Alkali–Silica Reactions: Literature Review on the Influence of Moisture and Temperature and the Knowledge Gap
Abstract
:1. Introduction
1.1. Background
1.2. The Mechanism of the ASR
2. Review Objectives
3. Moisture in Concrete
4. The Role of Moisture in the Alkali–Silica Reaction
4.1. Relative Humidity Threshold
4.2. The Role of Alternate Drying and Wetting on the Availability of Moisture for the Alkali–Silica Reaction
5. The Role of Temperature in the Kinetics of the ASR
6. The Influence of Moisture and Temperature on the Microstructure Properties of ASR-Affected Concrete
7. Conclusions
- Moisture is an important factor for the reaction and an ample amount is needed. The utilization of relative humidity, an indirect technique measuring the quantity of water vapour in the air, has been both commonly employed and scrutinized. However, it has been preferred as a means of moisture measurement in ASR-affected concrete due to the ease of measurement compared to other methods like the degree of capillary saturation.
- A moisture threshold of 80% RH has been generally agreed to be required to initiate and sustain the reaction. However, based on reviewed works in the literature, there exist studies that report thresholds lower and higher than this value. The threshold could be dependent on the reactivity of the aggregate, temperature, or other factors that differed among the existing studies.
- Concrete subjected to wet and dry cycles are prone to exhibiting moisture gradients, and their impact on ASR expansion may vary depending on the size of the concrete member, with slender members experiencing a quicker drop in RH across their depth. In managing the reaction, minimizing the number of wetting and drying cycles is critical. Furthermore, understanding the combined influence of moisture availability, cyclic conditions, and concrete properties is essential for developing effective strategies to mitigate the deleterious effects of the ASR.
- The influence of temperature on the ASR is multifaceted and extends beyond its direct inclusion among the primary factors (alkalis, silica, moisture) necessary for the initiation and continuation of the reaction. An increase in temperature has been identified as the most efficient factor for accelerating ASR-induced expansion in laboratory conditions. However, the ultimate expansion can be lower at higher temperatures. Efforts to address these complexities have not consistently proven to be effective. Hence, the relationship between temperature and ASR-induced expansion is complex and non-linear.
- The assessment of the ASR in concrete has evolved to include a detailed assessment of the influence of induced expansion on microstructural properties, recognizing that ASR induces microstructural degradation before visible signs appear at the macroscale. This shift in focus emphasizes the need to comprehend the influence of moisture and temperature in ASR-affected concrete from a microstructural standpoint rather than just the induced expansion. The availability of moisture and temperature has been reviewed to have a possible influence on the properties of cracks induced by the ASR.
8. Recommendations
- The ambiguity surrounding the behaviour of the ASR due to variations in constituents and exposure conditions results in uncertainties when comparing studies. These variations hinder the establishment of a universal RH threshold. Different thresholds have been reported by authors and it is likely that the threshold is influenced by factors such as temperature, aggregate reactivity, and the sample sizes used to determine reported thresholds. Further research is required to determine the most important influencing factor.
- Existing studies failed to consider the effect of initial moisture content/internal RH in determining the critical RH for the ASR. Considering this effect on the evolution of RH in affected concrete over time would further our understanding of the reaction.
- Temperature has been proven to enhance the rate of ASR-induced expansion; however, there could be a reduction in ultimate expansion due to factors like alkali leaching, the drying of gel, and improved pores. This can influence the assessment of the full reactivity potential of aggregates being assessed. Thus, more research is needed to develop fast and reliable tests that can withstand these challenges.
- Most microscopic assessments have been performed at high and constant moisture levels. However, such conditions are not frequent in practice. Understanding the role of low moisture levels and the impact of cyclic conditions on the microstructural properties of ASR-affected concrete is crucial.
- Moisture and temperature have been confirmed to exacerbate ASR in concrete. However, the damage responses and the reduction in mechanical properties over a large range of moisture and temperature conditions and how these responses change over time are unknown. Carrying out more research in this area would improve our understanding of the internal behaviour of concrete at different exposure conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajabipour, F.; Giannini, E.; Dunant, C.; Ideker, J.H.; Thomas, M.D.A. Alkali–Silica Reaction: Current Understanding of the Reaction Mechanisms and the Knowledge Gaps. Cem. Concr. Res. 2015, 76, 130–146. [Google Scholar] [CrossRef]
- Tragardh, J.; Lagerblad, B. Influence of ASR Expansion on the Frost Resistance of Concrete. In Proceedings of the 10th International Conference on Alkali Aggregate Reaction in Concrete, Melbourne, Australia, 18–23 August 1996; pp. 853–860. [Google Scholar]
- Deschenes, R.A.; Giannini, E.; Drimalas, T.; Fournier, B.; Hale, W.M. Effects of Moisture, Temperature, and Freezing and Thawing on Alkali-Silica Reaction. ACI Mater. J. 2018, 115, 575–584. [Google Scholar] [CrossRef]
- Boddy, A.M.; Hooton, R.D.; Thomas, M.D.A. The Effect of the Silica Content of Silica Fume on Its Ability to Control Alkali–Silica Reaction. Cem. Concr. Res. 2003, 33, 1263–1268. [Google Scholar] [CrossRef]
- Diamond, S. Effects of Two Danish Flyashes on Alkali Contents of Pore Solutions of Cement-Flyash Pastes. Cem. Concr. Res. 1981, 11, 383–394. [Google Scholar] [CrossRef]
- Duchesne, J.; Bérubé, M.A. The Effectiveness of Supplementary Cementing Materials in Suppressing Expansion Due to ASR: Another Look at the Reaction Mechanisms Part 2: Pore Solution Chemistry. Cem. Concr. Res. 1994, 24, 221–230. [Google Scholar] [CrossRef]
