Optimizing the Mechanoluminescent Properties of CaZnOS:Tb via Microwave-Assisted Synthesis: A Comparative Study with Conventional Thermal Methods
Abstract
1. Introduction
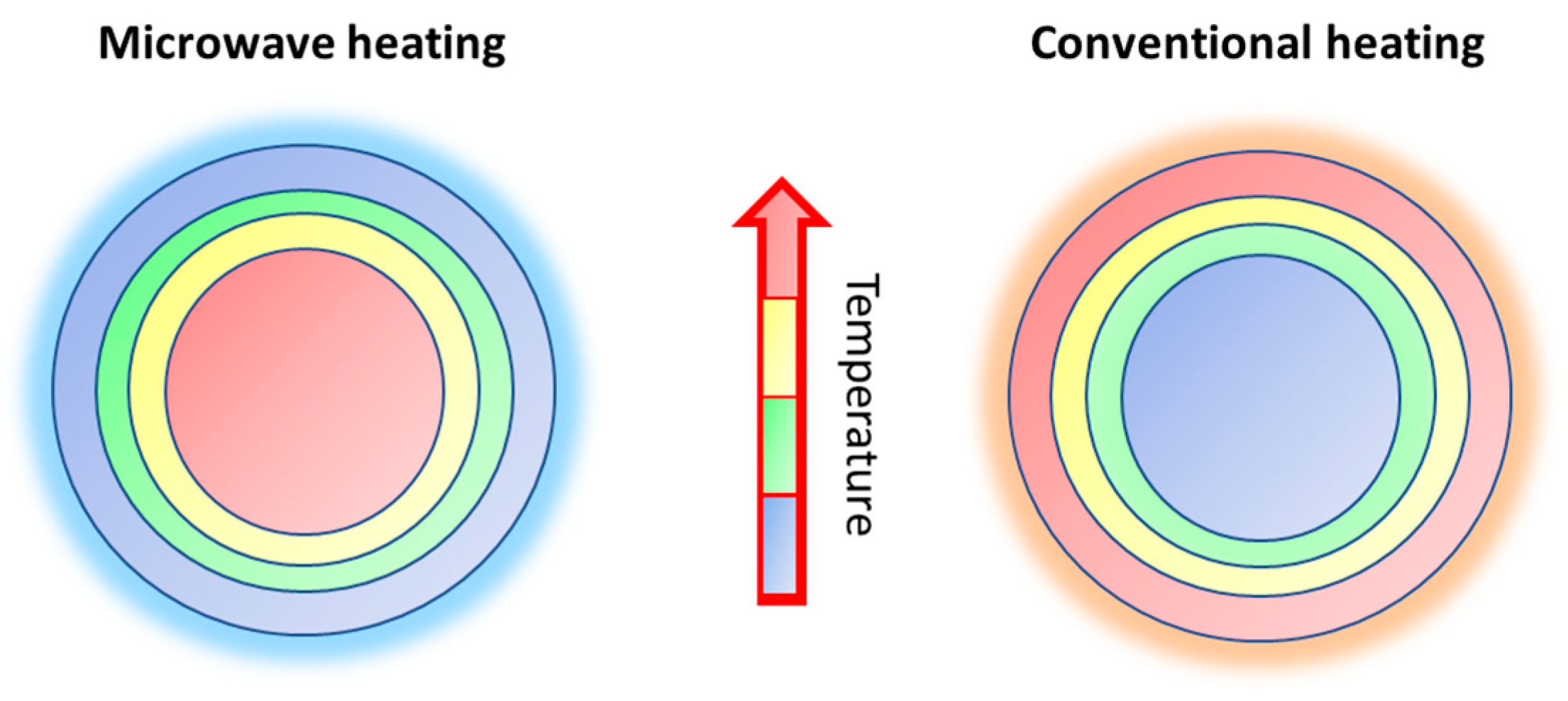
- Reduced synthesis time;
- Consistent and uniform heating of the material;
- High-quality materials with high yield;
- Eco-friendly, as it consumes less energy and produces less waste than traditional methods;
- Cost-effective, as it requires less equipment and facilities than traditional methods.
