A Review of Oxygen Carrier Materials and Related Thermochemical Redox Processes for Concentrating Solar Thermal Applications
Abstract
1. Introduction
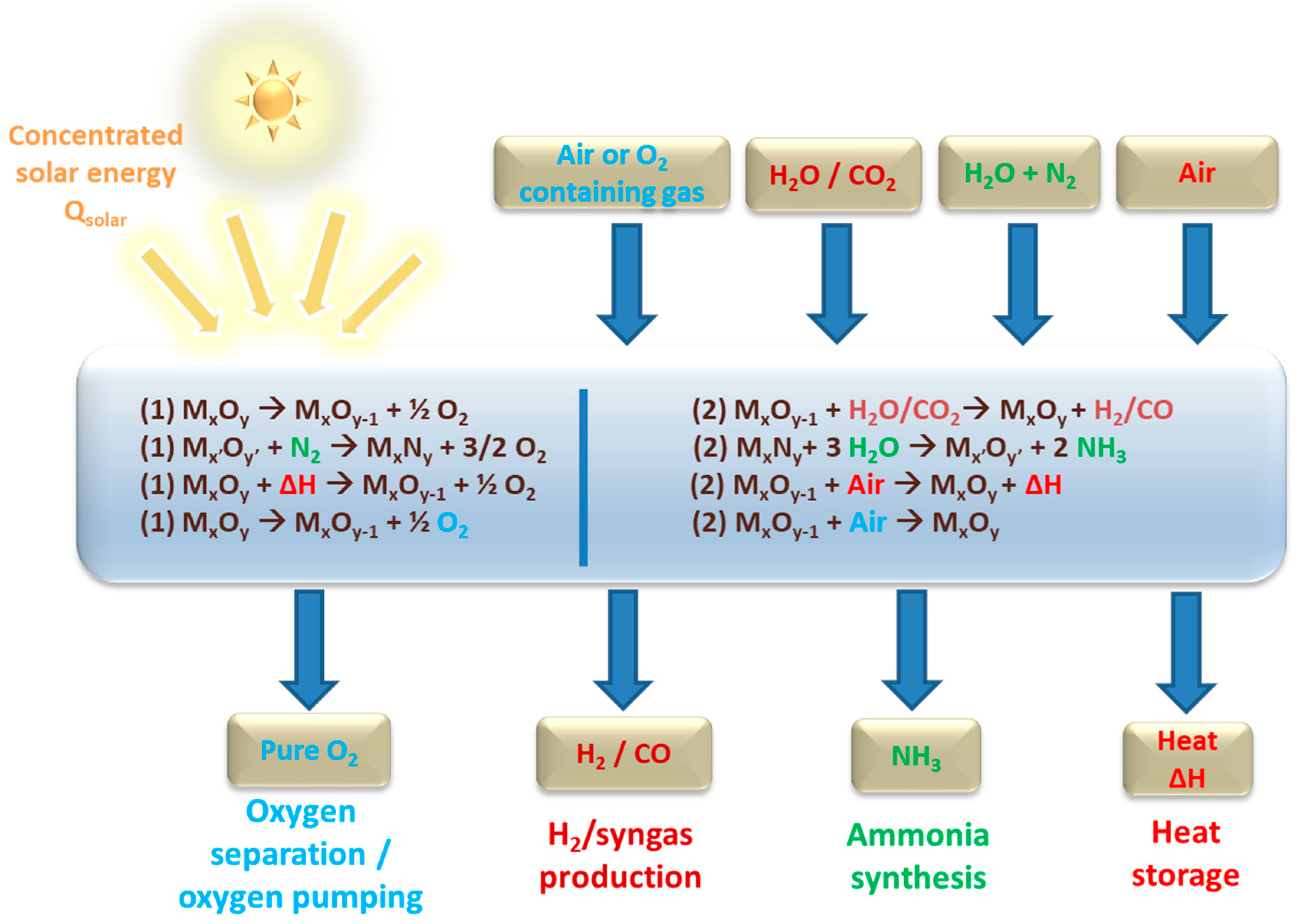
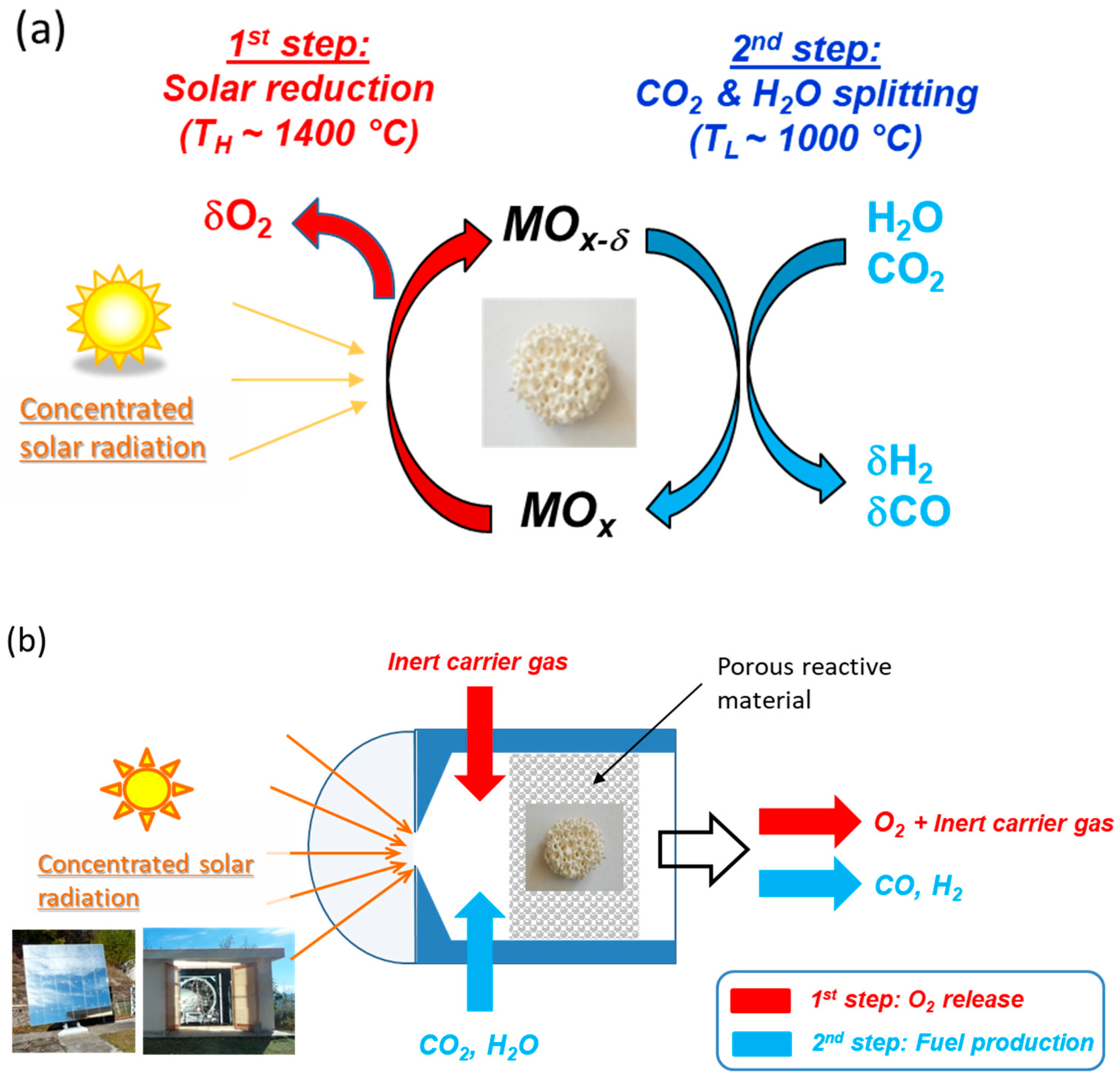
2. Solar Thermochemical Fuels from Two-Step H2O and CO2-Splitting Redox Cycles
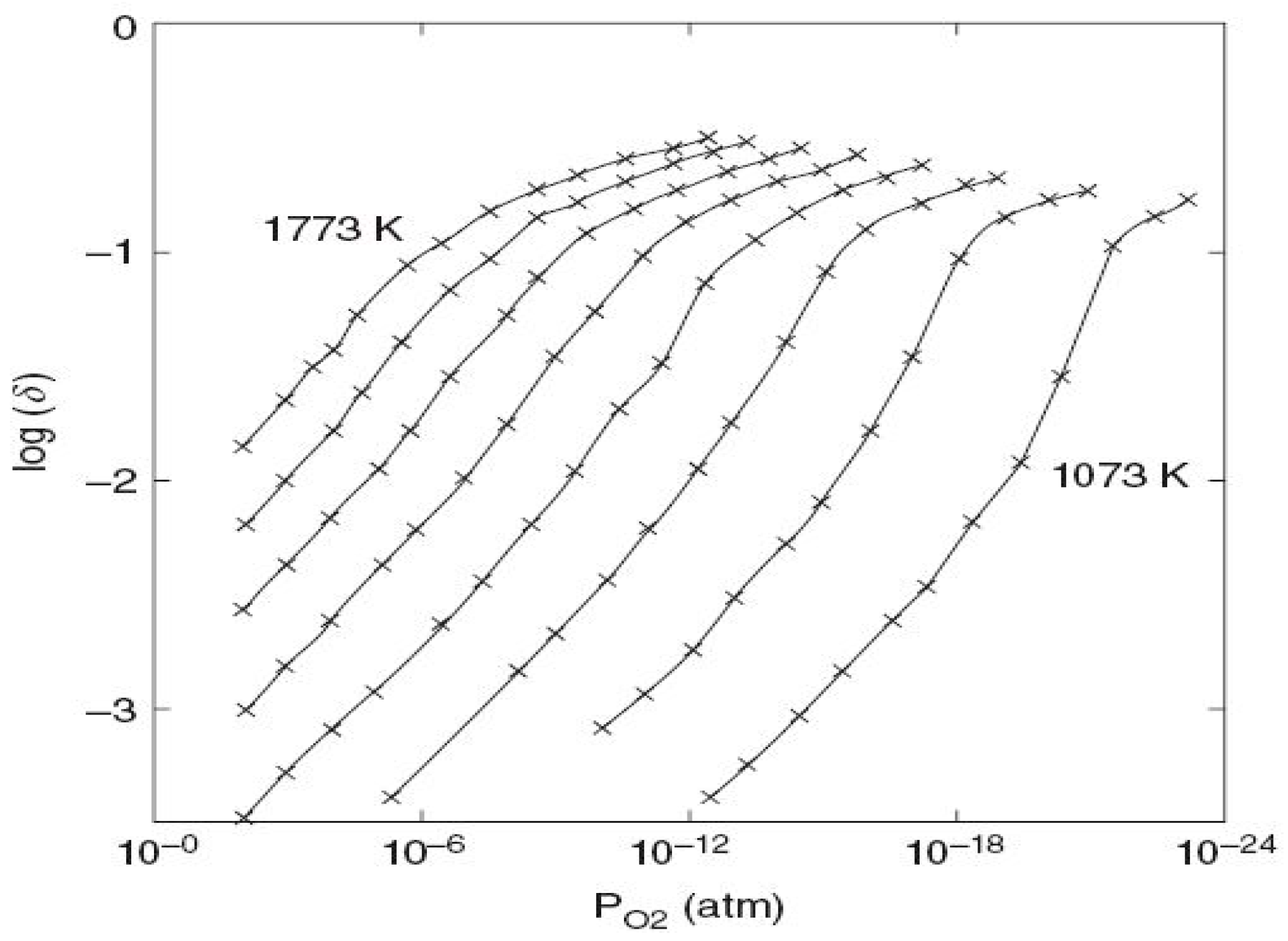
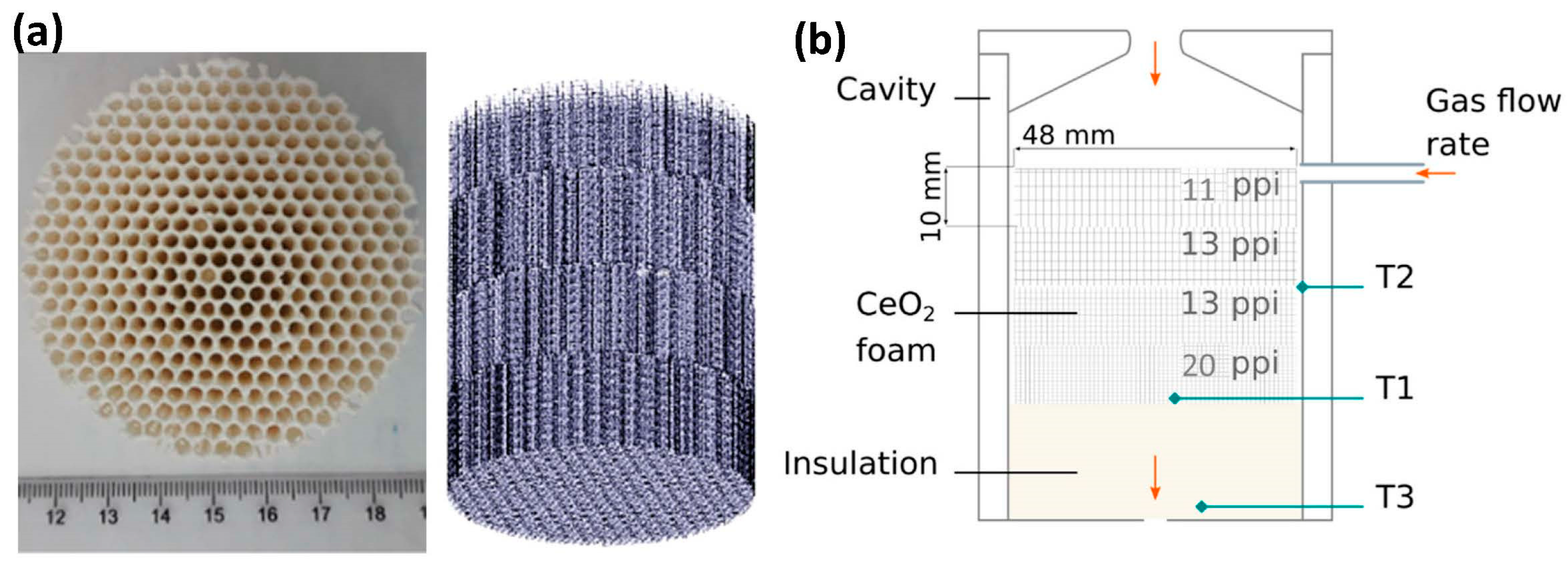
2.1. Ceria-Based Cycles
2.2. Perovskite-Based Cycles
2.3. Dual-Phase Materials, Membranes, and High-Entropy Oxides
3. Ammonia Synthesis via Metal Oxide/Metal Nitride Chemical-Looping Cycles
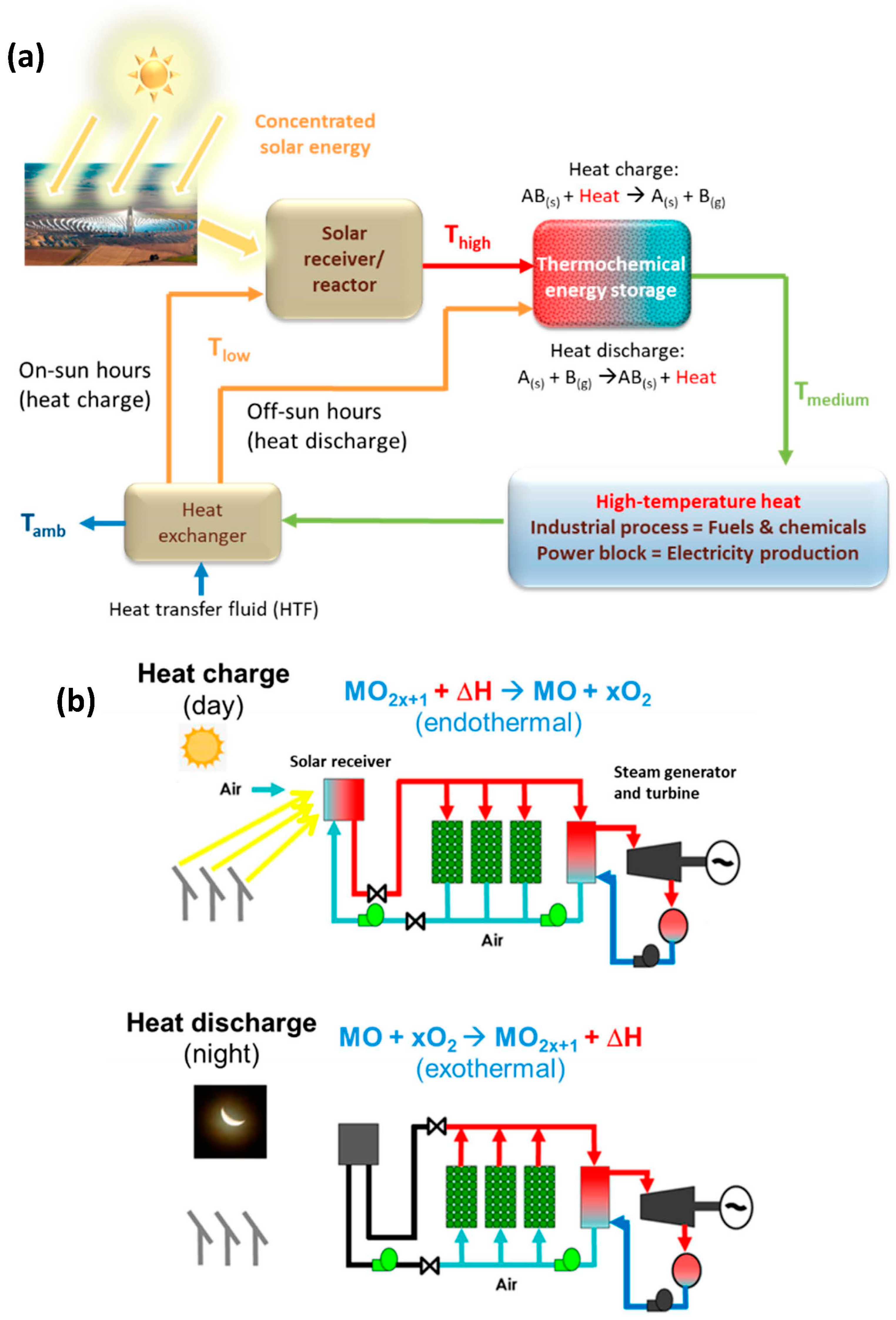
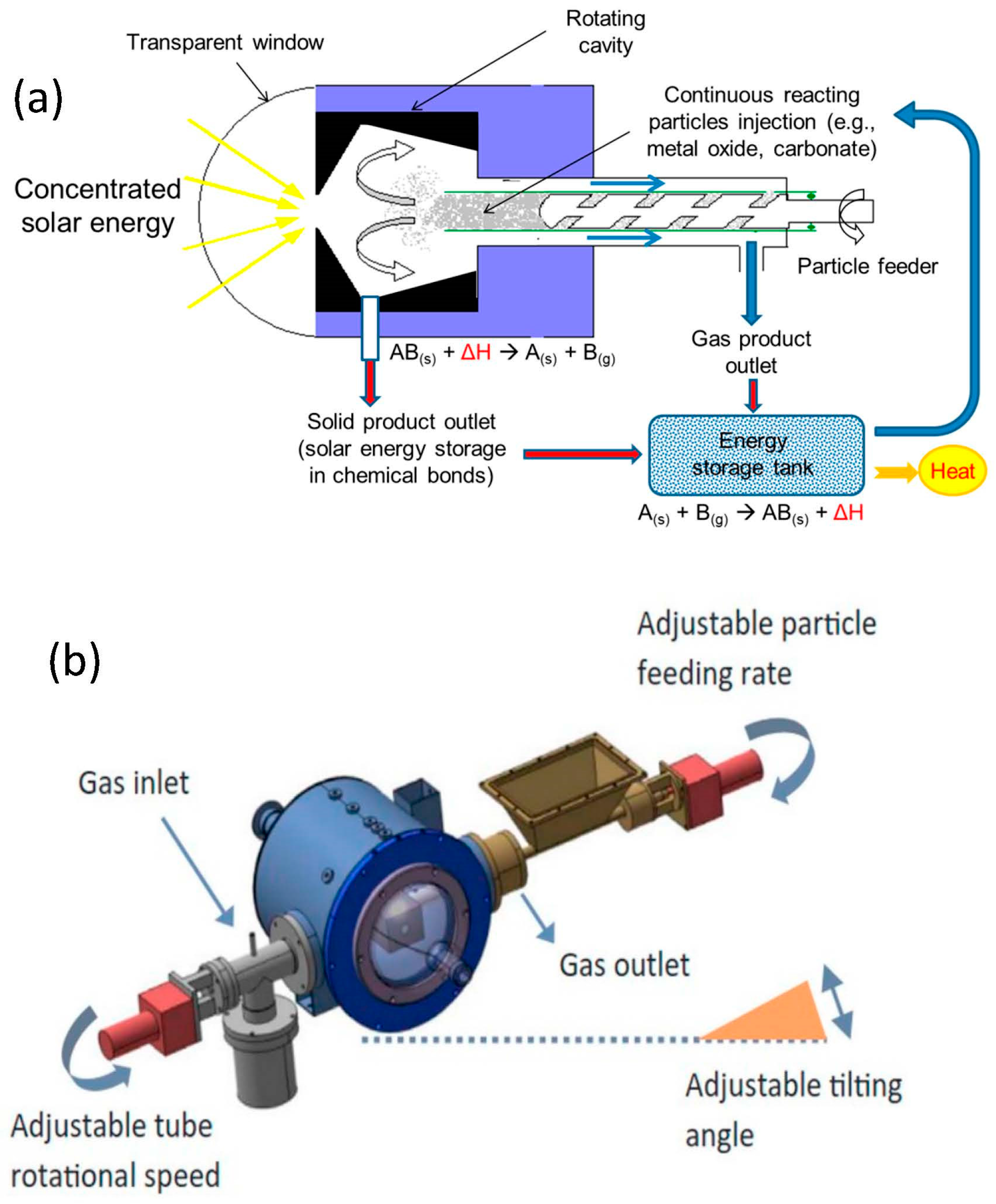
4. Thermochemical Energy Storage at High Temperature
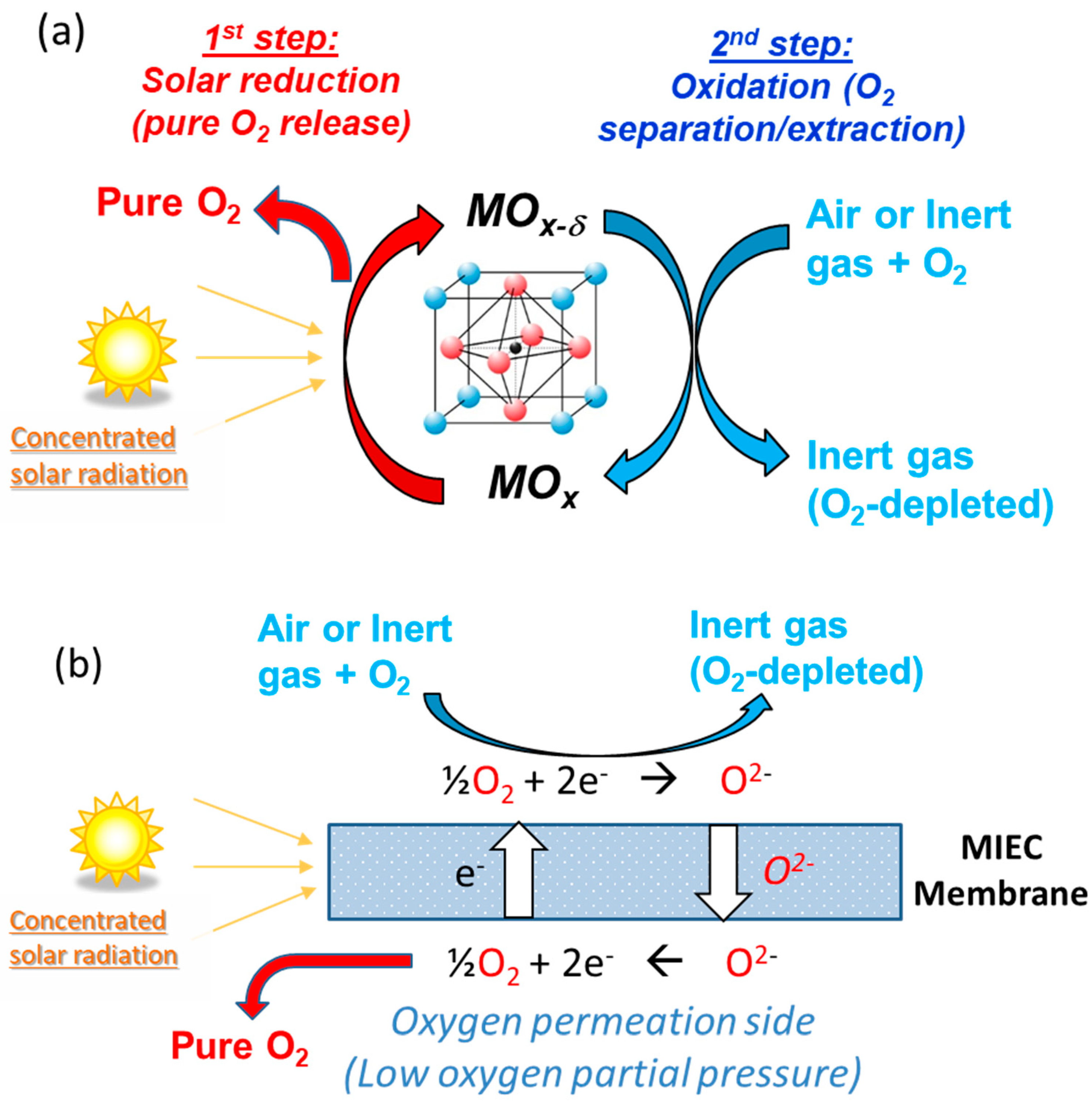
5. Thermochemical Air Separation and Oxygen Pumping
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Imtiaz, Q.; Donat, F.; Müller, C.R.; Li, F. Chemical Looping beyond Combustion—A Perspective. Energy Environ. Sci. 2020, 13, 772–804. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming Technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A.; Mendiara, T.; Gayán, P.; de Diego, L.F.; García-Labiano, F. Chemical Looping Combustion of Solid Fuels. Prog. Energy Combust. Sci. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Luo, M.; Yi, Y.; Wang, S.; Wang, Z.; Du, M.; Pan, J.; Wang, Q. Review of Hydrogen Production Using Chemical-Looping Technology. Renew. Sustain. Energy Rev. 2018, 81, 3186–3214. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, K.; Clough, P.T.; Anthony, E.J. Developments in Calcium/Chemical Looping and Metal Oxide Redox Cycles for High-Temperature Thermochemical Energy Storage: A Review. Fuel Process. Technol. 2020, 199, 106280. [Google Scholar] [CrossRef]
- Teng, L.; Xuan, Y.; Da, Y.; Sun, C.; Liu, X.; Ding, Y. Direct Solar-Driven Reduction of Greenhouse Gases into Hydrocarbon Fuels Incorporating Thermochemical Energy Storage via Modified Calcium Looping. Chem. Eng. J. 2022, 440, 135955. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Combined ZnO Reduction and Methane Reforming for Co-Production of Pure Zn and Syngas in a Prototype Solar Thermochemical Reactor. Fuel Process. Technol. 2021, 211, 106572. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Solar-Driven Chemical Looping Methane Reforming Using ZnO Oxygen Carrier for Syngas and Zn Production in a Cavity-Type Solar Reactor. Catalysts 2020, 10, 1356. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Stepwise Solar Methane Reforming and Water-Splitting via Lattice Oxygen Transfer in Iron and Cerium Oxides. Energy Technol. 2020, 8, 1900415. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Thermochemical Performance Assessment of Solar Continuous Methane-Driven ZnO Reduction for Co-Production of Pure Zinc and Hydrogen-Rich Syngas. Chem. Eng. J. 2022, 429, 132356. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Solar Chemical Looping Gasification of Biomass with the ZnO/Zn Redox System for Syngas and Zinc Production in a Continuously-Fed Solar Reactor. Fuel 2018, 215, 66–79. [Google Scholar] [CrossRef]
- Wang, X.; Abanades, S.; Chuayboon, S.; Zhang, J.; Wei, J. Solar-Driven Chemical Looping Reforming of Methane over SrFeO3-δ-Ca0.5Mn0.5O Nanocomposite Foam. Int. J. Hydrogen Energy 2022, 47, 33664–33676. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Tailoring Hybrid Nonstoichiometric Ceria Redox Cycle for Combined Solar Methane Reforming and Thermochemical Conversion of H2O/CO2. Energy Fuels 2016, 30, 6050–6058. [Google Scholar] [CrossRef]
- Nair, M.; Abanades, S. Solid-State Redox Kinetics of CeO2 in Two-Step Solar CH4 Partial Oxidation and Thermochemical CO2 Conversion. Catalysts 2021, 11, 723. [Google Scholar] [CrossRef]
- Krenzke, P.T.; Fosheim, J.R.; Davidson, J.H. Solar Fuels via Chemical-Looping Reforming. Sol. Energy 2017, 156, 48–72. [Google Scholar] [CrossRef]
- Fosheim, J.R.; Hathaway, B.J.; Davidson, J.H. High Efficiency Solar Chemical-Looping Methane Reforming with Ceria in a Fixed-Bed Reactor. Energy 2019, 169, 597–612. [Google Scholar] [CrossRef]
- Warren, K.J.; Reim, J.; Randhir, K.; Greek, B.; Carrillo, R.; Hahn, D.W.; Scheffe, J.R. Theoretical and Experimental Investigation of Solar Methane Reforming through the Nonstoichiometric Ceria Redox Cycle. Energy Technol. 2017, 5, 2138–2149. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Solar Chemical Looping Reforming of Methane Combined with Isothermal H2O/CO2 Splitting Using Ceria Oxygen Carrier for Syngas Production. J. Energy Chem. 2020, 41, 60–72. [Google Scholar] [CrossRef]
- Warren, K.J.; Carrillo, R.J.; Greek, B.; Hill, C.M.; Scheffe, J.R. Solar Reactor Demonstration of Efficient and Selective Syngas Production via Chemical-Looping Dry Reforming of Methane over Ceria. Energy Technol. 2020, 8, 2000053. [Google Scholar] [CrossRef]
- Hill, C.M.; Hernaiz, E.A.; Furler, P.; Ackermann, S.; Scheffe, J.R. Characterization of Zr-Doped Ceria and Sr-Doped La−Mn Perovskites as Redox Intermediates for Solar Chemical-Looping Reforming of Methane. Energy Technol. 2022, 10, 2100473. [Google Scholar] [CrossRef]
- Islam, M.T.; Huda, N.; Abdullah, A.B.; Saidur, R. A Comprehensive Review of State-of-the-Art Concentrating Solar Power (CSP) Technologies: Current Status and Research Trends. Renew. Sustain. Energy Rev. 2018, 91, 987–1018. [Google Scholar] [CrossRef]
- Weimer, A.W. Solarthermal Chemical Processing Challenges and Commercial Path Forward. Curr. Opin. Chem. Eng. 2012, 1, 211–217. [Google Scholar] [CrossRef]
- Wijewardane, S. Inventions, Innovations and New Technologies—Solar Thermochemical Fuels. Sol. Compass 2022, 2, 100024. [Google Scholar] [CrossRef]
- Warren, K.J.; Weimer, A.W. Solar Thermochemical Fuels: Present Status and Future Prospects. Sol. Compass 2022, 1, 100010. [Google Scholar] [CrossRef]
- Onigbajumo, A.; Swarnkar, P.; Will, G.; Sundararajan, T.; Taghipour, A.; Couperthwaite, S.; Steinberg, T.; Rainey, T. Techno-Economic Evaluation of Solar-Driven Ceria Thermochemical Water-Splitting for Hydrogen Production in a Fluidized Bed Reactor. J. Clean. Prod. 2022, 371, 133303. [Google Scholar] [CrossRef]
- Falter, C.; Valente, A.; Habersetzer, A.; Iribarren, D.; Dufour, J. An Integrated Techno-Economic, Environmental and Social Assessment of the Solar Thermochemical Fuel Pathway. Sustain. Energy Fuels 2020, 4, 3992–4002. [Google Scholar] [CrossRef]
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipiński, W. Techno-Economic Assessment of Solid–Gas Thermochemical Energy Storage Systems for Solar Thermal Power Applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]
- Boujjat, H.; Rodat, S.; Abanades, S. Techno-Economic Assessment of Solar-Driven Steam Gasification of Biomass for Large-Scale Hydrogen Production. Processes 2021, 9, 462. [Google Scholar] [CrossRef]
- Gorman, B.T.; Lanzarini-Lopes, M.; Johnson, N.G.; Miller, J.E.; Stechel, E.B. Techno-Economic Analysis of a Concentrating Solar Power Plant Using Redox-Active Metal Oxides as Heat Transfer Fluid and Storage Media. Front. Energy Res. 2021, 9, 734288. [Google Scholar] [CrossRef]
- Ma, Z.; Davenport, P.; Saur, G. System and Technoeconomic Analysis of Solar Thermochemical Hydrogen Production. Renew. Energy 2022, 190, 294–308. [Google Scholar] [CrossRef]
- Moser, M.; Pecchi, M.; Fend, T. Techno-Economic Assessment of Solar Hydrogen Production by Means of Thermo-Chemical Cycles. Energies 2019, 12, 352. [Google Scholar] [CrossRef]
- Stechel, E.B.; Miller, J.E. Re-Energizing CO2 to Fuels with the Sun: Issues of Efficiency, Scale, and Economics. J. CO2 Util. 2013, 1, 28–36. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. A Review of Solar Thermochemical Processes. Renew. Sustain. Energy Rev. 2016, 54, 497–532. [Google Scholar] [CrossRef]
- Bulfin, B.; Vieten, J.; Agrafiotis, C.; Roeb, M.; Sattler, C. Applications and Limitations of Two Step Metal Oxide Thermochemical Redox Cycles; a Review. J. Mater. Chem. A 2017, 5, 18951–18966. [Google Scholar] [CrossRef]
- Vieten, J.; Bulfin, B.; Huck, P.; Horton, M.; Guban, D.; Zhu, L.; Lu, Y.; Persson, K.A.; Roeb, M.; Sattler, C. Materials Design of Perovskite Solid Solutions for Thermochemical Applications. Energy Environ. Sci. 2019, 12, 1369–1384. [Google Scholar] [CrossRef]
- Abanades, S.; Kimura, H.; Otsuka, H. Hydrogen Production from Thermo-Catalytic Decomposition of Methane Using Carbon Black Catalysts in an Indirectly-Irradiated Tubular Packed-Bed Solar Reactor. Int. J. Hydrogen Energy 2014, 39, 18770–18783. [Google Scholar] [CrossRef]
- Abanades, S.; Rodat, S.; Boujjat, H. Solar Thermochemical Green Fuels Production: A Review of Biomass Pyro-Gasification, Solar Reactor Concepts and Modelling Methods. Energies 2021, 14, 1494. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. An Overview of Solar Decarbonization Processes, Reacting Oxide Materials, and Thermochemical Reactors for Hydrogen and Syngas Production. Int. J. Hydrogen Energy 2020, 45, 25783–25810. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Thermodynamic and Experimental Investigation of Solar-Driven Biomass Pyro-Gasification Using H2O, CO2, or ZnO Oxidants for Clean Syngas and Metallurgical Zn Production. Processes 2021, 9, 687. [Google Scholar] [CrossRef]
