Encapsulation of Silicon Nano Powders via Electrospinning as Lithium Ion Battery Anode Materials
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.3. Fabrication of Nano Silicon-Containing Fibers
2.4. Electrochemical Measurements
3. Results and Discussion
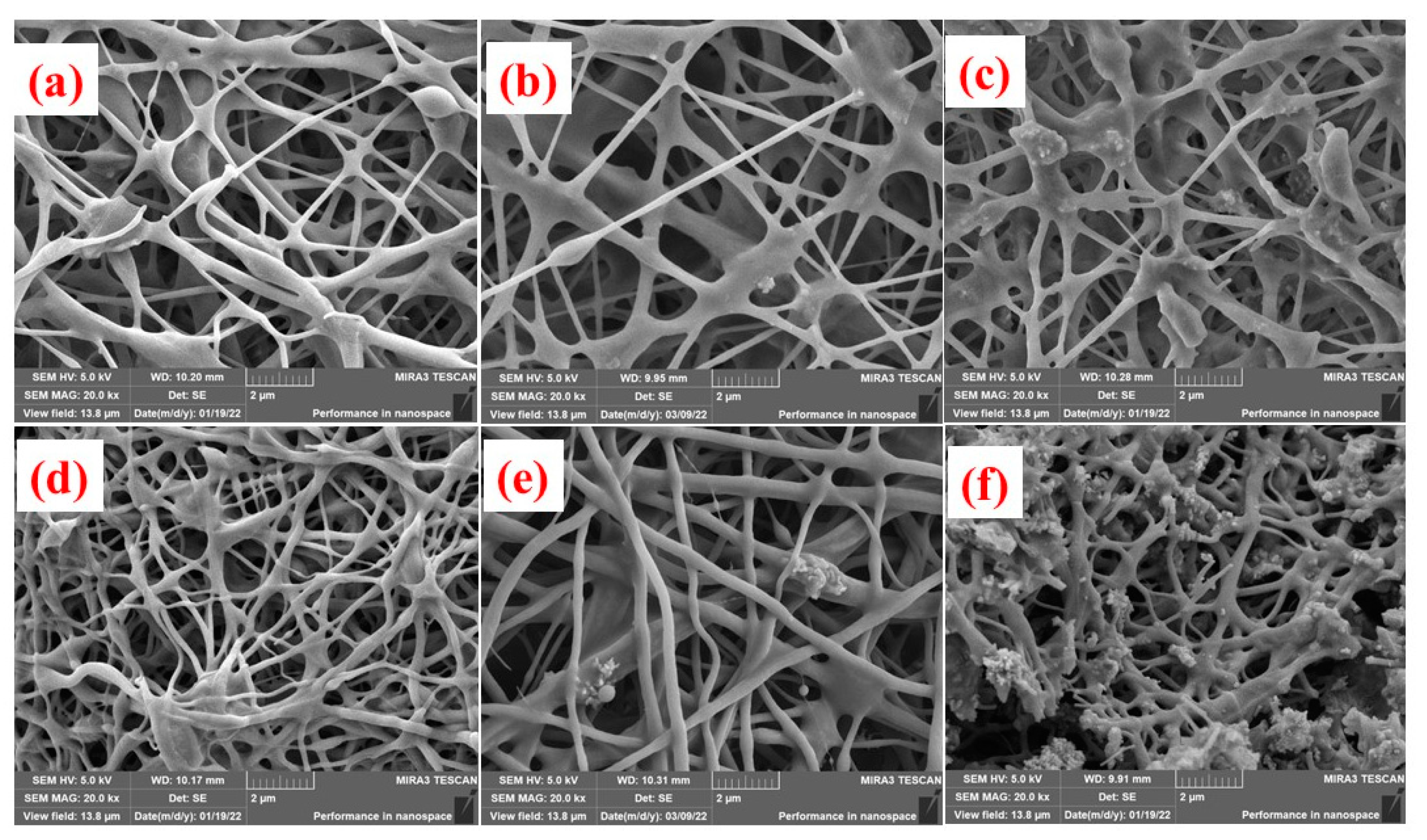
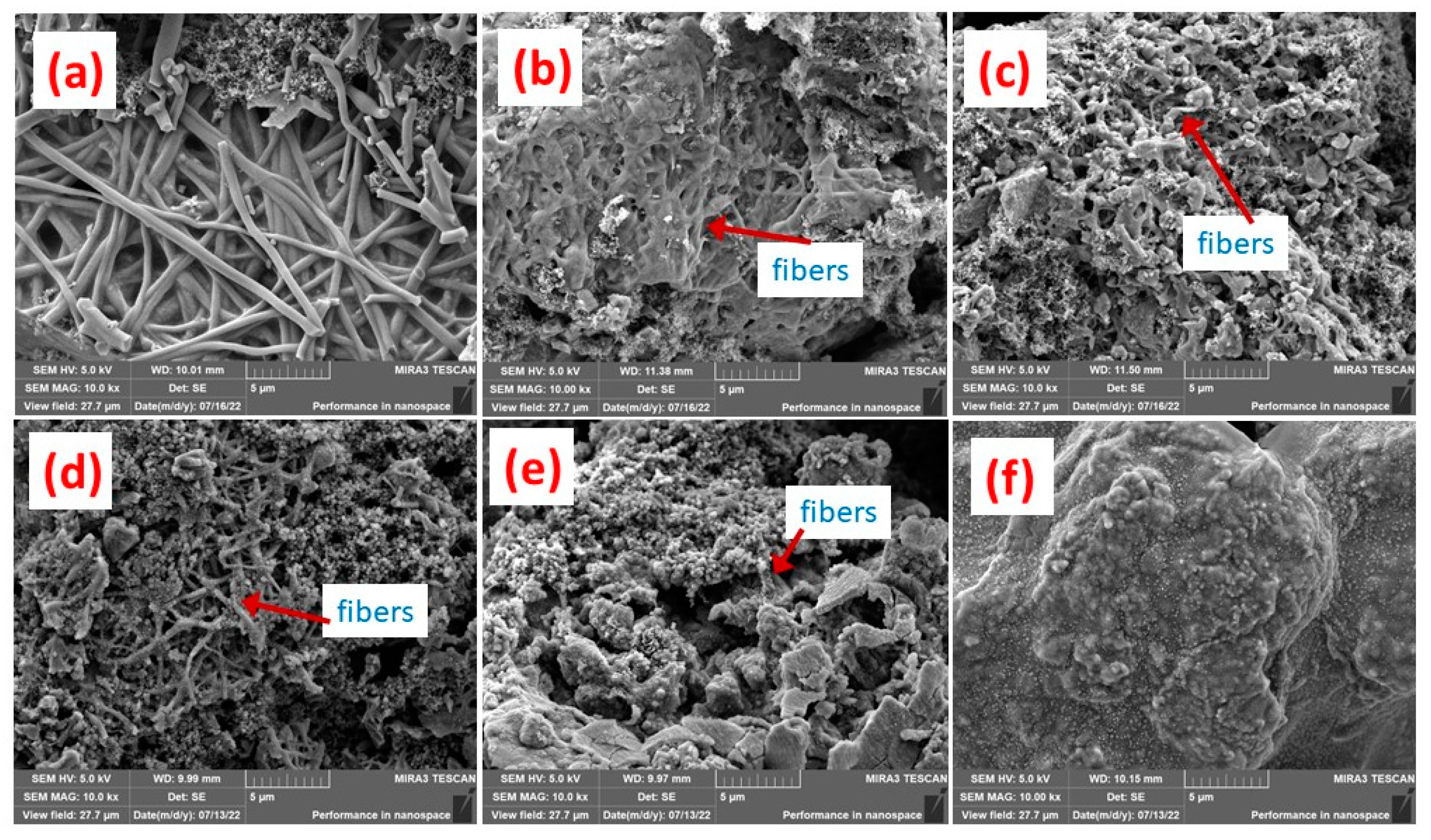
3.1. Micrograph Structure of Fibers
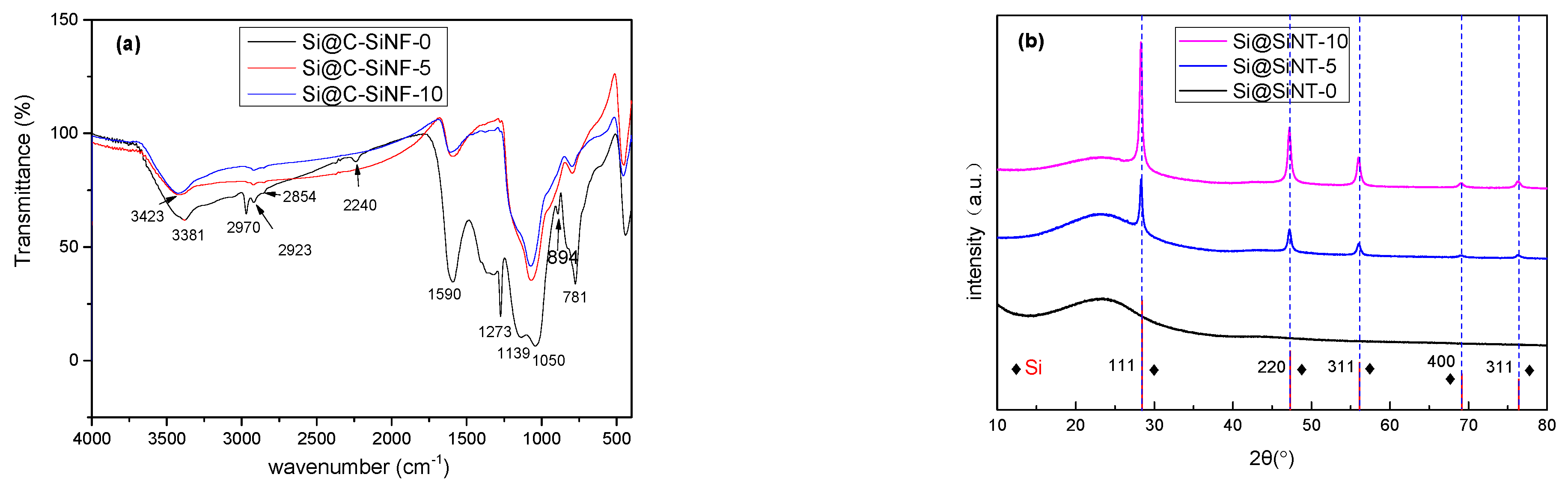
3.2. Thermal Analysis of Fibers
3.3. X-ray Diffraction of Fibers
3.4. Electrochemical Performance of Fibers as Anodes and Influences
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.J.; Wu, S.X.; Zhang, Y.P.; Liu, C.B.; Wei, X.J.; Luo, D.; Lin, Z. Towards efficient binders for silicon based lithium-ion battery anodes. Chem. Eng. J. 2021, 406, 126807. [Google Scholar] [CrossRef]
- Manj, R.Z.A.; Zhang, F.Z.; Rehman, W.U.; Luo, W.; Yang, J.P. Toward understanding the interaction within Silicon-based anodes for stable lithium storage. Chem. Eng. J. 2020, 385, 123821. [Google Scholar] [CrossRef]
- Golmon, S.; Maute, K.; Lee, S.-H.; Dunn, M.L. Stress generation in silicon particles during lithium insertion. Appl. Phys. Lett. 2010, 97, 033111. [Google Scholar] [CrossRef]
- Yuda, A.P.; Koraag, P.Y.E.; Iskandar, F.; Wasisto, H.S.; Sumboj, A. Advances of the top-down synthesis approach for high-performance silicon anodes in Li-ion batteries. J. Mater. Chem. A 2021, 9, 18906. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, W.; Wang, S.C.; Cui, Y. The effect of metallic coatings and crystallinity on the volume expansion of silicon during electrochemical lithiation/delithiation. Nano Energy 2012, 1, 401–410. [Google Scholar] [CrossRef]
- Salah, M.; Hall, C.; Eulate, E.A.D. Compressively stressed silicon nanoclusters as an antifracture mechanism for high-performance lithium-ion battery anodes. ACS Appl. Mater. Interfaces 2020, 12, 39195–39204. [Google Scholar] [CrossRef]
- Liu, L.; Lyu, J.; Li, T.; Zhao, T. Well-constructed silicon-based materials as high-performance lithium-ion battery anodes. Nanoscale 2016, 8, 701–722. [Google Scholar] [CrossRef]