2. Experimental
2.1. Materials and Methods
2.2. Characterization Techniques
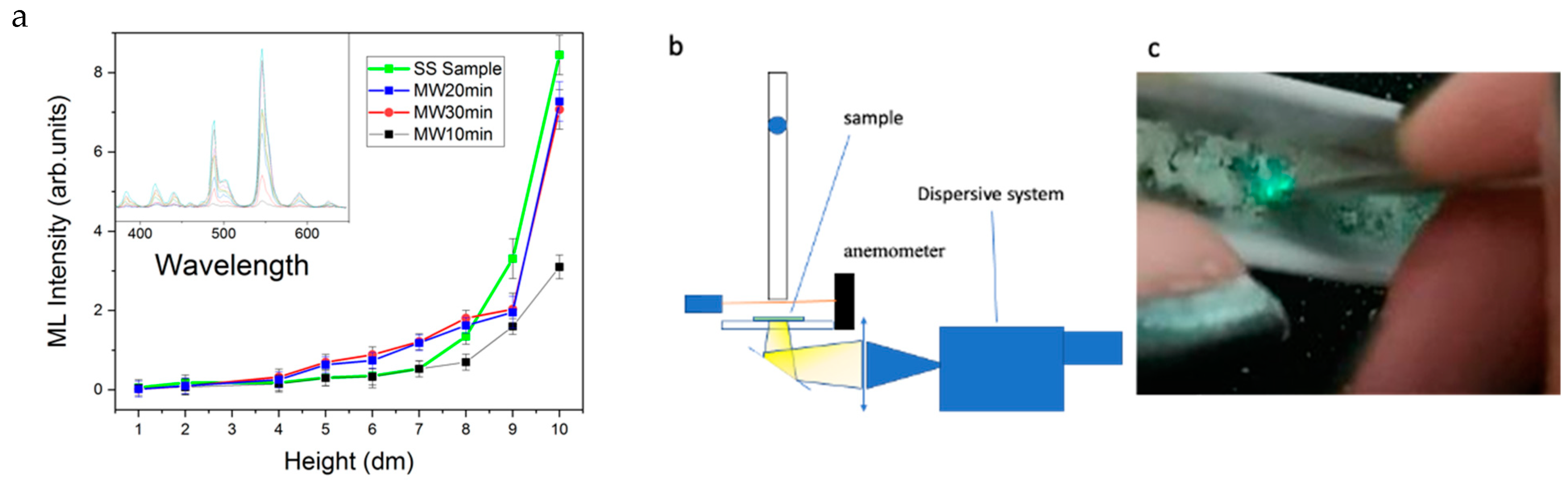
2.3. Mechanoluminescence Test
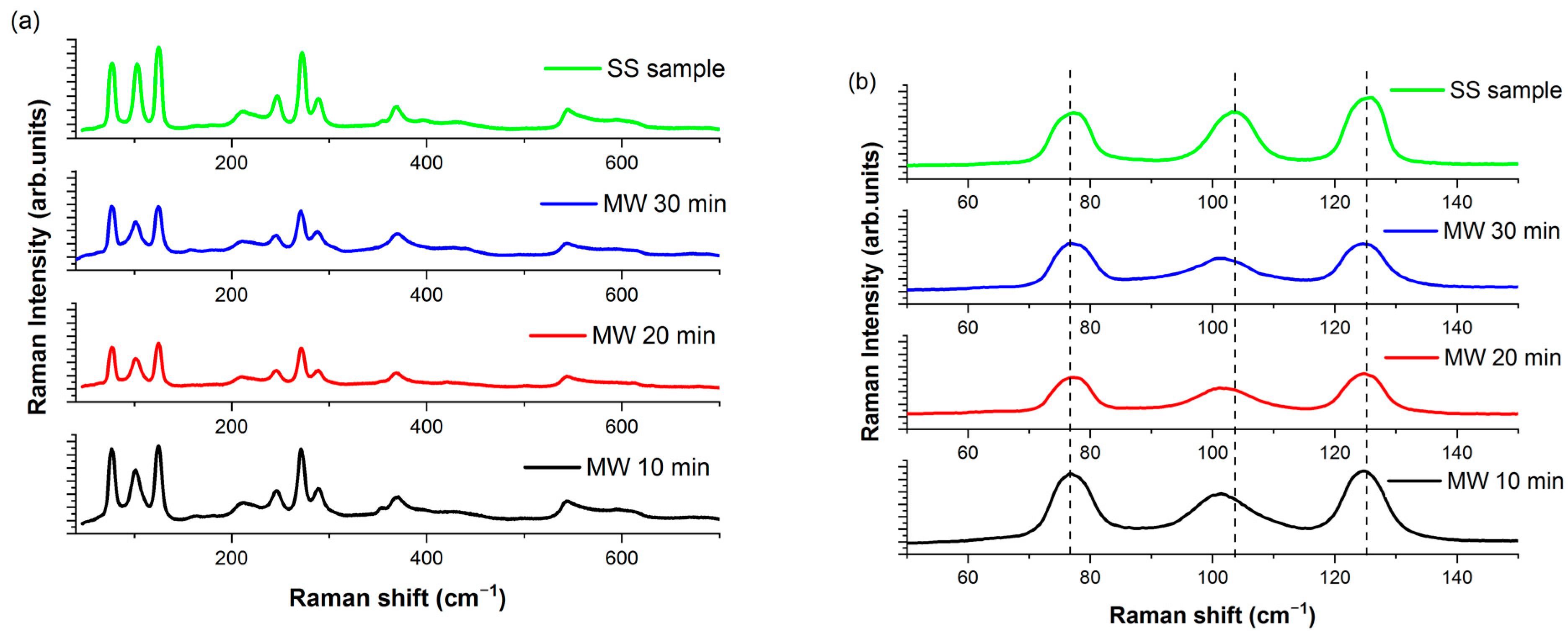
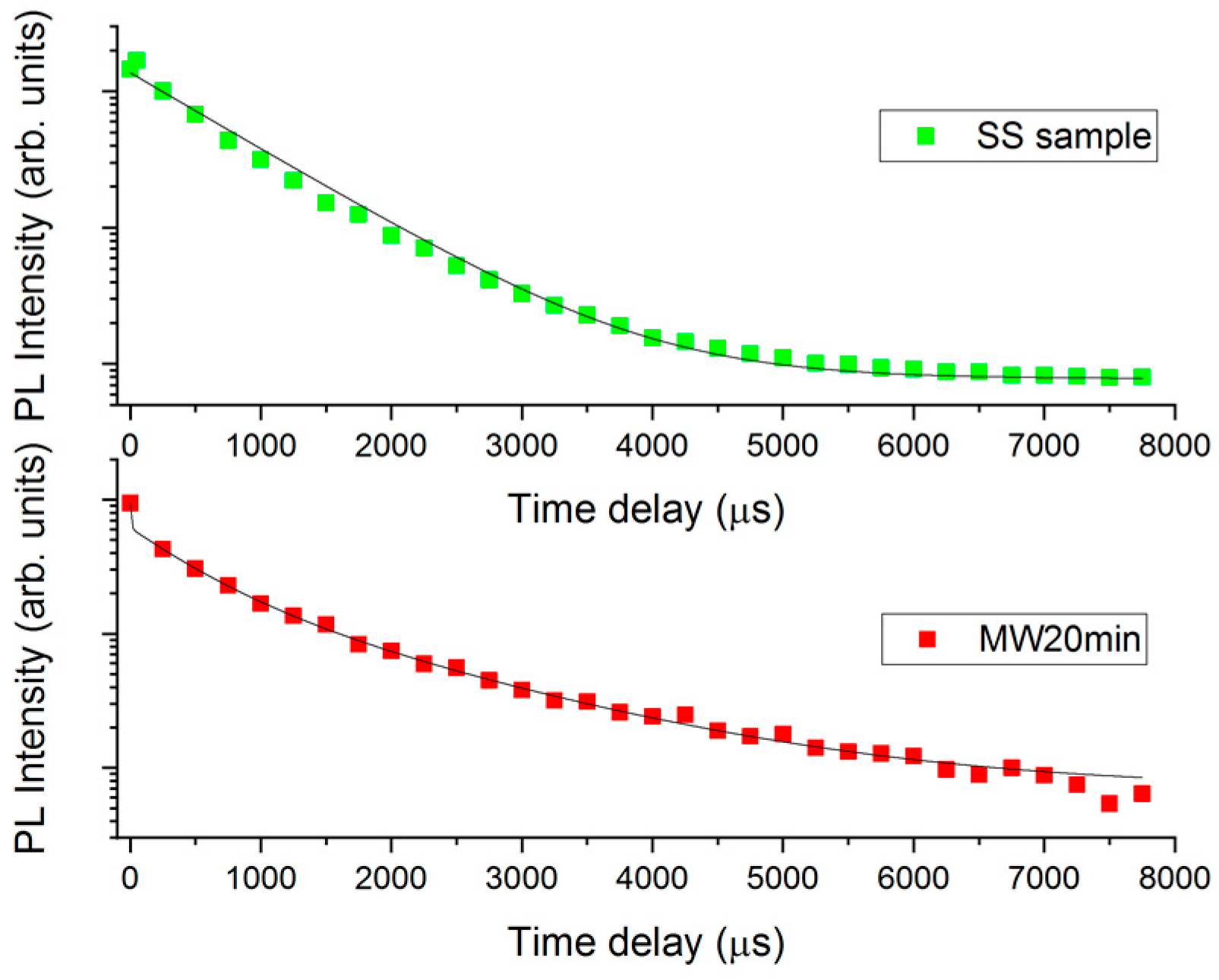
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Colacino, E.; Carta, M.; Pia, G.; Porcheddu, A.; Ricci, P.C.; Delogu, F. Processing and Investigation Methods in Mechanochemical Kinetics. ACS Omega 2018, 3, 9196–9209. [Google Scholar] [CrossRef]
- Fiore, C.; Sovic, I.; Lukin, S.; Halasz, I.; Martina, K.; Delogu, F.; Ricci, P.C.; Porcheddu, A.; Shemchuk, O.; Braga, D.; et al. Kabachnik-Fields Reaction by Mechanochemistry: New Horizons from Old Methods. ACS Sustain. Chem. Eng. 2020, 8, 18889–18902. [Google Scholar] [CrossRef]
- Sović, I.; Lukin, S.; Meštrović, E.; Halasz, I.; Porcheddu, A.; Delogu, F.; Ricci, P.C.; Caron, F.; Perilli, T.; Dogan, A.; et al. Mechanochemical Preparation of Active Pharmaceutical Ingredients Monitored by in Situ Raman Spectroscopy. ACS Omega 2020, 5, 28663–28672. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.; Smet, P.F. A review of mechanoluminescence in inorganic solids: Compounds, mechanisms, models and applications. Materials 2018, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Xie, R.J. Mechanoluminescence Rebrightening the Prospects of Stress Sensing: A Review. Adv. Mater. 2021, 33, 2005925. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, Y.; Xu, J.; Lin, H.; Wang, Y. Stress Sensing by Ratiometric Mechanoluminescence: A Strategy Based on Structural Probe. Laser Photonics Rev. 2022, 16, 2200365. [Google Scholar] [CrossRef]
- Chen, C.; Zhuang, Y.; Li, X.; Lin, F.; Peng, D.; Tu, D.; Xie, A.; Xie, R.J. Achieving Remote Stress and Temperature Dual-Modal Imaging by Double-Lanthanide-Activated Mechanoluminescent Materials. Adv. Funct. Mater. 2021, 31, 6. [Google Scholar] [CrossRef]
