Determination of the Effect of Wastewater on the Biological Activity of Mixtures of Fluoxetine and Its Metabolite Norfluoxetine with Nalidixic and Caffeic Acids with Use of E. coli Microbial Bioindicator Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Preparation
2.2. E. coli Strains Used in the Study
2.3. Wastewater Preparation
2.4. The Inhibition of E. coli Cell Viability Test in MM, RW and TW
2.5. Toxicity and Protein Damage Effect Determination in MM, RW and TW
2.6. Oxidative Stress Assay in MM, RW and TW
2.6.1. SodA Promoter Induction in MM, RW and TW
2.6.2. ROS Synthesis in MM, RW and TW
2.7. Statistical Analysis
3. Results
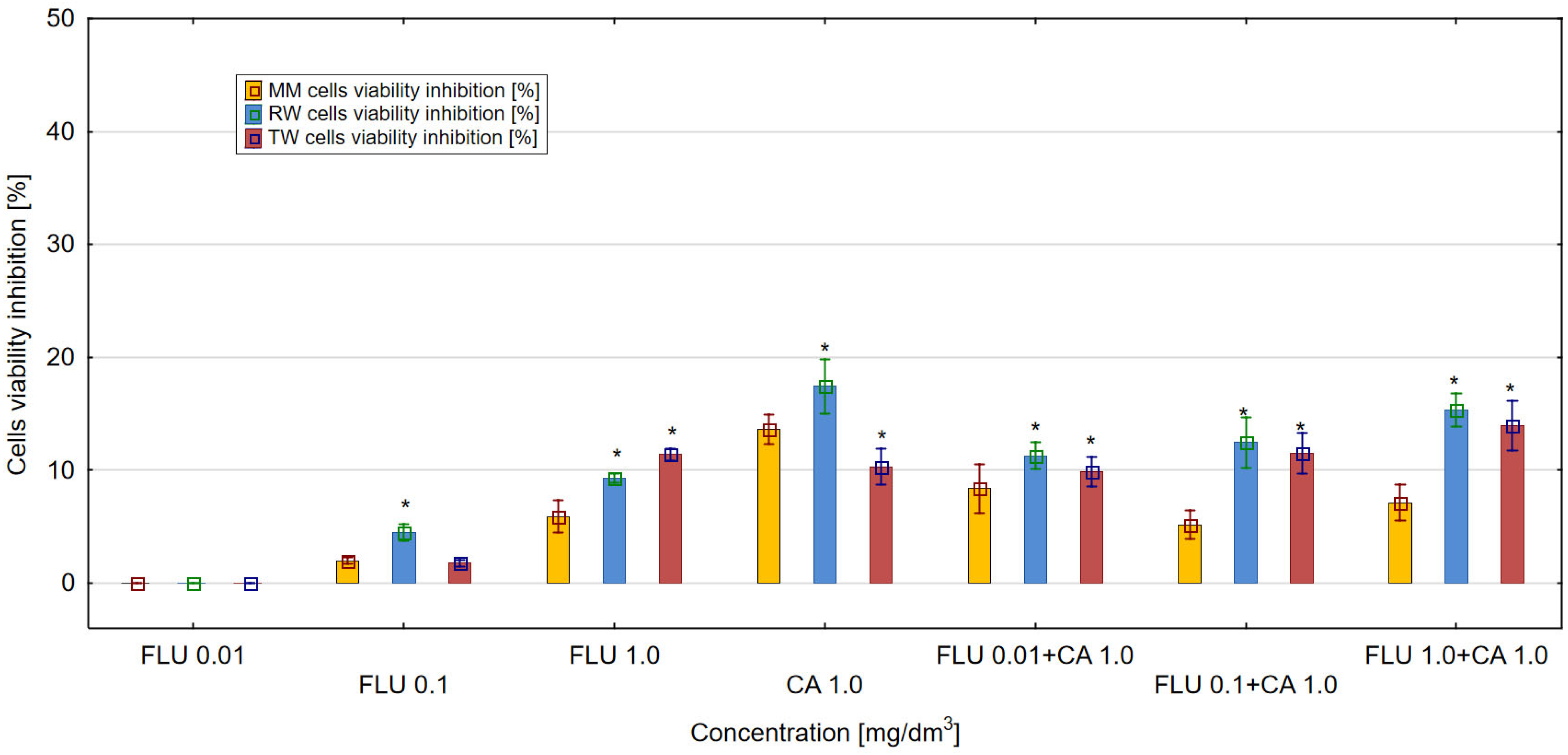
3.1. The Inhibition of E. coli Cell Viability in MM, RW and TW
3.2. Toxicity and Protein Damage Effect Determination in MM, RW and TW