- Zamfir, M.R.; Nguyen, H.T.; Moyen, E.; Lee, Y.H.; Pribat, D.J. Silicon nanowires for Li-based battery anodes: A review. Mater. Chem. A 2013, 1, 9566. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef]
- Choi, W.C.; Kim, H.J.; Lee, H.I. 5L-scale magnesio-milling reduction of nanostructured SiO2 for high capacity silicon anodes in lithium-ion batteries. Nano Lett. 2016, 16, 7261–7269. [Google Scholar] [CrossRef]
- Yang, J.H.; Ding, C.C.; Huang, L.L. The preparation of poly (γ-glutamic acid)-NHS ester as a natural cross-linking agent of collagen. Int. J. Biol. Macromol. 2017, 97, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kim, H.; Lim, H. Surfactant-based selective assembly approach for Si-embedded silicon oxycarbide composite materials in lithium-ion batteries. Chem. Eng. J. 2020, 401, 126091. [Google Scholar] [CrossRef]
- Wang, L.B.; Lin, N.; Zhou, J.B. Silicon nanoparticles obtained via a low temperature chemical “metathesis” synthesis route and their lithium-ion battery properties. Chem. Commun. 2015, 51, 2345–2348. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Xia, J.L.; Lee, J.H. Arrays of sealed silicon nanotubes as anodes for lithium ion batteries. Nano Lett. 2010, 10, 1710–1716. [Google Scholar] [CrossRef]
- Kennedy, T.; Brandon, M.; Ryan, K.M. Advances in the application of silicon and germanium nanowires for high-performance lithium-ion batteries. Adv. Mater. 2016, 28, 5696–5704. [Google Scholar] [CrossRef]
- Liu, L.H.; Zuo, X.X.; Cheng, Y.J.; Xia, Y.G. In Situ Synthesis and Dual Functionalization of Nano Silicon Enabled by a Semisolid Lithium Rechargeable Flow Battery. ACS Appl. Mater. Interfaces 2022, 14, 28748–28759. [Google Scholar] [CrossRef]
- Lee, J.K.; Oh, C.G.; Kim, N.Y.; Hwang, J.Y.; Sun, Y.K. Rational design of silicon-based composites for high-energy storage devices. J. Mater. Chem. A 2016, 4, 5366. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef]
- Ryu, J.; Hong, D.; Lee, H.-W.; Park, S. Practical considerations of Si-based anodes for lithium-ion battery applications. Nano Res. 2017, 10, 3970–4002. [Google Scholar] [CrossRef]
