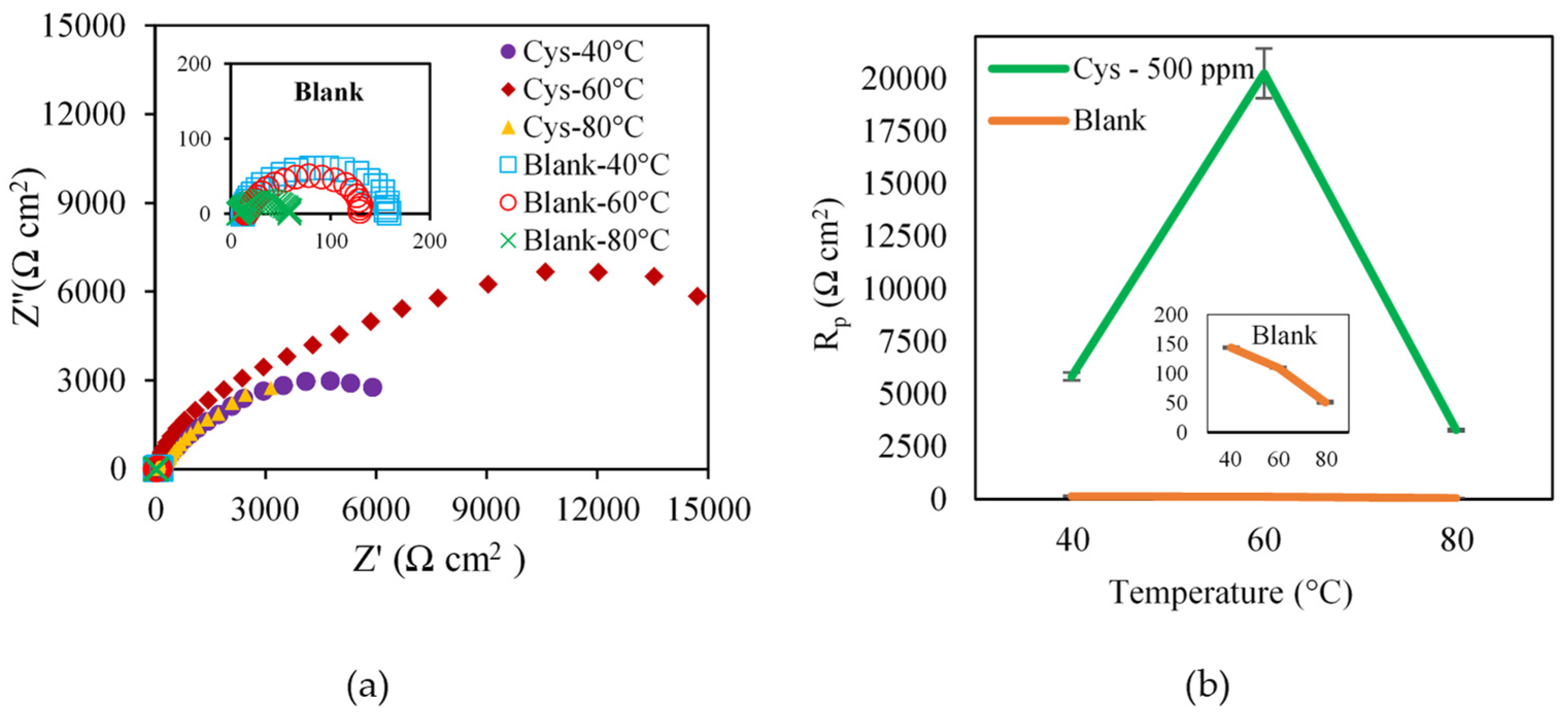
3.1.2. Effect of Temperature
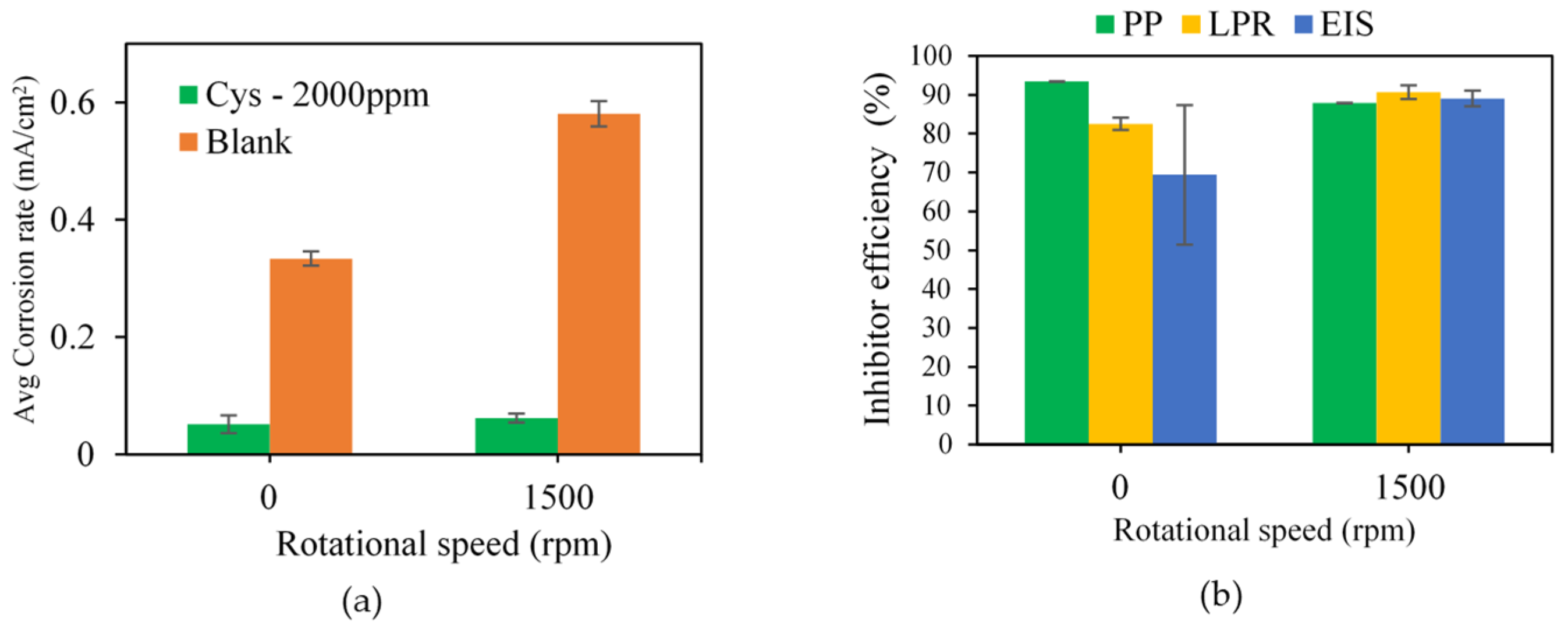
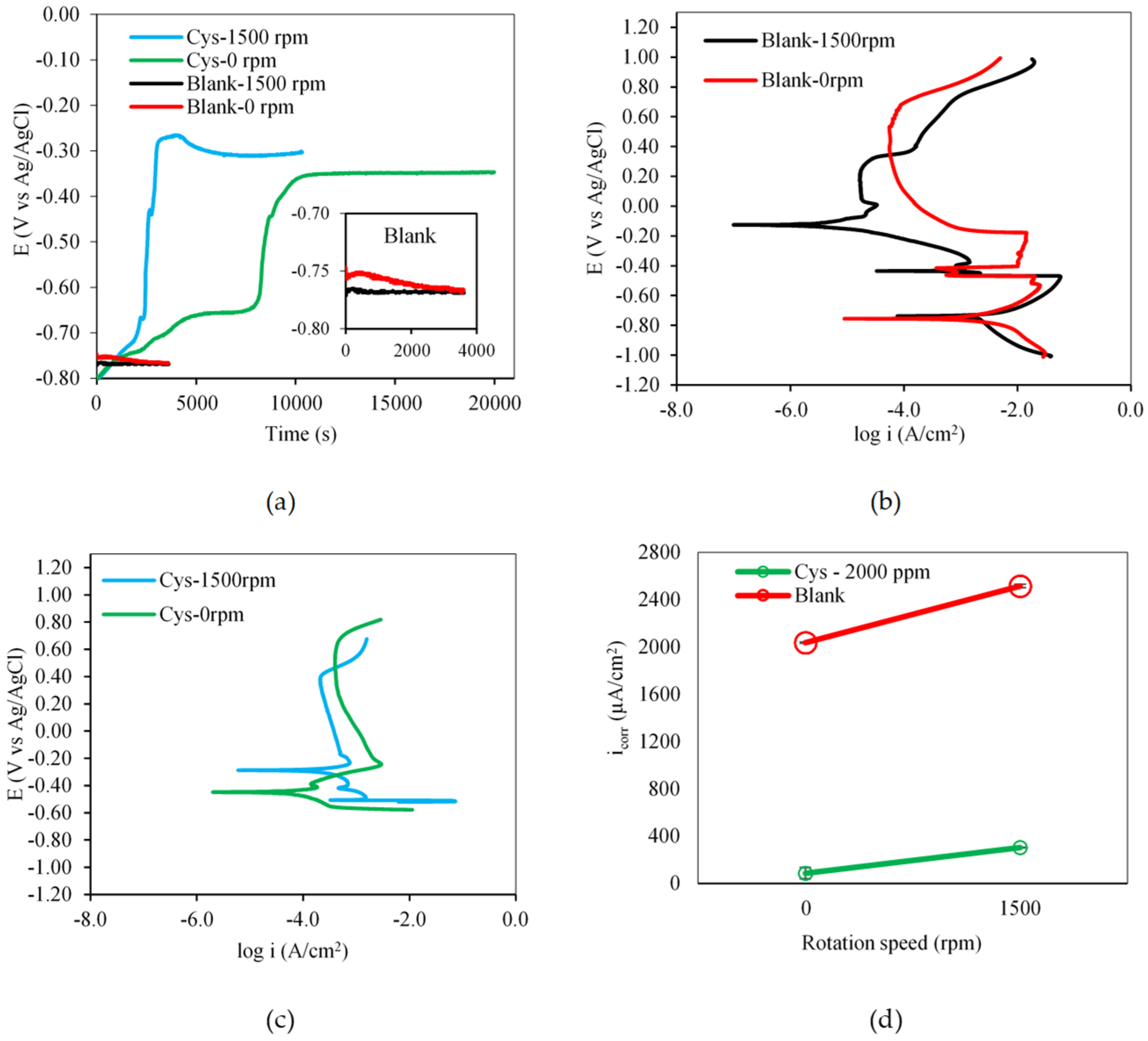
3.1.3. Effect of Flow Condition
3.1.4. Post Analysis
3.2. Weight Loss Inhibition Performance
3.3. Quantum Chemical Analysis
3.4. Corrosion Inhibition Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kohl, A.L.; Nielsen, R.B. Gas Purification. In Chemical Engineering Progress, 5th ed.; Gulf Publishing Company: Houston, TX, USA, 1997. [Google Scholar] [CrossRef]
- Sekkappan, S. Performance Evaluation of Condiments as Environmentally Friendly Corrosion Inhibitors for Amine-Based Carbon Dioxide Absorption Process. Master’s Thesis, University of Regina, Regina, SK, Canada, 2018. [Google Scholar]
- Suba, P.I.V. Corrsoion Inhibition Performance of Sulfur and Nitrogen Based Environmentally Friendly Inhibitor for CO2 Absorption Plants. Master’s Thesis, University of Regina, Regina, SK, Canada, 2020. [Google Scholar]
- Veawab, A.; Tontiwachwuthikul, P.; Chakma, A. Investigation of low-toxic organic corrosion inhibitors for CO2 separation process using aqueous MEA solvent. Ind. Eng. Chem. Res. 2001, 40, 4771–4777. [Google Scholar] [CrossRef]
- Srinivasan, S. Environmentally Friendly Corrosion Inhibitors for the Amine-Based CO2 Absorption Process. Master’s Thesis, University of Regina, Regina, SK, Canada, 2012. [Google Scholar]
- Talkhan, A.G.; Benamor, A.; Nasser, M.S. Corrosion study of carbon steel in CO2 loaded amine-amino acid solutions—Case of mixtures of N-Methyldiethanolamine and L-Arginine. IOP Conf. Ser. Earth Environ. Sci. 2018, 164, 012028. [Google Scholar] [CrossRef]
- Udayappan, B.; Veawab, A. Performance Analysis of Methionine as an Environmentally Friendly Corrosion Inhibitor for Carbon Steel in the Amine Based Carbon Capture Process. Int. J. Greenh. Gas Control 2022, 114, 103565. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Srivastava, V.; Haque, J.; Ibrahimi, B.E. Virgin and chemically functionalized amino acids as green corrosion inhibitors: Influence of molecular structure through experimental and in silico studies. J. Mol. Struct. 2021, 1226, 129259. [Google Scholar] [CrossRef]
- Pubchem. Chemical Information Database. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 August 2022).