- Agrafiotis, C.; von Storch, H.; Roeb, M.; Sattler, C. Solar Thermal Reforming of Methane Feedstocks for Hydrogen and Syngas Production—A Review. Renew. Sustain. Energy Rev. 2014, 29, 656–682. [Google Scholar] [CrossRef]
- Abanades, S. Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef]
- Abanades, S. Redox Cycles, Active Materials, and Reactors Applied to Water and Carbon Dioxide Splitting for Solar Thermochemical Fuel Production: A Review. Energies 2022, 15, 7061. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Sattler, C. A Review on Solar Thermal Syngas Production via Redox Pair-Based Water/Carbon Dioxide Splitting Thermochemical Cycles. Renew. Sustain. Energy Rev. 2015, 42, 254–285. [Google Scholar] [CrossRef]
- Mao, Y.; Gao, Y.; Dong, W.; Wu, H.; Song, Z.; Zhao, X.; Sun, J.; Wang, W. Hydrogen Production via a Two-Step Water Splitting Thermochemical Cycle Based on Metal Oxide—A Review. Appl. Energy 2020, 267, 114860. [Google Scholar] [CrossRef]
- Oudejans, D.; Offidani, M.; Constantinou, A.; Albonetti, S.; Dimitratos, N.; Bansode, A. A Comprehensive Review on Two-Step Thermochemical Water Splitting for Hydrogen Production in a Redox Cycle. Energies 2022, 15, 3044. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Lemort, F.; Flamant, G. Analysis of Solar Chemical Processes for Hydrogen Production from Water Splitting Thermochemical Cycles. Energy Convers. Manag. 2008, 49, 1547–1556. [Google Scholar] [CrossRef]
- Abanades, S.; Charvin, P.; Flamant, G.; Neveu, P. Screening of Water-Splitting Thermochemical Cycles Potentially Attractive for Hydrogen Production by Concentrated Solar Energy. Energy 2006, 31, 2805–2822. [Google Scholar] [CrossRef]
- Abanades, S. Thermogravimetry Analysis of CO2 and H2O Reduction from Solar Nanosized Zn Powder for Thermochemical Fuel Production. Ind. Eng. Chem. Res. 2012, 51, 741–750. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Solar Thermal Reduction of ZnO and SnO2: Characterization of the Recombination Reaction with O2. Chem. Eng. Sci. 2010, 65, 3671–3680. [Google Scholar] [CrossRef]
- Abanades, S.; Chambon, M. CO2 Dissociation and Upgrading from Two-Step Solar Thermochemical Processes Based on ZnO/Zn and SnO2/SnO Redox Pairs. Energy Fuels 2010, 24, 6667–6674. [Google Scholar] [CrossRef]
- Venstrom, L.J.; Hilsen, P.; Davidson, J.H. Heterogeneous Oxidation of Zinc Vapor by Steam and Mixtures of Steam and Carbon Dioxide. Chem. Eng. Sci. 2018, 183, 223–230. [Google Scholar] [CrossRef]
- Abanades, S. CO2 and H2O Reduction by Solar Thermochemical Looping Using SnO2/SnO Redox Reactions: Thermogravimetric Analysis. Int. J. Hydrogen Energy 2012, 37, 8223–8231. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S.; Jumas, J.-C.; Olivier-Fourcade, J. Characterization of Two-Step Tin-Based Redox System for Thermochemical Fuel Production from Solar-Driven CO2 and H2O Splitting Cycle. Ind. Eng. Chem. Res. 2014, 53, 5668–5677. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S. Kinetic Analysis of High-Temperature Solid–Gas Reactions by an Inverse Method Applied to ZnO and SnO2 Solar Thermal Dissociation. Chem. Eng. J. 2013, 217, 139–149. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S. Thermodynamic and Kinetic Study of the Carbothermal Reduction of SnO2 for Solar Thermochemical Fuel Generation. Energy Fuels 2014, 28, 1396–1405. [Google Scholar] [CrossRef]
- Abanades, S.; Villafan-Vidales, H.I. CO2 Valorisation Based on Fe3O4/FeO Thermochemical Redox Reactions Using Concentrated Solar Energy. Int. J. Energy Res. 2013, 37, 598–608. [Google Scholar] [CrossRef]
- Abanades, S.; Villafan-Vidales, H.I. CO2 and H2O Conversion to Solar Fuels via Two-Step Solar Thermochemical Looping Using Iron Oxide Redox Pair. Chem. Eng. J. 2011, 175, 368–375. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Lemort, F.; Flamant, G. Hydrogen Production by Three-Step Solar Thermochemical Cycles Using Hydroxides and Metal Oxide Systems. Energy Fuels 2007, 21, 2919–2928. [Google Scholar] [CrossRef]
- Kostoglou, M.; Lorentzou, S.; Konstandopoulos, A.G. Improved Kinetic Model for Water Splitting Thermochemical Cycles Using Nickel Ferrite. Int. J. Hydrogen Energy 2014, 39, 6317–6327. [Google Scholar] [CrossRef]
- Fresno, F.; Fernández-Saavedra, R.; Belén Gómez-Mancebo, M.; Vidal, A.; Sánchez, M.; Isabel Rucandio, M.; Quejido, A.J.; Romero, M. Solar Hydrogen Production by Two-Step Thermochemical Cycles: Evaluation of the Activity of Commercial Ferrites. Int. J. Hydrogen Energy 2009, 34, 2918–2924. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Beche, E.; Lemont, F.; Flamant, G. Hydrogen Production from Mixed Cerium Oxides via Three-Step Water-Splitting Cycles. Solid State Ion. 2009, 180, 1003–1010. [Google Scholar] [CrossRef]
- Chueh, W.C.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.M.; Steinfeld, A. High-Flux Solar-Driven Thermochemical Dissociation of CO2 and H2O Using Nonstoichiometric Ceria. Science 2010, 330, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Chueh, W.C.; Haile, S.M. A Thermochemical Study of Ceria: Exploiting an Old Material for New Modes of Energy Conversion and CO2 Mitigation. Phil. Trans. R. Soc. A 2010, 368, 3269–3294. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Drobek, M.; Ayral, A.; Julbe, A. Recent Progress on Ceria Doping and Shaping Strategies for Solar Thermochemical Water and CO2 Splitting Cycles. AIMS Mater. Sci. 2019, 6, 657–684. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Julbe, A.; Jouannaux, J.; Drobek, M.; Ayral, A.; Cartoixa, B. Remarkable Performance of Microstructured Ceria Foams for Thermochemical Splitting of H2O and CO2 in a Novel High–Temperature Solar Reactor. Chem. Eng. Res. Des. 2020, 156, 311–323. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, L.; Agrafiotis, C.; Vieten, J.; Roeb, M.; Sattler, C. Solar Fuels Production: Two-Step Thermochemical Cycles with Cerium-Based Oxides. Prog. Energy Combust. Sci. 2019, 75, 100785. [Google Scholar] [CrossRef]
- Bayon, A.; de la Calle, A.; Ghose, K.K.; Page, A.; McNaughton, R. Experimental, Computational and Thermodynamic Studies in Perovskites Metal Oxides for Thermochemical Fuel Production: A Review. Int. J. Hydrogen Energy 2020, 45, 12653–12679. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Julbe, A. Non-Stoichiometric Redox Active Perovskite Materials for Solar Thermochemical Fuel Production: A Review. Catalysts 2018, 8, 611. [Google Scholar] [CrossRef]
- Haeussler, A.; Julbe, A.; Abanades, S. Investigation of Reactive Perovskite Materials for Solar Fuel Production via Two-Step Redox Cycles: Thermochemical Activity, Thermodynamic Properties and Reduction Kinetics. Mater. Chem. Phys. 2022, 276, 125358. [Google Scholar] [CrossRef]
- Kubicek, M.; Bork, A.H.; Rupp, J.L.M. Perovskite Oxides—A Review on a Versatile Material Class for Solar-to-Fuel Conversion Processes. J. Mater. Chem. A 2017, 5, 11983–12000. [Google Scholar] [CrossRef]
- McDaniel, A.H.; Miller, E.C.; Arifin, D.; Ambrosini, A.; Coker, E.N.; O’Hayre, R.; Chueh, W.C.; Tong, J. Sr- and Mn-Doped LaAlO3−δ for Solar Thermochemical H2 and CO Production. Energy Environ. Sci. 2013, 6, 2424. [Google Scholar] [CrossRef]
- Zhang, D.; De Santiago, H.A.; Xu, B.; Liu, C.; Trindell, J.A.; Li, W.; Park, J.; Rodriguez, M.A.; Coker, E.N.; Sugar, J.D.; et al. Compositionally Complex Perovskite Oxides for Solar Thermochemical Water Splitting. Chem. Mater. 2023, 35, 1901–1915. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Solar Metallurgy for Sustainable Zn and Mg Production in a Vacuum Reactor Using Concentrated Sunlight. Sustainability 2020, 12, 6709. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Clean Magnesium Production Using Concentrated Solar Heat in a High-Temperature Cavity-Type Thermochemical Reactor. J. Clean. Prod. 2019, 232, 784–795. [Google Scholar] [CrossRef]
- Osinga, T.; Frommherz, U.; Steinfeld, A.; Wieckert, C. Experimental Investigation of the Solar Carbothermic Reduction of ZnO Using a Two-Cavity Solar Reactor. J. Sol. Energy Eng. 2004, 126, 633–637. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Solar Carbo-Thermal and Methano-Thermal Reduction of MgO and ZnO for Metallic Powder and Syngas Production by Green Extractive Metallurgy. Processes 2022, 10, 154. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Solar Metallurgical Process for High-Purity Zn and Syngas Production Using Carbon or Biomass Feedstock in a Flexible Thermochemical Reactor. Chem. Eng. Sci. 2023, 271, 118579. [Google Scholar] [CrossRef]
- Villafán-Vidales, H.I.; Abanades, S.; Montiel-González, M.; Romero-Paredes-Rubio, H. Carbo- and Methanothermal Reduction of Tungsten Trioxide into Metallic Tungsten for Thermochemical Production of Solar Fuels. Energy Technol. 2017, 5, 692–702. [Google Scholar] [CrossRef]