- Ramlochan, T.; Thomas, M.; Gruber, K.A. The Effect of Metakaolin on Alkali–Silica Reaction in Concrete. Cem. Concr. Res. 2000, 30, 339–344. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Raja, K.; Vishnuvardhan, K.; Suchithra, S.; Maniarasan, S.K.; Saravanan, M.M.; Miruna, M.; Prabanjan, S. The ASR Mechanism in Concrete and the Influence of Lithium in Mitigating It: A Critical Review. Mater. Today Proc. 2022, 65, A1–A6. [Google Scholar] [CrossRef]
- Kaladharan, G.; Szeles, T.; Stoffels, S.M.; Rajabipour, F. Novel Admixtures for Mitigation of Alkali-Silica Reaction in Concrete. Cem. Concr. Compos. 2021, 120, 104028. [Google Scholar] [CrossRef]
- Feng, X.; Thomas, M.D.A.; Bremner, T.W.; Balcom, B.J.; Folliard, K.J. Studies on Lithium Salts to Mitigate ASR-Induced Expansion in New Concrete: A Critical Review. Cem. Concr. Res. 2005, 35, 1789–1796. [Google Scholar] [CrossRef]
- Leemann, A.; Lörtscher, L.; Bernard, L.; Le Saout, G.; Lothenbach, B.; Espinosa-Marzal, R.M. Mitigation of ASR by the Use of LiNO3—Characterization of the Reaction Products. Cem. Concr. Res. 2014, 59, 73–86. [Google Scholar] [CrossRef]
- Diaz-Loya, I.; Juenger, M.; Seraj, S.; Minkara, R. Extending Supplementary Cementitious Material Resources: Reclaimed and Remediated Fly Ash and Natural Pozzolans. Cem. Concr. Compos. 2019, 101, 44–51. [Google Scholar] [CrossRef]
- Oey, T.; La Plante, E.C.; Falzone, G.; Hsiao, Y.-H.; Wada, A.; Monfardini, L.; Bauchy, M.; Bullard, J.W.; Sant, G. Calcium Nitrate: A Chemical Admixture to Inhibit Aggregate Dissolution and Mitigate Expansion Caused by Alkali-Silica Reaction. Cem. Concr. Compos. 2020, 110, 103592. [Google Scholar] [CrossRef]
- Xiao, R.; Huang, B.; Zhou, H.; Ma, Y.; Jiang, X. A State-of-the-Art Review of Crushed Urban Waste Glass Used in OPC and AAMs (Geopolymer): Progress and Challenges. Clean. Mater. 2022, 4, 100083. [Google Scholar] [CrossRef]
- Bérubé, M.-A.; Chouinard, D.; Pigeon, M.; Frenette, J.; Rivest, M.; Vézina, D. Effectiveness of Sealers in Counteracting Alkali–Silica Reaction in Highway Median Barriers Exposed to Wetting and Drying, Freezing and Thawing, and Deicing Salt. Can. J. Civ. Eng. 2002, 29, 329–337. [Google Scholar] [CrossRef]
- Lute, R.D.; Folliard, K.J.; Drimalas, T.; Rust, C.K. Coatings and Sealers for Mitigation of Alkali-Silica Reaction And/or Delayed Ettringite Formation. In Proceedings of the 15th International Conference on Alkali-Aggregate Reaction–ICAAR, Sao Paulo, Brazil, 3–7 July 2016; p. 10. [Google Scholar]
- Murray, C.D. Durability of Silane Sealer in a Highly Alkaline Environment. Bachelor’s Thesis, University of Arkansas, Fayetteville, NC, USA, 2014. [Google Scholar]
- Schindler, A.; Johnson, D.; Warnock, R.; Barnes, R. Effectiveness of Silane to Mitigate Alkali-Silica Reaction in a Historical Bridge. MATEC Web Conf. 2018, 199, 03009. [Google Scholar] [CrossRef]
- Thomas, M.; Folliard, K.; Fournier, B.; Rivard, P.; Drimalas, T. Methods for Evaluating and Treating ASR-Affected Structures: Results of Field Application and Demonstration Projects Volume I: Summary of Findings and Recommendations Final Report; FHWA Office of Pavement Technology: Washington, DC, USA, 2013.
- Ludwig, U. Effects of Environmental Conditions on Alkali-Aggregate Reaction. In Proceedings of the 8th International Conference on Alkali-Aggregate Reaction, Kyoto, Japan, 17–20 July 1989; pp. 583–596. [Google Scholar]
- Stark, D. The Moisture Condition of Field Concrete Exhibiting Alkali–Silica Reactivity. In Proceedings of the Second International Conference on Durability of Concrete, Montreal, QC, Canada, 4–9 August 1991; ACI Publication SP: Detroit, MI, USA, 1991; pp. 973–987. [Google Scholar]
- Poyet, S.; Sellier, A.; Capra, B.; Thèvenin-Foray, G.; Torrenti, J.-M.; Tournier-Cognon, H.; Bourdarot, E. Influence of Water on Alkali-Silica Reaction: Experimental Study and Numerical Simulations. J. Mater. Civ. Eng. 2006, 18, 588–596. [Google Scholar] [CrossRef]
- Pedneault, A. Development of Testing and Analytical Procedures for the Evaluation of the Residual Potential of Reaction, Expansion, and Deterioration of Concrete Affected by ASR. Master’s Thesis, Laval University, Quebec City, QC, Canada, 1996. [Google Scholar]
- Kurihara, T.; Katawaki, K. Effects of Moisture Control and Inhibition on Alkali Silica Reaction. In Proceedings of the 8th International Conference on Alkali-Aggregate Reaction, Kyoto, Japan, 17–20 July 1989; pp. 629–634. [Google Scholar]
- Tomosawa, F.; Tamura, K.; Abe, M. Influence of Water Content of Concrete on Alkali-Aggregate Reaction. In Proceedings of the 8th International Conference on Alkali-Aggregate Reaction, Kyoto, Japan, 17–20 July 1989; pp. 881–885. [Google Scholar]
- Stark, D.; Okamoto, P.; Diamond, S. Eliminating or Minimizing Alkali-Silica Reaktivity; Strategic Highway Research Program; SHRP-C: Washington, DC, USA, 1993; ISBN 978-0-309-05603-8. [Google Scholar]
- Sanchez, L.; Fournier, B.; Drimalas, T.; Bastien, J.; Mitchell, D.; Noel, M. Semi-Quantitative Condition Assessment of Concrete Distress through the Damage Rating Index. In Proceedings of the 15th International Conference on Alkali-Aggregate Reaction, Sao Paulo, Brazil, 3–7 July 2016; pp. 1–10. [Google Scholar]
- Sanchez, L.F.M.; Drimalas, T.; Fournier, B. Assessing Condition of Concrete Affected by Internal Swelling Reactions (ISR) through the Damage Rating Index (DRI). Cement 2020, 1–2, 100001. [Google Scholar] [CrossRef]
- Owsiak, Z.; Zapała-Sławeta, J.; Czapik, P. Diagnosis of Concrete Structures Distress Due to Alkali-Aggregate Reaction. Bull. Pol. Acad. Sci. Tech. Sci. 2015, 63, 23–29. [Google Scholar] [CrossRef]
- Juliana, M.M.d.F.; Vanessa Karla Barbosa, d.S.; Deborah Grasielly Cipriano, d.S.; Dione Luiza, d.S.; Eliana Cristina, B.M. Alkali-Aggregate Reaction: Definition, Influence and Control. Eng. Appl. Sci. 2018, 3, 12–20. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Effect of Particle Size on Alkali–Silica Reaction in Recycled Glass Mortars. Constr. Build. Mater. 2014, 66, 275–285. [Google Scholar] [CrossRef]
- Sanchez, L.; Fournier, B.; Jolin, M.; Duchesne, J. Reliable Quantification of AAR Damage through Assessment of the Damage Rating Index (DRI). Cem. Concr. Res. 2015, 67, 74–92. [Google Scholar] [CrossRef]