- Sigma Aldrich. L-Cysteine—Material Safety Data Sheet. 2020. Available online: https://www.sigmaaldrich.com/CA/en/sds/aldrich/168149 (accessed on 9 July 2022).
- Thermo Fisher Scientific. Material Safety Data Sheet; Thermo Fisher Scientific: Waltham, MA, USA, 2021; Volume 4, Available online: https://us.vwr.com/assetsvc/asset/en_US/id/16490607/contents (accessed on 2 July 2022).
- Hamadi, L.; Mansouri, S.; Oulmi, K.; Kareche, A. The use of amino acids as corrosion inhibitors for metals: A review. Egypt. J. Pet. 2018, 27, 1157–1165. [Google Scholar] [CrossRef]
- Ibrahimi, B.E.; Jmiai, A.; Bazzi, L.; Issami, S.E. Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arab. J. Chem. 2020, 13, 740–771. [Google Scholar] [CrossRef]
- Fu, J.-J.; Li, S.-N.; Wang, Y.; Liu, X.-D.; Lu, L.-D. Computational and electrochemical studies on the inhibition of corrosion of mild steel by L -Cysteine and its derivatives. J. Mater. Sci. 2011, 46, 3550–3559. [Google Scholar] [CrossRef]
- Raja, A.S.; Venkatesan, R.; Sonisheeba, R.; Raj, J.; Sivakumar, S.; Angel, P.; Sathiyabama, J. Corrosion Inhibition by Cysteine—An Overview. Int. J. Adv. Res. Chem. Sci. 2014, 1, 101–109. [Google Scholar]
- Zhang, C.; Duan, H.; Zhao, J. Synergistic inhibition effect of imidazoline derivative and l-cysteine on carbon steel corrosion in a CO2-saturated brine solution. Corros. Sci. 2016, 112, 160–169. [Google Scholar] [CrossRef]
- ASTM G1-03(2017)e1; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of Cyclic Potentiodynamic Polarization Test Results for Study of Corrosion Behavior of Metals: A Review. Prot. Met. Phys. Chem. Surf. 2018, 54, 976–989. [Google Scholar] [CrossRef]
- ASTM F2129-15(2015); Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices. ASTM International: West Conshohocken, PA, USA, 2015; pp. 1–8. [CrossRef]
