Improved Oxidation Resistance of Graphite Block by Introducing Curing Process of Phenolic Resin
Abstract
:1. Introduction
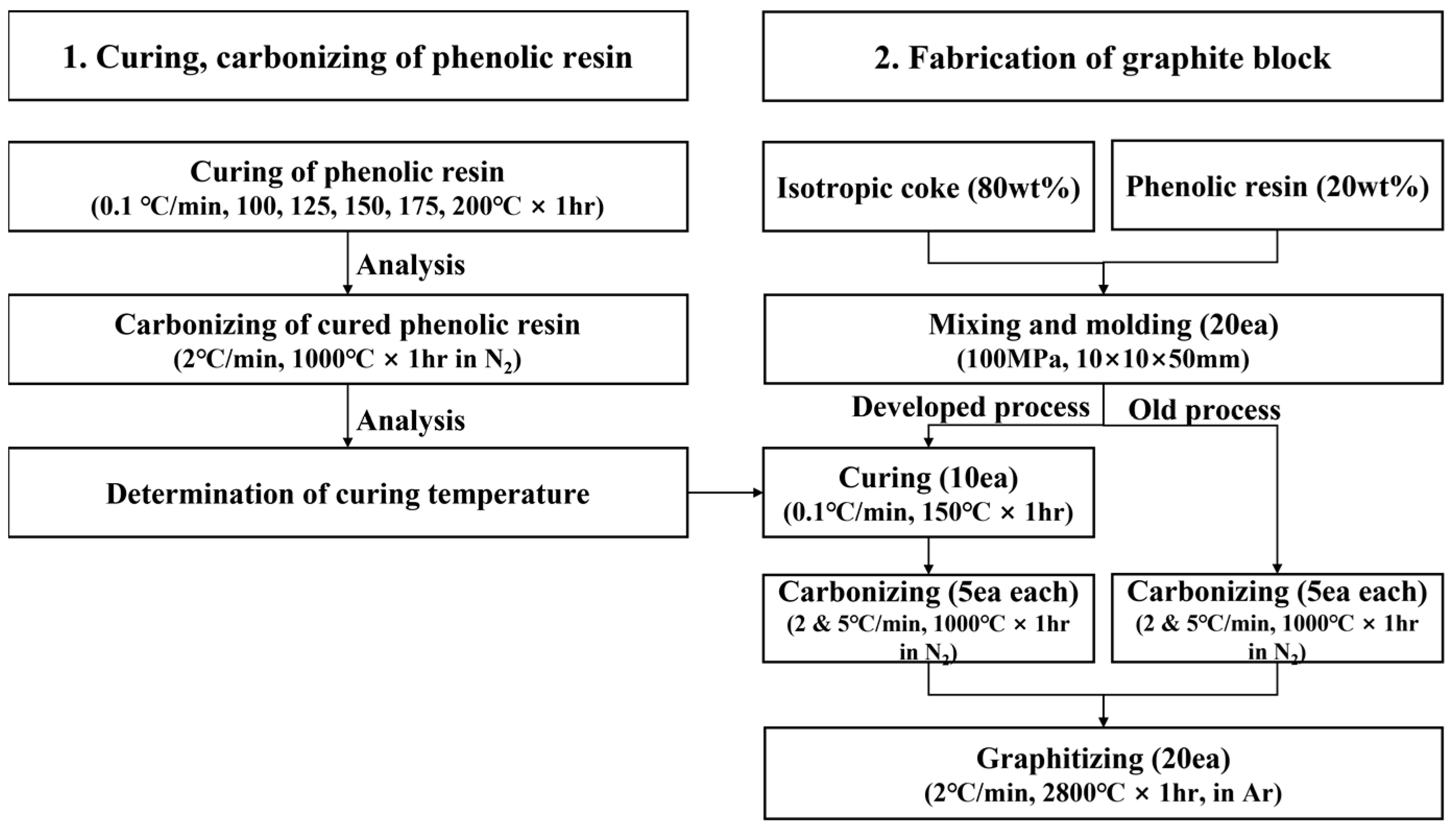
2. Experimental Procedure
2.1. Curing, Carbonizing, and Analysis of Phenolic Resin
2.1.1. Curing and Carbonizing of Phenolic Resin
2.1.2. Methylene Index from FTIR
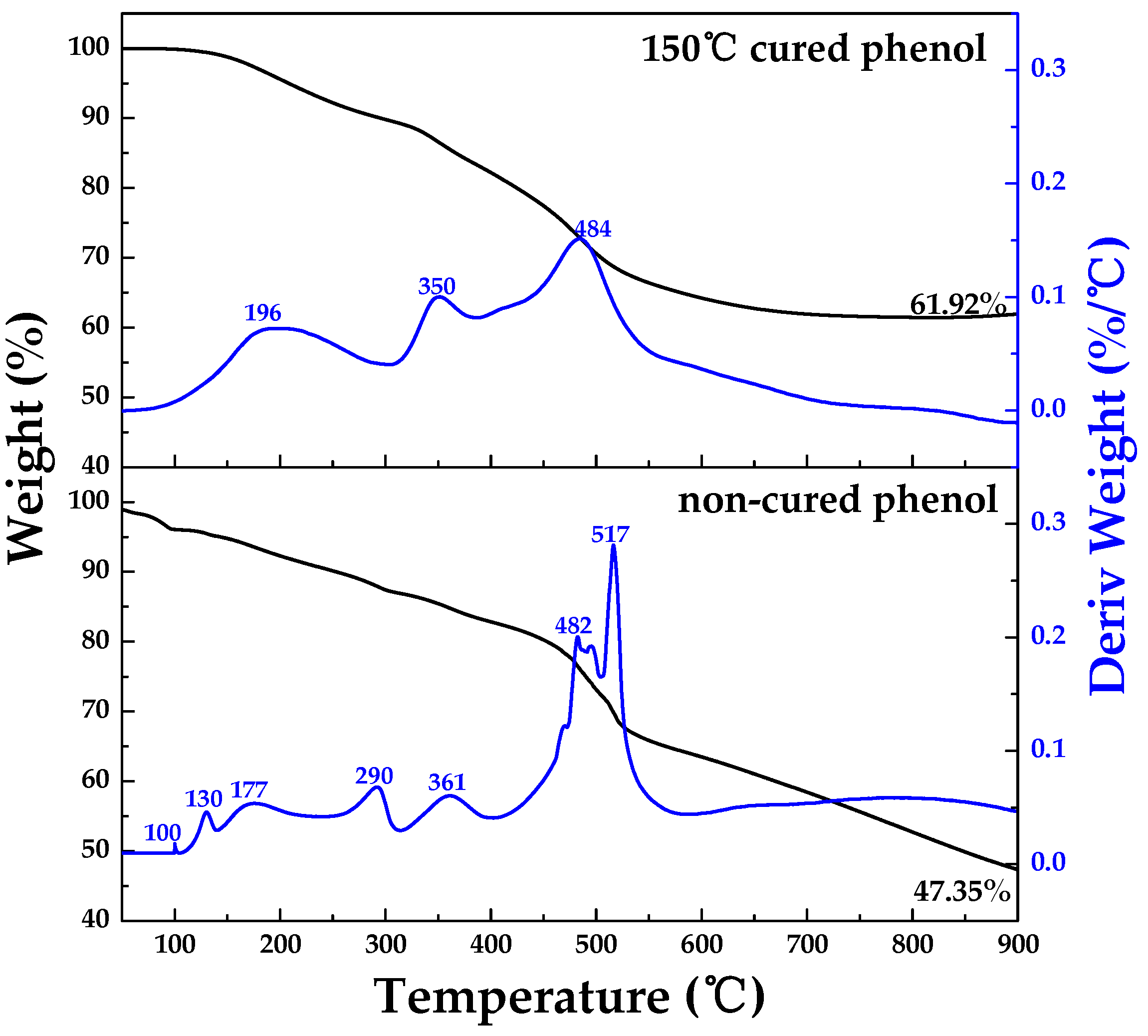
2.1.3. Weight Change with Regard to Temperature from TG-DTG
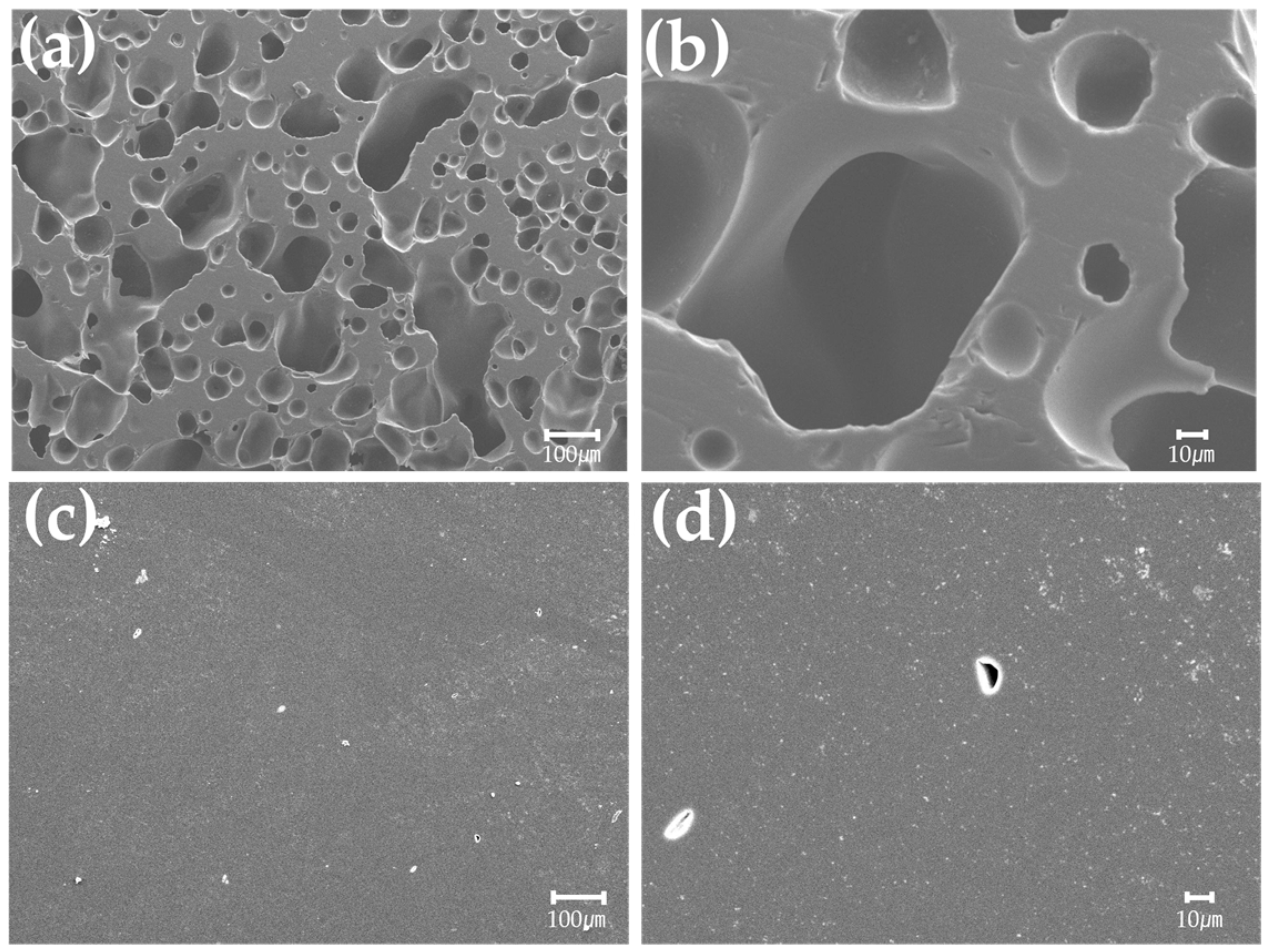
2.1.4. Observation of Pores
2.2. Fabrication and Analysis of Graphite Block
2.2.1. Fabrication of Graphite Block
2.2.2. Bulk Density and Porosity of Graphite Block
Porosity (%) = {(Saturated weight − dry weight)/(Saturated weight − underwater weight)} × 100
2.2.3. Electrical Resistivity of Graphite Block
2.2.4. Flexural Strength of Graphite Block
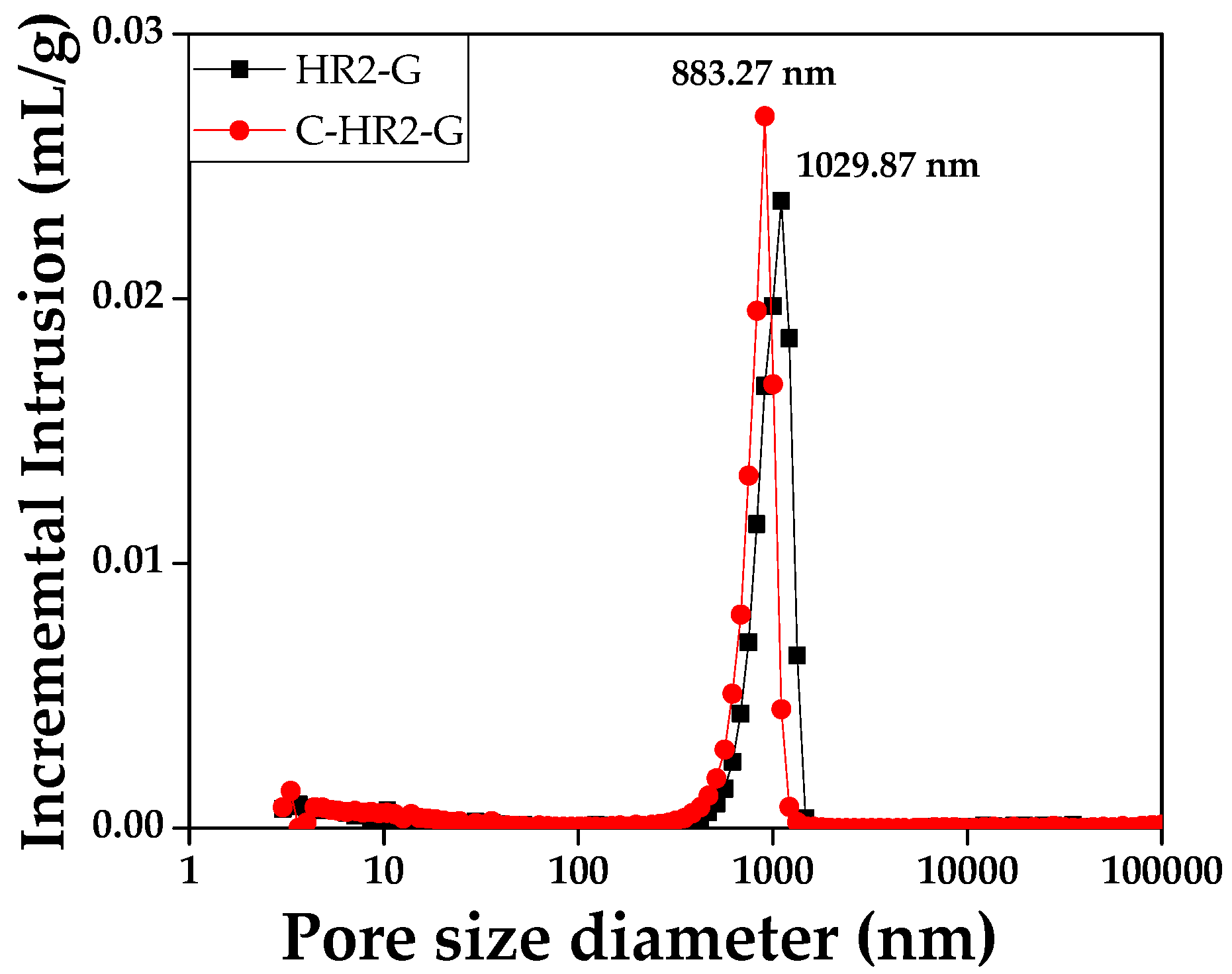
2.2.5. Pore Analysis of Graphite Blocks
2.2.6. Oxidation Resistance Test
3. Results and Discussion
3.1. Analysis of Cured and Carbonized Phenol
3.1.1. Methylene Index from FTIR
3.1.2. Weight Change with Regard to Temperature from TG-DTG
3.1.3. Pore Observation of Carbonized Phenol
3.2. Analysis of Graphite Block
3.2.1. Bulk density and porosity of graphite block
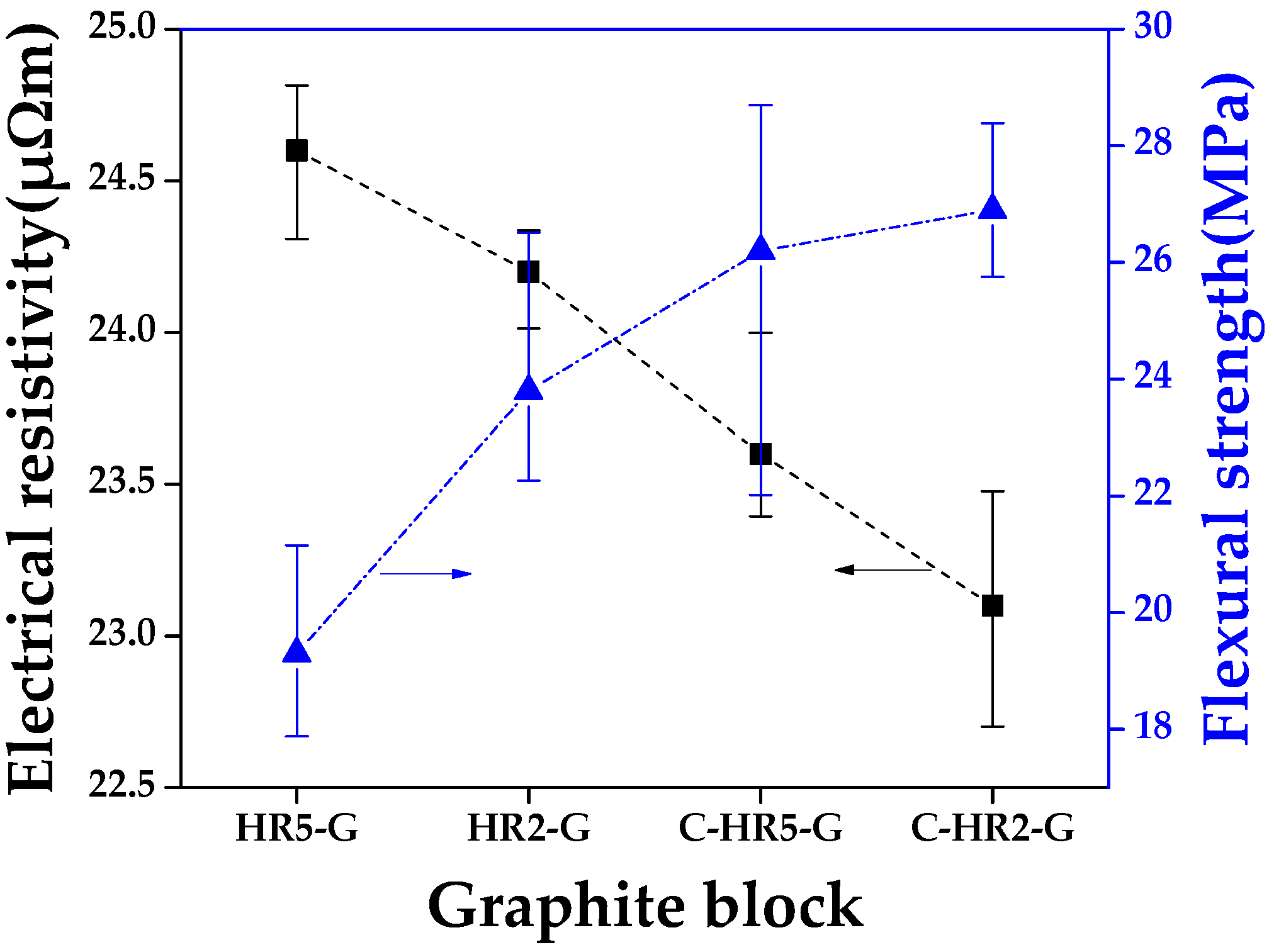
3.2.2. Electrical Resistivity and Flexural Strength of Graphite Block
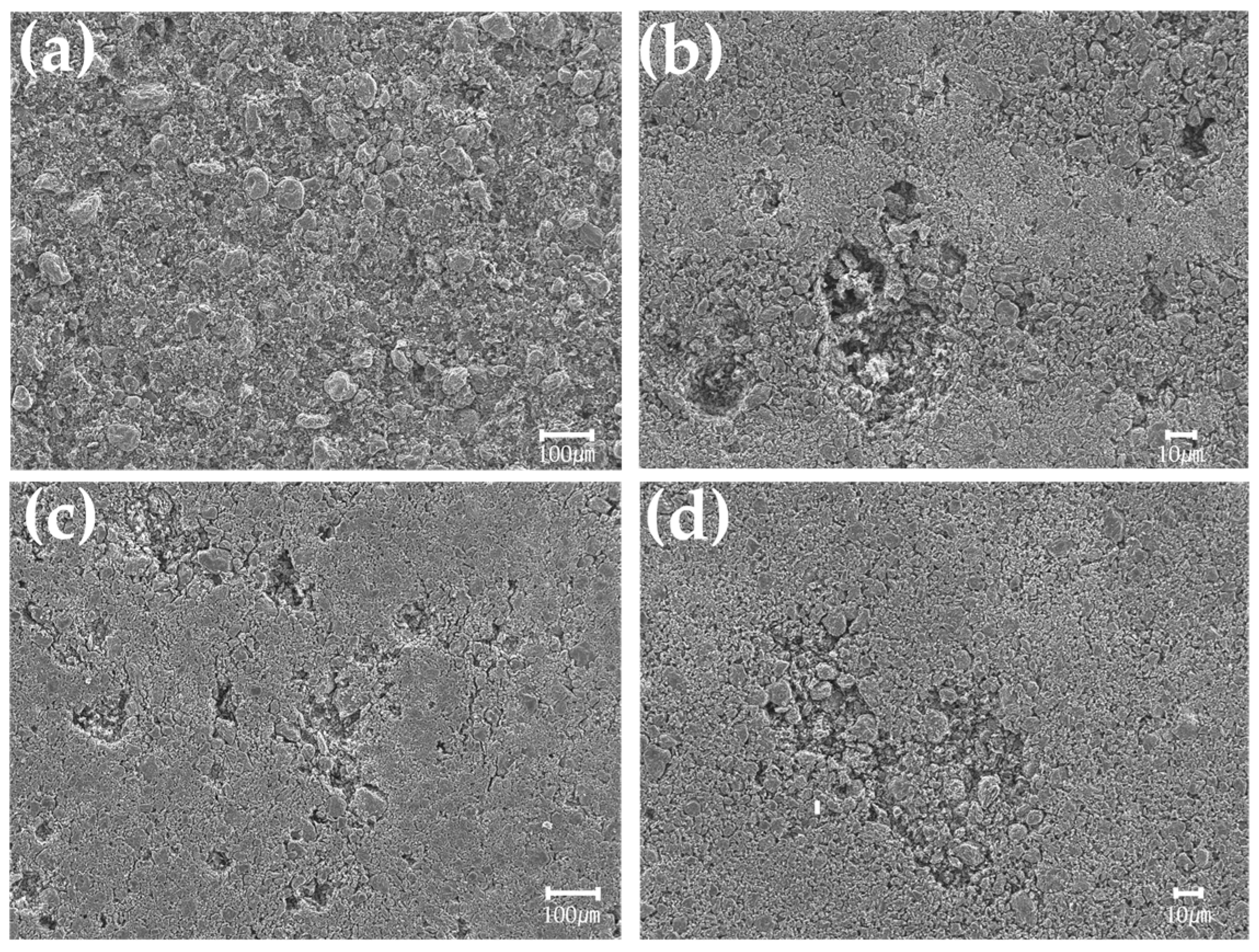
3.2.3. Pore Observation of Graphite Block
3.2.4. Oxidation Resistance Analysis