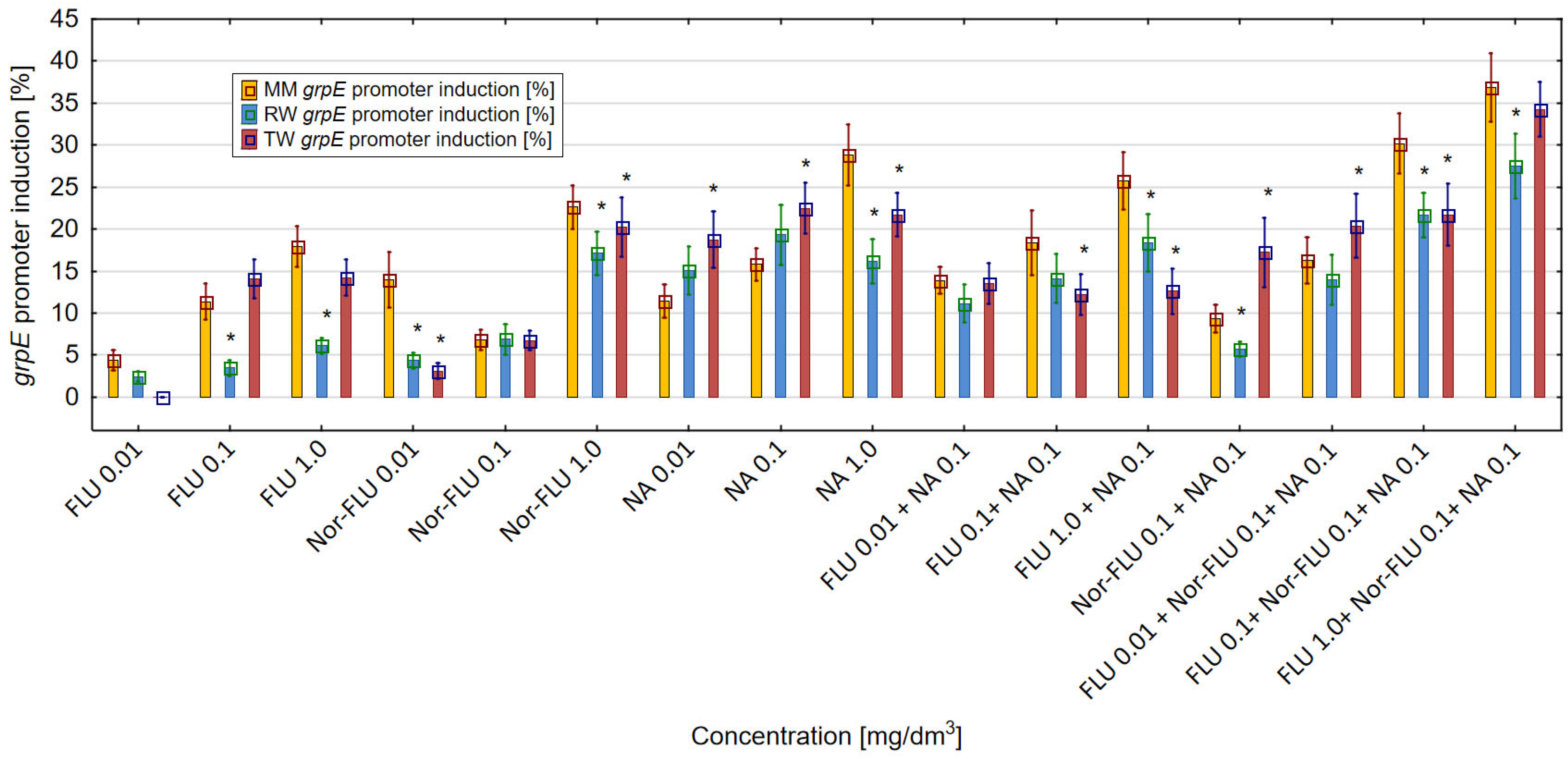
3.3. Oxidative Stress Assay in MM, RW and TW
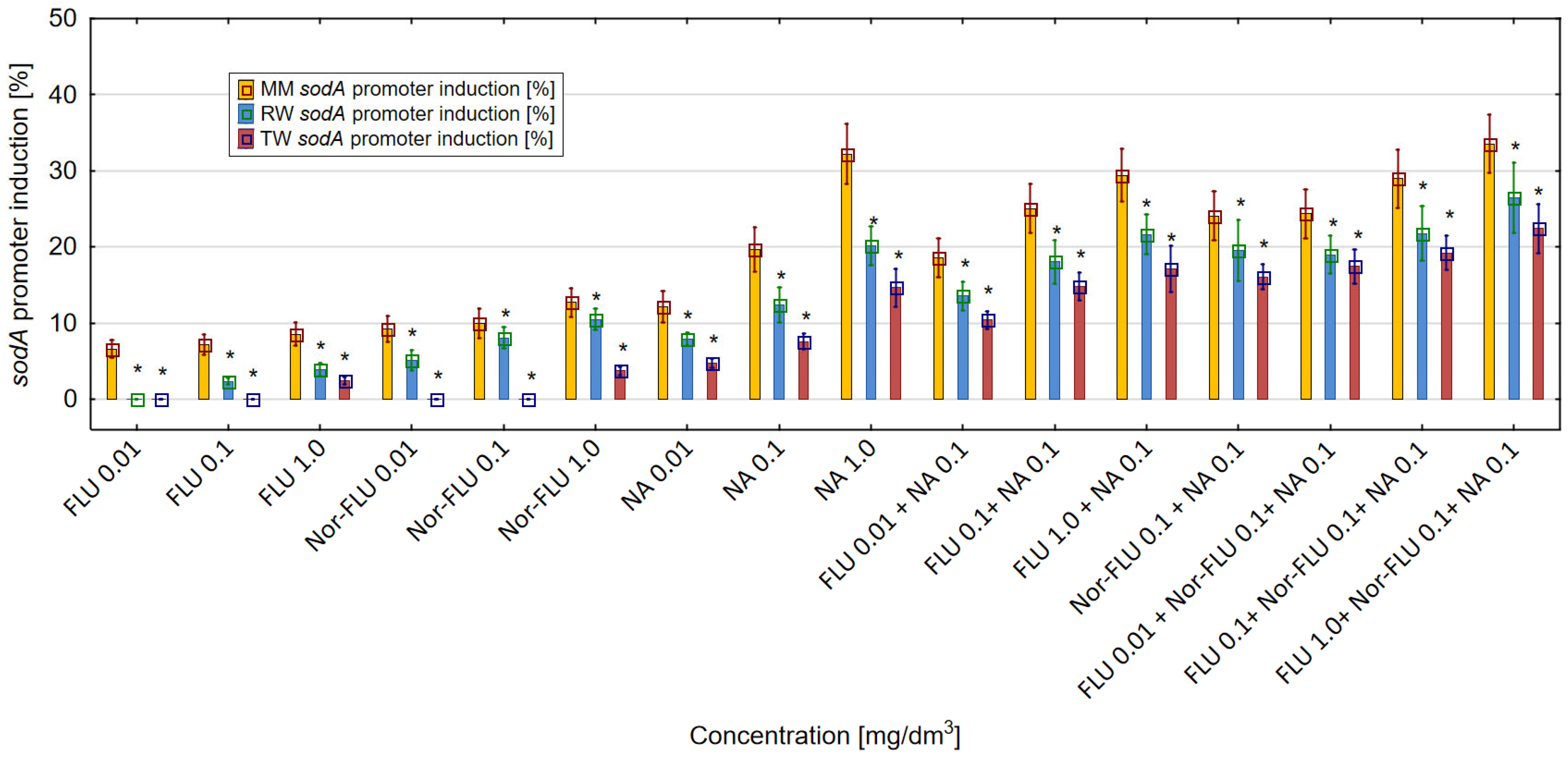
SodA Promoter Induction in MM, RW and TW
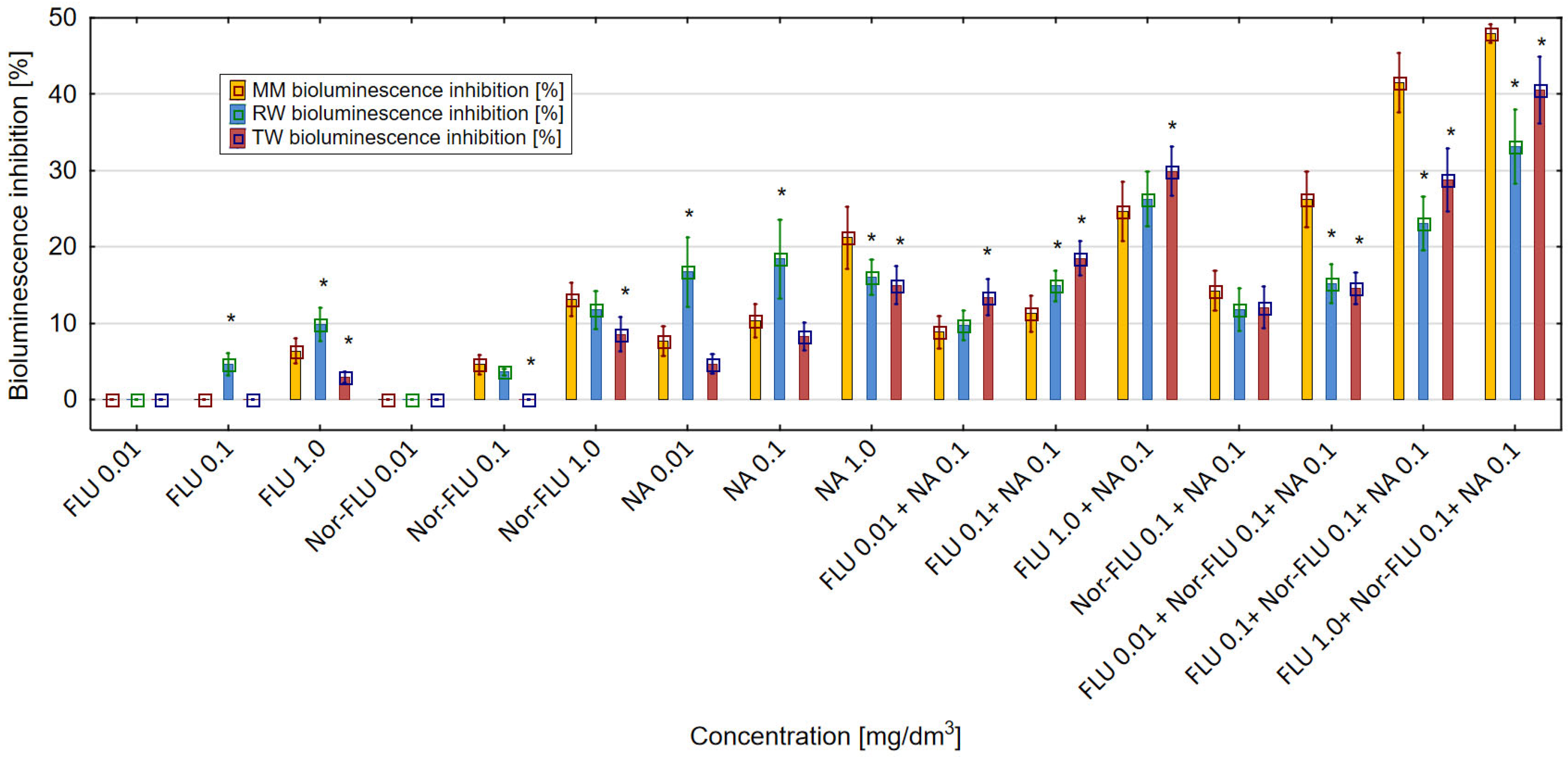
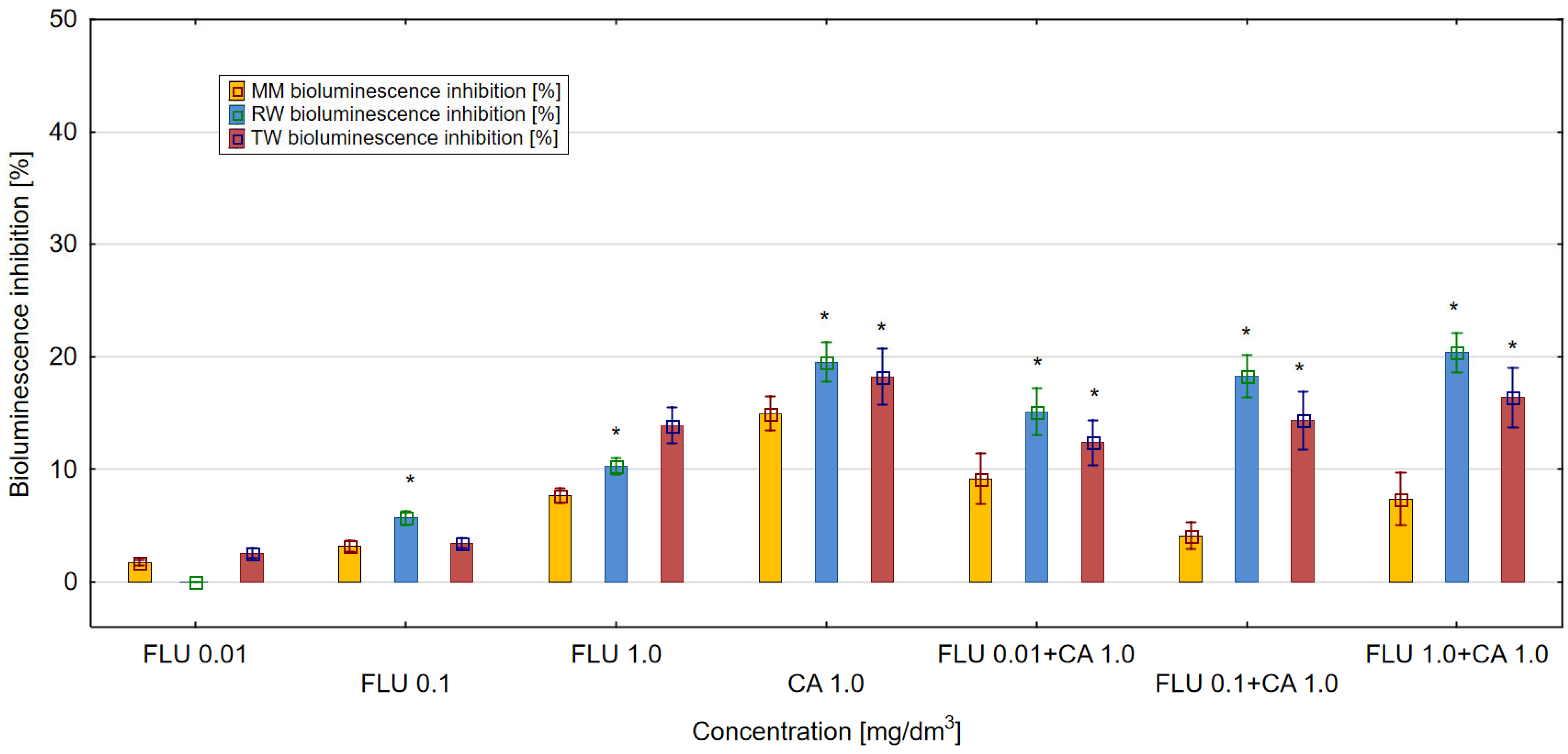
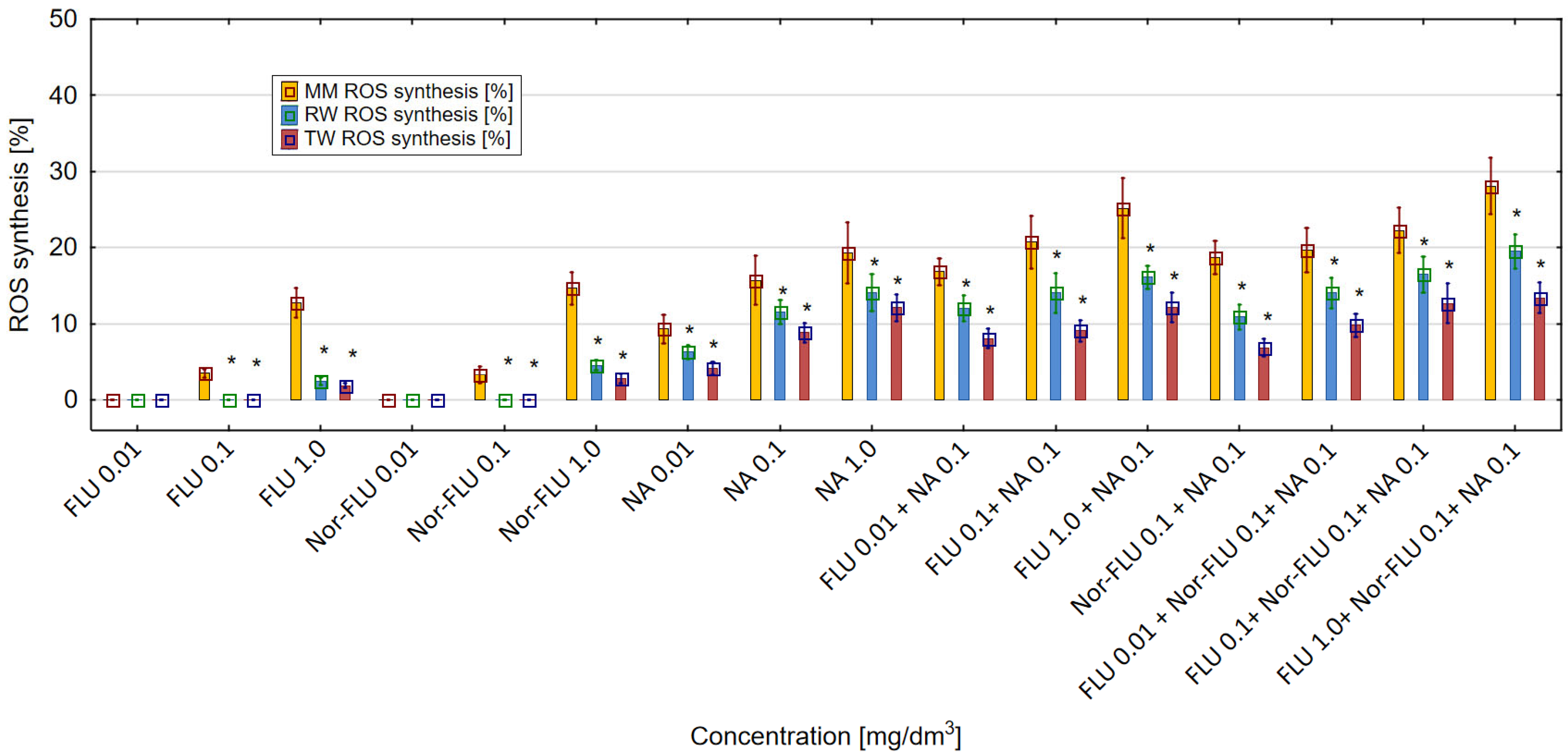
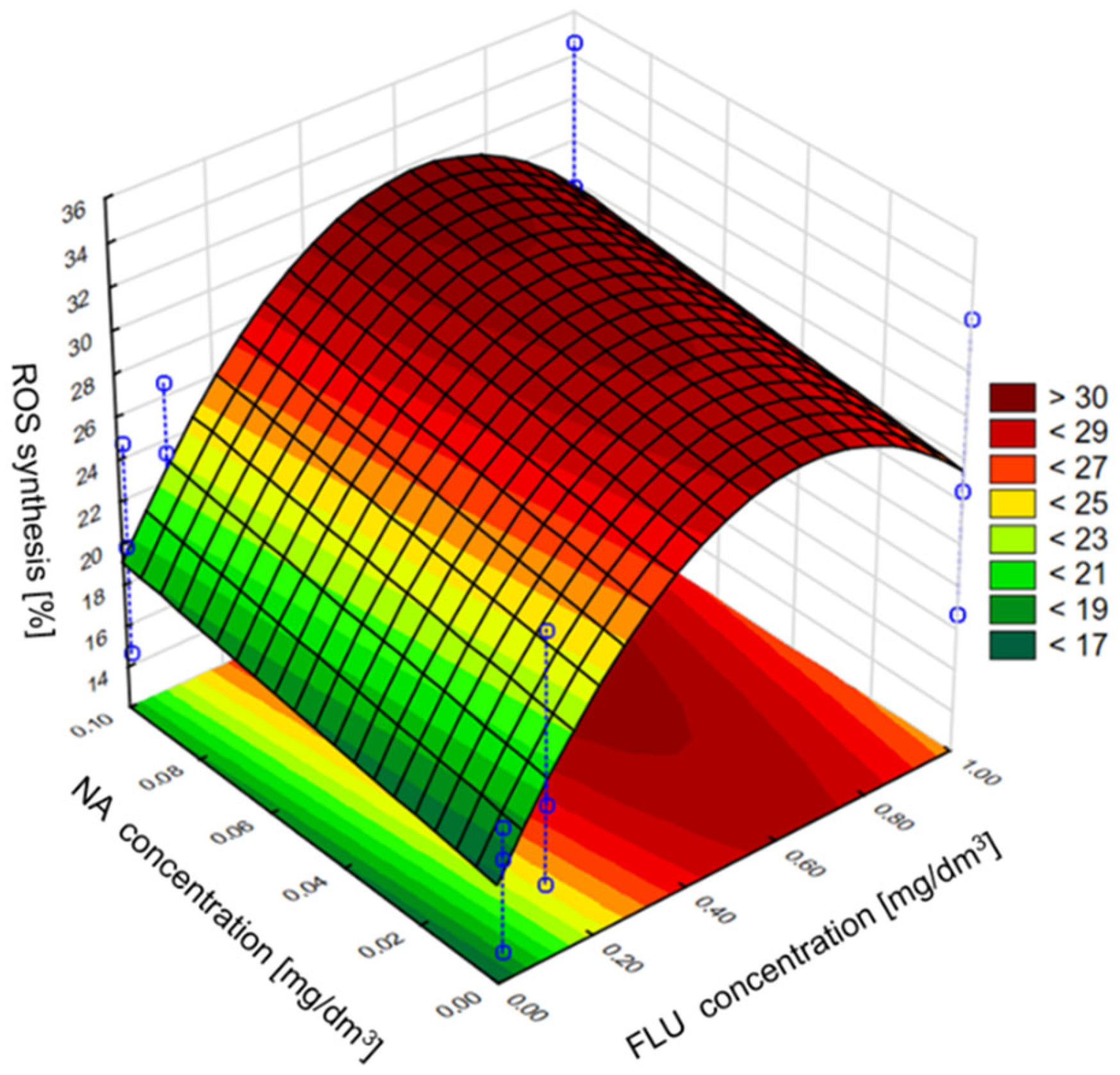
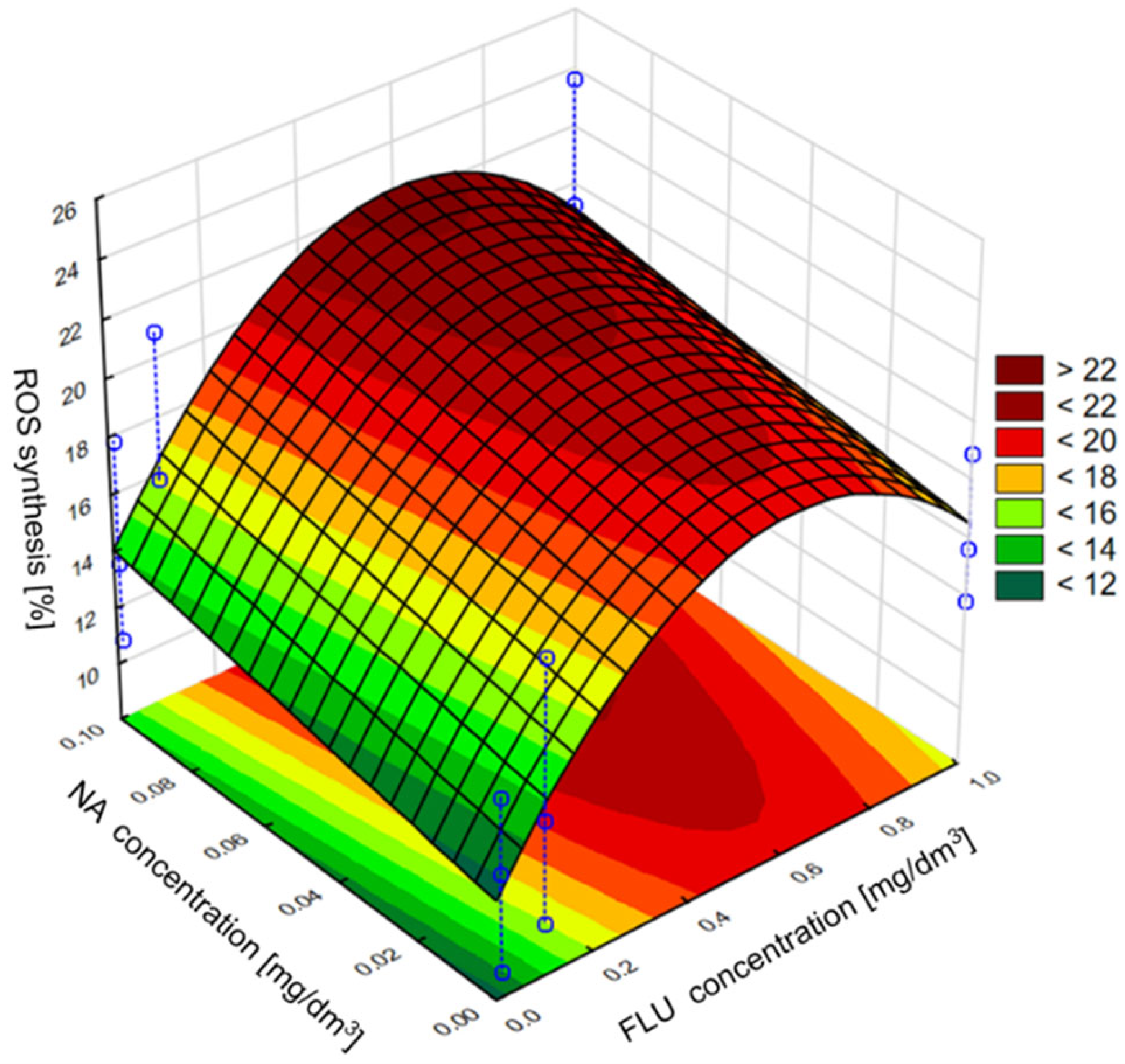
3.4. ROS Generation in MM, RW and TW