- Wang, L.; Ma, T.; Chang, Z.; Li, H.; Fu, M.; Li, X. Solar Fuels Production via Two-Step Thermochemical Cycle Based on Fe3O4/Fe with Methane Reduction. Sol. Energy 2019, 177, 772–781. [Google Scholar] [CrossRef]
- Bellouard, Q.; Rodat, S.; Grateau, M.; Abanades, S. Solar Biomass Gasification Combined With Iron Oxide Reduction for Syngas Production and Green Iron Metallurgy. Front. Energy Res. 2020, 8, 66. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Kinetic Investigation of Hydrogen Generation from Hydrolysis of SnO and Zn Solar Nanopowders. Int. J. Hydrogen Energy 2009, 34, 5326–5336. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Lemont, F.; Flamant, G. Experimental Study of SnO2/SnO/Sn Thermochemical Systems for Solar Production of Hydrogen. AIChE J. 2008, 54, 2759–2767. [Google Scholar] [CrossRef]
- Abanades, S.; Charvin, P.; Lemont, F.; Flamant, G. Novel Two-Step SnO2/SnO Water-Splitting Cycle for Solar Thermochemical Production of Hydrogen. Int. J. Hydrogen Energy 2008, 33, 6021–6030. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Flamant, G.; Lemort, F. Two-Step Water Splitting Thermochemical Cycle Based on Iron Oxide Redox Pair for Solar Hydrogen Production. Energy 2007, 32, 1124–1133. [Google Scholar] [CrossRef]
- Miller, J.E.; McDaniel, A.H.; Allendorf, M.D. Considerations in the Design of Materials for Solar-Driven Fuel Production Using Metal-Oxide Thermochemical Cycles. Adv. Energy Mater. 2014, 4, 1300469. [Google Scholar] [CrossRef]
- Fernández-Saavedra, R.; Gómez-Mancebo, M.B.; Caravaca, C.; Sánchez, M.; Quejido, A.J.; Vidal, A. Hydrogen Production by Two-Step Thermochemical Cycles Based on Commercial Nickel Ferrite: Kinetic and Structural Study. Int. J. Hydrogen Energy 2014, 39, 6819–6826. [Google Scholar] [CrossRef]
- Scheffe, J.R.; McDaniel, A.H.; Allendorf, M.D.; Weimer, A.W. Kinetics and Mechanism of Solar-Thermochemical H2 Production by Oxidation of a Cobalt Ferrite–Zirconia Composite. Energy Environ. Sci. 2013, 6, 963. [Google Scholar] [CrossRef]
- Tamaura, Y.; Ueda, Y.; Matsunami, J.; Hasegawa, N.; Nezuka, M.; Sano, T.; Tsuji, M. Solar Hydrogen Production by Using Ferrites. Sol. Energy 1999, 65, 55–57. [Google Scholar] [CrossRef]
- Diver, R.B.; Miller, J.E.; Allendorf, M.D.; Siegel, N.P.; Hogan, R.E. Solar Thermochemical Water-Splitting Ferrite-Cycle Heat Engines. J. Sol. Energy Eng. 2008, 130, 041001. [Google Scholar] [CrossRef]
- Gokon, N.; Takahashi, S.; Yamamoto, H.; Kodama, T. Thermochemical Two-Step Water-Splitting Reactor with Internally Circulating Fluidized Bed for Thermal Reduction of Ferrite Particles. Int. J. Hydrogen Energy 2008, 33, 2189–2199. [Google Scholar] [CrossRef]
- Tong, J.; Jiang, Q.; Chen, Z.; Jiang, Z.; Li, C. Two-Step Thermochemical Cycles for CO2 Splitting on Zr-Doped Cobalt Ferrite Supported on Silica. Sol. Energy 2015, 116, 133–143. [Google Scholar] [CrossRef]
- Gokon, N.; Hasegawa, T.; Takahashi, S.; Kodama, T. Thermochemical Two-Step Water-Splitting for Hydrogen Production Using Fe-YSZ Particles and a Ceramic Foam Device. Energy 2008, 33, 1407–1416. [Google Scholar] [CrossRef]
- Gokon, N.; Murayama, H.; Nagasaki, A.; Kodama, T. Thermochemical Two-Step Water Splitting Cycles by Monoclinic ZrO2-Supported NiFe2O4 and Fe3O4 Powders and Ceramic Foam Devices. Sol. Energy 2009, 83, 527–537. [Google Scholar] [CrossRef]
- Roeb, M.; Säck, J.-P.; Rietbrock, P.; Prahl, C.; Schreiber, H.; Neises, M.; de Oliveira, L.; Graf, D.; Ebert, M.; Reinalter, W.; et al. Test Operation of a 100kW Pilot Plant for Solar Hydrogen Production from Water on a Solar Tower. Sol. Energy 2011, 85, 634–644. [Google Scholar] [CrossRef]
- Lorentzou, S.; Dimitrakis, D.; Zygogianni, A.; Karagiannakis, G.; Konstandopoulos, A.G. Thermochemical H2O and CO2 Splitting Redox Cycles in a NiFe2O4 Structured Redox Reactor: Design, Development and Experiments in a High Flux Solar Simulator. Sol. Energy 2017, 155, 1462–1481. [Google Scholar] [CrossRef]
- Lorentzou, S.; Pagkoura, C.; Zygogianni, A.; Karagiannakis, G.; Konstandopoulos, A.G. Thermochemical Cycles over Redox Structured Reactors. Int. J. Hydrogen Energy 2017, 42, 19664–19682. [Google Scholar] [CrossRef]
- Carrillo, R.J.; Scheffe, J.R. Advances and Trends in Redox Materials for Solar Thermochemical Fuel Production. Sol. Energy 2017, 156, 3–20. [Google Scholar] [CrossRef]
- McDaniel, A.H. Renewable Energy Carriers Derived from Concentrating Solar Power and Nonstoichiometric Oxides. Curr. Opin. Green Sustain. Chem. 2017, 4, 37–43. [Google Scholar] [CrossRef]
- Abanades, S.; Flamant, G. Thermochemical Hydrogen Production from a Two-Step Solar-Driven Water-Splitting Cycle Based on Cerium Oxides. Sol. Energy 2006, 80, 1611–1623. [Google Scholar] [CrossRef]
- Panlener, R.J.; Blumenthal, R.N.; Garnier, J.E. A Thermodynamic Study of Nonstoichiometric Cerium Dioxide. J. Phys. Chem. Solids 1975, 36, 1213–1222. [Google Scholar] [CrossRef]
- Zinkevich, M.; Djurovic, D.; Aldinger, F. Thermodynamic Modelling of the Cerium–Oxygen System. Solid State Ion. 2006, 177, 989–1001. [Google Scholar] [CrossRef]
- Takacs, M.; Scheffe, J.R.; Steinfeld, A. Oxygen Nonstoichiometry and Thermodynamic Characterization of Zr Doped Ceria in the 1573–1773 K Temperature Range. Phys. Chem. Chem. Phys. 2015, 17, 7813–7822. [Google Scholar] [CrossRef] [PubMed]
- Chuayboon, S.; Abanades, S.; Rodat, S. High-Purity and Clean Syngas and Hydrogen Production From Two-Step CH4 Reforming and H2O Splitting Through Isothermal Ceria Redox Cycle Using Concentrated Sunlight. Front. Energy Res. 2020, 8, 128. [Google Scholar] [CrossRef]
- Haeussler, A.; Chuayboon, S.; Abanades, S. Solar Redox Cycling of Ceria in a Monolithic Reactor for Two-Step H2O/CO2 Splitting: Isothermal Methane-Induced Reduction versus Temperature-Swing Cycle. AIP Conf. Proc. 2020, 2303, 170009. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Syngas Production via Solar-Driven Chemical Looping Methane Reforming from Redox Cycling of Ceria Porous Foam in a Volumetric Solar Reactor. Chem. Eng. J. 2019, 356, 756–770. [Google Scholar] [CrossRef]
- Marxer, D.; Furler, P.; Takacs, M.; Steinfeld, A. Solar Thermochemical Splitting of CO2 into Separate Streams of CO and O2 with High Selectivity, Stability, Conversion, and Efficiency. Energy Environ. Sci. 2017, 10, 1142–1149. [Google Scholar] [CrossRef]
- Pullar, R.C.; Novais, R.M.; Caetano, A.P.F.; Barreiros, M.A.; Abanades, S.; Oliveira, F.A.C. A Review of Solar Thermochemical CO2 Splitting Using Ceria-Based Ceramics With Designed Morphologies and Microstructures. Front. Chem. 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lu, Y.; Li, F. Reactivity of Ni, Cr and Zr Doped Ceria in CO2 Splitting for CO Production via Two-Step Thermochemical Cycle. Int. J. Hydrogen Energy 2018, 43, 13754–13763. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Y. Reactivity and Efficiency of Ceria-Based Oxides for Solar CO2 Splitting via Isothermal and Near-Isothermal Cycles. Energy Fuels 2018, 32, 736–746. [Google Scholar] [CrossRef]
- Le Gal, A.; Abanades, S.; Bion, N.; Le Mercier, T.; Harlé, V. Reactivity of Doped Ceria-Based Mixed Oxides for Solar Thermochemical Hydrogen Generation via Two-Step Water-Splitting Cycles. Energy Fuels 2013, 27, 6068–6078. [Google Scholar] [CrossRef]
- Call, F.; Roeb, M.; Schmücker, M.; Sattler, C.; Pitz-Paal, R. Ceria Doped with Zirconium and Lanthanide Oxides to Enhance Solar Thermochemical Production of Fuels. J. Phys. Chem. C 2015, 119, 6929–6938. [Google Scholar] [CrossRef]
- Arifin, D.; Ambrosini, A.; Wilson, S.A.; Mandal, B.; Muhich, C.L.; Weimer, A.W. Investigation of Zr, Gd/Zr, and Pr/Zr—Doped Ceria for the Redox Splitting of Water. Int. J. Hydrogen Energy 2020, 45, 160–174. [Google Scholar] [CrossRef]
- Ackermann, S.; Scheffe, J.R.; Steinfeld, A. Diffusion of Oxygen in Ceria at Elevated Temperatures and Its Application to H2O/CO2 Splitting Thermochemical Redox Cycles. J. Phys. Chem. C 2014, 118, 5216–5225. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S. Additive Manufacturing and Two-Step Redox Cycling of Ordered Porous Ceria Structures for Solar-Driven Thermochemical Fuel Production. Chem. Eng. Sci. 2021, 246, 116999. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Julbe, A.; Jouannaux, J.; Cartoixa, B. Two-Step CO2 and H2O Splitting Using Perovskite-Coated Ceria Foam for Enhanced Green Fuel Production in a Porous Volumetric Solar Reactor. J. CO2 Util. 2020, 41, 101257. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Julbe, A.; Jouannaux, J.; Cartoixa, B. Solar Thermochemical Fuel Production from H2O and CO2 Splitting via Two-Step Redox Cycling of Reticulated Porous Ceria Structures Integrated in a Monolithic Cavity-Type Reactor. Energy 2020, 201, 117649. [Google Scholar] [CrossRef]
- Thanda, V.K.; Fend, T.; Laaber, D.; Lidor, A.; von Storch, H.; Säck, J.P.; Hertel, J.; Lampe, J.; Menz, S.; Piesche, G.; et al. Experimental Investigation of the Applicability of a 250 KW Ceria Receiver/Reactor for Solar Thermochemical Hydrogen Generation. Renew. Energy 2022, 198, 389–398. [Google Scholar] [CrossRef]