3.2.5. Physical Properties Change after Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.M.; Kang, D.S.; Kim, W.S.; Roh, J.S. Fabrication of Isotropic Bulk Graphite Using Artificial Graphite Scrap. Carbon Lett. 2014, 15, 142–145. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, D.S.; Roh, J.S. Bulk Graphite: Materials and Manufacturing Process. Carbon Lett. 2015, 16, 135–146. [Google Scholar] [CrossRef]
- An, D.H.; Kim, K.H.; Lim, C.H.; Lee, Y.S. Effect of Kneading and Carbonization Temperature on the Structure of the Carbon Block for Thermally Conductive Bulk Graphites. Carbon Lett. 2021, 31, 1357–1364. [Google Scholar] [CrossRef]
- Knop, A.; Plato, L.A. Phenolic Resins: Chemistry, Applications and Performance Future Directions; Springer: Berlin/Heidelberg, Germany, 1985; pp. 147–155. [Google Scholar]
- Irie, S.; James, R. Phenolic Resins: A Century of Progress; Springer: Berlin/Heidelberg, Germany, 2010; pp. 503–515. [Google Scholar]
- Yang, Z.; Liu, B.; Zhao, H.; Li, J.; Guo, X.; Zhang, D.; Liu, Z. Pyrolysis Mechanism of Composite Binder Composed of Coal Tar Pitch and Phenolic Resin for Carbon Materials. J. Anal. Appl. Pyrolysis 2023, 169, 105840. [Google Scholar] [CrossRef]
- Propp, W.A. Graphite Oxidation Thermodynamics/Reactions; Office of Scientific and Technical Information, Idaho National Lab. (INL): Idaho Falls, ID, USA, 1998. [Google Scholar]
- Choi, W.K.; Kim, B.J.; Chi, S.H.; Park, S.J. Nuclear graphites (I): Oxidation behaviors. Carbon Lett. 2009, 10, 239–249. [Google Scholar] [CrossRef]