- Fernandes, I.; Broekmans, M.A.T.M. Alkali–Silica Reactions: An Overview. Part I. Metallogr. Microstruct. Anal. 2013, 2, 257–267. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ghiasvand, E.; Nili, M. Relation between Mechanical Properties of Concrete and Alkali-Silica Reaction (ASR); A Review. Constr. Build. Mater. 2020, 258, 119567. [Google Scholar] [CrossRef]
- Hou, X.; Struble, L.J.; Kirkpatrick, R.J. Formation of ASR Gel and the Roles of C-S-H and Portlandite. Cem. Concr. Res. 2004, 34, 1683–1696. [Google Scholar] [CrossRef]
- Wang, H.; Gillott, J.E. Mechanism of Alkali-Silica Reaction and the Significance of Calcium Hydroxide. Cem. Concr. Res. 1991, 21, 647–654. [Google Scholar] [CrossRef]
- Frýbort, A.; Všianský, D.; Štulířová, J.; Stryk, J.; Gregerová, M. Variations in the Composition and Relations between Alkali-Silica Gels and Calcium Silicate Hydrates in Highway Concrete. Mater. Charact. 2018, 137, 91–108. [Google Scholar] [CrossRef]
- Leemann, A.; Shi, Z.; Lindgård, J. Characterization of Amorphous and Crystalline ASR Products Formed in Concrete Aggregates. Cem. Concr. Res. 2020, 137, 106190. [Google Scholar] [CrossRef]
- Powers, T.C.; Steinour, H.H. An Interpretation of Some Published Researches on the Alkali–Aggregate Reaction; Part 1—The Chemical Reactions and Mechanisms of Expansion. J. Am. Concr. Inst. 1955, 26, 497–516. [Google Scholar]
- Chatterji, S.; Jensen, A.D.; Thaulow, N.; Christensen, P.; Denmark, T. Studies of Alkali–Silica Reaction. Part 3. Mechanism by Which NaCl and Ca (OH)2 Affect the Reaction. Cem. Concr. Res. 1986, 16, 246–254. [Google Scholar] [CrossRef]
- Cai, Y.; Xuan, D.; Poon, C.S. Effects of Nano-SiO2 and Glass Powder on Mitigating Alkali-Silica Reaction of Cement Glass Mortars. Constr. Build. Mater. 2019, 201, 295–302. [Google Scholar] [CrossRef]
- Gause, G.R.; Tucker, J. Method for Determining the Moisture Condition in Hardened Concrete. J. Res. Natl. Bur. Stan. 1940, 25, 403. [Google Scholar] [CrossRef]
- González, J.A.; López, W.; Rodríguez, P. Effects of Moisture Availability on Corrosion Kinetics of Steel Embedded in Concrete. Corrosion 1993, 49, 1004–1010. [Google Scholar] [CrossRef]
- Chen, X.; Huang, W.; Zhou, J. Effect of Moisture Content on Compressive and Split Tensile Strength of Concrete. Indian J. Eng. Mater. Sci. 2012, 9, 427–435. [Google Scholar]
- Lura, P.; Winnefeld, F.; Fang, X. A Simple Method for Determining the Total Amount of Physically and Chemically Bound Water of Different Cements. J. Therm. Anal. Calorim. 2017, 130, 653–660. [Google Scholar] [CrossRef]
- Hundt, J.; Buschmann, J. Moisture Measurement in Concrete: Analysis of the Results of a RILEM Inquiry Carried out by the B.A.M. Mat. Constr. 1971, 4, 253–256. [Google Scholar] [CrossRef]
- Kumara, W.A.S.; Halvorsen, B.M.; Melaaen, M.C. Single-Beam Gamma Densitometry Measurements of Oil–Water Flow in Horizontal and Slightly Inclined Pipes. Int. J. Multiph. Flow 2010, 36, 467–480. [Google Scholar] [CrossRef]
- De Jong, S.M.; Heijenk, R.A.; Nijland, W.; van der Meijde, M. Monitoring Soil Moisture Dynamics Using Electrical Resistivity Tomography under Homogeneous Field Conditions. Sensors 2020, 20, 5313. [Google Scholar] [CrossRef]
- Andrade, C.; Sarría, J.; Alonso, C. Relative Humidity in the Interior of Concrete Exposed to Natural and Artificial Weathering. Cem. Concr. Res. 1999, 29, 1249–1259. [Google Scholar] [CrossRef]
- Nilsson, L.-O. (Ed.) Methods of Measuring Moisture in Building Materials and Structures; RILEM State-of-the-Art Reports; Springer International Publishing: Cham, Switzerland, 2018; Volume 26, ISBN 978-3-319-74230-4. [Google Scholar]
- Zeilinger, A.; Hübner, R. Measurement of Moisture Motion Under a Temperature Gradient in a Concrete for SNR-300 Using Thermal Neutrons. In Proceedings of the Concrete Properties Relevant to PCRV; IASMiRT: London, UK, 1975; pp. 1–9. [Google Scholar]
- Pandey, T.; Bhuiya, T.; Singh, B.; Harsh, R. A Review on Microwave Based Moisture Measurement System for Granular Materials. IOSR J. Electron. Commun. Eng. (IOSR-JECE) 2012, 3, 37–41. [Google Scholar] [CrossRef]
- Grinzato, E.; Ludwig, N.; Cadelano, G.; Bertucci, M.; Gargano, M.; Bison, P. Infrared Thermography for Moisture Detection: A Laboratory Study and In-Situ Test. Mater. Eval. 2011, 69, 97–104. [Google Scholar]
- Derome, D.; Fazio, P. Experimental Setup for the Study of Air Leakage Patterns. In Proceedings of the Thermal Performance of the Exterior Envelopes of Buildings VII, Clearwater Beach, FL, USA, 6–10 December 1998; American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc.: Atlanta, GA, USA, 1998; pp. 99–108. [Google Scholar]
- Lindgård, J.; Rodum, E.; Pedersen, B. Alkali-Silica Reactions in Concrete—Relationship between Water Content and Observed Damage on Structures. ACI Symp. Publ. 2006, 234, 147–166. [Google Scholar] [CrossRef]
- Yang, Q. Inner Relative Humidity and Degree of Saturation in High-Performance Concrete Stored in Water or Salt Solution for 2 Years. Cem. Concr. Res. 1999, 29, 45–53. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Han, Y. Simulation of Moisture Field of Concrete with Pre-Soaked Lightweight Aggregate Addition. Constr. Build. Mater. 2015, 96, 599–614. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y.; Han, Y.; Sun, W. Shrinkage and Interior Humidity of Concrete under Dry–Wet Cycles. Dry. Technol. 2012, 30, 583–596. [Google Scholar] [CrossRef]
- Geiker, M.R.; Laugesen, P. On the Effect of Laboratory Conditioning and Freeze/Thaw Exposure on Moisture Profiles in HPC. Cem. Concr. Res. 2001, 31, 1831–1836. [Google Scholar] [CrossRef]
- Lindgård, J.; Andiç-Çakır, Ö.; Fernandes, I.; Rønning, T.F.; Thomas, M.D.A. Alkali–Silica Reactions (ASR): Literature Review on Parameters Influencing Laboratory Performance Testing. Cem. Concr. Res. 2012, 42, 223–243. [Google Scholar] [CrossRef]
- Wardeh, G.; Perrin, B. Relative Permeabilities of Cement-Based Materials: Influence of the Tortuosity Function. J. Build. Phys. 2006, 30, 39–57. [Google Scholar] [CrossRef]
- Chaudhry, R.H. Determination of Air Voids, Capillary, and Gel Porosity in Hardened Concrete Using Mass-Based Saturation Techniques. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2018. [Google Scholar]
- Weiss, J. Relating Transport Properties to Performance in Concrete Pavements; Map Brief. CP Road Map. December 2014. National Concrete Pavement Technology Center, Ames, IA. Available online: https://intrans.iastate.edu/app/uploads/2018/08/MAPbriefDecember2014.pdf (accessed on 6 December 2023).