- Orfila, M.; Sanz, D.; Linares, M.; Molina, R.; Sanz, R.; Marugán, J.; Botas, J.Á. H2 Production by Thermochemical Water Splitting with Reticulated Porous Structures of Ceria-Based Mixed Oxide Materials. Int. J. Hydrogen Energy 2021, 46, 17458–17471. [Google Scholar] [CrossRef]
- Cho, H.-S.; Kodama, T.; Gokon, N.; Bellan, S.; Kim, J.-K. Development of Synthesis and Fabrication Process for Mn-CeO2 Foam via Two-Step Water-Splitting Cycle Hydrogen Production. Energies 2021, 14, 6919. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Abanades, S.; Salvado, I.M.M.; Ferreira, J.M.F.; Pullar, R.C. Robocasting of 3D Printed and Sintered Ceria Scaffold Structures with Hierarchical Porosity for Solar Thermochemical Fuel Production from the Splitting of CO 2. Nanoscale 2022, 14, 4994–5001. [Google Scholar] [CrossRef]
- Hoes, M.; Ackermann, S.; Theiler, D.; Furler, P.; Steinfeld, A. Additive-Manufactured Ordered Porous Structures Made of Ceria for Concentrating Solar Applications. Energy Technol. 2019, 7, 1900484. [Google Scholar] [CrossRef]
- Abanades, S.; Haeussler, A.; Julbe, A. Synthesis and Thermochemical Redox Cycling of Porous Ceria Microspheres for Renewable Fuels Production from Solar-Aided Water-Splitting and CO2 Utilization. Appl. Phys. Lett. 2021, 119, 023902. [Google Scholar] [CrossRef]
- Abanades, S.; Haeussler, A. Two-Step Thermochemical Cycles Using Fibrous Ceria Pellets for H2 Production and CO2 Reduction in Packed-Bed Solar Reactors. Sustain. Mater. Technol. 2021, 29, e00328. [Google Scholar] [CrossRef]
- Oliveira, F.A.C.; Barreiros, M.A.; Abanades, S.; Caetano, A.P.F.; Novais, R.M.; Pullar, R.C. Solar Thermochemical CO2 Splitting Using Cork-Templated Ceria Ecoceramics. J. CO2 Util. 2018, 26, 552–563. [Google Scholar] [CrossRef]
- Oliveira, F.A.C.; Barreiros, M.A.; Haeussler, A.; Caetano, A.P.F.; Mouquinho, A.I.; Oliveira e Silva, P.M.; Novais, R.M.; Pullar, R.C.; Abanades, S. High Performance Cork-Templated Ceria for Solar Thermochemical Hydrogen Production via Two-Step Water-Splitting Cycles. Sustain. Energy Fuels 2020, 4, 3077–3089. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Costa Oliveira, F.A.; Barreiros, M.A.; Caetano, A.P.F.; Novais, R.M.; Pullar, R.C. Solar Redox Cycling of Ceria Structures Based on Fiber Boards, Foams, and Biomimetic Cork-Derived Ecoceramics for Two-Step Thermochemical H2O and CO2 Splitting. Energy Fuels 2020, 34, 9037–9049. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Experimental Screening of Perovskite Oxides as Efficient Redox Materials for Solar Thermochemical CO2 Conversion. Sustain. Energy Fuels 2018, 2, 843–854. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Insights into the Redox Performance of Non-Stoichiometric Lanthanum Manganite Perovskites for Solar Thermochemical CO2 Splitting. ChemistrySelect 2016, 1, 4449–4457. [Google Scholar] [CrossRef]
- Muhich, C.L.; Blaser, S.; Hoes, M.C.; Steinfeld, A. Comparing the Solar-to-Fuel Energy Conversion Efficiency of Ceria and Perovskite Based Thermochemical Redox Cycles for Splitting H2O and CO2. Int. J. Hydrogen Energy 2018, 43, 18814–18831. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R.; AlMomani, F.; Rashid, S.; Qiblawey, H.; Saleh Saad, M.A.; Khraisheh, M.; Kumar, G.; Gupta, R.B.; Shende, R.V. Thermochemical Splitting of CO2 Using Solution Combustion Synthesized Lanthanum–Strontium–Manganese Perovskites. Fuel 2021, 285, 119154. [Google Scholar] [CrossRef]
- Şanlı, S.B.; Pişkin, B. Effect of B-Site Al Substitution on Hydrogen Production of La0.4Sr0.6Mn1−xAlx (x = 0.4, 0.5 and 0.6) Perovskite Oxides. Int. J. Hydrogen Energy 2022, 47, 19411–19421. [Google Scholar] [CrossRef]
- Sastre, D.; Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Exploring the Redox Behavior of La0.6Sr0.4Mn1−xAlxO3 Perovskites for CO2-Splitting in Thermochemical Cycles. Top Catal 2017, 60, 1108–1118. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Solid-State Reoxidation Kinetics of A/B-Site Substituted LaMnO3 During Solar Thermochemical CO2 Conversion. Energy Technol. 2021, 9, 2000885. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Correlating Oxygen Mobility with Thermochemical CO2-Splitting Efficiency in A-Site Substituted Manganite Perovskites. Sustain. Energy Fuels 2021, 5, 4570–4574. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Cation Synergy in Sr and Al Substituted LaMnO3 during Solar Thermochemical CO2 Splitting. Energy Adv. 2023, 2, 137–147. [Google Scholar] [CrossRef]
- Gager, E.; Frye, M.; McCord, D.; Scheffe, J.; Nino, J.C. Reticulated Porous Lanthanum Strontium Manganite Structures for Solar Thermochemical Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 31152–31164. [Google Scholar] [CrossRef]
- Barcellos, D.R.; Coury, F.G.; Emery, A.; Sanders, M.; Tong, J.; McDaniel, A.; Wolverton, C.; Kaufman, M.; O’Hayre, R. Phase Identification of the Layered Perovskite CexSr2–xMnO4 and Application for Solar Thermochemical Water Splitting. Inorg. Chem. 2019, 58, 7705–7714. [Google Scholar] [CrossRef] [PubMed]
- Bergeson-Keller, A.M.; Sanders, M.D.; O’Hayre, R.P. Reduction Thermodynamics of Sr1−xCexMnO3 and CexSr2−xMnO4 Perovskites for Solar Thermochemical Hydrogen Production. Energy Technol. 2022, 10, 2100515. [Google Scholar] [CrossRef]
- Sai Gautam, G.; Stechel, E.B.; Carter, E.A. Exploring Ca–Ce–M–O (M = 3d Transition Metal) Oxide Perovskites for Solar Thermochemical Applications. Chem. Mater. 2020, 32, 9964–9982. [Google Scholar] [CrossRef]
- Naik, J.M.; Ritter, C.; Bulfin, B.; Steinfeld, A.; Erni, R.; Patzke, G.R. Reversible Phase Transformations in Novel Ce-Substituted Perovskite Oxide Composites for Solar Thermochemical Redox Splitting of CO2. Adv. Energy Mater. 2021, 11, 2003532. [Google Scholar] [CrossRef]
- Naghavi, S.S.; He, J.; Wolverton, C. CeTi2O6—A Promising Oxide for Solar Thermochemical Hydrogen Production. ACS Appl. Mater. Interfaces 2020, 12, 21521–21527. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, T.; Dai, S.; Ren, T.; Chang, Z.; Fu, M.; Li, X.; Li, Y. Solar Thermochemical CO2 Splitting with Doped Perovskite LaCo0.7Zr0.3O3: Thermodynamic Performance and Solar-to-Fuel Efficiency. RSC Adv. 2020, 10, 35740–35752. [Google Scholar] [CrossRef] [PubMed]
- Azcondo, M.T.; Orfila, M.; Marugán, J.; Sanz, R.; Muñoz-Noval, A.; Salas-Colera, E.; Ritter, C.; García-Alvarado, F.; Amador, U. Novel Perovskite Materials for Thermal Water Splitting at Moderate Temperature. ChemSusChem 2019, 12, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Parvanian, A.M.; Salimijazi, H.; Shabaninejad, M.; Troitzsch, U.; Kreider, P.; Lipiński, W.; Saadatfar, M. Thermochemical CO2 Splitting Performance of Perovskite Coated Porous Ceramics. RSC Adv. 2020, 10, 23049–23057. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Bare, Z.J.L.; Morelock, R.J.; Rodriguez, M.A.; Ambrosini, A.; Musgrave, C.B.; McDaniel, A.H.; Coker, E.N. Computationally Accelerated Discovery and Experimental Demonstration of Gd0.5La0.5Co0.5Fe0.5O3 for Solar Thermochemical Hydrogen Production. Front. Energy Res. 2021, 9, 750600. [Google Scholar] [CrossRef]
- Gokon, N.; Hara, K.; Sugiyama, Y.; Bellan, S.; Kodama, T.; Hyun-seok, C. Thermochemical Two-Step Water Splitting Cycle Using Perovskite Oxides Based on LaSrMnO3 Redox System for Solar H2 Production. Thermochim. Acta 2019, 680, 178374. [Google Scholar] [CrossRef]
- Bork, A.H.; Povoden-Karadeniz, E.; Carrillo, A.J.; Rupp, J.L.M. Thermodynamic Assessment of the Solar-to-Fuel Performance of La0.6Sr0.4Mn1−yCryO3−δ Perovskite Solid Solution Series. Acta Mater. 2019, 178, 163–172. [Google Scholar] [CrossRef]
- Jouannaux, J.; Haeussler, A.; Drobek, M.; Ayral, A.; Abanades, S.; Julbe, A. Lanthanum Manganite Perovskite Ceramic Powders for CO2 Splitting: Influence of Pechini Synthesis Parameters on Sinterability and Reactivity. Ceram. Int. 2019, 45, 15636–15648. [Google Scholar] [CrossRef]
- Gao, K.; Liu, X.; Jiang, Z.; Zheng, H.; Song, C.; Wang, X.; Tian, C.; Dang, C.; Sun, N.; Xuan, Y. Direct Solar Thermochemical CO2 Splitting Based on Ca- and Al- Doped SmMnO3 Perovskites: Ultrahigh CO Yield within Small Temperature Swing. Renew. Energy 2022, 194, 482–494. [Google Scholar] [CrossRef]
- Gao, K.; Liu, X.; Wang, T.; Zhu, Z.; Li, P.; Zheng, H.; Song, C.; Xuan, Y.; Li, Y.; Ding, Y. Sr-Doped SmMnO3 Perovskites for High-Performance near-Isothermal Solar Thermochemical CO2-to-Fuel Conversion. Sustain. Energy Fuels 2021, 5, 4295–4310. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Gao, K.; Meng, X.; Xu, Q.; Song, C.; Zhu, Z.; Zheng, H.; Hao, Y.; Xuan, Y. Ca- and Ga-Doped LaMnO3 for Solar Thermochemical CO2 Splitting with High Fuel Yield and Cycle Stability. ACS Appl. Energy Mater. 2021, 4, 9000–9012. [Google Scholar] [CrossRef]
- Qian, X.; He, J.; Mastronardo, E.; Baldassarri, B.; Yuan, W.; Wolverton, C.; Haile, S.M. Outstanding Properties and Performance of CaTi0.5Mn0.5O3–δ for Solar-Driven Thermochemical Hydrogen Production. Matter 2021, 4, 688–708. [Google Scholar] [CrossRef]
