Phosphodiester Stationary Phases as Universal Chromatographic Materials for Separation in RP LC, HILIC, and Pure Aqueous Mobile Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment and Chemicals
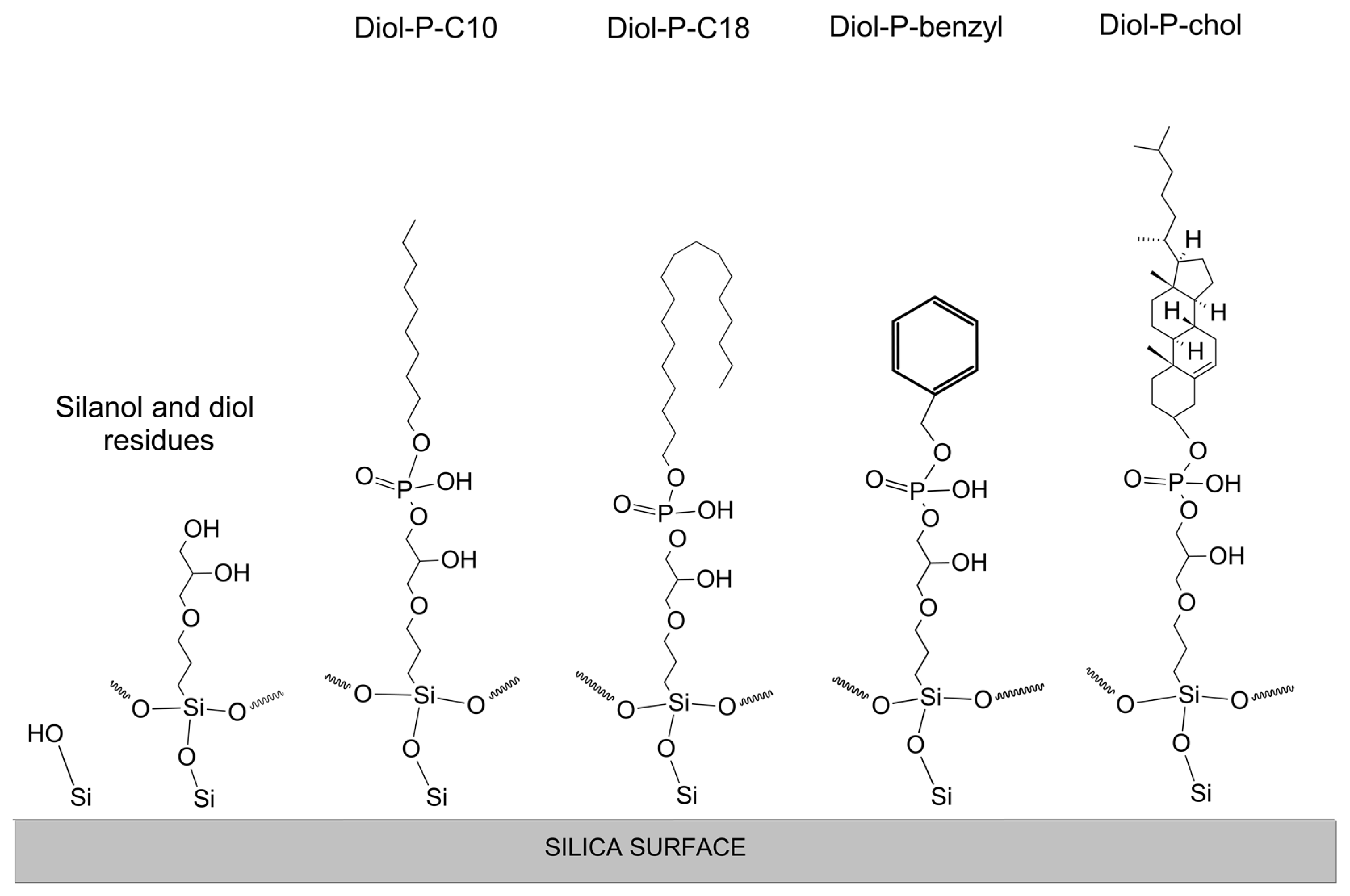
2.2. Materials
2.3. Methods
3. Results and Discussion
3.1. Hydrophobicity
3.2. Retention Analyses
3.3. Mixture Separation
3.3.1. Purine Alkaloids
3.3.2. Nucleosides
3.3.3. Benzene and Polycyclic Aromatic Hydrocarbons
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Płotka, J.; Tobiszewski, M.; Sulej, A.M.; Kupska, M.; Górecki, T.; Namieśnik, J. Green chromatography. J. Chromatogr. A 2013, 1307, 1–20. [Google Scholar] [CrossRef]
- Snyder, L.R.; Dolan, J.W.; Gant, J.R. Gradient elution in high-performance liquid chromatography. I. Theoretical basis for reversed-phase systems. J. Chromatogr. A 1979, 165, 3–30. [Google Scholar] [CrossRef]
- Kazakevich, Y.; Lobrutto, R. HPLC for Pharmaceuticals Scientist; John Wiley and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Jandera, P.; Janás, P. Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 2017, 967, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Bocian, S.; Skoczylas, M.; Goryńska, I.; Matyska, M.; Pesek, J.; Buszewski, B. Solvation processes on phenyl-bonded stationary phases—The influence of polar functional groups. J. Sep. Sci. 2016, 39, 4369–4376. [Google Scholar] [CrossRef] [PubMed]
- Noga, S.; Bocian, S.; Buszewski, B. Hydrophilic interaction liquid chromatography columns classification by effect of solvation and chemometric methods. J. Chromatogr. A 2013, 1278, 89–97. [Google Scholar] [CrossRef]
- Bocian, S.; Vajda, P.; Felinger, A.; Buszewski, B. Solvent excess adsorption on the stationary phases for reversed-phase liquid chromatography with polar functional groups. J. Chromatogr. A 2008, 1204, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. A 1990, 499, 177–196. [Google Scholar] [CrossRef]
- Noga, S.; Buszewski, B. Hydrophilic interaction liquid chromatography (HILIC)—A powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef]
- Jandera, P. Stationary and mobile phases in hydrophilic interaction chromatography: A review. Anal. Chim. Acta 2011, 692, 1–25. [Google Scholar] [CrossRef]
- Szumski, M.; Buszewski, B. Study of electroosmotic flow in packed capillary columns. J. Chromatogr. A 2004, 1032, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Soukup, J.; Jandera, P. Adsorption of water from aqueous acetonitrile on silica-based stationary phases in aqeous normal-phase liquid chromatography. J. Chromatogr. A 2014, 1374, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Dembek, M.; Bocian, S. Stationary Phases for Green Liquid Chromatography. Materials 2022, 15, 419. [Google Scholar] [CrossRef] [PubMed]
