Formulation and Biodegradation of Surface-Supported Biopolymer-Based Microgels Formed via Hard Templating onto Vaterite CaCO3 Crystals
Abstract
:1. Introduction
2. Experimental Section
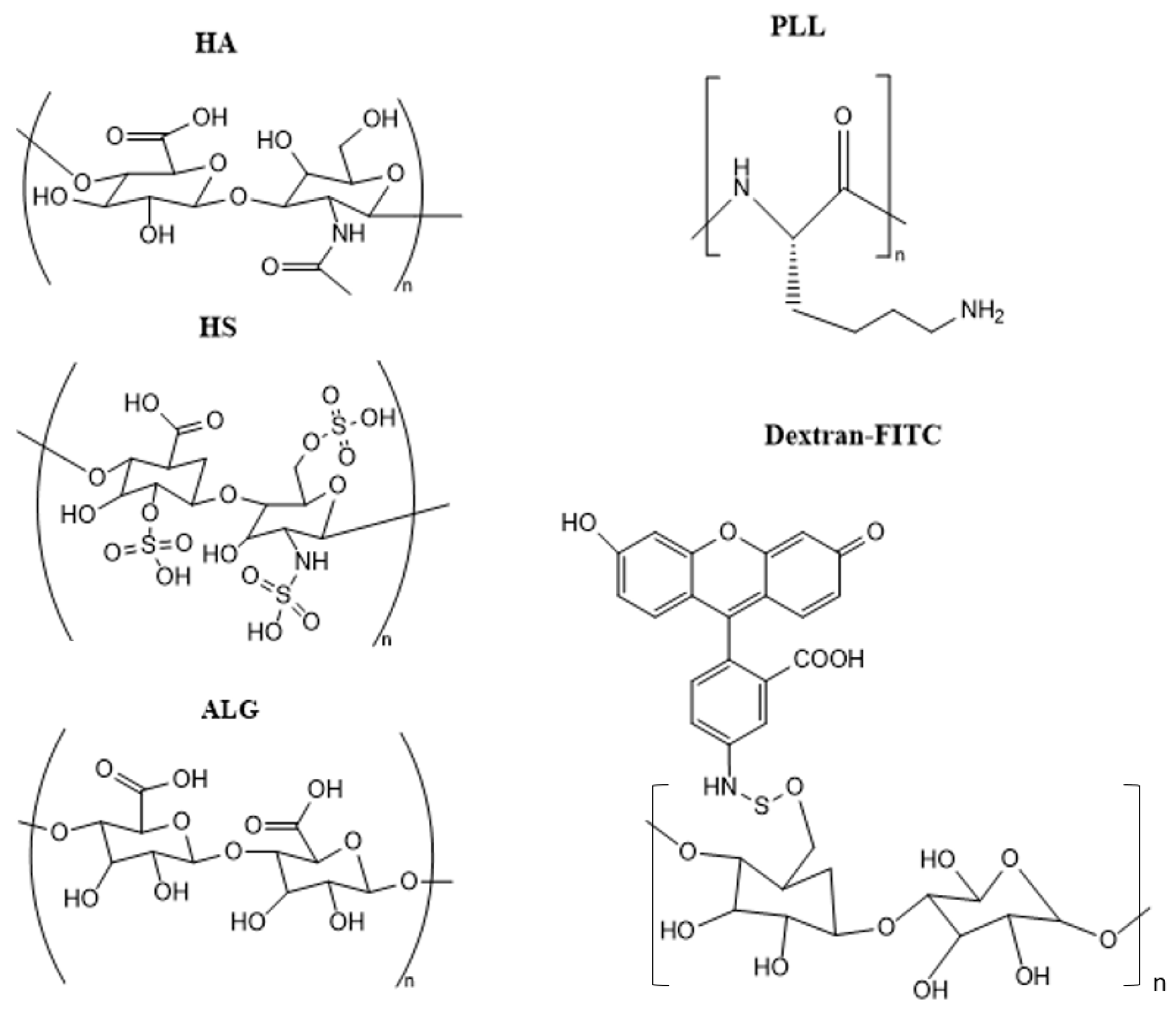
2.1. Materials