- Conejo, L.S.; Costa, M.L.; Oishi, S.S.; Botelho, E.C. Degradation Behavior of Carbon Nanotubes/Phenol-Furfuryl Alcohol Multifunctional Composites with Aerospace Application. Mater. Res. Express 2017, 4, 105701. [Google Scholar] [CrossRef]
- Khezrabadi, M.N.; Javadpour, J.; Rezaie, H.R.; Naghizadeh, R. The Effect of Additives on the Properties and Microstructures of Al2O3-C Refractories. J. Mater. Sci. 2006, 41, 3027–3032. [Google Scholar] [CrossRef]
- Dutta, S.; Das, P.; Das, A.; Mukhopadhyay, S. Significant Improvement of Refractoriness of Al2O3–C Castables Containing Calcium Aluminate Nano-Coatings on Graphite. Ceram. Int. 2014, 40, 4407–4414. [Google Scholar] [CrossRef]
- Zhang, S.; Lee, W.E. Improving the Water-Wettability and Oxidation Resistance of Graphite Using Al2O3/SiO2 Sol-Gel Coatings. J. Eur. Ceram. Soc. 2003, 23, 1215–1221. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, D.; Zhang, S.; Du, F. Study on Improving the High-Temperature Oxidation Resistance of Pyrolytic Carbons of Phenolic Resin Binder by in-Situ Formation of Carbon Nanotubes. React. Funct. Polym. 2020, 157, 104772. [Google Scholar] [CrossRef]
- Kang, D.S.; Kim, B.J.; Lee, K.J.; Kim, S.H.; Lee, S.W.; Roh, J.S. Developing hollow carbon balls by oxidation of carbon blacks. Carbon Lett. 2013, 14, 55–57. [Google Scholar] [CrossRef]
- Shin, Y.W. A study of mechanical properties on high density graphite products with expanded graphite(1). J. Power Syst. Eng. 2005, 9, 143–147. [Google Scholar]
