Hot Cracking Behaviors of Mg-Zn-Er Alloys with Different Er Contents
Abstract
1. Introduction
2. Experimental Method
2.1. Alloy Preparing
2.2. Hot Cracking Apparatus
2.3. Microstructure Analysis
3. Results
3.1. Hot Cracking Samples
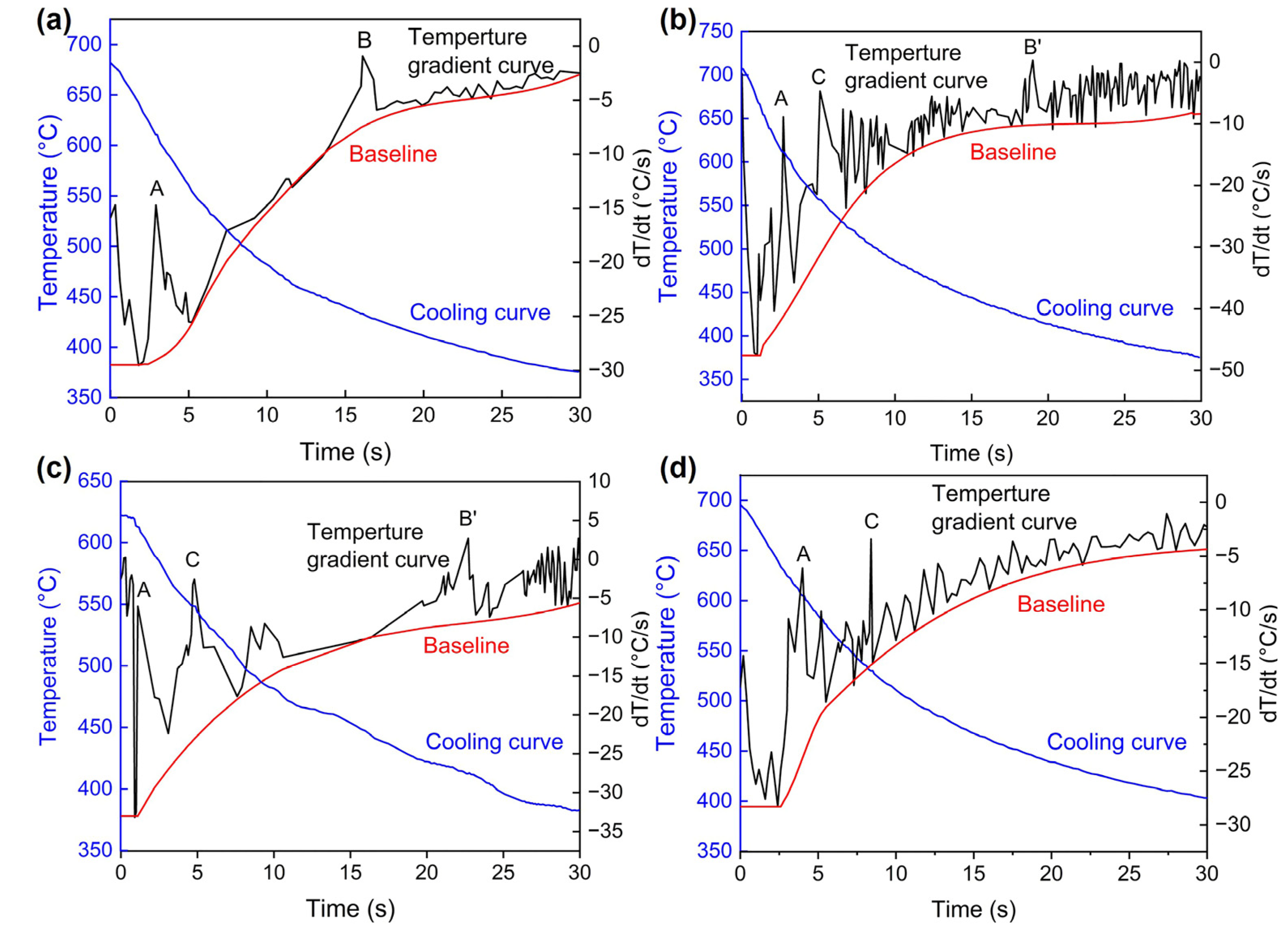
3.2. Contraction Behaviors
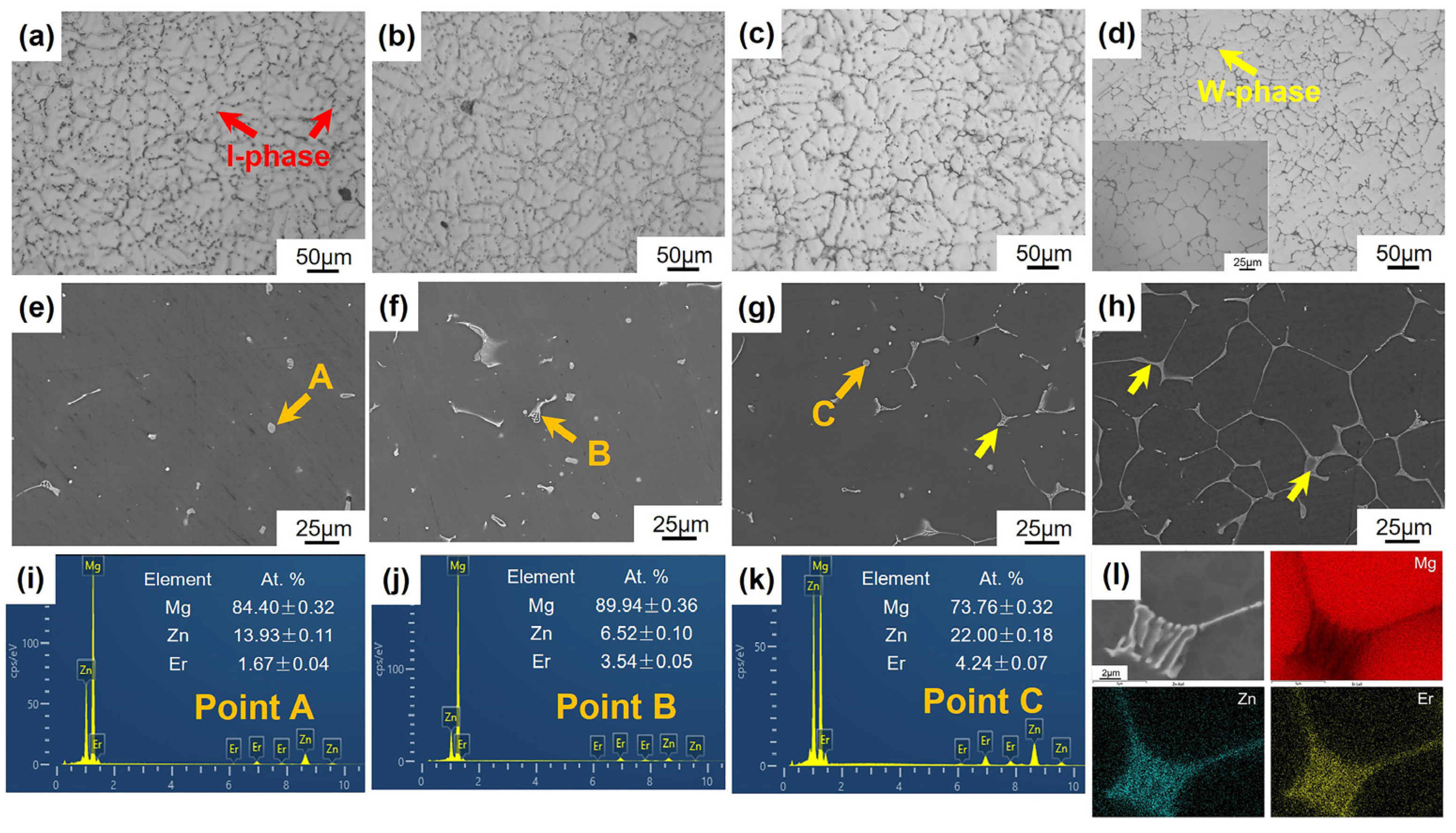
3.3. Microstructures
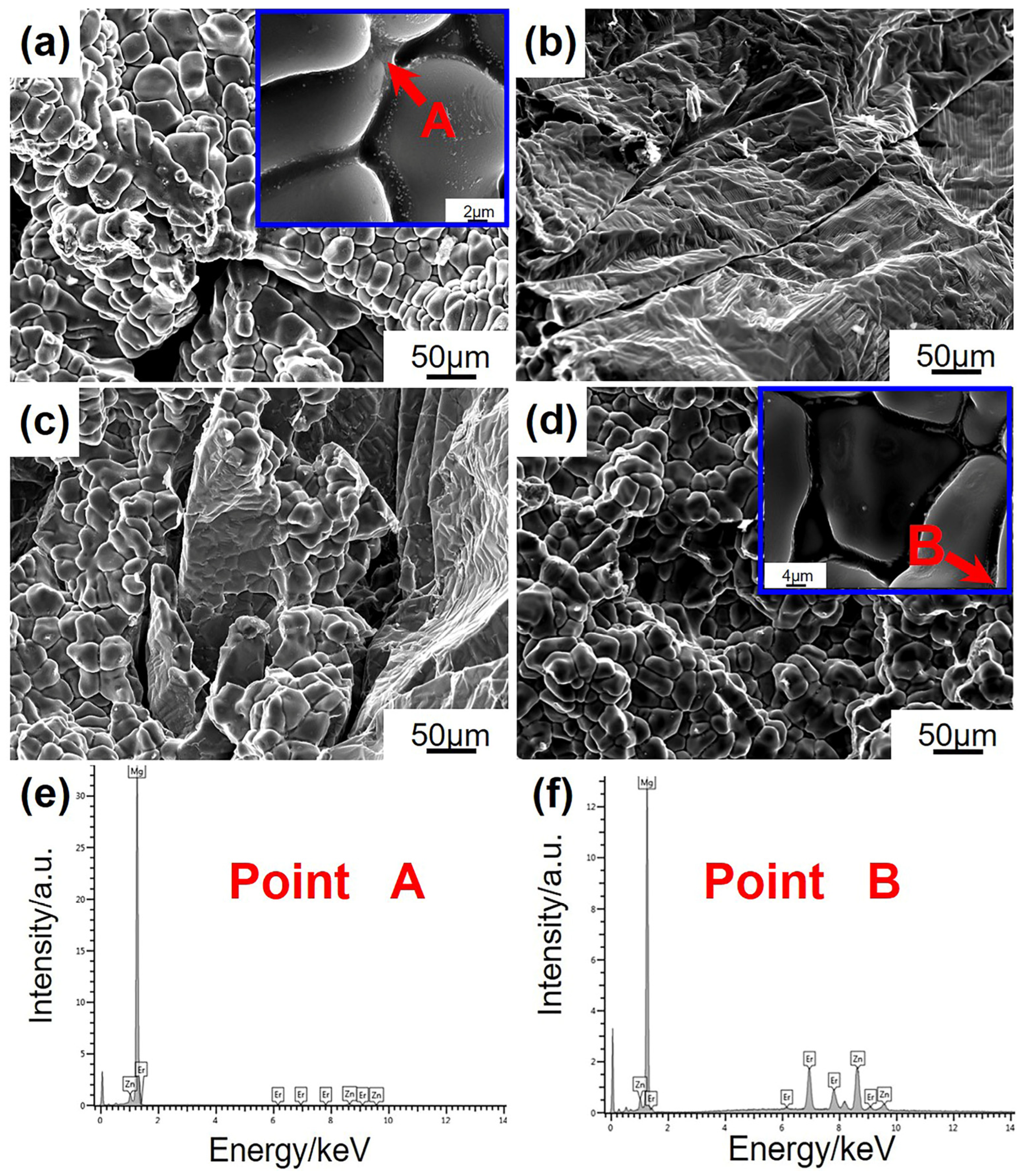
3.4. Hot cracking Fracture
4. Discussion
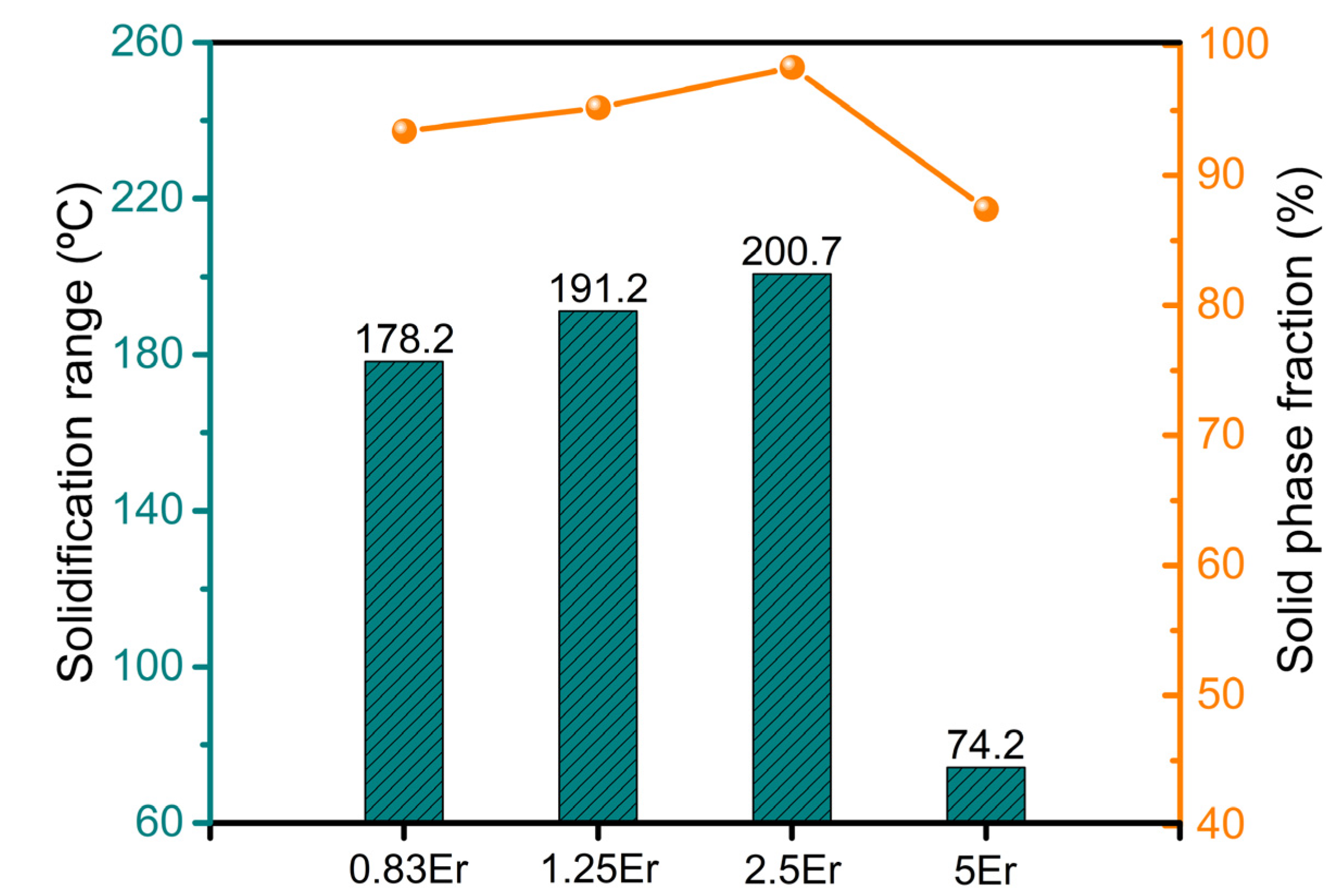
4.1. Freezing Range and Feeding Behaviors
4.2. Permeability of the Mushy Zone
5. Conclusions
- (1)
- The freezing range and solid phase fraction at hot cracking initiation increase with increasing Er content up to 2.5 wt.% and then decrease with concentrations up to 5 wt.%. The Mg-5Zn-5Er alloy exhibits the highest liquid phase fraction and reduced freezing range, contributing to the decreased hot cracking tendency, which shows minimal hot cracking susceptibility. Conversely, the Mg-5Zn-2.5Er alloy exhibits the maximal hot cracking susceptibility;
- (2)
- The Mg-5Zn-xEr alloys with different Er contents form the W phase and/or I-phase during solidification. The I-phase of the Mg-5Zn-0.83Er alloy is formed by the eutectic reaction. When the Er content is 1.25 wt.% or 2.5 wt.%, the W phase precipitates first, and the remaining liquids still contribute to the subsequent peritectic reaction to generate the I-phase. The lack of surplus liquids leads to a higher hot cracking tendency. For the Mg-5Zn-5Er alloy, the more effective liquids by the eutectic reaction (L → α-Mg + W phase) and high phase precipitation temperature lead to the lowest freezing range;