- Olajide, O.; Nokken, M.; Sanchez, L. A Review on the Role of Moisture and Temperature in Alkali-Silica Reaction (ASR). In Proceedings of the 16th International Conference on Alkali-Aggregate Reaction in Concrete (ICAAR), Lisbon, Portugal, 31 May 2022. [Google Scholar]
- Saccani, A.; Bonora, V.; Monari, P. Laboratory Short-Term Evaluation of ASR: A Contribution. Cem. Concr. Res. 2001, 31, 739–742. [Google Scholar] [CrossRef]
- Lenzner, D. Influence of the Amount of Mixing Water on the Alkali-Silica Reaction. In Proceedings of the 5th International Conference on Alkali-Aggregate Reaction, Cape Town, South Africa, 30 March–3 April 1981; pp. 1–6. [Google Scholar]
- Nilsson, L.-O.; Peterson, O. A Moisture Problem Causing Pop-Outs in Concrete Floors; Alkali Silica Reactions in Scania, Sweden; Division of Building Materials, LTH, Lund University: Lund, Sweden, 1983; pp. 1–106. [Google Scholar]
- Multon, S.; Toutlemonde, F. Effect of Moisture Conditions and Transfers on Alkali Silica Reaction Damaged Structures. Cem. Concr. Res. 2010, 40, 924–934. [Google Scholar] [CrossRef]
- Multon, S.; Seignol, J.-F.; Toutlemonde, F. Structural Behavior of Concrete Beams Affected by Alkali-Silica Reaction. ACI Mater. J. 2005, 102, 67–76. [Google Scholar] [CrossRef]
- Olafsson, H. The Effect of Relative Humidity and Temperature on Alkali Expansion of Mortar Bars. In Proceedings of the 7th International Conference on Alkali Aggregate Reaction in Concrete, Ottawa, ON, Canada, 22–24 August 1986; pp. 461–465. [Google Scholar]
- Lindgård, J.; Thomas, M.D.A.; Sellevold, E.J.; Pedersen, B.; Andiç-Çakır, Ö.; Justnes, H.; Rønning, T.F. Alkali–Silica Reaction (ASR)—Performance Testing: Influence of Specimen Pre-Treatment, Exposure Conditions and Prism Size on Alkali Leaching and Prism Expansion. Cem. Concr. Res. 2013, 53, 68–90. [Google Scholar] [CrossRef]
- Bažant, Z.P.; Steffens, A. Mathematical Model for Kinetics of Alkali–Silica Reaction in Concrete. Cem. Concr. Res. 2000, 30, 419–428. [Google Scholar] [CrossRef]
- Hashemi, A.; Donnell, K.M.; Zoughi, R.; Kurtis, K.E. Effect of Humidity on Dielectric Properties of Mortars with Alkali-Silica Reaction (ASR) Gel. In Proceedings of the 2015 IEEE International Instrumentation and Measurement Technology Conference (I2MTC) Proceedings, Pisa, Italy, 11–14 May 2015; IEEE: Pisa, Italy, 2015; pp. 1502–1506. [Google Scholar]
- BCA. The Diagnosis of Alkali–Silica Reaction-Report of a Working Party; British Cement Association: Slough, UK, 1992; p. 36. [Google Scholar]
- Reed, R.G. Measuring Relative Humidity in Concrete Pavements as a Method to Assess ASR Mitigation Measures. Bachelor’s Thesis, University of Arkansas, Fayetteville, NC, USA, 2016. [Google Scholar]
- Gudmundsson, B.; Asgeirsson, H. Some Investigations on Alkali Aggregate Reaction. Cem. Concr. Res. 1975, 5, 211–220. [Google Scholar] [CrossRef]
- Sinno, N.; Shehata, M.H. Role of Temperature on Alkali-Silica Reaction and the Efficacy of Supplementary Cementitious Materials. Constr. Build. Mater. 2021, 313, 125427. [Google Scholar] [CrossRef]
- Guo, J.-J.; Liu, P.-Q.; Wu, C.-L.; Wang, K. Effect of Dry–Wet Cycle Periods on Properties of Concrete under Sulfate Attack. Appl. Sci. 2021, 11, 888. [Google Scholar] [CrossRef]
- Farny, J.A.; Kerkhoff, B. Diagnosis and Control of Alkali-Aggregate Reactions in Concrete; Portland Cement Association: Skokie, IL, USA, 2007; pp. 1–26. [Google Scholar]
- Kagimoto, H.; Kawamura, M. Measurements of Strain and Humidity within Massive Concrete Cylinders Related to the Formation of ASR Surface Cracks. Cem. Concr. Res. 2011, 41, 808–816. [Google Scholar] [CrossRef]
- Kagimoto, H.; Yasuda, Y.; Kawamura, M. Effects of Expansion Behavior of ASR-Affected Concrete in Atmospheres with Various Values of Relative Humidity on Surface Cracking. In Proceedings of the 15th International Conference on Alkali Aggregate Reaction in Concrete, Sao Paulo, Brazil, 3–7 July 2016; pp. 1–10. [Google Scholar]