- Idris, M.N.; Daud, A.R.; Othman, N.K. Electrochemical impedance spectroscopy study on corrosion inhibition of benzyltriethylammonium chloride. Proc. AIP Conf. 2013, 1571, 23–28. [Google Scholar] [CrossRef]
- ASTM G102-89(2015)e1; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2015; pp. 1–7. [CrossRef]
- Srinivasan, S.; Veawab, A.; Aroonwilas, A. Screening and Evaluating Environmentally-Friendly Corrosion Inhibitors for Amine-Based CO2 Absorption Process. In Corrosion Inhibitors, Principles and Recent Applications; InTech: London, UK, 2018; pp. 225–251. [Google Scholar] [CrossRef]
- Papavinasam, S.; Revie, R.W. Review of testing methods and standards for oil field corrosion inhibitors. In Proceedings of the EUROCORR 2004—European Corrosion Conference: Long Term Prediction and Modelling of Corrosion, Nice, France, 12–16 September 2004; pp. 1–10. [Google Scholar]
- Hosseini, M.; Mertens, S.F.L.; Arshadi, M.R. Synergism and antagonism in mild steel corrosion inhibition by sodium dodecylbenzene sulphonate and hexamethylenetetramine. Corros. Sci. 2003, 45, 1473–1489. [Google Scholar] [CrossRef]
- Tchoumene, R.; Dedzo, G.K.; Ngameni, E. Intercalation of 1,2,4-triazole in methanol modified-kaolinite: Application for copper corrosion inhibition in concentrated sodium chloride aqueous solution. J. Solid State Chem. 2022, 311, 123103. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Saji, V.S. 2—Experimental methods of inhibitor evaluation. In Heterocyclic Organic Corrosion Inhibitors: Principles and Applications; Quraishi, M.A., Chauhan, D.S., Saji, V.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 21–57. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Rameshkumar, S.; Danaee, I.; Rashvandavei, M.; Vijayan, M. Quantum chemical and experimental investigations on equipotent effects of (+)R and (−)S enantiomers of racemic amisulpride as eco-friendly corrosion inhibitors for mild steel in acidic solution. J. Mol. Liq. 2015, 212, 168–186. [Google Scholar] [CrossRef]