- (3)
- The Mg-5Zn-5Er alloy exhibits the best permeability of the mushy zone due to the refined grain size, which is beneficial to feed the emerging cavities and micro-pores. Meanwhile, a large number of eutectic phases at the fracture would heal the cracking, which increases the hot cracking resistance of the alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, M.Y.; Zhang, Z.W.; Cui, Y.; Liu, L.Y.; Liu, Y.W.; Liaw, K.P. Achieving strength and ductility synergy via a nanoscale superlattice precipitate in a cast Mg-Y-Zn-Er alloy. Int. J. Plast. 2023, 163, 103558. [Google Scholar] [CrossRef]
- Lin, J.M.; Fu, P.H.; Wang, Y.X.; Liu, H.; Zheng, Y.; Peng, L.M.; Ding, W.J. Effect of La addtion on microstructure, meachanical behavior, strengthening and toughening mechanisms of cast Mg-Gd-Zn alloy. Mater. Sci. Eng. A 2023, 866, 144688. [Google Scholar] [CrossRef]
- Wang, G.G.; Weuker, J.P. Recent developments in high-pressure die-cast magnesium alloys for automotive and future applications. J. Magnes. Alloys 2023, 11, 78–87. [Google Scholar] [CrossRef]
- Pan, F.S.; Yang, M.B.; Chen, X.H. A review on casting magnesium alloys: Modification of commercial alloys and development of new alloys. J. Mater. Sci. Technol. 2016, 32, 1211–1221. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.X.; Katgerman, L.; Du, Q.; Zhang, J.S.; Zhuang, L.Z. Recent advances in hot tearing during casting of aluminium alloys. Prog. Mater. Sci. 2021, 117, 100741. [Google Scholar] [CrossRef]
- Song, J.F.; Pan, F.S.; Jiang, B.; Atrens, A.; Zhang, M.X.; Lu, Y. A review on hot tearing of magnesium alloys. J. Magnes. Alloys 2016, 4, 151–172. [Google Scholar] [CrossRef]