- Gautam, B.P.; Panesar, D.K. The Effect of Elevated Conditioning Temperature on the ASR Expansion, Cracking and Properties of Reactive Spratt Aggregate Concrete. Constr. Build. Mater. 2017, 140, 310–320. [Google Scholar] [CrossRef]
- Deschenes, R.A., Jr. Mitigation and Evaluation of Alkali-Silica Reaction (ASR) and Freezing and Thawing in Concrete Transportation Structures. Theses Diss. 2017, 2467, 273. [Google Scholar]
- Gillott, J.E. Alkali-Aggregate Reactions in Concrete. Eng. Geol. 1975, 9, 303–326. [Google Scholar] [CrossRef]
- Helmuth, R.; Stark, D.; Diamond, S.; Moranville-regourd, M. Alkali-Silica Reactivity: An Overview of Research. In Proceedings of the National Research Council, Washington, DC, USA, 19 June 1993. [Google Scholar]
- Lu, D.; Zhou, X.; Xu, Z.; Lan, X.; Tang, M.; Fournier, B. Evaluation of Laboratory Test Method for Determining the Potential Alkali Contribution from Aggregate and the ASR Safety of the Three-Gorges Dam Concrete. Cem. Concr. Res. 2006, 36, 1157–1165. [Google Scholar] [CrossRef]
- Bérubé, M.-A.; Duchesne, J.; Dorion, J.F.; Rivest, M. Laboratory Assessment of Alkali Contribution by Aggregates to Concrete and Application to Concrete Structures Affected by Alkali–Silica Reactivity. Cem. Concr. Res. 2002, 32, 1215–1227. [Google Scholar] [CrossRef]
- Drolet, C.; Duchesne, J.; Fournier, B. Effect of Alkali Release by Aggregates on Alkali-Silica Reaction. Constr. Build. Mater. 2017, 157, 263–276. [Google Scholar] [CrossRef]
- Maia Neto, F.M.; Andrade, T.W.C.O.; Gomes, R.M.; Leal, A.F.; Almeida, A.N.F.; Lima Filho, M.R.F.; Torres, S.M. Considerations on the Effect of Temperature, Cation Type and Molarity on Silica Degradation and Implications to ASR Assessment. Constr. Build. Mater. 2021, 299, 123848. [Google Scholar] [CrossRef]
- Ideker, J.H.; East, B.L.; Folliard, K.J.; Thomas, M.D.A.; Fournier, B. The Current State of the Accelerated Concrete Prism Test. Cem. Concr. Res. 2010, 40, 550–555. [Google Scholar] [CrossRef]
- Fournier, B.; Chevrier, R.; Grosbois, M.; Lisella, R.; Folliard, K.; Ideker, J.; Shehatad, M.; Thomas, M.; Baxter, S. The Accelerated Concrete Prism Test (60 °C): Variability of the Test Method and Proposed Expansion Limits. Researchgate, 2014. [Google Scholar]
- Sanchez, L.; Kuperman, S.C.; Helene, P. Using the Accelerated Brazilian Concrete Prism Test (ABCPT) to Evaluate Alkali Aggregate Reaction (AAR). IBRACON Struct. Mater. J. 2011, 4, 575–581. [Google Scholar] [CrossRef]
- Kawabata, Y.; Dunant, C.; Yamada, K.; Scrivener, K. Impact of Temperature on Expansive Behavior of Concrete with a Highly Reactive Andesite Due to the Alkali–Silica Reaction. Cem. Concr. Res. 2019, 125, 105888. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J.; Jeong, H. Alkali–Silica Reaction: Kinetics of Chemistry of Pore Solution and Calcium Hydroxide Content in Cementitious System. Cem. Concr. Res. 2015, 71, 36–45. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.-R.; Liu, H.-B.; Liu, X.-Z.; Li, H.; Chen, X. Meso-Mechanical Research on Alkali-Silica Reaction Expansion in Pyrex Glass and Silica Sand at Different Temperatures and Curing Times. Constr. Build. Mater. 2019, 223, 377–393. [Google Scholar] [CrossRef]
- Folliard, K.J.; Ideker, J.H.; Thomas, M.D.; Fournier, B. Assessing Aggregate Reactivity Using the Accelerated Concrete Prism Test. In Proceedings of the 7th CANMET/ACI International Conference on Recent Advances in Concrete Technology, Ottawa, ON, Canada, 1 July 2004; pp. 269–283. [Google Scholar]
- Li, B.; Baingam, L.; Kurumisawa, K.; Nawa, T.; Liu, X.Z. Micro-Mechanical Modelling for the Prediction of Alkali-Silica Reaction (ASR) Expansion: Influence of Curing Temperature Conditions. Constr. Build. Mater. 2018, 164, 554–569. [Google Scholar] [CrossRef]