- Branzoi, I.V.; Codescu, M. Electrochemical studies on the stability and corrosion resistance of new zirconium-based alloys for biomedical applications. Surf. Interface Anal. 2008, 40, 167–173. [Google Scholar] [CrossRef]
- Desimone, M.P.; Grundmeier, G.; Gordillo, G.; Simison, S.N. Amphiphilic amido-amine as an effective corrosion inhibitor for mild steel exposed to CO2 saturated solution: Polarization, EIS and PM-IRRAS studies. Electrochim. Acta 2011, 56, 2990–2998. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Yu, L.; Zou, Y.; Wang, Y. An investigation of a combined thiourea and hexamethylenetetramine as inhibitors for corrosion of N80 in 15% HCl solution: Electrochemical experiments and quantum Chemical Calculation. Int. J. Corros. 2015, 2015, 548031. [Google Scholar] [CrossRef]
- Solmaz, R. Investigation of corrosion inhibition mechanism and stability of vitamin B1 on mild steel in 0.5 M HCl solution. Corros. Sci. 2014, 81, 75–84. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Fu, H. Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corros. Sci. 2012, 62, 163–175. [Google Scholar] [CrossRef]
- Abderrahim, K.; Chouchane, T.; Selatnia, I.; Sid, A.; Mosset, P. Evaluation of the effect of Tetramethylammonium hydroxide on the corrosion inhibition of A9M steel in industrial water: An experimental, morphological and MD simulation insights. Chem. Data Collect. 2020, 28, 100391. [Google Scholar] [CrossRef]
- Ghames, A. Theoretical and experimental studies of adsorption characteristics of newly synthesized Schiff bases and their evaluation as corrosion inhibitors for mild steel in 1 M HCl. Int. J. Electrochem. Sci. 2017, 12, 4867–4897. [Google Scholar] [CrossRef]