- Du, X.D.; Wang, F.; Wang, Z.; Zhou, L.; Wei, Z.Q.; Liu, Z.; Mao, P.L. Effect of Ca/Al ratio on hot tearing susceptibility of Mg-Al-Ca alloy. J. Alloys Compd. 2022, 911, 165113. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Mu, W.P.; Liu, S.M.; Wang, F.; Zhou, L.; Wang, Z.; Mao, P.L.; Liu, Z. Effects of Gd on hot tearing susceptibility of as-cast Mg96.94-Zn1-Y(2−x)-Gdx-Zr0.06 alloys reinforced with LPSO phase. J. Alloys Compd. 2022, 926, 166895. [Google Scholar] [CrossRef]
- Easton, M.A.; Gibson, M.A.; Zhu, S.M.; Abbott, T.B. An a prior hot-tearing indicator applied to die-cast magnesium-rare earth alloys. Metall. Mater. Trans. A 2014, 45, 3586. [Google Scholar] [CrossRef]
- Srinivasan, A.; Wang, Z.; Huang, Y.D.; Beckmann, F.; Kainer, K.U.; Hort, N. Hot tearing characteristics of binary Mg-Gd alloy castings. Metall. Mater. Trans. A 2013, 44, 2285–2298. [Google Scholar] [CrossRef]
- Zhang, G.J.; Wang, Y.; Liu, Z.; Liu, S.M. Influence of Al addition on solidification path and hot tearing susceptibility of Mg-2Zn-(3 + 0.5x)Y-xAl alloys. J. Magnes. Alloys 2019, 7, 272–282. [Google Scholar] [CrossRef]
- Du, X.D.; Wang, F.; Wang, Z.; Zhou, L.; Liu, Z.; Mao, P.L. Effect of addition of minor amounts of Sb and Gd on hot tearing susceptibility of Mg-5Al-3Ca alloy. J. Magnes. Alloys 2023, 11, 694–705. [Google Scholar] [CrossRef]
- Vinodh, G.; Jafari-Nndooshan, H.R.; Li, D.J.; Zeng, X.Q.; Hu, B.; Carter, J.T.; Sachdev, A.K. Effect of Al Content on hot-tearing susceptibility of Mg-10Zn-xAl Alloys. Metall. Mater. Trans. A 2020, 51, 1897–1910. [Google Scholar] [CrossRef]