- Golmakani, F.; Hooton, R.D. Impact of Pore Solution Concentration on the Accelerated Mortar Bar Alkali-Silica Reactivity Test. Cem. Concr. Res. 2019, 121, 72–80. [Google Scholar] [CrossRef]
- Kawabata, Y.; Yamada, K.; Sagawa, Y.; Ogawa, S. Alkali-Wrapped Concrete Prism Test (AW-CPT)—New Testing Protocol toward a Performance Test against Alkali-Silica Reaction–. J. Adv. Concr. Technol. 2018, 16, 441–460. [Google Scholar] [CrossRef]
- Lindgård, J.; Nixon, P.J.; Borchers, I.; Schouenborg, B.; Wigum, B.J.; Haugen, M.; Åkesson, U. The EU “PARTNER” Project—European Standard Tests to Prevent Alkali Reactions in Aggregates: Final Results and Recommendations. Cem. Concr. Res. 2010, 40, 611–635. [Google Scholar] [CrossRef]
- De Grazia, M.T.; Goshayeshi, N.; Gorga, R.; Sanchez, L.F.M.; Santos, A.C.; Souza, D.J. Comprehensive Semi-Empirical Approach to Describe Alkali Aggregate Reaction (AAR) Induced Expansion in the Laboratory. J. Build. Eng. 2021, 40, 102298. [Google Scholar] [CrossRef]
- Cukierski, D. Quantifying Alkali-Silica Reaction in Concrete: Damage Rating Index. In Proceedings of the Concrete Institute of Australia’s Biennial National Conference, Perth, Australia, 5–8 September 2021; pp. 1–6. [Google Scholar]
- Rivard, P.; Ballivy, G. Assessment of the Expansion Related to Alkali-Silica Reaction by the Damage Rating Index Method. Constr. Build. Mater. 2005, 19, 83–90. [Google Scholar] [CrossRef]
- Sanchez, L.; Fournier, B.; Jolin, M.; Sanchez, L.F.M.; Fournier, B.; Jolin, M.; Bustamante, M.A.B. Evaluation of the Microscopic ASR Features through the Damage Rating Index (DRI) for Different Concrete Strengths and Aggregate Types (Fine and Coarse Reactive Aggregates). In Proceedings of the 14th Euroseminar on Microscopy Applied to Building Materials, Copenhagen, Denmark, 1 June 2013. [Google Scholar]
- Wang, Y.; Gao, P.; Su, H.; Qin, Y.; Wang, Y.; Xue, G. Failure Criteria and Microstructure Evolution Mechanism of the Alkali–Silica Reaction of Concrete. Rev. Adv. Mater. Sci. 2023, 62, 20230102. [Google Scholar] [CrossRef]
- Shakoorioskooie, M.; Griffa, M.; Leemann, A.; Zboray, R.; Lura, P. Quantitative Analysis of the Evolution of ASR Products and Crack Networks in the Context of the Concrete Mesostructure. Cem. Concr. Res. 2022, 162, 106992. [Google Scholar] [CrossRef]
- Glinicki, M.A.; Jóźwiak-Niedźwiedzka, D.; Antolik, A.; Dziedzic, K.; Dąbrowski, M.; Bogusz, K. Diagnosis of ASR Damage in Highway Pavement after 15 Years of Service in Wet-Freeze Climate Region. Case Stud. Constr. Mater. 2022, 17, e01226. [Google Scholar] [CrossRef]
- Antolik, A.; Jóźwiak-Niedźwiedzka, D. Assessment of the Alkali-Silica Reactivity Potential in Granitic Rocks. Constr. Build. Mater. 2021, 295, 123690. [Google Scholar] [CrossRef]
- Fernandes, I. Composition of Alkali–Silica Reaction Products at Different Locations within Concrete Structures. Mater. Charact. 2009, 60, 655–668. [Google Scholar] [CrossRef]
- Rößler, C.; Möser, B.; Giebson, C.; Ludwig, H.-M. Application of Electron Backscatter Diffraction to Evaluate the ASR Risk of Concrete Aggregates. Cem. Concr. Res. 2017, 95, 47–55. [Google Scholar] [CrossRef]
- Boehm-Courjault, E.; Barbotin, S.; Leemann, A.; Scrivener, K. Microstructure, Crystallinity and Composition of Alkali-Silica Reaction Products in Concrete Determined by Transmission Electron Microscopy. Cem. Concr. Res. 2020, 130, 105988. [Google Scholar] [CrossRef]
- Sanchez, L.F.M.; Drimalas, T.; Fournier, B.; Mitchell, D.; Bastien, J. Comprehensive Damage Assessment in Concrete Affected by Different Internal Swelling Reaction (ISR) Mechanisms. Cem. Concr. Res. 2018, 107, 284–303. [Google Scholar] [CrossRef]
- Joo, H.E.; Takahashi, Y. Analytical and Experimental Studies on Alkali-Silica Reaction Mechanism: Aggregate Cracking and Chemical Composition Change of Gel. Cem. Concr. Compos. 2023, 139, 105003. [Google Scholar] [CrossRef]