- Ituen, E.; Akaranta, O.; James, A.O. Evaluation of performance of corrosion inhibitors using adsorption isotherm models: An overview. Chem. Sci. Int. J. 2017, 18, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Saadi, R.; Saadi, Z.; Fazaeli, R.; Fard, N.E. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Hazazi, O.A.; Fawzy, A.; Awad, M.I. Sulfachloropyridazine as an eco-friendly inhibitor for corrosion of mild steel in H2SO4 solution. Chem. Sci. Rev. Lett. 2015, 4, 67–79. [Google Scholar]
- Pournazari, S.; Moayed, M.H.; Rahimizadeh, M. In situ inhibitor synthesis from admixture of benzaldehyde and benzene-1,2-diamine along with FeCl3 catalyst as a new corrosion inhibitor for mild steel in 0.5 M sulphuric acid. Corros. Sci. 2013, 71, 20–31. [Google Scholar] [CrossRef]
- Ituen, E.; Mkpenie, V.; Ekemini, E. Corrosion inhibition of X80 steel in simulated acid wash solution using glutathione and its blends: Experimental and theoretical studies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123597. [Google Scholar] [CrossRef]
- Obot, I.B.; Macdonald, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors: Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Mehmeti, V.V.; Berisha, A.R. Corrosion study of mild steel in aqueous sulfuric acid solution using 4-methyl-4h-1,2,4-triazole-3-thiol and 2-mercaptonicotinic acid—An experimental and theoretical study. Front. Chem. 2017, 5, 61. [Google Scholar] [CrossRef]
- Lukovits, I.; Kosztolányi, T.; Kálmán, E.; Pálinkás, G. Corrosion inhibitors: Correlation between chemical structure and efficiency. In Proceedings of the Corrosion 99, San Antonio, TX, USA, 25–30 April 1999. Paper Number: NACE-99242. [Google Scholar]
- Zheng, L.; Matin, N.S.; Landon, J.; Thomas, G.A.; Liu, K. CO2 loading-dependent corrosion of carbon steel and formation of corrosion products in anoxic 30 wt.% monoethanolamine-based solutions. Corros. Sci. 2016, 102, 44–54. [Google Scholar] [CrossRef]