- Zhou, Y.; Mao, P.L.; Wang, Z.; Zhou, L.; Wang, F.; Liu, Z. Experimental investigation and simulation assessment on fluidity and hot tearing of Mg-Zn-Cu system alloys. J. Mater. Process. Technol. 2021, 297, 117259. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Liu, S.M.; Liu, Z.; Wang, F.; Mao, P.L.; Wang, X.X.; Li, X.X. Effects of Zn content on hot tearing susceptibility of Mg-7Gd-5Y-0.5Zr Alloy. Metals 2020, 10, 414. [Google Scholar] [CrossRef]
- Song, J.F.; Wang, Z.; Huang, Y.D.; Srinivasan, A.; Beckmann, F.; Kainer, K.U.; Hort, N. Effect of Zn addition on hot tearing behaviour of Mg-0.5Ca-xZn alloys. Mater. Design 2015, 87, 157–170. [Google Scholar] [CrossRef]
- Wang, W.Z.; Zhang, M.M.; Yang, Z.Q.; Hu, W.W. Dynamic precipitation and strengthening in a Mg-Zn-Gd alloy during hot deformation. J. Alloys Compd. 2022, 905, 164219. [Google Scholar] [CrossRef]
- Itakura, M.; Yamaguchi, M.; Egusa, D.; Abe, E. Density functional theory study of solute cluster growth processes in Mg-Y-Zn LPSO alloys. Acta Mater. 2021, 203, 116491. [Google Scholar] [CrossRef]
- Zhou, Y.; Mao, P.L.; Zhou, L.; Wang, Z.; Wang, F.; Liu, Z. Effect of long-period stacking ordered phase on hot tearing susceptibility of Mg-1Zn-xY alloys. J. Magnes. Alloys 2020, 8, 1176–1185. [Google Scholar] [CrossRef]
- Liao, H.X.; Kim, J.; Lee, T.; Song, J.F.; Peng, J.; Jiang, B.; Pan, F.S. Effect of heat treatment on LPSO morphology and mechanical properties of Mg-Zn-Y-Gd alloys. J. Magnes. Alloys 2020, 8, 1120–1127. [Google Scholar] [CrossRef]
- Bae, D.H.; Kim, S.H.; Kim, D.H.; Kim, W.T. Deformation behavior of Mg-Zn-Y alloys reinforced by icosahedral quasicrystalline particles. Acta Mater. 2002, 50, 1356–2343. [Google Scholar] [CrossRef]