- Matzinos, P.D.; Patrick, J.W.; Walker, A. Coal-tar pitch as a matrix precursor for 2-DC/C composites. Carbon 1996, 34, 639–644. [Google Scholar] [CrossRef]
- Youm, H.N.; Kim, K.J.; Lee, J.M.; Chung, Y.J. Effects of impregnation on the manufacture of high density carbon materials. Yoop Hakhoechi 1993, 30, 852–858. [Google Scholar]
- Zhao, Y.; Li, X.; Jia, X.; Gao, S. Why and How to Tailor the Vertical Coordinate of Pore Size Distribution to Construct ORR-Active Carbon Materials? Nano Energy 2019, 58, 384–391. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Roh, J.S. The Pore-Filling Effect of Bulk Graphite According to Viscosity of Impregnant. Korean J. Mater. Res. 2021, 31, 101–107. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.H.; Kim, W.S.; Roh, J.S. The Effect of the Heating Rate during Carbonization on the Porosity, Strength, and Electrical Resistivity of Graphite Blocks Using Phenolic Resin as a Binder. Materials 2022, 15, 3259. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.M.; Lim, Y.S.; Park, H.S.; Kim, M.S. Effect of KOH addition on pore structure of glassy carbon prepared by polymerization of phenolic resin. Polym. Korea 2002, 26, 477–482. [Google Scholar]
- Maleki, H.; Holland, L.R.; Jenkins, G.M.; Zimmerman, R.L. Determining the shortest production time for glassy carbon ware. Carbon 1997, 35, 227–234. [Google Scholar] [CrossRef]