- Ruderisch, A.; Iwanek, W.; Pfeiffer, J.; Fischer, G.; Albert, K.; Schurig, V. Synthesis and characterization of a novel resorcinarene-based stationary phase bearing polar headgroups for use in reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2005, 1095, 40–49. [Google Scholar] [CrossRef]
- Jandera, P.; Hájek, T. Utilization of dual retention mechanism on columns with bonded PEG and diol stationary phases for adjusting the separation selectivity of phenolic and flavone natural antioxidants. J. Sep. Sci. 2009, 32, 3603–3619. [Google Scholar] [CrossRef] [PubMed]
- Coym, J.W. Comparison of retention on traditional alkyl, Polar endcapped, and Polar embedded group stationary phases. J. Sep. Sci. 2008, 31, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, K.; Bocian, S. The versatility of N, O -dialkylphosphoramidate stationary phase-separations in HILIC, highly aqueous RP LC conditions and purely aqueous mobile phase. Analyst 2018, 143, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Bocian, S.; Krzemińska, K. The separations using pure water as a mobile phase in liquid chromatography using polar-embedded stationary phases. Green Chem. Lett. Rev. 2019, 12, 69–78. [Google Scholar] [CrossRef]
- Krzemińska, K.; Dembek, M.; Bocian, S. The competitiveness of solvent adsorption on polar-embedded stationary phases. J. Sep. Sci. 2018, 41, 4296–4303. [Google Scholar] [CrossRef]
- Krzemińska, K.; Bocian, S.; Pluskota, R.; Buszewski, B. Surface properties of stationary phases with embedded polar group based on secondary interaction, zeta potential measurement and linear solvatation energy relationship studies. J. Chromatogr. A 2021, 1637, 461853. [Google Scholar] [CrossRef]
- Jandera, P.; Hájek, T.; Škeřiková, V.; Soukup, J. Dual hydrophilic interaction-RP retention mechanism on polar columns: Structural correlations and implementation for 2-D separations on a single column. J. Sep. Sci. 2010, 33, 841–852. [Google Scholar] [CrossRef]
- Gritti, F.; dos Santos Pereira, A.; Sandra, P.; Guiochon, G. Efficiency of the same neat silica column in hydrophilic interaction chromatography and per aqueous liquid chromatography. J. Chromatogr. A 2010, 1217, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.J.; Wu, N.; Biba, M.; Hartman, R.; Brkovic, T.; Gong, X.; Helmy, R.; Schafer, W.; Cuff, J.; Pirzada, Z.; et al. Greening analytical chromatography. TrAC Trends Anal. Chem. 2010, 29, 667–680. [Google Scholar] [CrossRef]
- Dembek, M.; Bocian, S. Pure water as a mobile phase in liquid chromatography techniques. TrAC Trends Anal. Chem. 2020, 123, 115793. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, M.; Dai, X.; Yang, H.; Yang, Y.; Ou, J.; Liao, M.; Liu, J.; Wang, L. Polar-embedded phenyl dendritic stationary phase for multi-mode chromatographic separation. Microchem. J. 2023, 185, 108303. [Google Scholar] [CrossRef]