- Amin, M.A.; Khaled, K.F.; Mohsen, Q.; Arida, H.A. A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros. Sci. 2010, 52, 1684–1695. [Google Scholar] [CrossRef]
- Farahati, R.; Mousavi-khoshdel, S.M.; Ghaffarinejad, A.; Behzadi, H. Experimental and computational study of penicillamine drug and cysteine as water-soluble green corrosion inhibitors of mild steel. Prog. Org. Coat. 2020, 142, 105567. [Google Scholar] [CrossRef]
- Preethi, K.; Shetty, P.; Sunil, D. Effect of cysteine as environmentally friendly inhibitor on AA6061-T6 corrosion in 0.5 M HCl: Electrochemical and surface studies. Surf. Eng. Appl. Electrochem. 2020, 56, 624–634. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Wu, Y. The inhibition effect and mechanism of L -cysteine on the corrosion of bronze covered with a CuCl patina. Corros. Sci. 2015, 97, 89–99. [Google Scholar] [CrossRef]
- Morad, M.S. Effect of amino acids containing sulfur on the corrosion of mild steel in phosphoric acid solutions containing Cl−, F− and Fe3+ ions: Behavior under polarization conditions. J. Appl. Electrochem. 2005, 35, 889–895. [Google Scholar] [CrossRef]

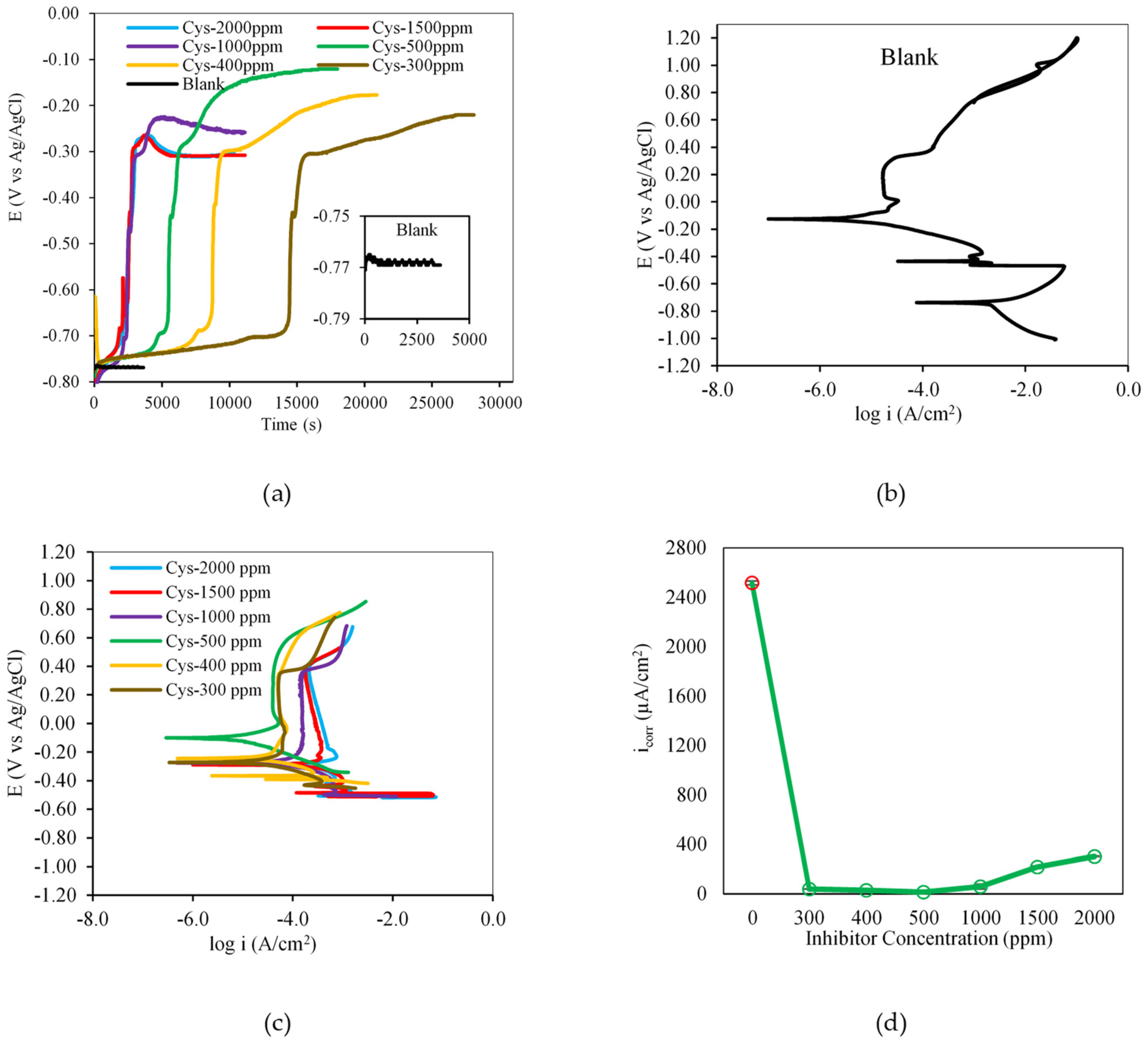
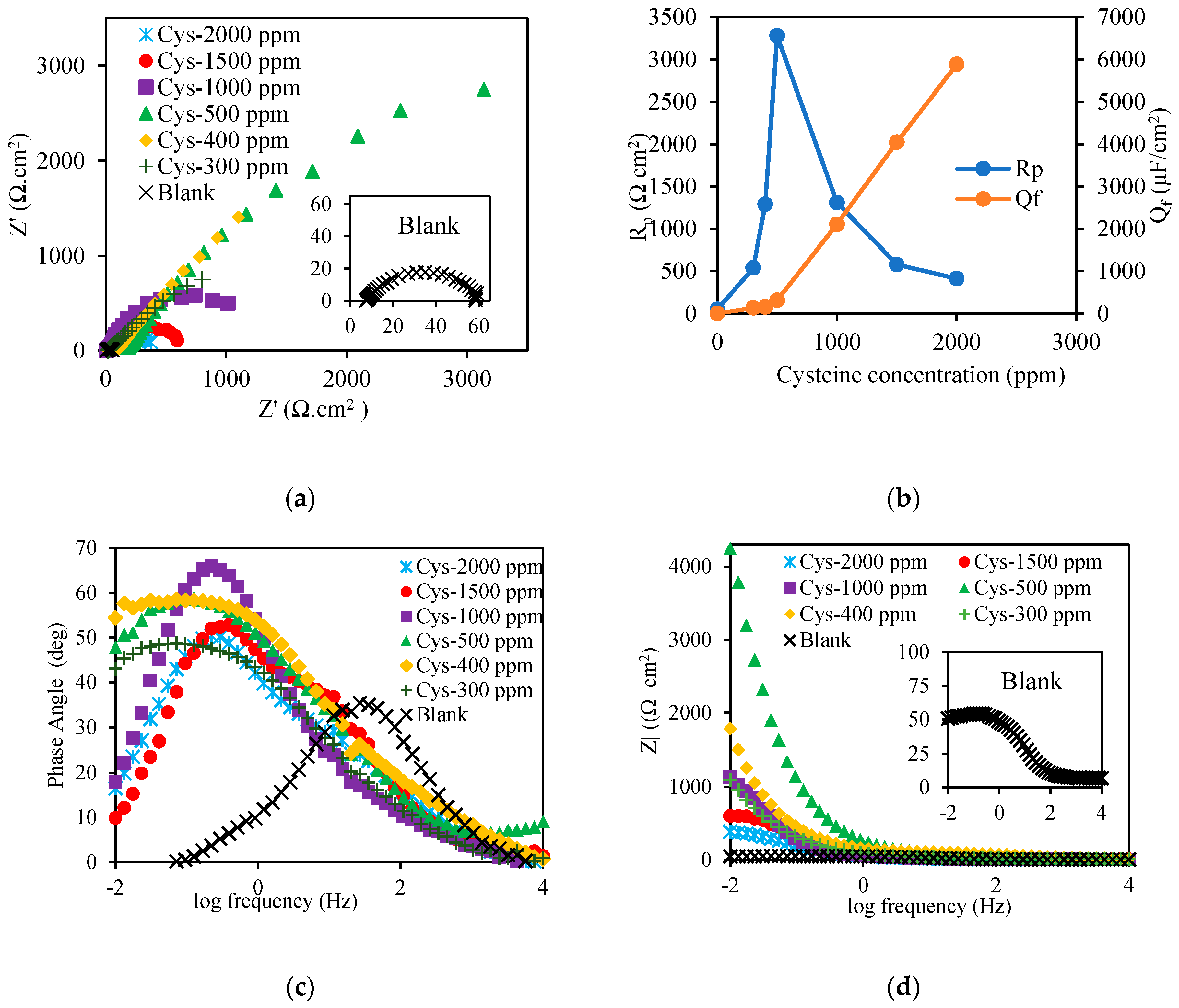
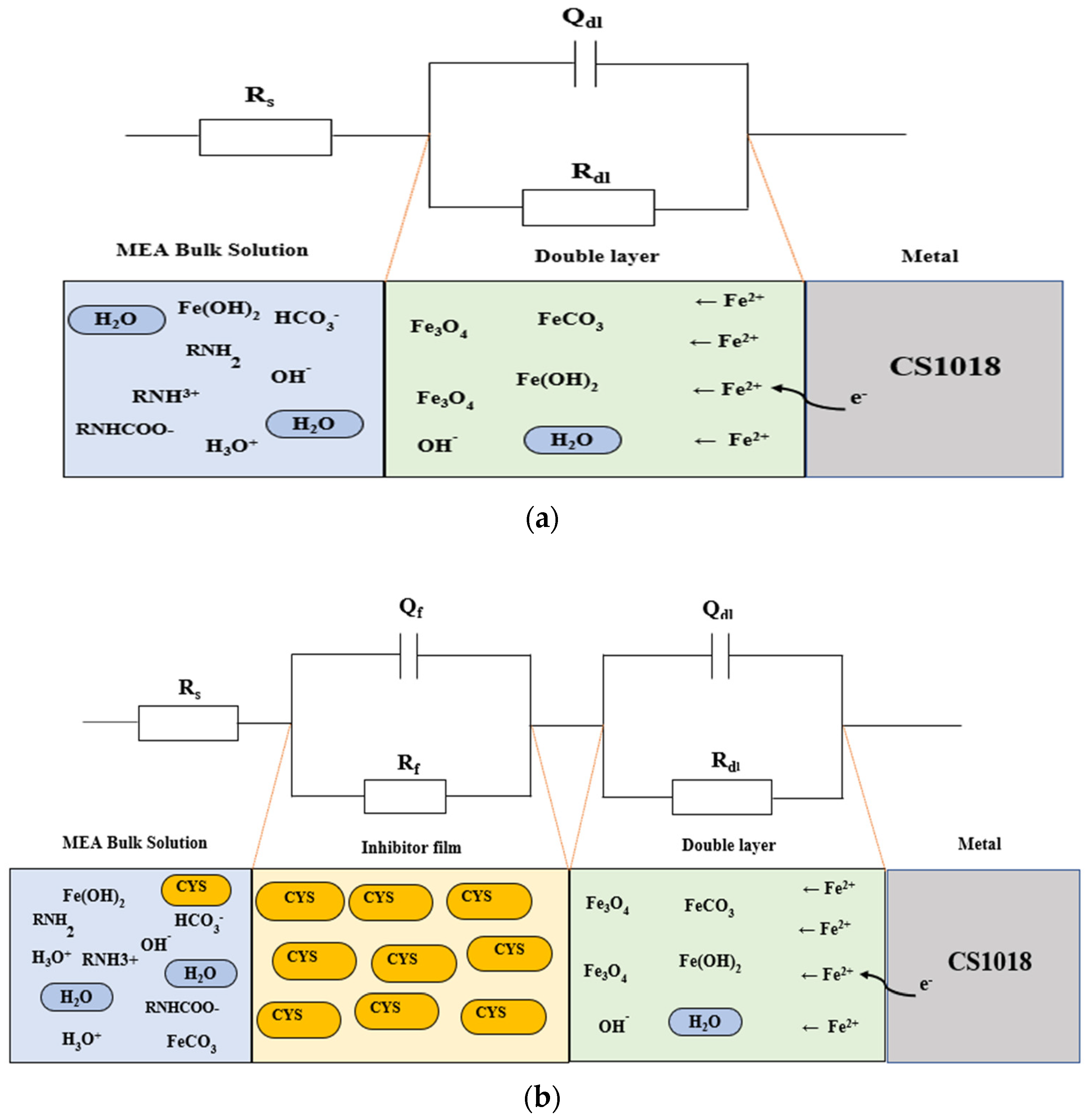


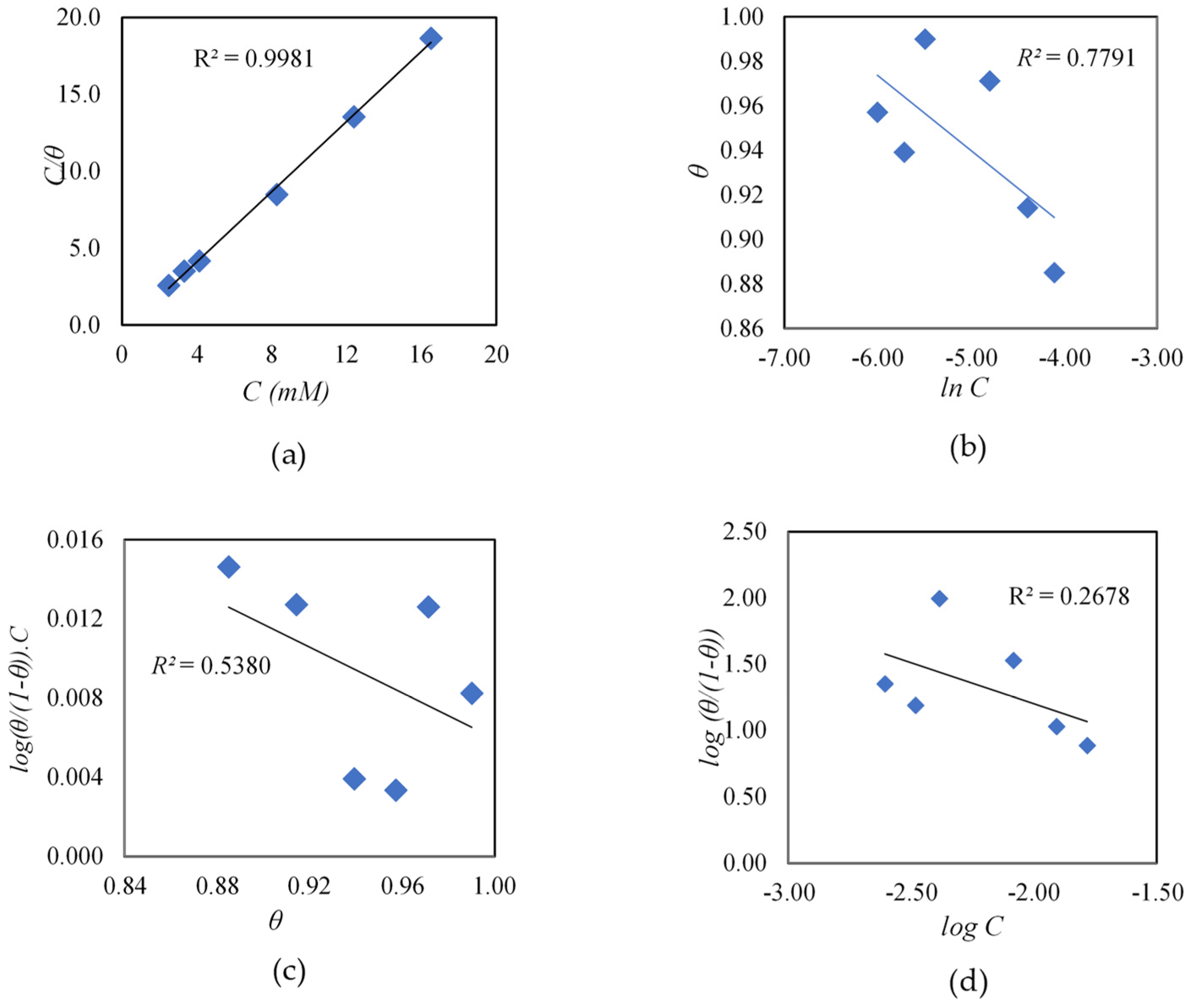
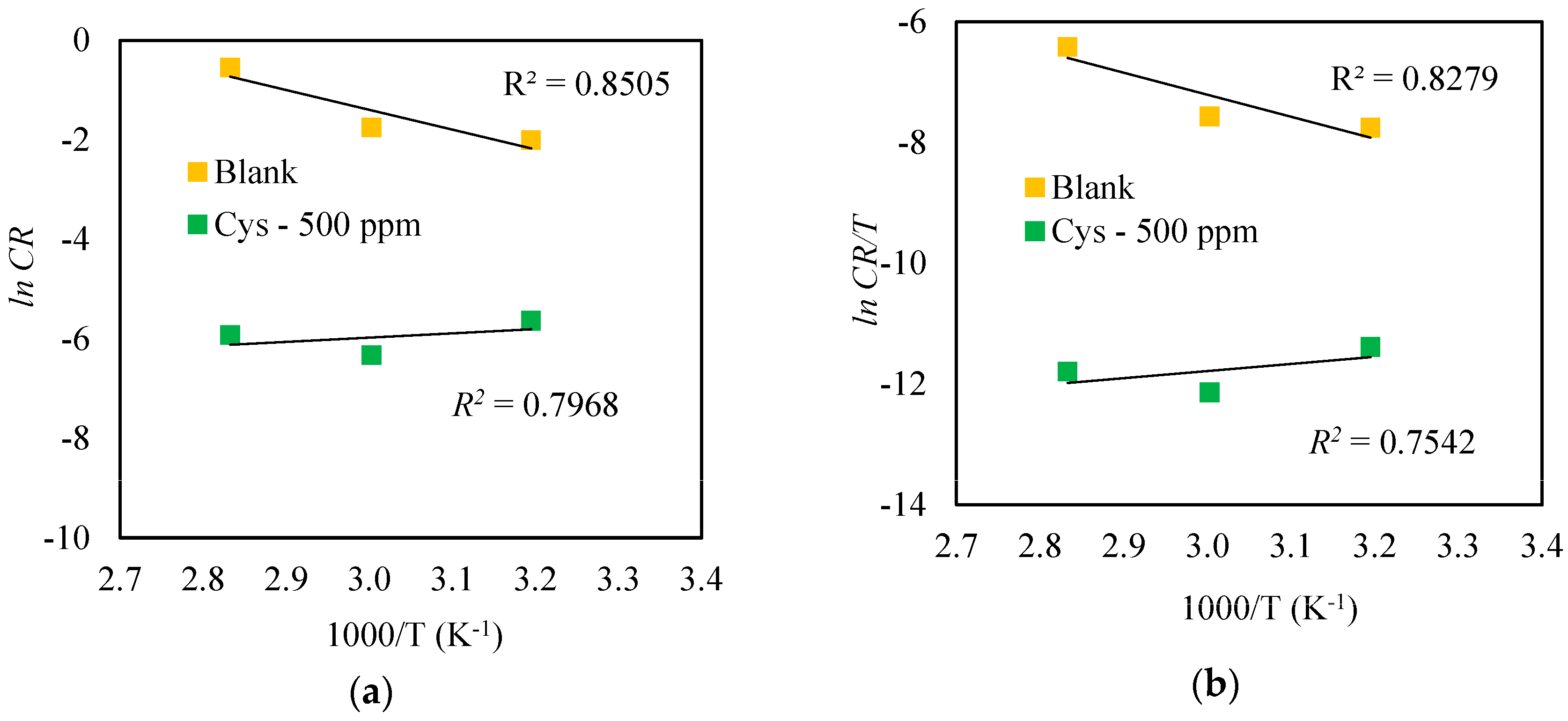


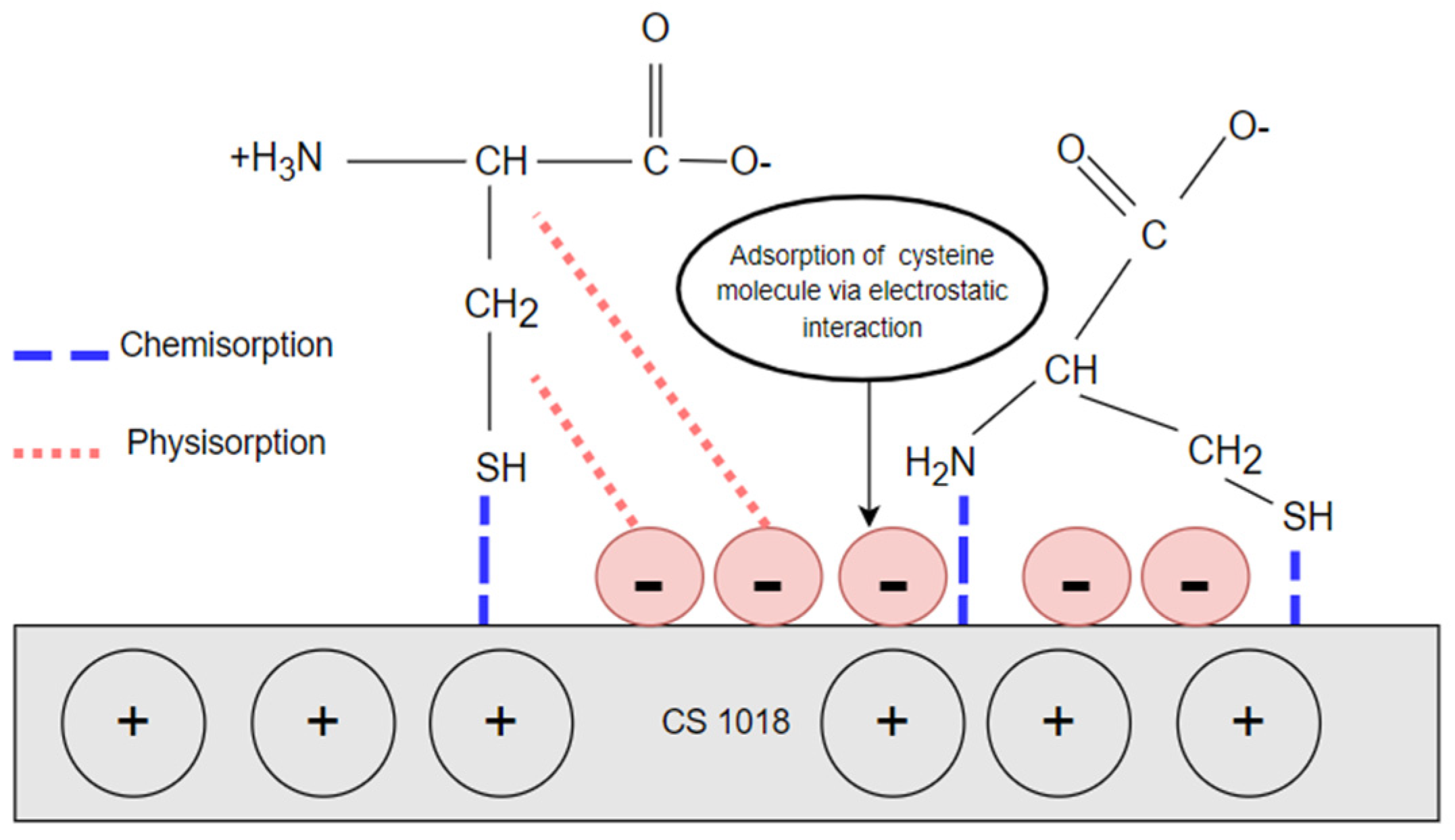
| Chemical | LD50 (mg/kg)—Oral—Rat | Biodegradability | Bioaccumulation (as log pow) | |
|---|---|---|---|---|
| Tested corrosion inhibitors | Cysteine (Cys) | 5850 | 98% Readily biodegradable—After 28 days in aerobic environment. | −3.05 |
| CO2 absorption solvents | Monoethanolamine (MEA) | 1720 | Readily biodegradable. In the strum test after 28 days, 91% of the MEA degrades. | −1.31 |
| Diethanolamine (DEA) | 780 | Depending on the soil conditions, biodegradable in soil. | −1.43 | |