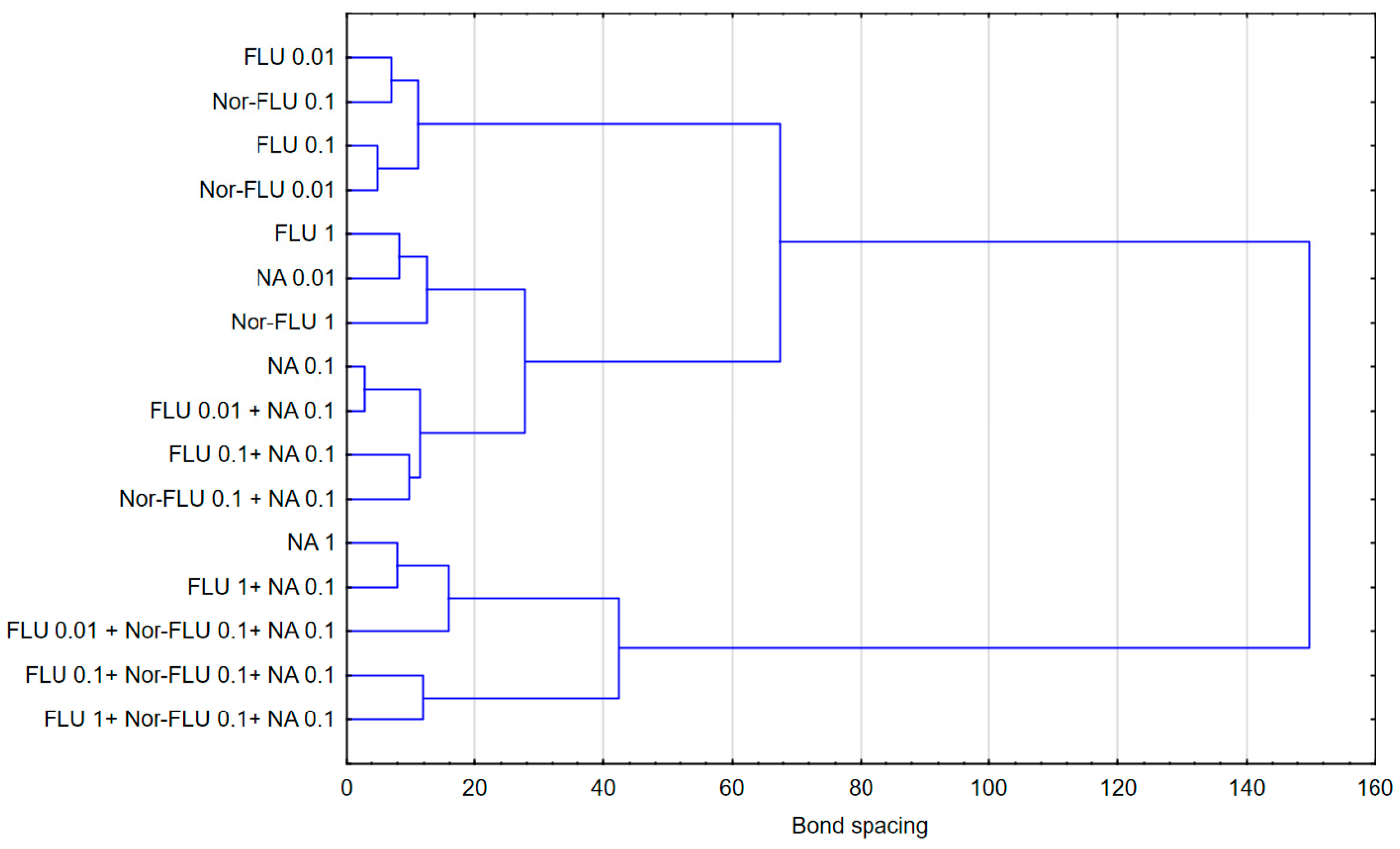
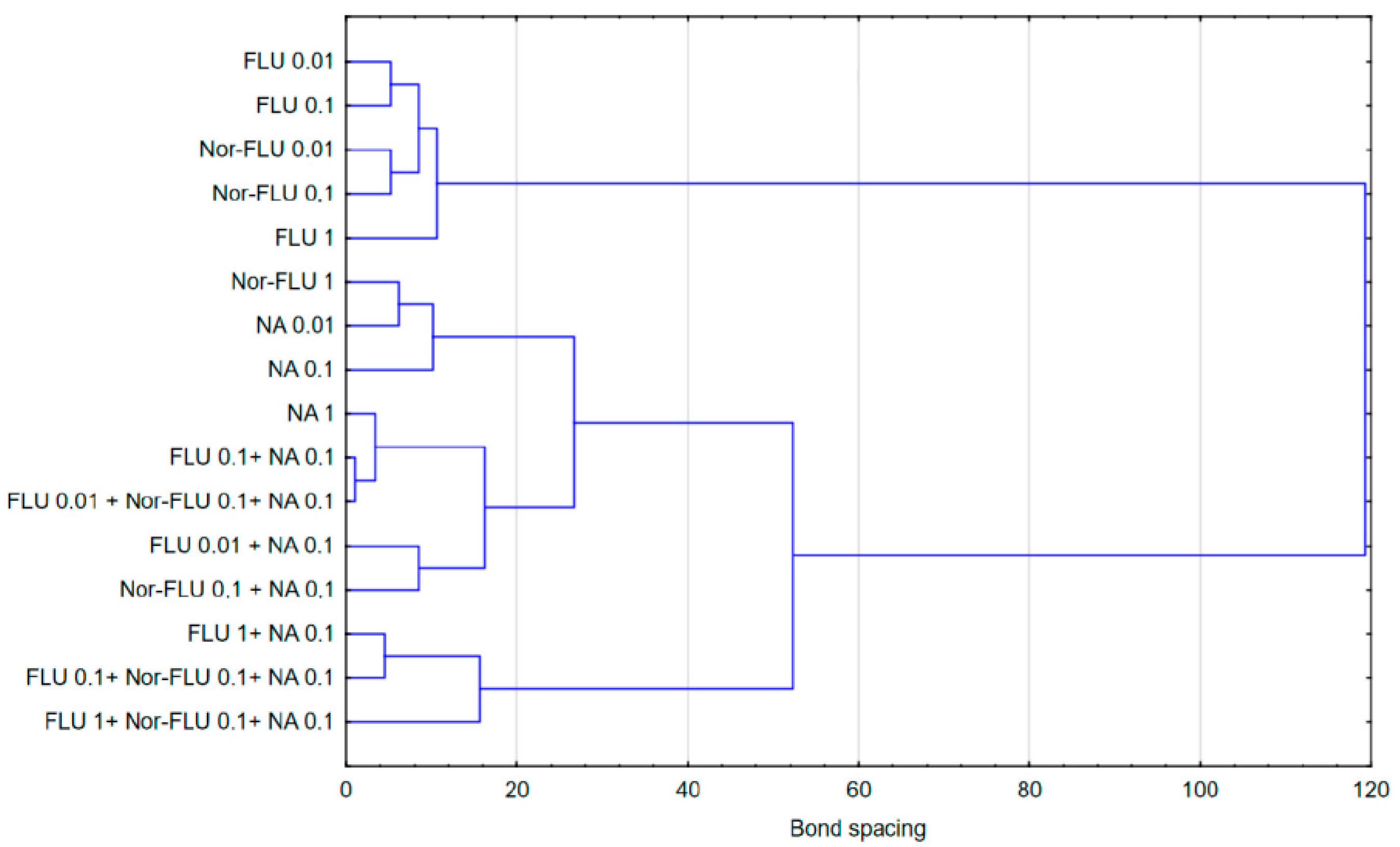
3.5. Statistical Analysis
4. Discussion
5. Conclusions
- (1)
- FLU, Nor-FLU, NA, CA and their mixtures in MM, RW and TW and with varying intensities affect the expression level of the luxCDABE reporter gene in E. coli strains.
- (2)
- Wastewater significantly influenced the toxicity and oxidative stress generation in E. coli cultures exposed to the tested chemical compounds and their mixtures.
- (3)
- The metabolite Nor-FLU showed higher toxicity compared to the parent drug FLU and a slightly higher oxidative stress synthesis.
- (4)
- Regardless of the tested environment, NA showed higher toxicity and ROS synthesis as compared to FLU and Nor-FLU.
- (5)
- Regardless of the tested environment, CA showed higher toxicity compared to FLU.
- (6)
- Among the tested mixtures, the highest toxicity and level of oxidative stress generation were characterized by those containing FLU at a concentration of 1 mg/dm3. The most toxic mixture was FLU (1 mg/dm3) with Nor-FLU (0.1 mg/dm3) and NA (0.1 mg/dm3).
- (7)
- An assessment of the interactions between the components of the mixtures in wastewater revealed synergism, potentiation and antagonism.