- Xiao, N.; Zeng, Y.Z.; Lu, X.L.; Sun, Y.H.; Sun, B.Z. On the γ′ and W strengthening precipitates in Dy and Zn microalloyed magnesium alloys. Mater. Lett. 2022, 307, 131028. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, Z.H.; Liu, K.; Li, S.B.; Du, W.B. Effects of Er on hot cracking susceptibility of Mg-5Zn-xEr magnesium alloys. Acta Metall. Sin. 2019, 55, 389–398. [Google Scholar]
- Li, H.; Du, W.B.; Li, S.B.; Wang, Z.H. Effect of Zn/Er weight ratio on phase formation and mechanical properties of as-cast Mg-Zn-Er alloys. Mater. Design 2012, 35, 259–265. [Google Scholar] [CrossRef]
- Kou, S.D. A criterion for cracking during solidification. Acta Mater. 2015, 88, 366–374. [Google Scholar] [CrossRef]
- Soysal, T.; Kou, S.D. A simple test for assessing solidification cracking susceptibility and checking validity of susceptibility prediction. Acta Mater. 2018, 143, 181–197. [Google Scholar] [CrossRef]
- Haq, I.U.; Shin, J.S.; Lee, Z.H. Computer-aided cooling curve analysis of A356 aluminum alloy. Met. Mater. Int. 2004, 10, 89–96. [Google Scholar]
- Li, J.H.; Du, W.B.; Li, S.B.; Wang, Z.H. Icosahedral quasicrystalline phase in an as-cast Mg-Zn-Er alloy. Rare Met. 2009, 28, 297. [Google Scholar] [CrossRef]
- Wang, Q.F.; Du, W.B.; Liu, K.; Wang, Z.H.; Li, S.B. Effect of Zn addition on microstructure and mechanical properties of as-cast Mg-2Er alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 3792–3796. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification; Trans Tech Publications: Wollerau, Switzerland, 1984. [Google Scholar]