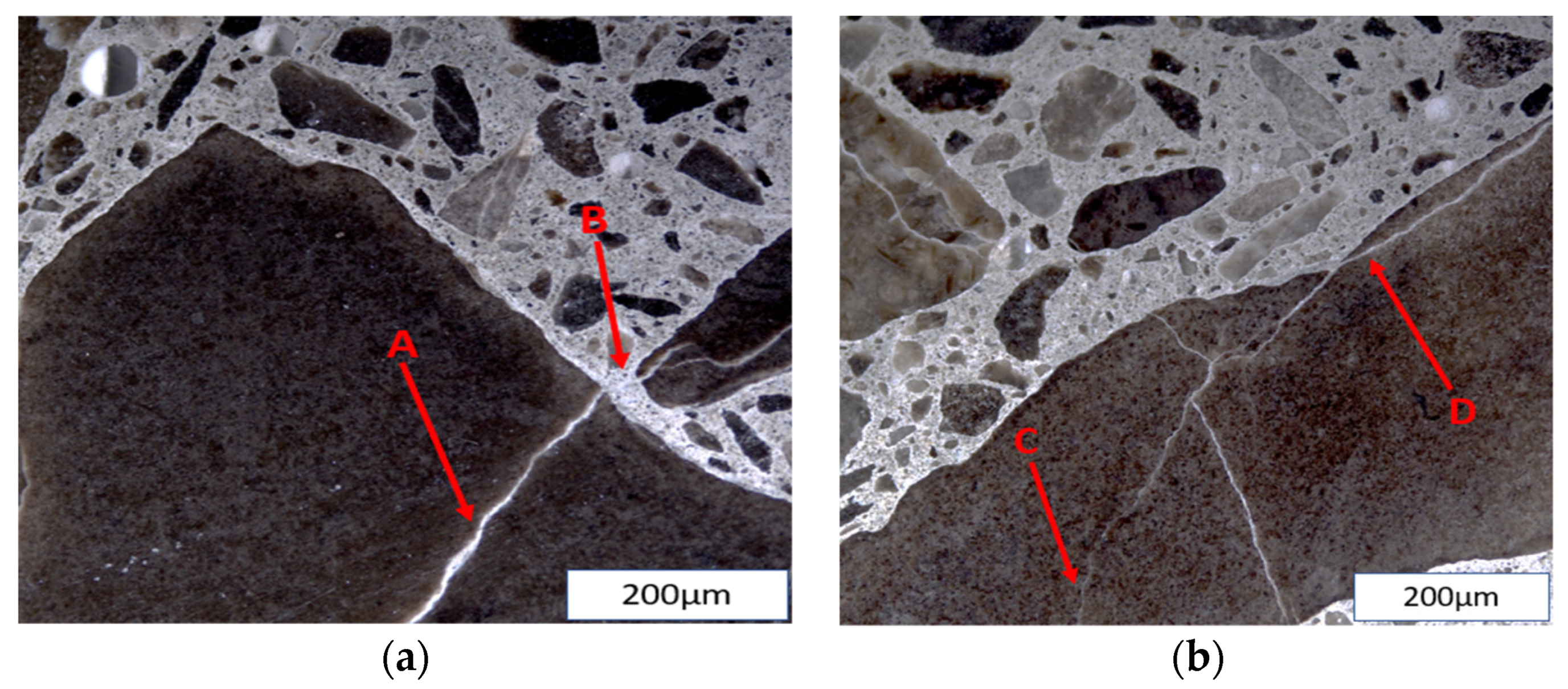
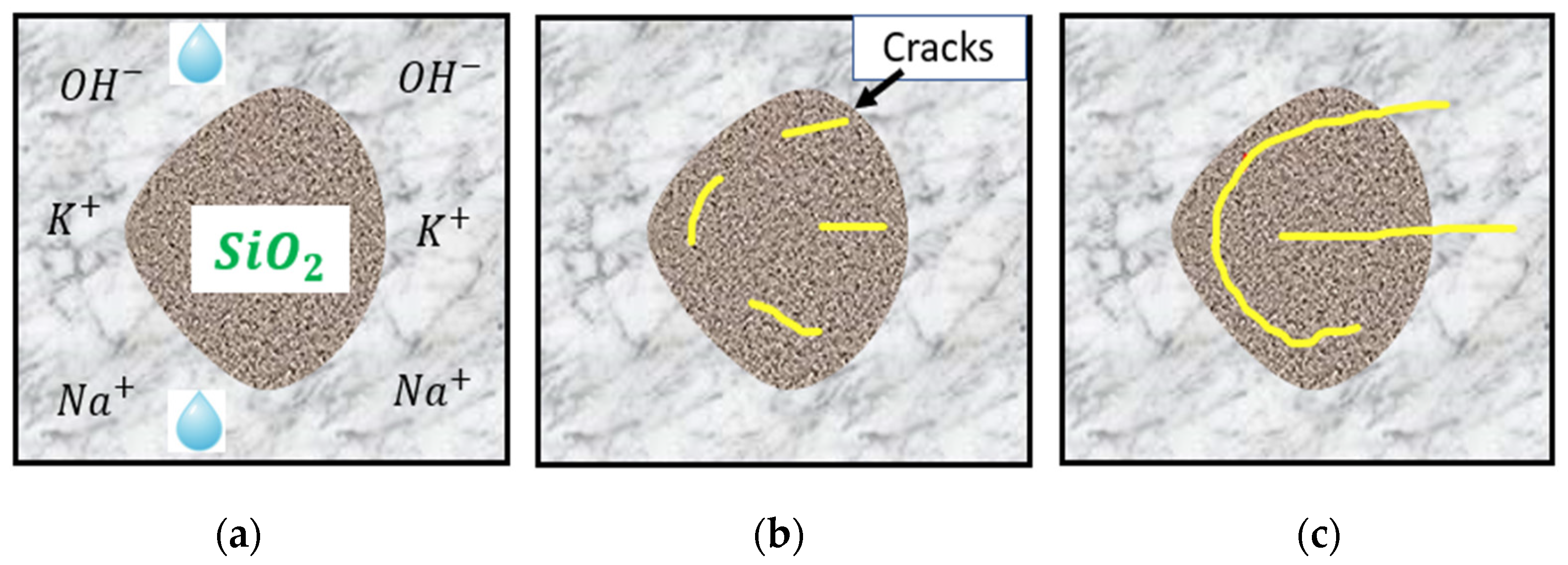


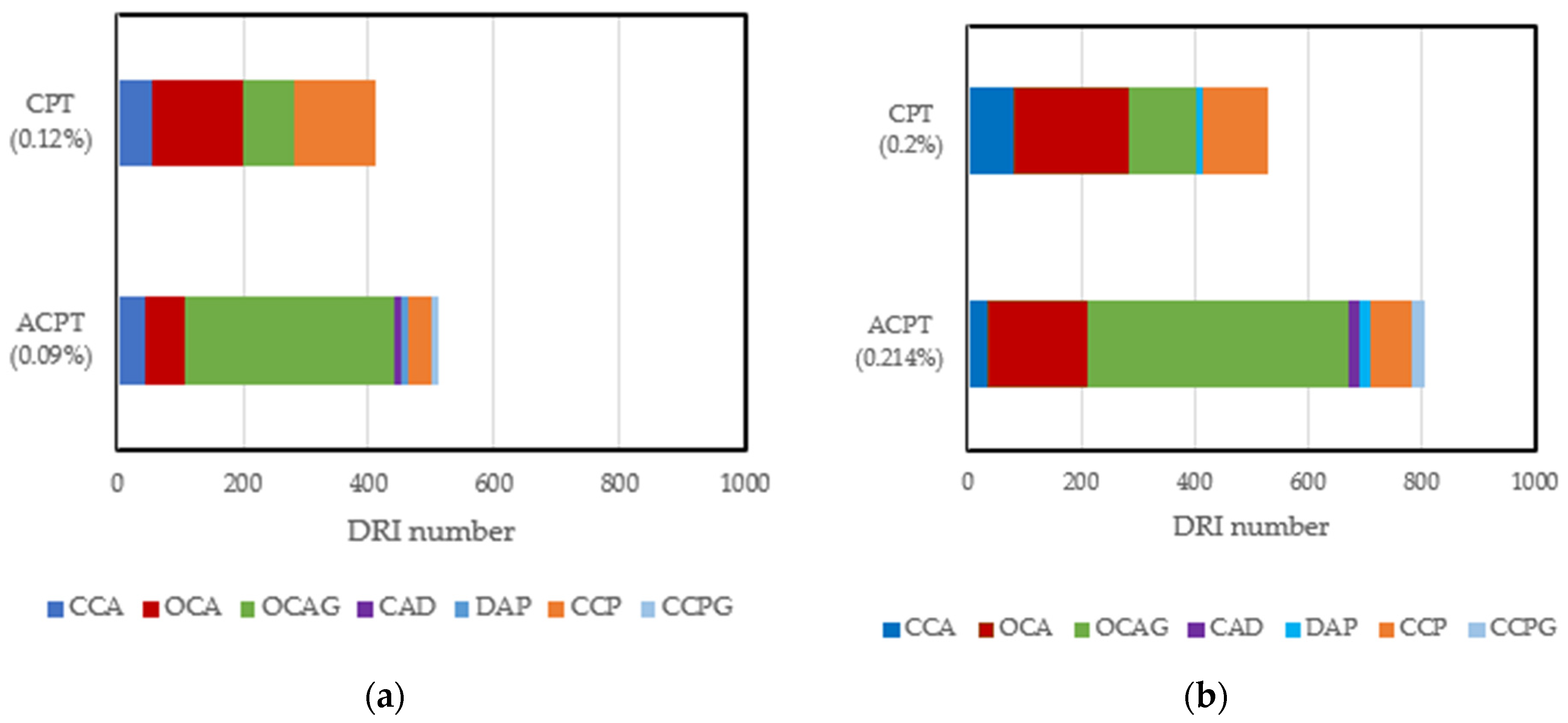
| Test | NDT | Location | Influencing Factor | Output |
|---|---|---|---|---|
| Gamma densitometry [47] | Yes | Different depth | Geometry | Density of concrete |
| Electrical resistance [48] | Yes | Surface | Degree of maturity | Electrical conductivity |
| Hygrometry [49] | Yes | Location of choice | Calibration, temperature | Relative humidity |
| Chemical reactions [50] | No | Representative sample | Evaporation | Reaction with water |
| Thermalized neutrons [51] | Yes | Surface | Chemical composition | Thermal neutron detector |
| Microwave absorption [52] | Yes | Few inches deep | Mix proportion | Electromagnetic waves |
| Infrared thermography [53] | Yes | No contact | Concrete density | Surface temperature |
| Gravimetric [54] | No | Representative sample | Depth of collection, incomplete drying | Surface temperature |
| Ref. | Aggregate Type | Sample Size | Reactive Content | Alkali | Temp. | Test Duration | Final Expansion | Expansion at 28 Days |
|---|---|---|---|---|---|---|---|---|
| [3] | Highly reactive fine | 75 × 75 × 285 mm (prism) | Fine agg. | 1.25% | 21 °C | 167 days | 0.14% | 0.010% |
| 40 °C | 0.55% | 0.020% | ||||||
| [22] | Reactive limestone | 16 × 2 cm (cylinder) | Fine agg. | 1.84% | 60 °C | 200 days | 0.24% | 0.220% |
| [71] | Highly reactive quartz | 75 × 75 × 285 mm (prism) | Coarse agg. | 0.925% | 38 °C | 365 days | 0.24% | 0.010% |
| 60 °C | 273 days | 0.18% | 0.040% | |||||
| [77] | Sudbury | 100 × 285 mm (cylinder) | Coarse agg. | 1.25% | 38 °C | 365 days | 0.20% | 0.010% |
| 60 °C | 0.16% | 0.040% | ||||||
| 75 × 75 × 285 mm (prism) | 38 °C | 0.170% | 0.015% | |||||
| 60 °C | 0.080% | 0.030% | ||||||
| Spratt | 100 × 285 mm (cylinder) | Coarse agg. | 1.25% | 38 °C | 365 days | 0.26% | 0.018% | |
| 60 °C | 0.18% | 0.110% | ||||||
| 75 × 75 × 285 mm (prism) | 38 °C | 0.21% | 0.020% | |||||
| 60 °C | 0.17% | 0.100% | ||||||
| [82] | Spratt | 75 × 75 × 285 mm (prism) | Coarse agg. | 1.25% | 38 °C | 730 days | 0.26% | 0.013% |
| 50 °C | 187 days | 0.248% | 0.085% | |||||
| [88] | Spratt | 75 × 150 mm (cylinder) | Fine agg. | 1.07% | 38 °C | 365 days | 0.160% | 0.033% |
| 60 °C | 0.074% | 0.058% | ||||||
| Springhill | 38 °C | 0.230% | 0.028% | |||||
| 60 °C | 0.103% | 0.050% | ||||||
| [90] | Spratt | 75 × 75 × 285 mm (prism) | Coarse agg. | 1.25% | 38 °C | 365 days | 0.257% | ------- |
| 60 °C | 91 days | 0.160% | ------- | |||||
| Sudbury | 38 °C | 365 days | 0.171% | ------- | ||||
| 60 °C | 91 days | 0.138% | ------- | |||||
| [91] | Spratt | 75 × 75 × 285 mm (prism) | Coarse agg. | 1.25% | 38 °C | 365 days | 0.229% | ------- |
| 60 °C | 182 days | 0.167% | 0.103% | |||||
| Sudbury | 38 °C | 365 days | 0.150% | ------- | ||||
| 60 °C | 182 days | 0.187% | 0.022% | |||||
| [92] | Limestone | 75 × 75 × 285 mm (prism) | Coarse agg. | 1.25% | 38 °C | 365 days | 0.22% | 0.000% |
| 60 °C | 150 days | 0.19% | 0.065% | |||||
| [93] | Highly reactive andesite | 75 × 75 × 250 mm (prism) | Coarse agg. | 0.94% | 40 °C | 365 days | 0.140% | 0.035% |
| 60 °C | 0.110% | 0.070% | ||||||
| [94] | Highly reactive Jobe sand | 25 × 25 × 285 mm (prism) | Fine agg. | 1.04% | 38 °C | 600 days | 0.800% | 0.050% |
| 55 °C | 0.790% | 0.350% | ||||||
| [95] | Silica sand and Pyrex glass | 28 × 28 × 180 mm (prism) | Fine agg. | 1.20% | 30 °C | 100 days | 0.650% | 0.350% |
| 60 °C | 0.420% | 0.410% |
| Ref. | Study | Test and Methods | Findings |
|---|---|---|---|
| [38] | Characterization of amorphous and crystalline ASR products formed in concrete aggregates | SEM, X-ray spectroscopy, and Raman microscopy | The morphology of crystalline ASR products is influenced by temperature. |