- Qian, X.; He, J.; Mastronardo, E.; Baldassarri, B.; Wolverton, C.; Haile, S.M. Favorable Redox Thermodynamics of SrTi0.5Mn0.5O3−δ in Solar Thermochemical Water Splitting. Chem. Mater. 2020, 32, 9335–9346. [Google Scholar] [CrossRef]
- Emery, A.A.; Saal, J.E.; Kirklin, S.; Hegde, V.I.; Wolverton, C. High-Throughput Computational Screening of Perovskites for Thermochemical Water Splitting Applications. Chem. Mater. 2016, 28, 5621–5634. [Google Scholar] [CrossRef]
- Ezbiri, M.; Takacs, M.; Stolz, B.; Lungthok, J.; Steinfeld, A.; Michalsky, R. Design Principles of Perovskites for Solar-Driven Thermochemical Splitting of CO2. J. Mater. Chem. A 2017, 5, 15105–15115. [Google Scholar] [CrossRef]
- Ezbiri, M.; Takacs, M.; Theiler, D.; Michalsky, R.; Steinfeld, A. Tunable Thermodynamic Activity of LaxSr1−x MnyAl1−yO3−δ (0 ≤ x ≤ 1, 0 ≤ y ≤ 1) Perovskites for Solar Thermochemical Fuel Synthesis. J. Mater. Chem. A 2017, 5, 4172–4182. [Google Scholar] [CrossRef]
- Bork, A.H.; Carrillo, A.J.; Hood, Z.D.; Yildiz, B.; Rupp, J.L.M. Oxygen Exchange in Dual-Phase La0.65Sr0.35MnO3–CeO2 Composites for Solar Thermochemical Fuel Production. ACS Appl. Mater. Interfaces 2020, 12, 32622–32632. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Julbe, A. Demonstration of a Ceria Membrane Solar Reactor Promoted by Dual Perovskite Coatings for Continuous and Isothermal Redox Splitting of CO2 and H2O. J. Membr. Sci. 2021, 634, 119387. [Google Scholar] [CrossRef]
- Abanades, S.; Haeussler, A.; Julbe, A. Thermochemical Solar-Driven Reduction of CO2 into Separate Streams of CO and O2 via an Isothermal Oxygen-Conducting Ceria Membrane Reactor. Chem. Eng. J. 2021, 422, 130026. [Google Scholar] [CrossRef]
- Tou, M.; Jin, J.; Hao, Y.; Steinfeld, A.; Michalsky, R. Solar-Driven Co-Thermolysis of CO2 and H2O Promoted by in Situ Oxygen Removal across a Non-Stoichiometric Ceria Membrane. React. Chem. Eng. 2019, 4, 1431–1438. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Ghoniem, A.F. Mixed Ionic-Electronic Conducting (MIEC) Membranes for Thermochemical Reduction of CO2: A Review. Prog. Energy Combust. Sci. 2019, 74, 1–30. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-Stabilized Oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, Y.; Wang, Q.; Schweidler, S.; Botros, M.; Fu, T.; Hahn, H.; Brezesinski, T.; Breitung, B. High-Entropy Energy Materials: Challenges and New Opportunities. Energy Environ. Sci. 2021, 14, 2883–2905. [Google Scholar] [CrossRef]
- Zhai, S.; Rojas, J.; Ahlborg, N.; Lim, K.; Toney, M.F.; Jin, H.; Chueh, W.C.; Majumdar, A. The Use of Poly-Cation Oxides to Lower the Temperature of Two-Step Thermochemical Water Splitting. Energy Environ. Sci. 2018, 11, 2172–2178. [Google Scholar] [CrossRef]
- Le Gal, A.; Vallès, M.; Julbe, A.; Abanades, S. Thermochemical Properties of High Entropy Oxides Used as Redox-Active Materials in Two-Step Solar Fuel Production Cycles. Catalysts 2022, 12, 1116. [Google Scholar] [CrossRef]
- Gao, Y.; Mao, Y.; Song, Z.; Zhao, X.; Sun, J.; Wang, W.; Chen, G.; Chen, S. Efficient Generation of Hydrogen by Two-Step Thermochemical Cycles: Successive Thermal Reduction and Water Splitting Reactions Using Equal-Power Microwave Irradiation and a High Entropy Material. Appl. Energy 2020, 279, 115777. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Mao, Y.; Cao, H.; Zhang, S.; Wang, W.; Sun, C.; Song, Z.; Sun, J.; Zhao, X. Microwave-Triggered Low Temperature Thermal Reduction of Zr-Modified High Entropy Oxides with Extraordinary Thermochemical H2 Production Performance. Energy Convers. Manag. 2022, 252, 115125. [Google Scholar] [CrossRef]
- Luo, J. New High Entropy Perovskite Oxides with Increased Reducibility and Stability for Thermochemical Hydrogen Generation, Online Project Presentation. 2020. Available online: https://www.hydrogen.energy.gov/Pdfs/Review20/P194_luo_2020_p.Pdf (accessed on 1 January 2023).
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Liu, X.; Elgowainy, A.; Wang, M. Life Cycle Energy Use and Greenhouse Gas Emissions of Ammonia Production from Renewable Resources and Industrial By-Products. Green Chem. 2020, 22, 5751–5761. [Google Scholar] [CrossRef]
- Chen, T.; Ying, H.; Zhang, C.; Bi, J.; Li, Z.; Hao, J. Engineering an Fe2O3/FeS Hybrid Catalyst from a Deep Eutectic Solvent for Highly Efficient Electrocatalytic N2 Fixation. Chem. Commun. 2021, 57, 6688–6691. [Google Scholar] [CrossRef]
- Klaas, L.; Guban, D.; Roeb, M.; Sattler, C. Recent Progress towards Solar Energy Integration into Low-Pressure Green Ammonia Production Technologies. Int. J. Hydrogen Energy 2021, 46, 25121–25136. [Google Scholar] [CrossRef]
- Wang, L.; Xia, M.; Wang, H.; Huang, K.; Qian, C.; Maravelias, C.T.; Ozin, G.A. Greening Ammonia toward the Solar Ammonia Refinery. Joule 2018, 2, 1055–1074. [Google Scholar] [CrossRef]
- Michalsky, R.; Avram, A.M.; Peterson, B.A.; Pfromm, P.H.; Peterson, A.A. Chemical Looping of Metal Nitride Catalysts: Low-Pressure Ammonia Synthesis for Energy Storage. Chem. Sci. 2015, 6, 3965–3974. [Google Scholar] [CrossRef]
- Hunter, S.M.; Mckay, D.; Smith, R.I.; Hargreaves, J.S.J.; Gregory, D.H. Topotactic Nitrogen Transfer: Structural Transformation in Cobalt Molybdenum Nitrides. Chem. Mater. 2010, 22, 2898–2907. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Frei, A.; Halmann, M.; Steinfeld, A. Ammonia Production via a Two-Step Al2O3/AlN Thermochemical Cycle. 2. Kinetic Analysis. Ind. Eng. Chem. Res. 2007, 46, 2047–2053. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Halmann, M.; Steinfeld, A. Ammonia Production via a Two-Step Al2O3/AlN Thermochemical Cycle. 1. Thermodynamic, Environmental, and Economic Analyses. Ind. Eng. Chem. Res. 2007, 46, 2042–2046. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Hischier, I.; Frei, A.; Steinfeld, A. Ammonia Production via a Two-Step Al2O3/AlN Thermochemical Cycle. 3. Influence of the Carbon Reducing Agent and Cyclability. Ind. Eng. Chem. Res. 2008, 47, 2231–2237. [Google Scholar] [CrossRef]
- Murray, J.; Steinfeld, A.; Fletcher, E. Metals, Nitrides, and Carbides via Solar Carbothermal Reduction of Metal Oxides. Energy 1995, 20, 695–704. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Kumar, S.; Ichikawa, T.; Kojima, Y. A New Synthesis Route of Ammonia Production through Hydrolysis of Metal—Nitrides. Int. J. Hydrogen Energy 2017, 42, 24897–24903. [Google Scholar] [CrossRef]
- Michalsky, R.; Pfromm, P.H. Chromium as Reactant for Solar Thermochemical Synthesis of Ammonia from Steam, Nitrogen, and Biomass at Atmospheric Pressure. Sol. Energy 2011, 85, 2642–2654. [Google Scholar] [CrossRef]
- Michalsky, R.; Pfromm, P.H.; Steinfeld, A. Rational Design of Metal Nitride Redox Materials for Solar-Driven Ammonia Synthesis. Interface Focus. 2015, 5, 20140084. [Google Scholar] [CrossRef]
- Bartel, C.J.; Rumptz, J.R.; Weimer, A.W.; Holder, A.M.; Musgrave, C.B. High-Throughput Equilibrium Analysis of Active Materials for Solar Thermochemical Ammonia Synthesis. ACS Appl. Mater. Interfaces 2019, 11, 24850–24858. [Google Scholar] [CrossRef]
- Michalsky, R.; Steinfeld, A. Computational Screening of Perovskite Redox Materials for Solar Thermochemical Ammonia Synthesis from N2 and H2O. Catal. Today 2017, 286, 124–130. [Google Scholar] [CrossRef]
- Prieto, C.; Cooper, P.; Fernández, A.I.; Cabeza, L.F. Review of Technology: Thermochemical Energy Storage for Concentrated Solar Power Plants. Renew. Sustain. Energy Rev. 2016, 60, 909–929. [Google Scholar] [CrossRef]
- Cot-Gores, J.; Castell, A.; Cabeza, L.F. Thermochemical Energy Storage and Conversion: A-State-of-the-Art Review of the Experimental Research under Practical Conditions. Renew. Sustain. Energy Rev. 2012, 16, 5207–5224. [Google Scholar] [CrossRef]
- Carrillo, A.J.; González-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Qi, C.; Ling, X.; Peng, H. State of the Art on the High-Temperature Thermochemical Energy Storage Systems. Energy Convers. Manag. 2018, 177, 792–815. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A Review on High Temperature Thermochemical Heat Energy Storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Sunku Prasad, J.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A Critical Review of High-Temperature Reversible Thermochemical Energy Storage Systems. Appl. Energy 2019, 254, 113733. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A Review of Promising Candidate Reactions for Chemical Heat Storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- Liu, M.; Steven Tay, N.H.; Bell, S.; Belusko, M.; Jacob, R.; Will, G.; Saman, W.; Bruno, F. Review on Concentrating Solar Power Plants and New Developments in High Temperature Thermal Energy Storage Technologies. Renew. Sustain. Energy Rev. 2016, 53, 1411–1432. [Google Scholar] [CrossRef]