- Jurkiewicz, K.; Pawlyta, M.; Zygadło, D.; Chrobak, D.; Duber, S.; Wrzalik, R.; Burian, A. Evolution of glassy carbon under heat treatment: Correlation structure–mechanical properties. J. Mater. Sci. 2018, 53, 3509–3523. [Google Scholar] [CrossRef]
- Walker, P.L. Chemistry and Physics of Carbon: A Series of Advances; Marcel Dekker, Inc.: New York, NY, USA, 1987; Volume 16. [Google Scholar]
- Pesin, L.A. Review Structure and properties of glass-like carbon. J. Mater. Sci. 2002, 37, 1–28. [Google Scholar] [CrossRef]
- Hajihosseini, S.; Nasirizadeh, N.; Hejazi, M.S.; Yaghmaei, P. A Sensitive DNA Biosensor Fabricated from Gold Nanoparticles and Graphene Oxide on a Glassy Carbon Electrode. Mater. Sci. Eng. C 2016, 61, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Tadyszak, K.; Litowczenko, J.; Majchrzycki, Ł.; Jeżowski, P.; Załęski, K.; Scheibe, B. Sucrose Based Cellular Glassy Carbon for Biological Applications. Mater. Chem. Phys. 2020, 239, 122033. [Google Scholar] [CrossRef]
- Acuña, N.T.; Güiza, A.V.; Córdoba, T.E. Reticulated Vitreous Carbon Foams from Sucrose: Promising Materials for Bone Tissue Engineering Applications. Macromol. Res. 2020, 28, 888–895. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, L.; Zhang, H.; Zhai, H.; Ren, H. Research Status, Industrial Application Demand and Prospects of Phenolic Resin. RSC Adv. 2019, 9, 28924–28935. [Google Scholar] [CrossRef] [PubMed]
- Chow, S. Thermal Analysis of Liquid Phenol-Formaldehyde Resin Curing. Holzforschung 1972, 26, 229–232. [Google Scholar] [CrossRef]