- Wan, M.; Luo, Q.; Ren, X.; Zheng, Y.; Gao, D.; Fu, Q.; Zu, F.; Xia, Z.; Wang, L. Preparation and performance of a poly(ethyleneimine) embedded N-acetyl-L-phenylalanine mixed-mode stationary phase for HPLC. Microchem. J. 2020, 157, 105021. [Google Scholar] [CrossRef]
- Ohyama, K.; Inoue, Y.; Kishikawa, N.; Kuroda, N. Preparation and characterization of surfactin-modified silica stationary phase for reversed-phase and hydrophilic interaction liquid chromatography. J. Chromatogr. A 2014, 1371, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Feng, Y.; Liu, X.; Chen, T.; Zhang, H. Preparation and evaluation of poly-l-lysine stationary phase for hydrophilic interaction/reversed-phase mixed-mode chromatography. Chromatographia 2011, 74, 523–530. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Tang, X.; Jia, Y.; Li, G.; Sun, X.; Wen, A. Synthesis and characterization of novel polar-embedded silica stationary phases for use in reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2013, 1271, 153–162. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, C.; Liang, T.; Liang, X. Polar-copolymerized approach based on horizontal polymerization on silica surface for preparation of polar-modified stationary phases. J. Chromatogr. A 2010, 1217, 4555–4560. [Google Scholar] [CrossRef]
- Dembek, M.; Bocian, S.; Buszewski, B. Solvent Influence on Zeta Potential of Stationary Phase—Mobile Phase Interface. Molecules 2022, 27, 968. [Google Scholar] [CrossRef]
- Dembek, M.; Szumski, M.; Bocian, S.; Buszewski, B. Optimization of the packing process of microcolumns with the embedded phosphodiester stationary phases. J. Sep. Sci. 2022, 45, 3310–3318. [Google Scholar] [CrossRef]
- Galushko, S.V. The calculation of retention and selectivity in reversed-phase liquid chromatography II. Methanol-water eluents. Chromatographia 1993, 36, 39–42. [Google Scholar] [CrossRef]
- Vissers, J.P.C.; Hoeben, M.A.; Laven, J.; Claessens, H.A.; Cramers, C.A. Hydrodynamic aspects of slurry packing processes in microcolumn liquid chromatography. J. Chromatogr. A 2000, 883, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Strickland, Z.; Kapalavavi, B.; Marple, R.; Gamsky, C. Industrial application of green chromatography—I. Separation and analysis of niacinamide in skincare creams using pure water as the mobile phase. Talanta 2011, 84, 169–174. [Google Scholar] [CrossRef]
- Tajuddin, R.; Smith, R.M. On-line coupled superheated water extraction (SWE) and superheated water chromatography (SWC). Analyst 2002, 127, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Dolan, J.W. Temperature selectivity in reversed-phase high performance liquid chromatography. J. Chromatogr. A 2002, 965, 195–205. [Google Scholar] [CrossRef] [PubMed]





| Stationary Phase | Carbon Load [%] | Coverage Density [µmol/m2] | Hydrophobicity (Hg) |
|---|---|---|---|
| Diol-P-C10 | 3.43 | 0.56 | 0.149 |
| Diol-P-C18 | 4.18 | 0.42 | 0.303 |
| Diol-P-Benzyl | 2.86 | 0.56 | 0.044 |
| Diol-P-Chol | 9.31 | 0.87 | 0.221 |