| [82] | The effect of elevated conditioning temperature on the ASR expansion, cracking, and properties of reactive Spratt aggregate concrete | Damage rating index | Similar ASR-induced expansion can result in different levels of damage as temperature increases. |
| [105] | Failure criteria and microstructure evolution mechanism of the alkali–silica reaction of concrete | Scanning electron microscopy (SEM) and X-ray computed microtomography | Microcracks propagate from voids in the aggregate into the cement paste. Presence of ASR gel in the pores leads to a reduction in porosity. |
| [106] | Quantitative analysis of the evolution of ASR products and crack networks in the context of the concrete mesostructure | Time-lapse X-ray tomography | Visualization of the movement of ASR products from the aggregates into the cement paste in 4D. |
| [107] | Diagnosis of ASR damage in highway pavement after 15 years of service in wet–freeze climate region | Thin section, SEM, and electron dispersive spectroscopy (EDS) | Cracks were identified in the grains of coarse quartzite aggregate. Gel-like products in the cracks were confirmed to be ASR products by EDS. |
| [108] | Assessment of the alkali–silica reactivity potential in granitic rocks | X-ray diffraction (XRD) and SEM/EDS | Contents of strained quartz in quartz were determined using image analysis and correlated with AMBT results. There existed a linear correlation between the two observations. |
| [109] | Composition of alkali–silica reaction products at different locations within concrete structures | Thin section and SEM/EDS | Structure and qualitative properties of ASR gel in different structures were verified. Composition of gel varied with location. Gels that had propagated into the cement paste contained a higher amount of calcium than those in the aggregates. |
| [110] | Application of electron backscatter diffraction to evaluate the ASR risk of concrete aggregates | SEM and electron backscatter diffraction (EBSD) | The dissolution of quartz at high pH was observed to occur along its grain and sub-grain boundaries. This technique can be used to assess the properties of slow-late reactive aggregates. |
| [111] | Microstructure, crystallinity, and composition of alkali–silica reaction products in concrete determined by transmission electron microscopy | SEM, EDS, focused ion beam (FIB), and transmission electron microscopy (TEM) | The morphology of ASR products differs with location. Products located in thin grains are amorphous while those in larger widths are crystalline. |
| Features | Weighting Factors |
|---|---|
| CCA: Closed cracks in aggregates | 0.25 |
| OCA: Open cracks in aggregates | 2 |
| OCAG: Open cracks in aggregates with reaction products | 2 |
| CAD: Coarse aggregate debonded | 3 |
| DAP: Disaggregated/corroded aggregate particle | 2 |
| CCP: Cracks in cement paste | 3 |
| CCPG: Cracks in cement paste with reaction products | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olajide, O.D.; Nokken, M.R.; Sanchez, L.F.M. Alkali–Silica Reactions: Literature Review on the Influence of Moisture and Temperature and the Knowledge Gap. Materials 2024, 17, 10. https://doi.org/10.3390/ma17010010
Olajide OD, Nokken MR, Sanchez LFM. Alkali–Silica Reactions: Literature Review on the Influence of Moisture and Temperature and the Knowledge Gap. Materials. 2024; 17(1):10. https://doi.org/10.3390/ma17010010
Chicago/Turabian StyleOlajide, Olusola D., Michelle R. Nokken, and Leandro F. M. Sanchez. 2024. "Alkali–Silica Reactions: Literature Review on the Influence of Moisture and Temperature and the Knowledge Gap" Materials 17, no. 1: 10. https://doi.org/10.3390/ma17010010
APA StyleOlajide, O. D., Nokken, M. R., & Sanchez, L. F. M. (2024). Alkali–Silica Reactions: Literature Review on the Influence of Moisture and Temperature and the Knowledge Gap. Materials, 17(1), 10. https://doi.org/10.3390/ma17010010