- Dou, F.; Shi, L.; Chen, G.; Zhang, D. Silicon/carbon composite anode materials for lithium-ion batteries. Energy Rev. 2019, 2, 149–198. [Google Scholar] [CrossRef]
- Yang, S.N.; Cheng, Y.; Xiao, X.; Pang, H. Development and application of carbon fiber in batteries. Chem. Eng. J. 2020, 384, 123294. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L. Synthesis and ethanol sensing properties of Fe-doped SnO2 nanofibers. Mater. Lett. 2009, 63, 917–919. [Google Scholar] [CrossRef]
- Bubel, K.; Zhang, Y.; Assem, Y.; Agarwal, S.; Greiner, A. Tenside-free biodegradable polymer nanofiber nonwovens by “green electrospinning”. Macromolecules 2013, 46, 7034–7042. [Google Scholar] [CrossRef]
- Sarang, K.; Zhao, X.; Holta, D.; Cao, H.; Arole, K.; Flouda, P.; Oh, E.-S.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. Carbon Additive-Free Crumpled Ti3C2TX MXene-Encapsulated Silicon Nanoparticle Anodes for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 10762–10773. [Google Scholar] [CrossRef]
- Bie, X.; Xiong, M.; Wang, B.; Dong, Y.W.; Chen, Z.X.; Huang, R.H. Glucose hydrothermal encapsulation of carbonized silicone polyester to prepare anode materials for lithium batteries with improved cycle stability. RSC Adv. 2022, 12, 9238–9248. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation study of waste polyethylene terephthalate (PET) under inert and oxidative environments. Thermochim. Acta 2019, 679, 178340. [Google Scholar] [CrossRef]
- Awad, R.; Mamaghani, A.H.; Boluk, Y.; Hashisho, Z. Synthesis and characterization of electrospun PAN-based activated carbon nanofibers reinforced with cellulose nanocrystals for adsorption of VOCs. Chem. Eng. J. 2021, 410, 128412. [Google Scholar] [CrossRef]
- Yin, C.X.; Weng, L.; Fei, Z.X.; Shi, L.Y.; Yang, K.K. Form-Stable phase change composites based on porous carbon derived from polyacrylonitrile hydrogel. Chem. Eng. J. 2022, 431, 134206. [Google Scholar] [CrossRef]