- Hou, Z.B.; Guo, D.W.; Cao, J.H.; Chang, Y. A method based on the centroid of segregation points: A voronoi polygon application to solidification of alloys. J. Alloys Compd. 2018, 762, 508–519. [Google Scholar] [CrossRef]
- Eskin, D.G.; Suyitno Katgerman, L. Mechanical properties in the semi-solid state and hot tearing of aluminium alloys. Prog. Mater. Sci. 2004, 49, 629–711. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.R.; Zhao, Z.Y.; Li, H.X.; Katgerman, L.; Zhang, J.S.; Zhuang, L.Z. Effect of main elements (Zn, Mg, and Cu) on hot tearing susceptibility during direct-chill casting of 7xxx aluminum alloys. Metall. Mater. Trans. A 2019, 50, 3603–3616. [Google Scholar] [CrossRef]
- Suyitno, S.; Kool, W.; Katgerman, L. Integrated approach for prediction of hot tearing. Metall. Mater. Trans. A 2009, 40, 2388–2400. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Q.L.; Liu, J.C.; Li, H.X.; Du, Q.; Zhang, J.S.; Zhuang, L.Z. The influences of grain size and morphology on the hot tearing susceptibility, contraction, and load behaviors of AA7050 alloy inoculated with Al-5Ti-1B master alloy. Metall. Mater. Trans. A 2016, 47, 4024–4037. [Google Scholar] [CrossRef]
- D’elia, F.; Ravindran, C.; Sediako, D. Interplay among solidification, microstructure, residual strain and hot tearing in B206 aluminum alloy. Mater. Sci. Eng. A 2015, 624, 169–180. [Google Scholar] [CrossRef]
- Easton, M.; Grandfield, J.F.; Stjohn, D.H.; Rinderer, B. The effect of grain refinement and cooling rate on the hot tearing of wrought aluminum alloys. Mater. Sci. Forum 2006, 519, 1675–1680. [Google Scholar] [CrossRef]







| Alloy | Zn (wt.%) | Er (wt.%) | Mg (wt.%) | Main Phases |