| Methyl diethanolamine (MDEA) | 1945 | After 28 days in activated sludge, it does not biodegrade, but it degraded more than 96% after 40 days in a continuous-flow study where the inoculum had time to acclimate. | −1.50 | |
| Inorganic corrosion inhibitors | Sodium metavanadate | 183 | (For Inorganic substance-Methods used for determining biodegradability are not applicable.) | Toxic to aquatic life with long lasting effects |
| Copper carbonate | 159 | Very toxic to aquatic life | ||
| Vanadium pentoxide | 10 | Toxic to aquatic life with long lasting effects | ||
| Nickel (II) sulfate hexahydrate | 362 | May cause long term adverse effect in the aquatic environment. |
| System | Ea (kJ mol−1) | ΔHa (kJ mol−1) | ΔSa (J mol−1 K−1) |
|---|---|---|---|
| Blank | 30.28 | 27.44 | −174.05 |
| 500 ppm Cysteine | 9.86 | 7.10 | −268.51 |
| Specimen | Corrosion Rate (mmpy) | Inhibition Efficiency (%) | |
|---|---|---|---|
| Blank | 500 ppm Cysteine | ||
| M1-Full immersion (dynamic-liquid phase) | 3.47 | 0.30 | 91.37 |
| M2-Full immersion (static-liquid phase) | 1.74 | 0.18 | 89.63 |
| M3-Partial Immersion (static -liquid & vapor phase) | 1.12 | 0.02 | 98.60 |
| M4-No immersion (static-vapor phase) | 0.08 | 0.02 | 81.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibullah, M.I.; Veawab, A. Cysteine as an Alternative Eco-Friendly Corrosion Inhibitor for Absorption-Based Carbon Capture Plants. Materials 2023, 16, 3496. https://doi.org/10.3390/ma16093496
Habibullah MI, Veawab A. Cysteine as an Alternative Eco-Friendly Corrosion Inhibitor for Absorption-Based Carbon Capture Plants. Materials. 2023; 16(9):3496. https://doi.org/10.3390/ma16093496
Chicago/Turabian StyleHabibullah, Mohamed Ishaq, and Amornvadee Veawab. 2023. "Cysteine as an Alternative Eco-Friendly Corrosion Inhibitor for Absorption-Based Carbon Capture Plants" Materials 16, no. 9: 3496. https://doi.org/10.3390/ma16093496