- Rodat, S.; Abanades, S.; Boujjat, H.; Chuayboon, S. On the Path toward Day and Night Continuous Solar High Temperature Thermochemical Processes: A Review. Renew. Sustain. Energy Rev. 2020, 132, 110061. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Recent Advances in Thermochemical Energy Storage via Solid–Gas Reversible Reactions at High Temperature. Energies 2020, 13, 5859. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A Review on High-Temperature Thermochemical Energy Storage Based on Metal Oxides Redox Cycle. Energy Convers. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of Thermochemical Systems Based on Solid-Gas Reversible Reactions for High Temperature Solar Thermal Energy Storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Dizaji, H.B.; Hosseini, H. A Review of Material Screening in Pure and Mixed-Metal Oxide Thermochemical Energy Storage (TCES) Systems for Concentrated Solar Power (CSP) Applications. Renew. Sustain. Energy Rev. 2018, 98, 9–26. [Google Scholar] [CrossRef]
- Block, T.; Schmücker, M. Metal Oxides for Thermochemical Energy Storage: A Comparison of Several Metal Oxide Systems. Sol. Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of Thermochemical Cycles Based on Solid Oxide Redox Systems for Thermochemical Storage of Solar Heat. Part 1: Testing of Cobalt Oxide-Based Powders. Sol. Energy 2014, 102, 189–211. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of Thermochemical Cycles Based on Solid Oxide Redox Systems for Thermochemical Storage of Solar Heat. Part 2: Redox Oxide-Coated Porous Ceramic Structures as Integrated Thermochemical Reactors/Heat Exchangers. Sol. Energy 2015, 114, 440–458. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Tescari, S.; Roeb, M.; Schmücker, M.; Sattler, C. Exploitation of Thermochemical Cycles Based on Solid Oxide Redox Systems for Thermochemical Storage of Solar Heat. Part 3: Cobalt Oxide Monolithic Porous Structures as Integrated Thermochemical Reactors/Heat Exchangers. Sol. Energy 2015, 114, 459–475. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Sattler, C. Exploitation of Thermochemical Cycles Based on Solid Oxide Redox Systems for Thermochemical Storage of Solar Heat. Part 4: Screening of Oxides for Use in Cascaded Thermochemical Storage Concepts. Sol. Energy 2016, 139, 695–710. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical Heat Storage Based on the Mn2O3/Mn3O4 Redox Couple: Influence of the Initial Particle Size on the Morphological Evolution and Cyclability. J. Mater. Chem. A 2014, 2, 19435–19443. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Revisiting the BaO2/BaO Redox Cycle for Solar Thermochemical Energy Storage. Phys. Chem. Chem. Phys. 2016, 18, 8039–8048. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, M.; Horvath, F.; Knoll, C.; Lager, D.; Gierl-Mayer, C.; Weinberger, P.; Winter, F. High-Temperature Energy Storage: Kinetic Investigations of the CuO/Cu2O Reaction Cycle. Energy Fuels 2017, 31, 2324–2334. [Google Scholar] [CrossRef]
- Haseli, P.; Jafarian, M.; Nathan, G.J. High Temperature Solar Thermochemical Process for Production of Stored Energy and Oxygen Based on CuO/Cu2O Redox Reactions. Sol. Energy 2017, 153, 1–10. [Google Scholar] [CrossRef]
- Lei, F.; Dyall, A.; AuYeung, N. An In-Depth Investigation of BaO2/BaO Redox Oxides for Reversible Solar Thermochemical Energy Storage. Sol. Energy Mater. Sol. Cells 2021, 223, 110957. [Google Scholar] [CrossRef]
- Schrader, A.J.; Muroyama, A.P.; Loutzenhiser, P.G. Solar Electricity via an Air Brayton Cycle with an Integrated Two-Step Thermochemical Cycle for Heat Storage Based on Co3O4/CoO Redox Reactions: Thermodynamic Analysis. Sol. Energy 2015, 118, 485–495. [Google Scholar] [CrossRef]
- Muroyama, A.P.; Schrader, A.J.; Loutzenhiser, P.G. Solar Electricity via an Air Brayton Cycle with an Integrated Two-Step Thermochemical Cycle for Heat Storage Based on Co3O4/CoO Redox Reactions II: Kinetic Analyses. Sol. Energy 2015, 122, 409–418. [Google Scholar] [CrossRef]
- Han, X.; Wang, L.; Ling, H.; Ge, Z.; Lin, X.; Dai, X.; Chen, H. Critical Review of Thermochemical Energy Storage Systems Based on Cobalt, Manganese, and Copper Oxides. Renew. Sustain. Energy Rev. 2022, 158, 112076. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. High-Temperature Thermochemical Energy Storage Based on Redox Reactions Using Co-Fe and Mn-Fe Mixed Metal Oxides. J. Solid State Chem. 2017, 253, 6–14. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. Experimental Investigation of Co–Cu, Mn–Co, and Mn–Cu Redox Materials Applied to Solar Thermochemical Energy Storage. ACS Appl. Energy Mater. 2018, 1, 3385–3395. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Appl. Sci. 2018, 8, 2618. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Improving the Thermochemical Energy Storage Performance of the Mn2O3/Mn3O4 Redox Couple by the Incorporation of Iron. ChemSusChem 2015, 8, 1947–1954. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Manganese Oxide-Based Thermochemical Energy Storage: Modulating Temperatures of Redox Cycles by Fe–Cu Co-Doping. J. Energy Storage 2016, 5, 169–176. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Moya, J.; Bayón, A.; Jana, P.; de la Peña O’Shea, V.A.; Romero, M.; Gonzalez-Aguilar, J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical Energy Storage at High Temperature via Redox Cycles of Mn and Co Oxides: Pure Oxides versus Mixed Ones. Sol. Energy Mater. Sol. Cells 2014, 123, 47–57. [Google Scholar] [CrossRef]
- Gigantino, M.; Sas Brunser, S.; Steinfeld, A. High-Temperature Thermochemical Heat Storage via the CuO/Cu2O Redox Cycle: From Material Synthesis to Packed-Bed Reactor Engineering and Cyclic Operation. Energy Fuels 2020, 34, 16772–16782. [Google Scholar] [CrossRef]
- Block, T.; Knoblauch, N.; Schmücker, M. The Cobalt-Oxide/Iron-Oxide Binary System for Use as High Temperature Thermochemical Energy Storage Material. Thermochim. Acta 2014, 577, 25–32. [Google Scholar] [CrossRef]
- Wokon, M.; Block, T.; Nicolai, S.; Linder, M.; Schmücker, M. Thermodynamic and Kinetic Investigation of a Technical Grade Manganese-Iron Binary Oxide for Thermochemical Energy Storage. Sol. Energy 2017, 153, 471–485. [Google Scholar] [CrossRef]
- Wokon, M.; Kohzer, A.; Linder, M. Investigations on Thermochemical Energy Storage Based on Technical Grade Manganese-Iron Oxide in a Lab-Scale Packed Bed Reactor. Sol. Energy 2017, 153, 200–214. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Understanding Redox Kinetics of Iron-Doped Manganese Oxides for High Temperature Thermochemical Energy Storage. J. Phys. Chem. C 2016, 120, 27800–27812. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Block, T.; Senholdt, M.; Tescari, S.; Roeb, M.; Sattler, C. Exploitation of Thermochemical Cycles Based on Solid Oxide Redox Systems for Thermochemical Storage of Solar Heat. Part 6: Testing of Mn-Based Combined Oxides and Porous Structures. Sol. Energy 2017, 149, 227–244. [Google Scholar] [CrossRef]
- Rahmatian, N.; Bo, A.; Randhir, K.; Klausner, J.F.; Petrasch, J. Bench-Scale Demonstration of Thermochemical Energy Storage Using the Magnesium-Manganese-Oxide Redox System. J. Energy Storage 2022, 45, 103682. [Google Scholar] [CrossRef]
- Zhang, Z.; Andre, L.; Abanades, S. Experimental Assessment of Oxygen Exchange Capacity and Thermochemical Redox Cycle Behavior of Ba and Sr Series Perovskites for Solar Energy Storage. Sol. Energy 2016, 134, 494–502. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Investigation of Metal Oxides, Mixed Oxides, Perovskites and Alkaline Earth Carbonates/Hydroxides as Suitable Candidate Materials for High-Temperature Thermochemical Energy Storage Using Reversible Solid-Gas Reactions. Mater. Today Energy 2018, 10, 48–61. [Google Scholar] [CrossRef]
- Cai, R.; Bektas, H.; Wang, X.; McClintock, K.; Teague, L.; Yang, K.; Li, F. Accelerated Perovskite Oxide Development for Thermochemical Energy Storage by a High-Throughput Combinatorial Approach. Adv. Energy Mater. 2023, 2203833. [Google Scholar] [CrossRef]
- Chen, X.; Kubota, M.; Yamashita, S.; Kita, H. Investigation of Sr-Based Perovskites for Redox-Type Thermochemical Energy Storage Media at Medium-High Temperature. J. Energy Storage 2021, 38, 102501. [Google Scholar] [CrossRef]
- Yuan, P.; Xu, H.; Ning, Z.; Xiao, G. Understanding Thermochemical Energy Storage Performance of Ba1−xSrxCoO3−δ Perovskite System: A Computational and Experimental Study. J. Energy Storage 2023, 61, 106695. [Google Scholar] [CrossRef]
- Babiniec, S.M.; Coker, E.N.; Miller, J.E.; Ambrosini, A. Investigation of LaxSr1−xCoyM1−yO3−d (M = Mn, Fe) Perovskite Materials as Thermochemical Energy Storage Media. Sol. Energy 2015, 118, 451–459. [Google Scholar] [CrossRef]
- Gokon, N.; Yawata, T.; Bellan, S.; Kodama, T.; Cho, H.-S. Thermochemical Behavior of Perovskite Oxides Based on LaxSr1−x(Mn, Fe, Co)O3−δ and BaySr1−yCoO3−δ Redox System for Thermochemical Energy Storage at High Temperatures. Energy 2019, 171, 971–980. [Google Scholar] [CrossRef]