- (8)
- The E. coli microbial biosensors with grpE, lac and sodA promoters and the luxCDABE reporter gene used in the research are useful tools in studies on the toxicity and oxidative stress generated by the analyzed chemical compounds and their mixtures in wastewater.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orozco-Hernández, J.M.; Gómez-Oliván, L.M.; Elizalde-Velázquez, G.A.; Heredia-García, G.; Cardoso-Vera, J.D.; Dublán-García, O.; Islas-Flores, H.; SanJuan-Reyes, N.; Galar-Martínez, M. Effects of oxidative stress induced by environmental relevant concentrations of fluoxetine on the embryonic development on Danio rerio. Sci. Total Environ. 2022, 807, 151048. [Google Scholar] [CrossRef] [PubMed]
- Rabeea, S.A.; Merchant, H.A.; Khan, M.U.; Kow, C.S.; Hasan, S.S. Surging trends in prescriptions and costs of antidepressants in England amid COVID-19. DARU J. Pharm. Sci. 2021, 29, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Camal, N.; Cardoso-Vera, J.D.; Islas-Flores, H.; Gómez-Oliván, L.M.; Mejía-García, A. Consumption and ocurrence of antidepressants (SSRIs) in pre- and post-COVID-19 pandemic, their environmental impact and innovative removal methods: A review. Sci. Total Environ. 2022, 829, 154656. [Google Scholar] [CrossRef]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Sagahón-Azúa, J.; Medellín-Garibay, S.E.; Chávez-Castillo, C.E.; González-Salinas, C.G.; Milán-Segovia, R.D.C.; Romano-Moreno, S. Factors associated with fluoxetine and norfluoxetine plasma concentrations and clinical response in Mexican patients with mental disorders. Pharmacol. Res. Perspect. 2021, 9, e00864. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Selective serotonin re-uptake inhibitors (SSRIs) in the aquatic environment: An ecopharmacovigilance approach. Sci. Total Environ. 2012, 437, 185–195. [Google Scholar] [CrossRef]
- Puckowski, A.; Mioduszewska, K.; Łukaszewicz, P.; Borecka, M.; Caban, M.; Maszkowska, J.; Stepnowski, P. Bioaccumulation and analytics of pharmaceutical residues in the environment: A review. J. Pharm. Biomed. Anal. 2016, 127, 232–255. [Google Scholar] [CrossRef]
- De Sousa, A.; Rocha, J.E.; de Souza, T.; de Freitas, T.; Ribeiro-Filho, J.; Coutinho, H.D. New roles of fluoxetine in pharmacology: Antibacterial effect and modulation of antibiotic activity. Microb. Pathog. 2018, 123, 368–371. [Google Scholar] [CrossRef]
- Bossus, M.C.; Guler, Y.Z.; Short, S.J.; Morrison, E.R.; Ford, A.T. Behavioural and transcriptional changes in the amphipod Echinogammarus marinus exposed to two antidepressants, fluoxetine and sertraline. Aquat. Toxicol. 2014, 151, 46–56. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Pereira, A.M.P.T.; Meisel, L.M.; Lino, C.M.; Pena, A. Reviewing the serotonin reuptake inhibitors (SSRIs) footprint in the aquatic biota: Uptake, bioaccumulation and ecotoxicology. Environ. Pollut. 2015, 197, 127–143. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Topp, E.; Grenni, P. Pharmaceuticals in the environment: Biodegradation and effects on natural microbial communities. A review. J. Pharm. Biomed. Anal. 2015, 106, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ad, I.; Zolokov, A.; Lomnitski, L.; Taler, M.; Bar, M.; Luria, D.; Ram, E.; Weizman, A. Evaluation of the potential anti-cancer activity of the antidepressant sertraline in human colon cancer cell lines and in colorectal cancer-xenografted mice. Int. J. Oncol. 2008, 33, 277–286. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G.; Wawryniuk, M.; Giebułtowicz, J.; Olkowski, A.; Drobniewska, A. Influence of Selected Antidepressants on the Ciliated Protozoan Spirostomum ambiguum: Toxicity, Bioaccumulation, and Biotransformation Products. Molecules 2020, 25, 1476. [Google Scholar] [CrossRef] [PubMed]
- Ergen, A.M.; Yalçın, S.S. Unexpected drug residuals in human milk in Ankara, capital of Turkey. BMC Pregnancy Childbirth 2019, 19, 348. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xi, X.; Xu, J.; Xie, R.; Jiang, J. Distribution patterns of antibiotic residues in an urban river catchment. Water Environ. J. 2019, 33, 31–39. [Google Scholar] [CrossRef]
- Patiño, Y.; Pilehvar, S.; Díaz, E.; Ordóñez, S.; De Wael, K. Electrochemical reduction of nalidixic acid at glassy carbon electrode modified with multi-walled carbon nanotubes. J. Hazard. Mater. 2017, 323, 621–631. [Google Scholar] [CrossRef]
- Gleckman, R.; Alvarez, S.; Joubert, D.W.; Matthews, S.J. Drug therapy reviews: Nalidixic acid. Am. J. Hosp. Pharm. 1979, 36, 1071–1076. [Google Scholar] [CrossRef]
- Jung, J.E.; Kim, H.S.; Lee, C.S.; Park, D.-H.; Kim, Y.-N.; Lee, M.-J.; Lee, J.W.; Park, J.-W.; Kim, M.-S.; Ye, S.K.; et al. Caffeic acid and its synthetic derivative CADPE suppress tumor angiogenesis by blocking STAT3-mediated VEGF expression in human renal carcinoma cells. Carcinogenesis 2007, 28, 1780–1787. [Google Scholar] [CrossRef]
- Yanez, E.; Santander, P.; Contreras, D.; Yanez, J.; Cornejo, L.; Mansilla, H.D. Homogeneous and heterogeneous degradation of caffeic acid using photocatalysis driven by UVA and solar light. J. Environ. Sci. Health Part A 2016, 51, 78–85. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, S.; Wang, L.; Zhang, N.; Shi, Y.; Zhou, H.; Liu, X.; Shao, L.; Liu, X.; Chen, J.; et al. Reproductive and developmental toxicity study of caffeic acid in mice. Food Chem. Toxicol. 2019, 123, 106–112. [Google Scholar] [CrossRef]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Sengar, A.; Vijayanandan, A. Effects of pharmaceuticals on membrane bioreactor: Review on membrane fouling mechanisms and fouling control strategies. Sci. Total Environ. 2022, 808, 152132. [Google Scholar] [CrossRef]
- Melamed, S.; Lalush, C.; Elad, T.; Yagur-Kroll, S.; Belkin, S.; Pedahzur, R. A bacterial reporter panel for the detection and classification of antibiotic substances. Microb. Biotechnol. 2012, 5, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.W.; Seo, H.B.; Belkin, S.; Gu, M.B. An optical detection module-based biosensor using fortified bacterial beads for soil toxicity assessment. Anal. Bioanal. Chem. 2020, 412, 3373–3381. [Google Scholar] [CrossRef] [PubMed]
- Matejczyk, M.; Ofman, P.; Dąbrowska, K.; Świsłocka, R.; Lewandowski, W. The study of biological activity of transformation products of diclofenac and its interaction with chlorogenic acid. J. Environ. Sci. 2020, 91, 128–141. [Google Scholar] [CrossRef]
- Matejczyk, M.; Ofman, P.; Dąbrowska, K.; Świsłocka, R.; Lewandowski, W. Evaluation of the biological impact of the mixtures of diclofenac with its biodegradation metabolites 4′-hydroxydiclofenac and 5-hydroxydiclofenacon Escherichia coli. DCF synergistic effect with caffeic acid. Arch. Environ. Prot. 2020, 46, 10–22. [Google Scholar] [CrossRef]