- Zhuang, Y.; Li, X.; Lin, F.; Chen, C.; Wu, Z.; Luo, H.; Jin, L.; Xie, R.J. Visualizing Dynamic Mechanical Actions with High Sensitivity and High Resolution by Near-Distance Mechanoluminescence Imaging. Adv. Mater. 2022, 34, 2202864. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Shin, H.G.; Timilsina, S.; Kim, J.; Kim, G.-W. Sound visualization based on mechanoluminescent diaphragms. In Nano-, Bio-, Info-Tech Sensors, and Wearable Systems; SPIE: Bellingham, DC, USA, 2022; Volume 12045, p. 34. [Google Scholar] [CrossRef]
- Zhou, H.; Du, Y.; Wu, C.; Jiang, Y.; Wang, F.; Zhang, J.; Wang, Z. Understanding the mechanoluminescent mechanisms of manganese doped zinc sulfide based on load effects. J. Lumin. 2018, 203, 683–688. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Karnaushenko, D.; Chen, L.; Hao, J.; Ding, F.; Schmidt, O.G. Addressable and Color-Tunable Piezophotonic Light-Emitting Stripes. Adv. Mater. 2017, 29, 1605165. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Xu, C.-N.; Kamimura, S.; Terasawa, Y.; Yamada, H.; Wang, X. An intense elastico-mechanoluminescence material CaZnOS:Mn2+ for sensing and imaging multiple mechanical stresses. Opt. Express 2013, 21, 12976. [Google Scholar] [CrossRef]
- Sambrook, T.; Smura, C.F.; Clarke, S.J.; Kang, M.O.; Halasyamani, P.S. Structure and physical properties of the polar oxysulfide CaZnOS. Inorg. Chem. 2007, 46, 2571–2574. [Google Scholar] [CrossRef] [PubMed]
- Joos, J.J.; Lejaeghere, K.; Korthout, K.; Feng, A.; Poelman, D.; Smet, P.F. Charge transfer induced energy storage in CaZnOS:Mn—Insight from experimental and computational spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 9075–9085. [Google Scholar] [CrossRef] [PubMed]
- Corpino, R.; Angioni, D.; Satta, J.; Ugbo, F.C.; Chiriu, D.; Carbonaro, C.M.; Melis, C.; Stagi, L.; Ricci, P.C. Emission mechanism in single and co-doped Tb:Eu:CaZnOS. J. Alloys Compd. 2021, 868, 159007. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Feng, A.; Chen, X.Y.; Zhao, J.T. Photoluminescence properties and energy levels of RE (RE = Pr, Sm, Er, Tm) in layered-CaZnOS oxysulfide. J. Appl. Phys. 2013, 114, 213518. [Google Scholar] [CrossRef]