- Yoon, S.B.; Kim, J.W.; Cho, D.H. Thermal Stability and Cure Behavior of Waterborne Phenol-Formaldehyde Resin. J. Adhes. Interface 2006, 7, 16–22. [Google Scholar]
- Hu, H.; Wang, W.; Jiang, L.; Liu, L.; Zhang, Y.; Yang, Y.; Wang, J. Curing Mechanism of Resole Phenolic Resin Based on Variable Temperature FTIR Spectra and Thermogravimetry-Mass Spectrometry. Polym. Polym. Compos. 2022, 30, 096739112211021. [Google Scholar] [CrossRef]
- Kejian, J.; Werhua, D.; Yusheng, Y. Determination of the hydroxymethyl index for phenolic resin by infrared spectroscopy. Chem. Anal. Meter. 2002, 11, 21–23. [Google Scholar]
- Ricci, A.; Oleja, K.J.; Parpinello, G.P.; Killmartin, P.A.; Versari, A. Application of furrier transform infrared (FTIR) spectroscopy in the characterization of tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, Z.; Shang, Z.; Zhao, J. Classification and identification of Rhodobryum roseum Limpr. And its adulterants based on furrier-transform infrared spectroscopy (FTIR) and chemometrics. PLoS ONE 2017, 12, e0172359. [Google Scholar]
- Chang, C.; Tackett, J.R. Characterization of phenolic resins with thermogravimetry-mass spectrometry. Thermochim. Acta 1991, 192, 181–190. [Google Scholar] [CrossRef]
- Oshida, K.; Ekinaga, N.; Endo, M.; Inagaki, M. Pore Analysis of Isotropic Graphite Using Image Processing of Optical Micrographs. TANSO 1996, 1996, 142–147. [Google Scholar] [CrossRef]