- Lei, Q.; Si, Q.; Zhang, J.; Jiang, Y.; Hu, L.; Qiao, L.; Zu, X.; Xiao, G.; Yang, J.; Li, S. Redox Cycle of Calcium Manganite for High Temperature Solar Thermochemical Storage Systems. Appl. Energy 2022, 305, 117958. [Google Scholar] [CrossRef]
- Jin, F.; Xu, C.; Yu, H.; Xia, X.; Ye, F.; Li, X.; Du, X.; Yang, Y. CaCo0.05Mn0.95O3−δ: A Promising Perovskite Solid Solution for Solar Thermochemical Energy Storage. ACS Appl. Mater. Interfaces 2021, 13, 3856–3866. [Google Scholar] [CrossRef] [PubMed]
- Mastronardo, E.; Qian, X.; Coronado, J.M.; Haile, S.M. Impact of La Doping on the Thermochemical Heat Storage Properties of CaMnO3-δ. J. Energy Storage 2021, 40, 102793. [Google Scholar] [CrossRef]
- Mastronardo, E.; Qian, X.; Coronado, J.M.; Haile, S.M. The Favourable Thermodynamic Properties of Fe-Doped CaMnO3 for Thermochemical Heat Storage. J. Mater. Chem. A 2020, 8, 8503–8517. [Google Scholar] [CrossRef]
- Babiniec, S.M.; Coker, E.N.; Miller, J.E.; Ambrosini, A. Doped Calcium Manganites for Advanced High-Temperature Thermochemical Energy Storage. Int. J. Energy Res. 2016, 40, 280–284. [Google Scholar] [CrossRef]
- Miller, J.E.; Babiniec, S.M.; Coker, E.N.; Loutzenhiser, P.G.; Stechel, E.B.; Ambrosini, A. Modified Calcium Manganites for Thermochemical Energy Storage Applications. Front. Energy Res. 2022, 10, 774099. [Google Scholar] [CrossRef]
- Imponenti, L.; Albrecht, K.J.; Wands, J.W.; Sanders, M.D.; Jackson, G.S. Thermochemical Energy Storage in Strontium-Doped Calcium Manganites for Concentrating Solar Power Applications. Sol. Energy 2017, 151, 1–13. [Google Scholar] [CrossRef]
- Schrader, A.J.; Schieber, G.L.; Ambrosini, A.; Loutzenhiser, P.G. Experimental Demonstration of a 5 KWth Granular-Flow Reactor for Solar Thermochemical Energy Storage with Aluminum-Doped Calcium Manganite Particles. Appl. Therm. Eng. 2020, 173, 115257. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Evaluation and Performances Comparison of Calcium, Strontium and Barium Carbonates during Calcination/Carbonation Reactions for Solar Thermochemical Energy Storage. J. Energy Storage 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Zare Ghorbaei, S.; Ale Ebrahim, H. Carbonation Reaction of Strontium Oxide for Thermochemical Energy Storage and CO2 Removal Applications: Kinetic Study and Reactor Performance Prediction. Appl. Energy 2020, 277, 115604. [Google Scholar] [CrossRef]
- Zare Ghorbaei, S.; Ale Ebrahim, H. Comparison of Kinetics and Thermochemical Energy Storage Capacities of Strontium Oxide, Calcium Oxide, and Magnesium Oxide during Carbonation Reaction. Renew. Energy 2022, 184, 765–775. [Google Scholar] [CrossRef]
- Rhodes, N.R.; Barde, A.; Randhir, K.; Li, L.; Hahn, D.W.; Mei, R.; Klausner, J.F.; AuYeung, N. Solar Thermochemical Energy Storage Through Carbonation Cycles of SrCO3/SrO Supported on SrZrO3. ChemSusChem 2015, 8, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Bagherisereshki, E.; Tran, J.; Lei, F.; AuYeung, N. Investigation into SrO/SrCO3 for High Temperature Thermochemical Energy Storage. Sol. Energy 2018, 160, 85–93. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P. Review of Carbonate-Based Systems for Thermochemical Energy Storage for Concentrating Solar Power Applications: State-of-the-Art and Outlook. Energy Fuels 2023, 37, 1777–1808. [Google Scholar] [CrossRef]
- Ammendola, P.; Raganati, F.; Miccio, F.; Murri, A.N.; Landi, E. Insights into Utilization of Strontium Carbonate for Thermochemical Energy Storage. Renew. Energy 2020, 157, 769–781. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Sarrion, B.; Perejon, A.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Manuel Valverde, J. Large-Scale High-Temperature Solar Energy Storage Using Natural Minerals. Sol. Energy Mater. Sol. Cells 2017, 168, 14–21. [Google Scholar] [CrossRef]
- Alonso, E.; Romero, M. Review of Experimental Investigation on Directly Irradiated Particles Solar Reactors. Renew. Sustain. Energy Rev. 2015, 41, 53–67. [Google Scholar] [CrossRef]
- Kodama, T.; Bellan, S.; Gokon, N.; Cho, H.S. Particle Reactors for Solar Thermochemical Processes. Sol. Energy 2017, 156, 113–132. [Google Scholar] [CrossRef]
- Bellan, S.; Kodama, T.; Gokon, N.; Matsubara, K. A Review on High-temperature Thermochemical Heat Storage: Particle Reactors and Materials Based on Solid–Gas Reactions. WIREs Energy Environ. 2022, 11, e440. [Google Scholar] [CrossRef]
- Alvarez Rivero, M.; Rodrigues, D.; Pinheiro, C.I.C.; Cardoso, J.P.; Mendes, L.F. Solid–Gas Reactors Driven by Concentrated Solar Energy with Potential Application to Calcium Looping: A Comparative Review. Renew. Sustain. Energy Rev. 2022, 158, 112048. [Google Scholar] [CrossRef]
- Zsembinszki, G.; Solé, A.; Barreneche, C.; Prieto, C.; Fernández, A.; Cabeza, L. Review of Reactors with Potential Use in Thermochemical Energy Storage in Concentrated Solar Power Plants. Energies 2018, 11, 2358. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Design of a Lab-Scale Rotary Cavity-Type Solar Reactor for Continuous Thermal Dissociation of Volatile Oxides Under Reduced Pressure. J. Sol. Energy Eng. 2010, 132, 021006. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Neveu, P.; Lemont, F.; Flamant, G. Dynamic Modeling of a Volumetric Solar Reactor for Volatile Metal Oxide Reduction. Chem. Eng. Res. Des. 2008, 86, 1216–1222. [Google Scholar] [CrossRef]
- Abanades, S.; Charvin, P.; Flamant, G. Design and Simulation of a Solar Chemical Reactor for the Thermal Reduction of Metal Oxides: Case Study of Zinc Oxide Dissociation. Chem. Eng. Sci. 2007, 62, 6323–6333. [Google Scholar] [CrossRef]
- Neises, M.; Tescari, S.; de Oliveira, L.; Roeb, M.; Sattler, C.; Wong, B. Solar-Heated Rotary Kiln for Thermochemical Energy Storage. Sol. Energy 2012, 86, 3040–3048. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Gallo, A.; Fuentealba, E.; Estrada, C.A. Experimental Aspects of CuO Reduction in Solar-Driven Reactors: Comparative Performance of a Rotary Kiln and a Packed-Bed. Renew. Energy 2017, 105, 665–673. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Fuentealba, E.; Estrada, C.A. First Experimental Studies of Solar Redox Reactions of Copper Oxides for Thermochemical Energy Storage. Sol. Energy 2015, 115, 297–305. [Google Scholar] [CrossRef]
- Abanades, S.; André, L. Design and Demonstration of a High Temperature Solar-Heated Rotary Tube Reactor for Continuous Particles Calcination. Appl. Energy 2018, 212, 1310–1320. [Google Scholar] [CrossRef]
- Pagkoura, C.; Karagiannakis, G.; Zygogianni, A.; Lorentzou, S.; Kostoglou, M.; Konstandopoulos, A.G.; Rattenburry, M.; Woodhead, J.W. Cobalt Oxide Based Structured Bodies as Redox Thermochemical Heat Storage Medium for Future CSP Plants. Sol. Energy 2014, 108, 146–163. [Google Scholar] [CrossRef]
- Karagiannakis, G.; Pagkoura, C.; Halevas, E.; Baltzopoulou, P.; Konstandopoulos, A.G. Cobalt/Cobaltous Oxide Based Honeycombs for Thermochemical Heat Storage in Future Concentrated Solar Power Installations: Multi-Cyclic Assessment and Semi-Quantitative Heat Effects Estimations. Sol. Energy 2016, 133, 394–407. [Google Scholar] [CrossRef]
- Tescari, S.; Singh, A.; Agrafiotis, C.; de Oliveira, L.; Breuer, S.; Schlögl-Knothe, B.; Roeb, M.; Sattler, C. Experimental Evaluation of a Pilot-Scale Thermochemical Storage System for a Concentrated Solar Power Plant. Appl. Energy 2017, 189, 66–75. [Google Scholar] [CrossRef]
- Brendelberger, S.; Vieten, J.; Roeb, M.; Sattler, C. Thermochemical Oxygen Pumping for Improved Hydrogen Production in Solar Redox Cycles. Int. J. Hydrogen Energy 2019, 44, 9802–9810. [Google Scholar] [CrossRef]
- Brendelberger, S.; Vieten, J.; Vidyasagar, M.J.; Roeb, M.; Sattler, C. Demonstration of Thermochemical Oxygen Pumping for Atmosphere Control in Reduction Reactions. Sol. Energy 2018, 170, 273–279. [Google Scholar] [CrossRef]
- Brendelberger, S.; von Storch, H.; Bulfin, B.; Sattler, C. Vacuum Pumping Options for Application in Solar Thermochemical Redox Cycles—Assessment of Mechanical-, Jet- and Thermochemical Pumping Systems. Sol. Energy 2017, 141, 91–102. [Google Scholar] [CrossRef]
- Bader, R.; Venstrom, L.J.; Davidson, J.H.; Lipiński, W. Thermodynamic Analysis of Isothermal Redox Cycling of Ceria for Solar Fuel Production. Energy Fuels 2013, 27, 5533–5544. [Google Scholar] [CrossRef]
- Ehrhart, B.D.; Muhich, C.L.; Al-Shankiti, I.; Weimer, A.W. System Efficiency for Two-Step Metal Oxide Solar Thermochemical Hydrogen Production—Part 3: Various Methods for Achieving Low Oxygen Partial Pressures in the Reduction Reaction. Int. J. Hydrogen Energy 2016, 41, 19904–19914. [Google Scholar] [CrossRef]
- Ermanoski, I.; Siegel, N.P.; Stechel, E.B. A New Reactor Concept for Efficient Solar-Thermochemical Fuel Production. J. Sol. Energy Eng. 2013, 135, 031002. [Google Scholar] [CrossRef]
- Lin, M.; Haussener, S. Solar Fuel Processing Efficiency for Ceria Redox Cycling Using Alternative Oxygen Partial Pressure Reduction Methods. Energy 2015, 88, 667–679. [Google Scholar] [CrossRef]