- Qiu, Z.; Rong, C.; Zhou, W.; Zhang, J.; Li, C.; Yu, L.; Liu, S.; Lian, S. A strategy for synthesizing CaZnOS:Eu2+ phosphor and comparison of optical properties with CaS:Eu2+. J. Alloys Compd. 2014, 583, 335–339. [Google Scholar] [CrossRef]
- Xu, Z.; Xia, Z.; Lei, B.; Liu, Q. Full color control and white emission from CaZnOS:Ce3+,Na+,Mn2+ phosphors: Via energy transfer. J. Mater. Chem. C 2016, 4, 9711–9716. [Google Scholar] [CrossRef]
- Yang, Y.L.; Zhou, Y.; Pan, D.J.; Zhang, Z.J.; Zhao, J.T. The luminescence properties of CaZnOS: Bi3+, Sm3+, Li+ phosphors with tunable emissions and energy transfer for white emission. J. Lumin. 2019, 206, 578–584. [Google Scholar] [CrossRef]
- Ricci, P.C.; Satta, J.; Chiriu, D.; Corpino, R.; Carbonaro, C.M.; Salis, M.; Melis, C.; Normile, P.S.; De Toro, J.A. Optical and vibrational properties of CaZnOS: The role of intrinsic defects. J. Alloys Compd. 2019, 777, 225–233. [Google Scholar] [CrossRef]
- Chiriu, D.; Stagi, L.; Carbonaro, C.M.; Corpino, R.; Ricci, P.C. Energy transfer mechanism between Ce and Tb ions in sol-gel synthesized YSO crystals. Mater. Chem. Phys. 2016, 171, 201–207. [Google Scholar] [CrossRef]
- Ricci, P.C.; Salis, M.; Corpino, R.; Carbonaro, C.M.; Fortin, E.; Anedda, A. A kinetics model for Tb3+ recombinations in low doped Tb: Lu1.8Y0.2SiO5 crystals. J. Appl. Phys. 2010, 108, 043512. [Google Scholar] [CrossRef]
- Tu, D.; Xu, C.N.; Fujio, Y.; Yoshida, A. Mechanism of mechanical quenching and mechanoluminescence in phosphorescent CaZnOS:Cu. Light Sci. Appl. 2015, 4, e356. [Google Scholar] [CrossRef]
- Miranda de Carvalho, J.; Pedroso, C.C.S.; Saula, M.S.d.N.; Felinto, M.C.F.C.; de Brito, H.F. Microwave-assisted preparation of luminescent inorganic materials: A fast route to light conversion and storage phosphors. Molecules 2021, 26, 2882. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.S.; Munasinghe, H.N.; Yatooma, E.N.; Rabuffetti, F.A. Microwave-assisted solid-state synthesis of NaRE(MO4)2 phosphors (RE = La, Pr, Eu, Dy; M = Mo, W). Dalt. Trans. 2020, 49, 7914–7919. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, H.J.; Vallance, S.R.; Kennedy, J.L.; Tapia-Ruiz, N.; Carassiti, L.; Harrison, A.; Whittaker, A.G.; Drysdale, T.D.; Kingman, S.W.; Gregory, D.H. Modern microwave methods in solid-state inorganic materials chemistry: From fundamentals to manufacturing. Chem. Rev. 2014, 114, 1170–1206. [Google Scholar] [CrossRef]
- Fathy, M.; Hassan, H.; Hafez, H.; Soliman, M.; Abulfotuh, F.; Kashyout, A.E.H.B. Simple and Fast Microwave-Assisted Synthesis Methods of Nanocrystalline TiO2and rGO Materials for Low-Cost Metal-Free DSSC Applications. ACS Omega 2022, 7, 16757–16765. [Google Scholar] [CrossRef]
- Bi, J.; Sun, L.; Wei, Q.; Zhang, K.; Zhu, L.; Wei, S.; Liao, D.; Sun, J. Rapid ultrasonic-microwave assisted synthesis of Eu3+ doped Y2O3 nanophosphors with enhanced luminescence properties. J. Mater. Res. Technol. 2020, 9, 9523–9530. [Google Scholar] [CrossRef]
- Cheng, Q.; Kang, M.; Wang, J.; Zhang, P.; Sun, R.; Song, L. Low temperature microwave solid-state synthesis of CaCO3: Eu3+, K+ phosphors. Adv. Powder Technol. 2015, 26, 848–852. [Google Scholar] [CrossRef]
- Lim, C.S.; Aleksandrovsky, A.; Molokeev, M.; Oreshonkov, A.; Atuchin, V. Microwave sol-gel synthesis and upconversion photoluminescence properties of CaGd2(WO4)4:Er3+/Yb3+ phosphors with incommensurately modulated structure. J. Solid State Chem. 2015, 228, 160–166. [Google Scholar] [CrossRef]
- He, C.; Xia, Z.; Liu, Q. Microwave solid state synthesis and luminescence properties of green-emitting Gd2O2S:Tb3+ phosphor. Opt. Mater. 2015, 42, 11–16. [Google Scholar] [CrossRef]
- Miranda De Carvalho, J.; Van Der Heggen, D.; Martin, L.I.D.J.; Smet, P.F. Microwave-assisted synthesis followed by a reduction step: Making persistent phosphors with a large storage capacity. Dalt. Trans. 2020, 49, 4518–4527. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, L. Total pattern fitting for the combined size–strain–stress–texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Ricci, P.C.; Carbonaro, C.M.; Corpino, R.; Cannas, C.; Salis, M. Optical and structural characterization of terbium-doped Y2SiO5 phosphor particles. J. Phys. Chem. C 2011, 115, 16630–16636. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Ran, W.; Deng, Z.; Shi, J.; Ren, C. Tunable Luminescence in Sr2MgSi2O7:Tb3+, Eu3+ Phosphors Based on Energy Transfer. Materials 2017, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhang, J.C.; Zhang, M.; Yan, X.; Long, Y.Z.; Wang, X. Intrinsic oxygen vacancies mediated multi-mechano-responsive piezoluminescence in undoped zinc calcium oxysulfide. Appl. Phys. Lett. 2017, 110, 233904. [Google Scholar] [CrossRef]
- Lakowitz, J.R. Energy Transfer. In Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; pp. 443–475. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugbo, F.C.; Porcu, S.; Corpino, R.; Pinna, A.; Carbonaro, C.M.; Chiriu, D.; Smet, P.F.; Ricci, P.C. Optimizing the Mechanoluminescent Properties of CaZnOS:Tb via Microwave-Assisted Synthesis: A Comparative Study with Conventional Thermal Methods. Materials 2023, 16, 3511. https://doi.org/10.3390/ma16093511
Ugbo FC, Porcu S, Corpino R, Pinna A, Carbonaro CM, Chiriu D, Smet PF, Ricci PC. Optimizing the Mechanoluminescent Properties of CaZnOS:Tb via Microwave-Assisted Synthesis: A Comparative Study with Conventional Thermal Methods. Materials. 2023; 16(9):3511. https://doi.org/10.3390/ma16093511
Chicago/Turabian StyleUgbo, Franca C., Stefania Porcu, Riccardo Corpino, Andrea Pinna, Carlo Maria Carbonaro, Daniele Chiriu, Philippe F. Smet, and Pier Carlo Ricci. 2023. "Optimizing the Mechanoluminescent Properties of CaZnOS:Tb via Microwave-Assisted Synthesis: A Comparative Study with Conventional Thermal Methods" Materials 16, no. 9: 3511. https://doi.org/10.3390/ma16093511
APA StyleUgbo, F. C., Porcu, S., Corpino, R., Pinna, A., Carbonaro, C. M., Chiriu, D., Smet, P. F., & Ricci, P. C. (2023). Optimizing the Mechanoluminescent Properties of CaZnOS:Tb via Microwave-Assisted Synthesis: A Comparative Study with Conventional Thermal Methods. Materials, 16(9), 3511. https://doi.org/10.3390/ma16093511