|---|---|---|---|---|
| Mg-5Zn-0.83Er | 5.3 ± 0.3 | 0.8 ± 0.1 | Bal. | α-Mg + I-phase |
| Mg-5Zn-1.25Er | 5.0 ± 0.1 | 1.1 ± 0.3 | Bal. | α-Mg + I-phase + W phase |
| Mg-5Zn-2.5Er | 5.1 ± 0.3 | 2.4 ± 0.1 | Bal. | α-Mg + I-phase + W phase |
| Mg-5Zn-5Er | 5.1 ± 0.2 | 5.3 ± 0.2 | Bal. | α-Mg + W phase |
| Alloy | fsht (%) | TL (°C) | Ts (°C) | ΔT (°C) | Tht (°C) |
|---|---|---|---|---|---|
| Mg-5Zn-0.83Er | 93.4 | 610.6 | 428.3 | 182.3 | 443 |
| Mg-5Zn-1.25Er | 95.2 | 611.6 | 420.2 | 191.4 | 426 |
| Mg-5Zn-2.5Er | 98.3 | 612.5 | 413.0 | 199.5 | 412 |
| Mg-5Zn-5Er | 87.4 | 605.0 | 530.8 | 74.2 | 543 |
| Point | At. % | Zn/Er Ratio | Phase | ||
|---|---|---|---|---|---|
| Mg | Zn | Er | |||
| A | 97.72 | 1.96 | 0.32 | 6.1 | I-phase |
| B | 64.81 | 23.78 | 11.41 | 2.1 | W phase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Z.; Li, S.; Ding, N.; Liu, K.; Du, W. Hot Cracking Behaviors of Mg-Zn-Er Alloys with Different Er Contents. Materials 2023, 16, 3546. https://doi.org/10.3390/ma16093546
Liu Y, Wang Z, Li S, Ding N, Liu K, Du W. Hot Cracking Behaviors of Mg-Zn-Er Alloys with Different Er Contents. Materials. 2023; 16(9):3546. https://doi.org/10.3390/ma16093546
Chicago/Turabian StyleLiu, Yaohong, Zhaohui Wang, Shubo Li, Ning Ding, Ke Liu, and Wenbo Du. 2023. "Hot Cracking Behaviors of Mg-Zn-Er Alloys with Different Er Contents" Materials 16, no. 9: 3546. https://doi.org/10.3390/ma16093546
APA StyleLiu, Y., Wang, Z., Li, S., Ding, N., Liu, K., & Du, W. (2023). Hot Cracking Behaviors of Mg-Zn-Er Alloys with Different Er Contents. Materials, 16(9), 3546. https://doi.org/10.3390/ma16093546