- Xiaowei, L.; Jean, C.R.; Suyuan, Y. Effect of Temperature on Graphite Oxidation Behavior. Nucl. Eng. Des. 2004, 227, 273–280. [Google Scholar] [CrossRef]
- Blanchard, A. The thermal oxidation of graphite-Irradiation damage in graphite due to fast neutrons in fission and fusion systems. IAEA-TECDOC 2000, 1154, 169–175. [Google Scholar]
- Chen, D.; Li, Z.; Miao, W.; Zhang, Z. Effects of porosity and temperature on oxidation behavior in air of selected nuclear graphites. Mater. Trans. 2012, 53, 1159–1163. [Google Scholar] [CrossRef]
- Matthews, A.C.; Kane, J.J.; Swank, W.D.; Windes, W.E. Nuclear graphite strength degradation under varying oxidizing conditions. Nucl. Eng. Des. 2021, 379, 111245. [Google Scholar] [CrossRef]





| Samples | Curing | Heating Rate during Carbonization (°C/min) | Graphitization |
|---|---|---|---|
| HR5-G | - | 5 | O |
| HR2-G | - | 2 | O |
| C-HR5-G | O | 5 | O |
| C-HR2-G | O | 2 | O |
| Observed Wave Number (cm−1) | Functional Group |
|---|---|
| 3272~3364 | OH stretch |
| 2923~2916 | aliphatic CH asymmetric stretch |
| 2849 | aliphatic CH symmetric stretch |
| 1594~1610 | C=C aromatic ring |
| 1508~1509 | C=C aromatic ring |
| 1438~1472 | aliphatic CH2 scissor bending |
| 1369 | phenolic OH in-plane deformation |
| 1206~1234 | alkyl-phenol C-O stretch |
| 1095~1099 | aromatic CH in-plane deformation |
| 1005~1050 | C-O stretch |
| Samples | Bulk Density (g/cm3) | Porosity (%) | Electrical Resistivity (μΩm) | Flexural Strength (MPa) | Weight Change after Oxidation (%) |
|---|---|---|---|---|---|