- Lim, J.H.; Sisco, P.; Mudalige, T.K.; Sanchez-Pomales, E.; Howard, P.C.; Linder, S.W. Detection and characterization of SiO2 and TiO2 nanostructures in dietary supplements. J. Agric. Food Chem. 2015, 63, 3144–3152. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.F.; Wang, Y.H.; Zhang, Z.L.; Tan, Q.Q.; Zhong, Z.Y.; Su, F.B. Carbon-coated porous silicon composites as high performance Li-ion battery anode materials: Can the production process be cheaper and greener? J. Mater. Chem. A 2016, 4, 552–560. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, F.Z.; Li, X.M.; Luo, W.; Li, L.; Zhang, H.; Yang, L.J. Engineering the distribution of carbon in silicon oxide nanospheres at the atomic level for highly stable anodes. Angew. Chem. Int. Ed. Engl. 2019, 58, 6669–6673. [Google Scholar] [CrossRef]
- Meekins, B.H.; Lin, Y.C.; Manser, J.S.; Manukyan, K.; Mukasyan, A.; Kamat, P.V.; McGinn, P.J. Photoactive porous silicon nanopowder. ACS Appl. Mater. Interfaces 2013, 5, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.F.; Zhang, F.; Hu, Y.S.; Sun, X.H.; Zhang, Y.C.; Lee, H.I.; Chen, L.Q.; Stucky, G.D. Low-temperature pseudomorphic transformation of ordered hierarchical macro-mesoporous SiO2/C nanocomposite to SiC via magnesiothermic reduction. J. Am. Chem. Soc. 2010, 132, 5552–5553. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Q.; Cheong, L.Z.; Wang, D.Y.; Shen, C. Nanostructured phosphorus doped silicon/graphite composite as anode for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 23672–23678. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, D.; Liu; Zhao, B.J. Binder-free Si nanoparticle electrode with 3D porous structure prepared by electrophoretic deposition for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 7497–7504. [Google Scholar] [CrossRef] [PubMed]
- Obrovac, M.N.; Krause, L.J. Reversible cycling of crystalline silicon powder. J. Electrochem. Soc. 2007, 154, A103–A108. [Google Scholar] [CrossRef]
- Guo, J.C.; Sun, A.; Chen, X.L.; Wang, C.S.; Manivannan, A. Cyclability study of silicon–carbon composite anodes for lithium-ion batteries using electrochemical impedance spectroscopy. Electrochim. Acta 2011, 56, 3981–3987. [Google Scholar] [CrossRef]