- Matejczyk, M.; Ofman, P.; Dąbrowska, K.; Świsłocka, R.; Lewandowski, W. Synergistic interaction of diclofenac and its metabolites with selected antibiotics and amygdalin in wastewaters. Environ. Res. 2020, 186, 109511. [Google Scholar] [CrossRef]
- Lee, S.; Amasia, M.; Madou, M.; Mitchell, R.J. Serum complement enhances the responses of genotoxin- and oxidative stress-sensitive Escherichia coli bioreporters. Biosens. Bioelectron. 2013, 46, 175–182. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Kessler, N.; Schauer, J.J.; Yagur-Kroll, S.; Melamed, S.; Tirosh, O.; Belkin, S.; Erel, Y. A bacterial bioreporter panel to assay the cytotoxicity of atmospheric particulate matter. Atmos. Environ. 2012, 63, 94–101. [Google Scholar] [CrossRef]
- Islas-Flores, H.; Manuel Gómez-Oliván, L.; Galar-Martínez, M.; Michelle Sánchez-Ocampo, E.; SanJuan-Reyes, N.; Ortíz-Reynoso, M.; Dublán-García, O. Cyto-genotoxicity and oxidative stress in common carp (Cyprinus carpio) exposed to a mixture of ibuprofen and diclofenac. Environ. Toxicol. 2017, 32, 1637–1650. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.S.; Cheow, Y.L.; Lee, S.M. The role of reactive oxygen species in the antimicrobial activity of pyochelin. J. Adv. Res. 2017, 8, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, D.; Ardiles, P.R.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.L.; Gómez-Ruiz, S. Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics 2019, 11, 30. [Google Scholar] [CrossRef]
- Bohnert, J.A.; Szymaniak-Vits, M.; Schuster, S.; Kern, W.V. Efflux inhibition by selective serotonin reuptake inhibitors in Escherichia coli. J. Antimicrob. Chemother. 2011, 66, 2057–2060. [Google Scholar] [CrossRef]
- Ayaz, M.; Subhan, F.; Ahmed, J.; Khan, A.-U.; Ullah, F.; Ullah, I.; Ali, G.; Syed, N.-I.-H.; Hussain, S. Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J. Biol. Res.-Thessalon. 2015, 22, 4. [Google Scholar] [CrossRef]
- Ayaz, M.; Subhan, F.; Ahmed, J.; Khan, A.; Ullah, F.; Sadiq, A.; Syed, N.-I.-H.; Ullah, I.; Hussain, S. Citalopram and venlafaxine differentially augments antimicrobial properties of antibiotics. Acta Pol. Pharm. 2015, 72, 1269–1278. [Google Scholar]
- Foletto, V.S.; Serafin, M.B.; Bottega, A.; da Rosa, T.F.; Machado, C.D.S.; Coelho, S.S.; Hörner, R. Repositioning of fluoxetine and paroxetine: Study of potential antibacterial activity and its combination with ciprofloxacin. Med. Chem. Res. 2020, 29, 556–563. [Google Scholar] [CrossRef]
- Khalil, O.M.; Gedawy, E.M.; El-Malah, A.A.; Adly, M.E. Novel nalidixic acid derivatives targeting topoisomerase II enzyme; Design, synthesis, anticancer activity and effect on cell cycle profile. Bioorganic Chem. 2019, 83, 262–276. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Alamo, C. Active metabolites as antidepressant drugs: The role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front. Psychiatry 2013, 4, 102. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Nava-Álvarez, R.; Razo-Estrada, A.C.; García-Medina, S.; Gómez-Olivan, L.M.; Galar-Martínez, M. Oxidative Stress Induced by Mixture of Diclofenac and Acetaminophen on Common Carp (Cyprinus carpio). Water Air Soil Pollut. 2014, 225, 1873. [Google Scholar] [CrossRef]
- Jajoo, A.; Donlon, C.; Shnayder, S.; Levin, M.; McVey, M. Sertraline induces DNA damage and cellular toxicity in Drosophila that can be ameliorated by antioxidants. Sci. Rep. 2020, 10, 4512. [Google Scholar] [CrossRef] [PubMed]
- Hazelton, P.D.; Cope, W.G.; Mosher, S.; Pandolfo, T.J.; Belden, J.B.; Barnhart, M.C.; Bringolf, R.B. Fluoxetine alters adult freshwater mussel behavior and larval metamorphosis. Sci. Total Environ. 2013, 445–446, 94–100. [Google Scholar] [CrossRef]
- Hazelton, P.D.; Du, B.; Haddad, S.P.; Fritts, A.K.; Chambliss, C.K.; Brooks, B.W.; Bringolf, R.B. Chronic fluoxetine exposure alters movement and burrowing in adult freshwater mussels. Aquat. Toxicol. 2014, 151, 27–35. [Google Scholar] [CrossRef]
- Mascolo, G.; Balest, L.; Cassano, D.; Laera, G.; Lopez, A.; Pollice, A.; Salerno, C. Biodegradability of pharmaceutical industrial wastewater and formation of recalcitrant organic compounds during aerobic biological treatment. Bioresour. Technol. 2010, 101, 2585–2591. [Google Scholar] [CrossRef]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Molina, R.; Martinez, F.; Melero, J.A.; Stathopoulou, P.M.; Tsiamis, G. Toxicity assessment of pharmaceutical compounds on mixed culture from activated sludge using respirometric technique: The role of microbial community structure. Sci. Total Environ. 2018, 630, 809–819. [Google Scholar] [CrossRef]
| Strain | Gene Promoter Acting as Sensing Element | Reporter Gene | Type of Stress Sensed | Reference |
|---|---|---|---|---|
| E. coli RFM443 | GrpE | Lux | Protein damage and general toxicity | [25] |
| E. coli RFM443 | Lac | Lux | General toxicity | [25] |
| E. coli SM345 | SodA | Lux | Oxidative stress | [24,26,27,28] |
| Parameter | Raw Wastewater | Treated Wastewater |
|---|---|---|
| BOD | 250 ± 100 | 3 ± 2 |
| COD | 450 ± 120 | 30 ± 10 |
| TSS | 250 ± 50 | 4 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matejczyk, M.; Ofman, P.; Wiater, J.; Świsłocka, R.; Kondzior, P.; Lewandowski, W. Determination of the Effect of Wastewater on the Biological Activity of Mixtures of Fluoxetine and Its Metabolite Norfluoxetine with Nalidixic and Caffeic Acids with Use of E. coli Microbial Bioindicator Strains. Materials 2023, 16, 3600. https://doi.org/10.3390/ma16093600
Matejczyk M, Ofman P, Wiater J, Świsłocka R, Kondzior P, Lewandowski W. Determination of the Effect of Wastewater on the Biological Activity of Mixtures of Fluoxetine and Its Metabolite Norfluoxetine with Nalidixic and Caffeic Acids with Use of E. coli Microbial Bioindicator Strains. Materials. 2023; 16(9):3600. https://doi.org/10.3390/ma16093600
Chicago/Turabian StyleMatejczyk, Marzena, Piotr Ofman, Józefa Wiater, Renata Świsłocka, Paweł Kondzior, and Włodzimierz Lewandowski. 2023. "Determination of the Effect of Wastewater on the Biological Activity of Mixtures of Fluoxetine and Its Metabolite Norfluoxetine with Nalidixic and Caffeic Acids with Use of E. coli Microbial Bioindicator Strains" Materials 16, no. 9: 3600. https://doi.org/10.3390/ma16093600
APA StyleMatejczyk, M., Ofman, P., Wiater, J., Świsłocka, R., Kondzior, P., & Lewandowski, W. (2023). Determination of the Effect of Wastewater on the Biological Activity of Mixtures of Fluoxetine and Its Metabolite Norfluoxetine with Nalidixic and Caffeic Acids with Use of E. coli Microbial Bioindicator Strains. Materials, 16(9), 3600. https://doi.org/10.3390/ma16093600