| Stationary Phase | Mobile Phase | Compound | Rt | k | NTP | Rs | As0.1 |
|---|---|---|---|---|---|---|---|
| Diol-P-C10 | 100% H2O | 1B | 1.720 | 0.025 | 2737 | 9.383 | 0.981 |
| 2B | 1.907 | 0.136 | 2467 | 1.311 | 0.939 | ||
| 3B | 2.655 | 0.582 | 2324 | 4.004 | 0.916 | ||
| 4% H2O | 1B | 5.774 | 2.441 | 62 | - | 2.141 | |
| 2B | 14.079 | 7.390 | 102 | 1.952 | 3.022 | ||
| 3B | 25.476 | 14.182 | 255 | 1.904 | 2.155 | ||
| Diol-P-C18 | 100% H2O | 1B | 1.782 | 0.087 | 9210 | - | 0.944 |
| 2B | 2.722 | 0.661 | 2667 | 6.591 | 1.061 | ||
| 3B | 20.373 | 11.430 | 1586 | 15.641 | 1.845 | ||
| 15% H2O | 1B | 2.126 | 0.297 | 8197 | - | 0.879 | |
| 2B | 4.357 | 1.658 | 8099 | 15.511 | 0.838 | ||
| 3B | 10.951 | 5.681 | 5292 | 16.572 | 1.397 | ||
| Diol-P-benzyl | 100% H2O | 1B | 2.727 | 0.606 | 659 | 2.668 | 2.36 |
| 2B | 4.949 | 1.914 | 366 | 3.042 | 5.398 | ||
| 3B | 8.487 | 3.998 | 102 | 1.612 | 7.274 | ||
| 15% H2O | 1B | 2.466 | 0.452 | 5638 | - | 1.203 | |
| 2B | 3.427 | 1.018 | 5064 | 5.933 | 1.249 | ||
| 3B | 4.204 | 1.476 | 3306 | 3.204 | 1.232 | ||
| Diol-P-Chol | 100% H2O | 1B | 1.800 | 0.068 | 14,503 | 15.136 | 0.921 |
| 2B | 2.828 | 0.679 | 3804 | 8.458 | 0.810 | ||
| 3B | 11.355 | 5.739 | 1403 | 12.216 | 1.478 | ||
| 25% H2O | 1B | 1.908 | 0.133 | 6656 | - | 0.821 | |
| 2B | 4.283 | 1.542 | 5240 | 14.382 | 0.730 | ||
| 3B | 21.023 | 11.477 | 2824 | 18.403 | 0.807 |
| Stationary Phase | Compound | Rt | k | NTP | Rs | As0.1 |
|---|---|---|---|---|---|---|
| Diol-P-C10 | 1C | 2.610 | 0.555 | 1168 | 2.949 | 2.624 |
| 2C | 4.942 | 1.945 | 434 | 3.718 | 3.440 | |
| 3C | 10.906 | 5.499 | 174 | 2.799 | 3.687 | |
| Diol-P-C18 | 1C | 2.632 | 0.606 | 1842 | 4.083 | 4.082 |
| 2C | 6.519 | 2.978 | 714 | 6.367 | 4.894 | |
| 3C | 33.867 | 19.663 | 1261 | 11.417 | 3.524 | |
| Diol-P-Benzyl | 1C | 2.727 | 0.606 | 659 | 2.668 | 2.360 |
| 2C | 4.949 | 1.914 | 366 | 3.042 | 5.398 | |
| 3C | 8.487 | 3.998 | 102 | 1.612 | 7.274 | |
| Diol-P-Chol | 1C | 3.088 | 0.832 | 1447 | 4.706 | 1.943 |
| 2C | 7.111 | 3.220 | 1725 | 7.971 | 1.941 | |
| 3C | 42.533 | 24.242 | 998 | 11.671 | 2.908 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembek, M.; Bocian, S. Phosphodiester Stationary Phases as Universal Chromatographic Materials for Separation in RP LC, HILIC, and Pure Aqueous Mobile Phase. Materials 2023, 16, 3539. https://doi.org/10.3390/ma16093539
Dembek M, Bocian S. Phosphodiester Stationary Phases as Universal Chromatographic Materials for Separation in RP LC, HILIC, and Pure Aqueous Mobile Phase. Materials. 2023; 16(9):3539. https://doi.org/10.3390/ma16093539
Chicago/Turabian StyleDembek, Mikołaj, and Szymon Bocian. 2023. "Phosphodiester Stationary Phases as Universal Chromatographic Materials for Separation in RP LC, HILIC, and Pure Aqueous Mobile Phase" Materials 16, no. 9: 3539. https://doi.org/10.3390/ma16093539