| HR5-G | 1.682 | 18.8 | 24.6 | 19.3 | −3.12 |
| HR2-G | 1.696 | 18.1 | 24.2 | 23.8 | −2.19 |
| C-HR5-G | 1.699 | 17.4 | 23.6 | 26.2 | −1.51 |
| C-HR2-G | 1.707 | 15.8 | 23.1 | 26.9 | −0.95 |
| Samples | Oxidation | Bulk Density (g/cm3) | Porosity (%) | Electrical Resistivity (μΩm) | Flexural Strength (MPa) |
|---|---|---|---|---|---|
| HR2-G | Before | 1.696 | 18.1 | 24.2 | 23.8 |
| After | 1.674 | 20.2 | 29.1 | 11.8 | |
| Δ% | −1.30 | +11.60 | +20.25 | −50.42 | |
| C-HR2-G | Before | 1.707 | 15.8 | 23.1 | 26.9 |
| After | 1.701 | 16.5 | 25.2 | 21.6 | |
| Δ% | −0.35 | +4.43 | +9.09 | −19.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.-H.; Lee, S.-H.; Roh, J.-S. Improved Oxidation Resistance of Graphite Block by Introducing Curing Process of Phenolic Resin. Materials 2023, 16, 3543. https://doi.org/10.3390/ma16093543
Ko J-H, Lee S-H, Roh J-S. Improved Oxidation Resistance of Graphite Block by Introducing Curing Process of Phenolic Resin. Materials. 2023; 16(9):3543. https://doi.org/10.3390/ma16093543
Chicago/Turabian StyleKo, Jong-Hwan, Sang-Hye Lee, and Jae-Seung Roh. 2023. "Improved Oxidation Resistance of Graphite Block by Introducing Curing Process of Phenolic Resin" Materials 16, no. 9: 3543. https://doi.org/10.3390/ma16093543
APA StyleKo, J.-H., Lee, S.-H., & Roh, J.-S. (2023). Improved Oxidation Resistance of Graphite Block by Introducing Curing Process of Phenolic Resin. Materials, 16(9), 3543. https://doi.org/10.3390/ma16093543