- Gan, C.H.; Zhang, C.K.; Wen, W.D.; Liu, Y.K.; Chen, J.; Xie, Q.S.; Luo, X.T. Enhancing delithiation reversibility of Li15Si4 alloy of silicon nanoparticles-carbon/graphite anode materials for stable-cycling lithium ion batteries by restricting the silicon particle size. ACS Appl. Mater. Interfaces 2019, 11, 35809–35819. [Google Scholar] [CrossRef]
- Jiang, S.S.; Hu, B.; Sahore, R.; Zhang, L.H.; Liu, H.H.; Zhang, L.; Lu, W.Q.; Zhao, B.; Zhang, Z.C. Surface-functionalized silicon nanoparticles as anode material for lithium-ion battery. ACS Appl. Mater. Interfaces 2018, 10, 44924–44931. [Google Scholar] [CrossRef]
- Dong, Z.; Du, W.B.; Yan, C.H.; Zhang, C.Y.; Chen, G.R.; Chen, J.; Sun, W.P.; Jiang, Y.Z.; Liu, Y.F.; Gao, M.; et al. A novel tin-bonded silicon anode for lithium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 45578–45588. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Xu, H.Y.; Zeng, Y.Y.; Ma, T.Y.; Wang, W.; Liu, L.M.; Wang, F.; Zhang, X.W.; Qiu, X.P. Quantification on growing mass of solid electrolyte interphase and deposited Mn (II) on the silicon anode of LiMn2O4 full lithium-ion cells. ACS Appl. Mater. Interfaces 2019, 11, 27839–27845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, F.Y.; Zhang, M.; Yu, Y.X.; Yuan, S.X.; Liu, Y.D. A highly efficient silicone-modified polyamide acid binder for silicon-based anode in lithium-ion batteries. ACS Appl. Energy Mater. 2021, 4, 7209–7218. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, M.; Bie, X.; Dong, Y.; Wang, B.; Zhang, Q.; Xie, X.; Liu, T.; Huang, R. Encapsulation of Silicon Nano Powders via Electrospinning as Lithium Ion Battery Anode Materials. Materials 2023, 16, 3566. https://doi.org/10.3390/ma16093566
Xiong M, Bie X, Dong Y, Wang B, Zhang Q, Xie X, Liu T, Huang R. Encapsulation of Silicon Nano Powders via Electrospinning as Lithium Ion Battery Anode Materials. Materials. 2023; 16(9):3566. https://doi.org/10.3390/ma16093566
Chicago/Turabian StyleXiong, Man, Xuan Bie, Yawei Dong, Ben Wang, Qunchao Zhang, Xuejun Xie, Tong Liu, and Ronghua Huang. 2023. "Encapsulation of Silicon Nano Powders via Electrospinning as Lithium Ion Battery Anode Materials" Materials 16, no. 9: 3566. https://doi.org/10.3390/ma16093566