- Brendelberger, S.; Sattler, C. Concept Analysis of an Indirect Particle-Based Redox Process for Solar-Driven H2O/CO2 Splitting. Sol. Energy 2015, 113, 158–170. [Google Scholar] [CrossRef]
- Brendelberger, S.; Roeb, M.; Lange, M.; Sattler, C. Counter Flow Sweep Gas Demand for the Ceria Redox Cycle. Sol. Energy 2015, 122, 1011–1022. [Google Scholar] [CrossRef]
- Vieten, J.; Bulfin, B.; Call, F.; Lange, M.; Schmücker, M.; Francke, A.; Roeb, M.; Sattler, C. Perovskite Oxides for Application in Thermochemical Air Separation and Oxygen Storage. J. Mater. Chem. A 2016, 4, 13652–13659. [Google Scholar] [CrossRef]
- Vieten, J.; Bulfin, B.; Senholdt, M.; Roeb, M.; Sattler, C.; Schmücker, M. Redox Thermodynamics and Phase Composition in the System SrFeO3−δ—SrMnO3−δ. Solid State Ion. 2017, 308, 149–155. [Google Scholar] [CrossRef]
- Bulfin, B.; Vieten, J.; Starr, D.E.; Azarpira, A.; Zachäus, C.; Hävecker, M.; Skorupska, K.; Schmücker, M.; Roeb, M.; Sattler, C. Redox Chemistry of CaMnO3 and Ca0.8Sr0.2MnO3 Oxygen Storage Perovskites. J. Mater. Chem. A 2017, 5, 7912–7919. [Google Scholar] [CrossRef]
- Motuzas, J.; Liu, S.; da Costa, J.C.D. Thermal Swing Reduction-Oxidation of Me(Ba, Ca, or Mg)SrCoCu Perovskites for Oxygen Separation from Air. Processes 2022, 10, 2239. [Google Scholar] [CrossRef]
- Capstick, S.; Bulfin, B.; Naik, J.M.; Gigantino, M.; Steinfeld, A. Oxygen Separation via Chemical Looping of the Perovskite Oxide Sr0.8Ca0.2FeO3 in Packed Bed Reactors for the Production of Nitrogen from Air. Chem. Eng. J. 2023, 452, 139289. [Google Scholar] [CrossRef]
- Jia, T.; Popczun, E.J.; Lekse, J.W.; Duan, Y. Effective Ca2+-Doping in Sr1−xCaxFeO3−δ Oxygen Carriers for Chemical Looping Air Separation: A Theoretical and Experimental Investigation. Appl. Energy 2021, 281, 116040. [Google Scholar] [CrossRef]
- Bush, H.E.; Datta, R.; Loutzenhiser, P.G. Aluminum-Doped Strontium Ferrites for a Two-Step Solar Thermochemical Air Separation Cycle: Thermodynamic Characterization and Cycle Analysis. Sol. Energy 2019, 188, 775–786. [Google Scholar] [CrossRef]
- Motuzas, J.; Diniz da Costa, J.C. Copper Aided Exchange in High Performance Oxygen Production by CuCo Binary Oxides for Clean Energy Delivery. J. Mater. Chem. A 2015, 3, 17344–17350. [Google Scholar] [CrossRef]
- Ni, C.; Ni, J.; Zhou, Z.; Jin, M. Structural and Chemical Stability of Sr-, Nb- and Zr-Doped Calcium Manganite as Oxygen-Storage Materials. J. Alloys Compd. 2017, 709, 789–795. [Google Scholar] [CrossRef]
- Dou, J.; Krzystowczyk, E.; Wang, X.; Richard, A.R.; Robbins, T.; Li, F. Sr1−xCaxFe1−yCoyO3−δ as Facile and Tunable Oxygen Sorbents for Chemical Looping Air Separation. J. Phys. Energy 2020, 2, 025007. [Google Scholar] [CrossRef]
- Ezbiri, M.; Reinhart, A.; Huber, B.; Allen, K.M.; Steinfeld, A.; Bulfin, B.; Michalsky, R. High Redox Performance of Y0.5Ba0.5CoO3−δ for Thermochemical Oxygen Production and Separation. React. Chem. Eng. 2020, 5, 685–695. [Google Scholar] [CrossRef]
- Pein, M.; Agrafiotis, C.; Vieten, J.; Giasafaki, D.; Brendelberger, S.; Roeb, M.; Sattler, C. Redox Thermochemistry of Ca-Mn-Based Perovskites for Oxygen Atmosphere Control in Solar-Thermochemical Processes. Sol. Energy 2020, 198, 612–622. [Google Scholar] [CrossRef]
- Motohashi, T.; Hirano, Y.; Masubuchi, Y.; Oshima, K.; Setoyama, T.; Kikkawa, S. Oxygen Storage Capability of Brownmillerite-Type Ca2AlMnO5+δ and Its Application to Oxygen Enrichment. Chem. Mater. 2013, 25, 372–377. [Google Scholar] [CrossRef]
- Görke, R.H.; Marek, E.J.; Donat, F.; Scott, S.A. Reduction and Oxidation Behavior of Strontium Perovskites for Chemical Looping Air Separation. Int. J. Greenh. Gas Control 2020, 94, 102891. [Google Scholar] [CrossRef]
- Yin, Q.; Kniep, J.; Lin, Y.S. Oxygen Sorption and Desorption Properties of Sr–Co–Fe Oxide. Chem. Eng. Sci. 2008, 63, 2211–2218. [Google Scholar] [CrossRef]
- Fujishiro, F.; Oshima, N.; Sakuragi, T.; Oishi, M. Oxygen Desorption Properties of Perovskite-Type SrFe1−xCoxO3−δ: B-Site Mixing Effect on the Reduction Properties of Fe and Co Ions. J. Solid State Chem. 2022, 312, 123254. [Google Scholar] [CrossRef]
- Ikeda, H.; Tsuchida, A.; Morita, J.; Miura, N. SrCoxFe1–xO3−δ Oxygen Sorbent Usable for High-Temperature Pressure-Swing Adsorption Process Operating at Approximately 300 °C. Ind. Eng. Chem. Res. 2016, 55, 6501–6505. [Google Scholar] [CrossRef]
- Rui, Z.; Ding, J.; Li, Y.; Lin, Y.S. SrCo0.8Fe0.2O3−δ Sorbent for High-Temperature Production of Oxygen-Enriched Carbon Dioxide Stream. Fuel 2010, 89, 1429–1434. [Google Scholar] [CrossRef]
- Bulfin, B.; Lapp, J.; Richter, S.; Gubàn, D.; Vieten, J.; Brendelberger, S.; Roeb, M.; Sattler, C. Air Separation and Selective Oxygen Pumping via Temperature and Pressure Swing Oxygen Adsorption Using a Redox Cycle of SrFeO3 Perovskite. Chem. Eng. Sci. 2019, 203, 68–75. [Google Scholar] [CrossRef]
- Ezbiri, M.; Allen, K.M.; Gàlvez, M.E.; Michalsky, R.; Steinfeld, A. Design Principles of Perovskites for Thermochemical Oxygen Separation. ChemSusChem 2015, 8, 1966–1971. [Google Scholar] [CrossRef]
- Bulfin, B.; Buttsworth, L.; Lidor, A.; Steinfeld, A. High-Purity Nitrogen Production from Air by Pressure Swing Adsorption Combined with SrFeO3 Redox Chemical Looping. Chem. Eng. J. 2021, 421, 127734. [Google Scholar] [CrossRef]
- Chen, W.; Chen, C.; Bouwmeester, H.J.M.; Nijmeijer, A.; Winnubst, L. Oxygen-Selective Membranes Integrated with Oxy-Fuel Combustion. J. Membr. Sci. 2014, 463, 166–172. [Google Scholar] [CrossRef]
- Rachadel, P.L.; Motuzas, J.; Machado, R.A.F.; Hotza, D.; Diniz da Costa, J.C. Influence of Porous Structures on O2 Flux of BSCF Asymmetric Membranes. Sep. Purif. Technol. 2017, 175, 164–169. [Google Scholar] [CrossRef]
- Schiestel, T.; Kilgus, M.; Peter, S.; Caspary, K.J.; Wang, H.; Caro, J. Hollow Fibre Perovskite Membranes for Oxygen Separation. J. Membr. Sci. 2005, 258, 1–4. [Google Scholar] [CrossRef]
- Song, J.; Feng, B.; Chu, Y.; Tan, X.; Gao, J.; Han, N.; Liu, S. One-Step Thermal Processing to Prepare BaCo0.95-Bi0.05ZrO3−δ Membranes for Oxygen Separation. Ceram. Int. 2019, 45, 12579–12585. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Li, D.; Zeng, L.; He, Y.; Boubeche, M.; Luo, H. High Oxygen Permeation Flux of Cobalt-Free Cu-Based Ceramic Dual-Phase Membranes. J. Membr. Sci. 2021, 633, 119403. [Google Scholar] [CrossRef]
- He, G.; Baumann, S.; Liang, F.; Hartmann, H.; Jiang, H.; Meulenberg, W.A. Phase Stability and Oxygen Permeability of Fe-Based BaFe0.9Mg0.05X0.05O3 (X = Zr, Ce, Ca) Membranes for Air Separation. Sep. Purif. Technol. 2019, 220, 176–182. [Google Scholar] [CrossRef]
- Haworth, P.; Smart, S.; Glasscock, J.; Diniz da Costa, J.C. High Performance Yttrium-Doped BSCF Hollow Fibre Membranes. Sep. Purif. Technol. 2012, 94, 16–22. [Google Scholar] [CrossRef]
- Tan, X.; Wang, Z.; Meng, B.; Meng, X.; Li, K. Pilot-Scale Production of Oxygen from Air Using Perovskite Hollow Fibre Membranes. J. Membr. Sci. 2010, 352, 189–196. [Google Scholar] [CrossRef]
- Baumann, S.; Serra, J.M.; Lobera, M.P.; Escolástico, S.; Schulze-Küppers, F.; Meulenberg, W.A. Ultrahigh Oxygen Permeation Flux through Supported Ba0.5Sr0.5Co0.8Fe0.2O3−δ Membranes. J. Membr. Sci. 2011, 377, 198–205. [Google Scholar] [CrossRef]
- Leo, A.; Motuzas, J.; Yacou, C.; Liu, S.; Serra, J.M.; Navarrete, L.; Drennan, J.; Julbe, A.; Diniz da Costa, J.C. Copper Oxide—Perovskite Mixed Matrix Membranes Delivering Very High Oxygen Fluxes. J. Membr. Sci. 2017, 526, 323–333. [Google Scholar] [CrossRef]
- Zeng, P.; Shao, Z.; Liu, S.; Xu, Z.P. Influence of M Cations on Structural, Thermal and Electrical Properties of New Oxygen Selective Membranes Based on SrCo0.95M0.05O3−δ Perovskite. Sep. Purif. Technol. 2009, 67, 304–311. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, Z.; Zhou, W.; Gu, H.; Shao, Z.; Liu, S. Re-Evaluation of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Perovskite as Oxygen Semi-Permeable Membrane. J. Membr. Sci. 2007, 291, 148–156. [Google Scholar] [CrossRef]
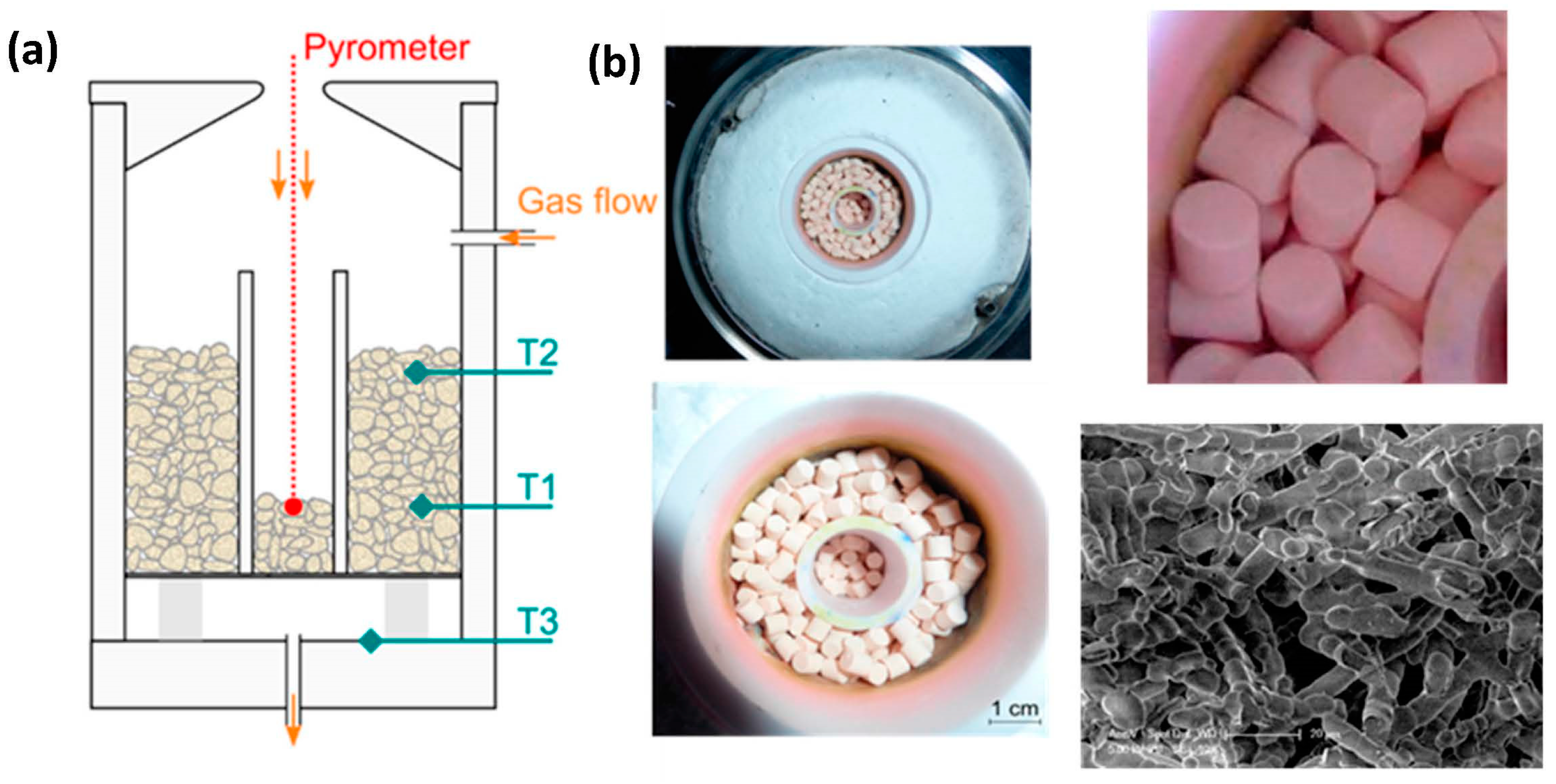
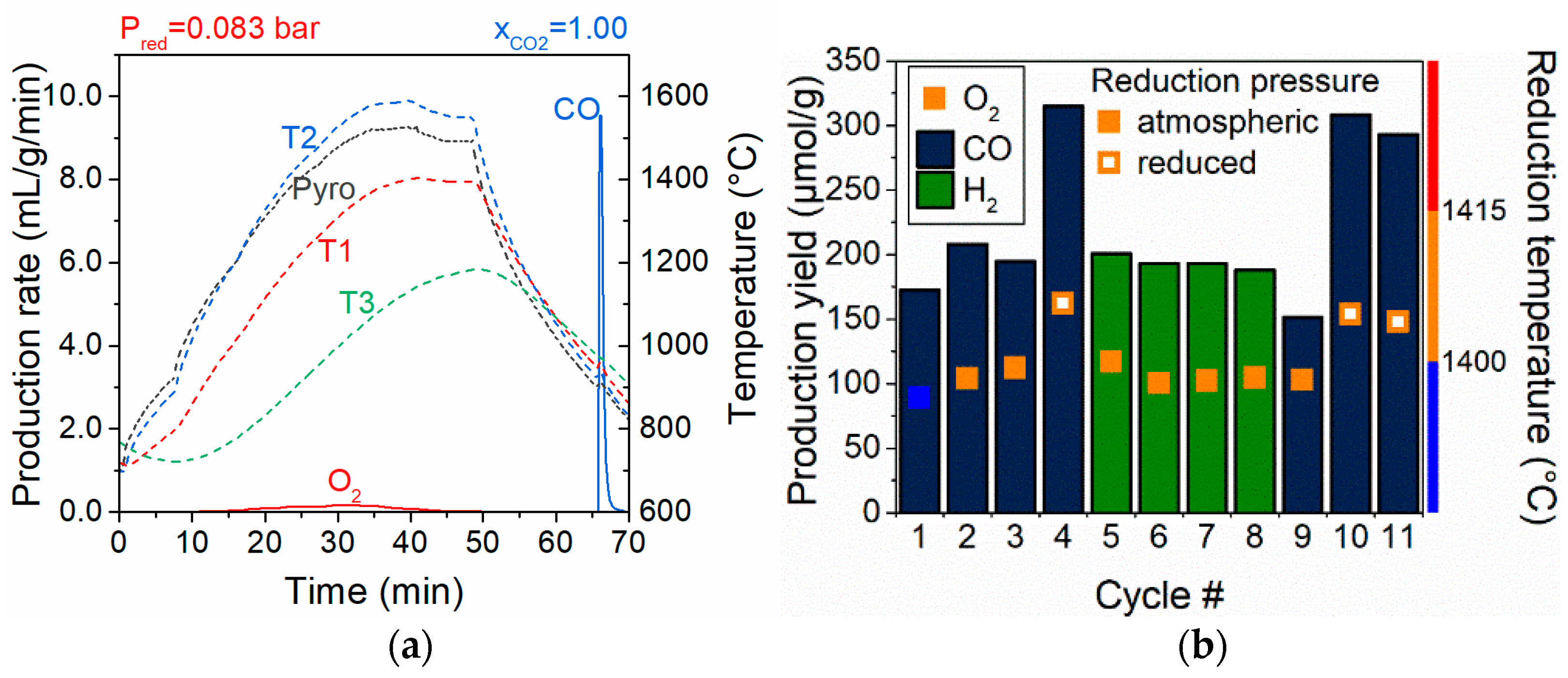
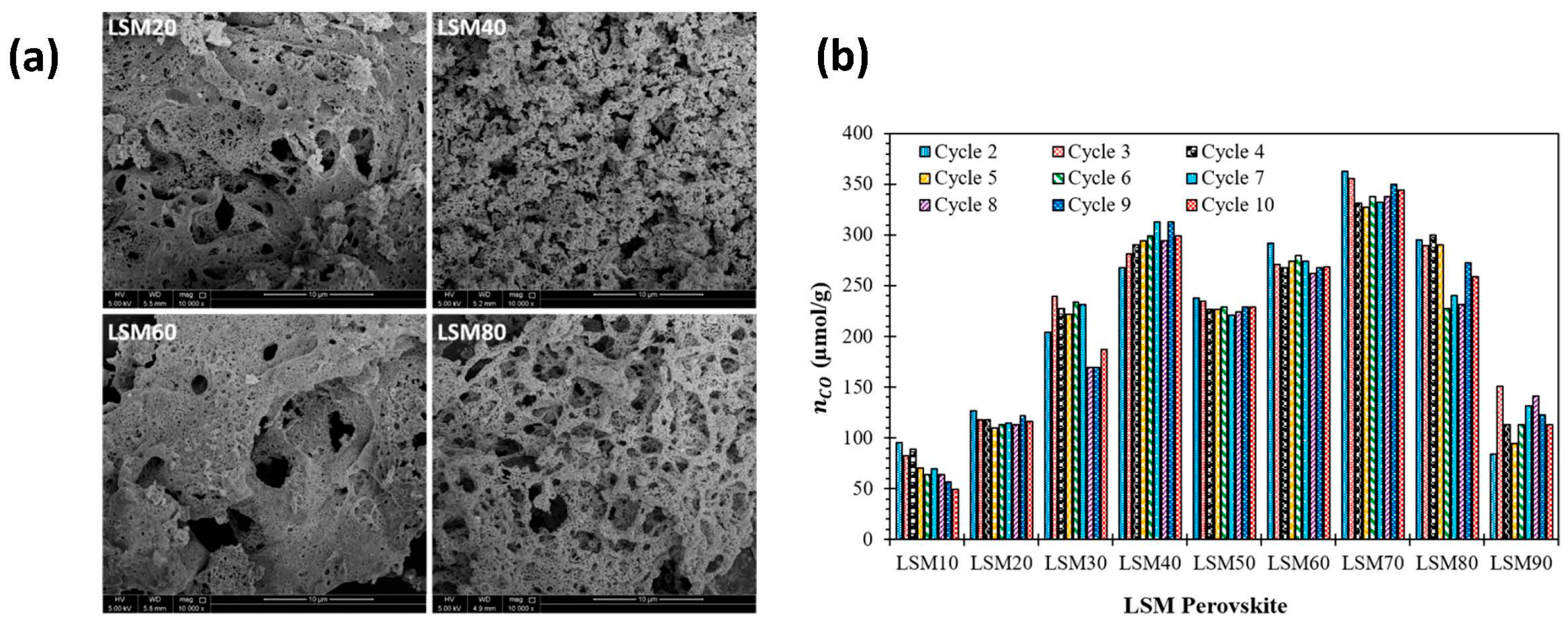

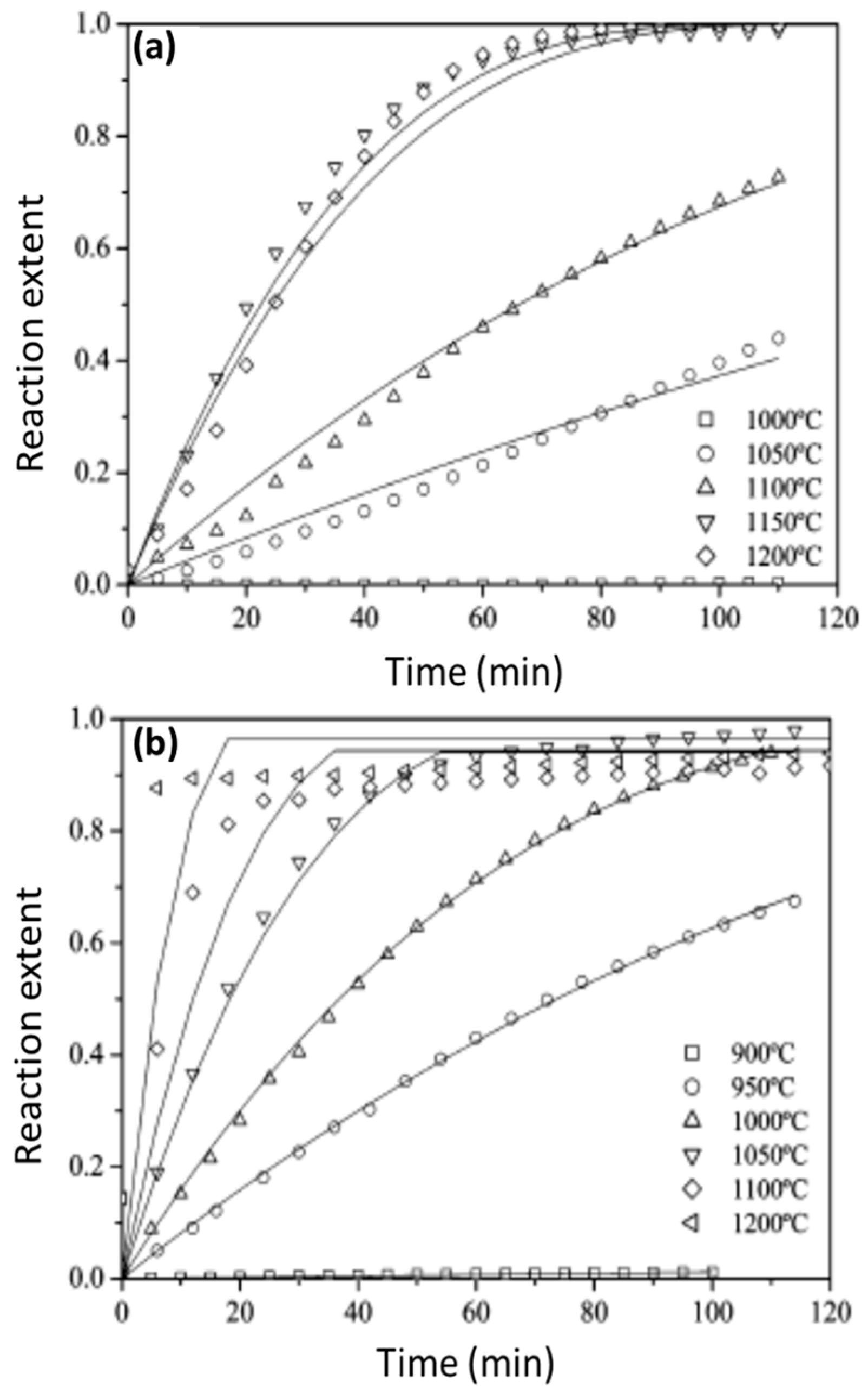
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abanades, S. A Review of Oxygen Carrier Materials and Related Thermochemical Redox Processes for Concentrating Solar Thermal Applications. Materials 2023, 16, 3582. https://doi.org/10.3390/ma16093582
Abanades S. A Review of Oxygen Carrier Materials and Related Thermochemical Redox Processes for Concentrating Solar Thermal Applications. Materials. 2023; 16(9):3582. https://doi.org/10.3390/ma16093582
Chicago/Turabian StyleAbanades, Stéphane. 2023. "A Review of Oxygen Carrier Materials and Related Thermochemical Redox Processes for Concentrating Solar Thermal Applications" Materials 16, no. 9: 3582. https://doi.org/10.3390/ma16093582
APA StyleAbanades, S. (2023). A Review of Oxygen Carrier Materials and Related Thermochemical Redox Processes for Concentrating Solar Thermal Applications. Materials, 16(9), 3582. https://doi.org/10.3390/ma16093582