2.2. Synthesis of ss-CaCO3 Vaterite Crystals
2.2.1. Two-Step Synthesis
2.2.2. One-Step Synthesis
2.3. Fabrication of ss-MGs
2.4. Biodegradation of MGs
2.5. Optical and Fluorescence Microscopy
2.6. Scanning Electron Microscopy (SEM)
2.7. Atomic Force Microscopy (AFM)
3. Results and Discussion
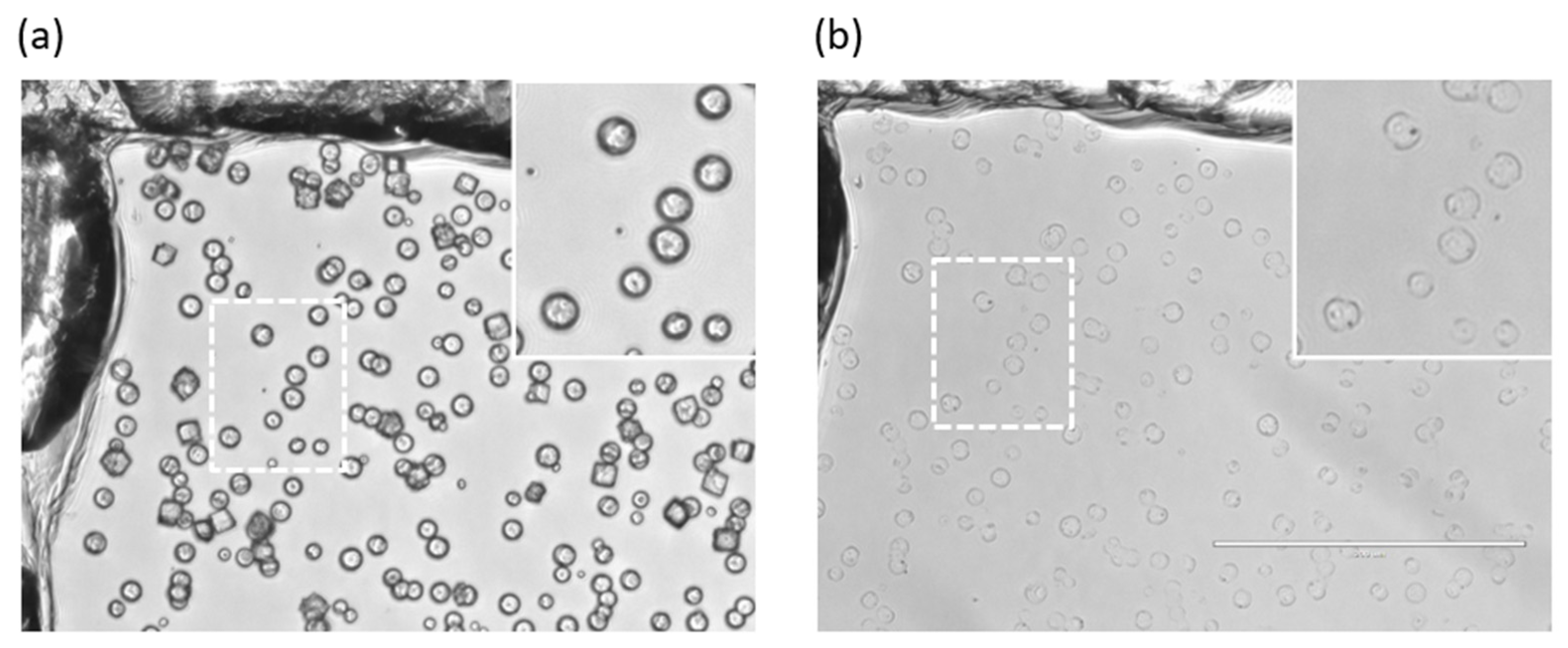
3.1. Synthesis of ss-CaCO3 Vaterite Crystals and ss-MGs
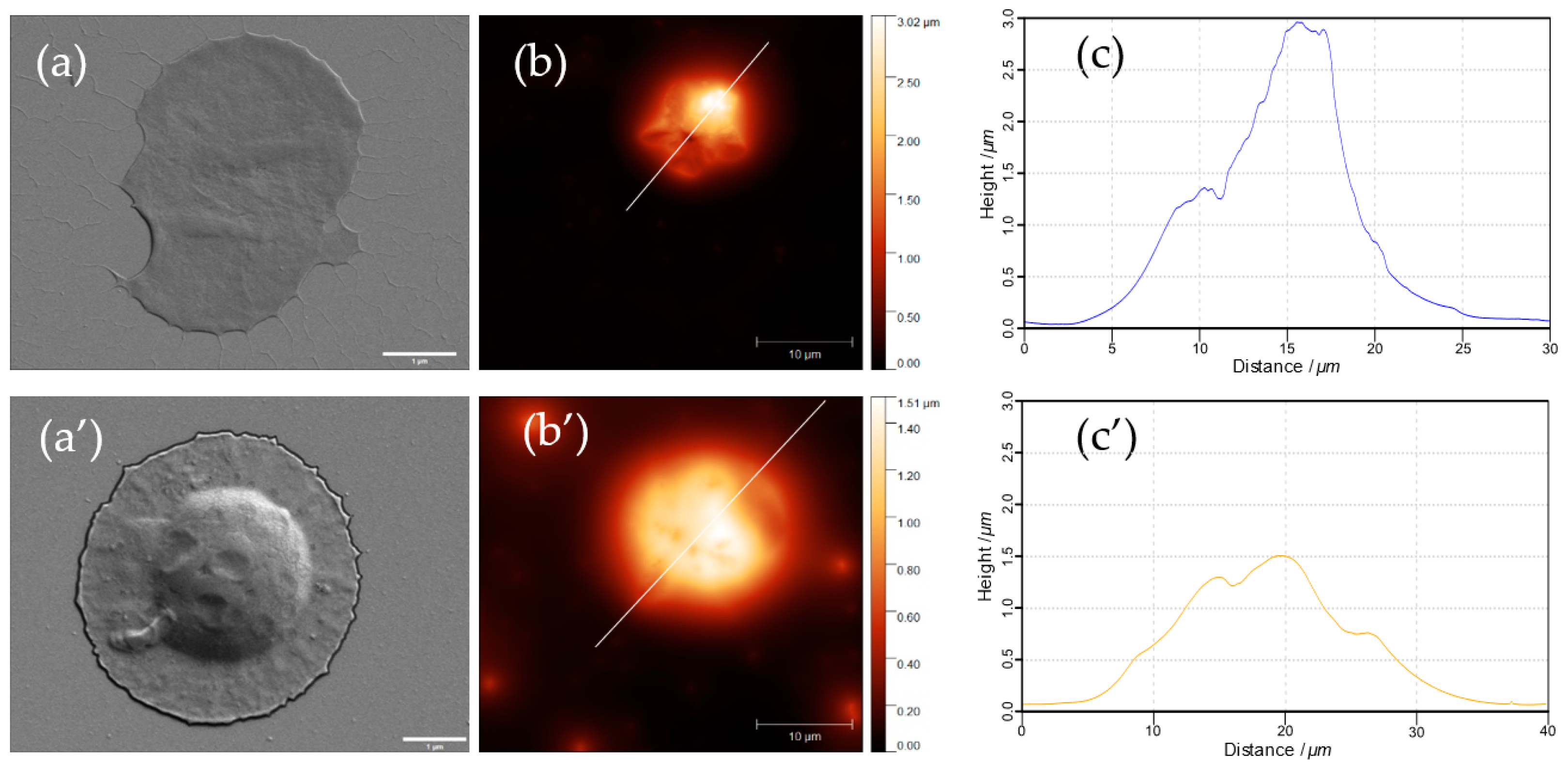
3.2. Properties of ss-CaCO3 and ss-MGs
3.3. Relation of Interpolymer Interactions in ss-MGs and Their Properties
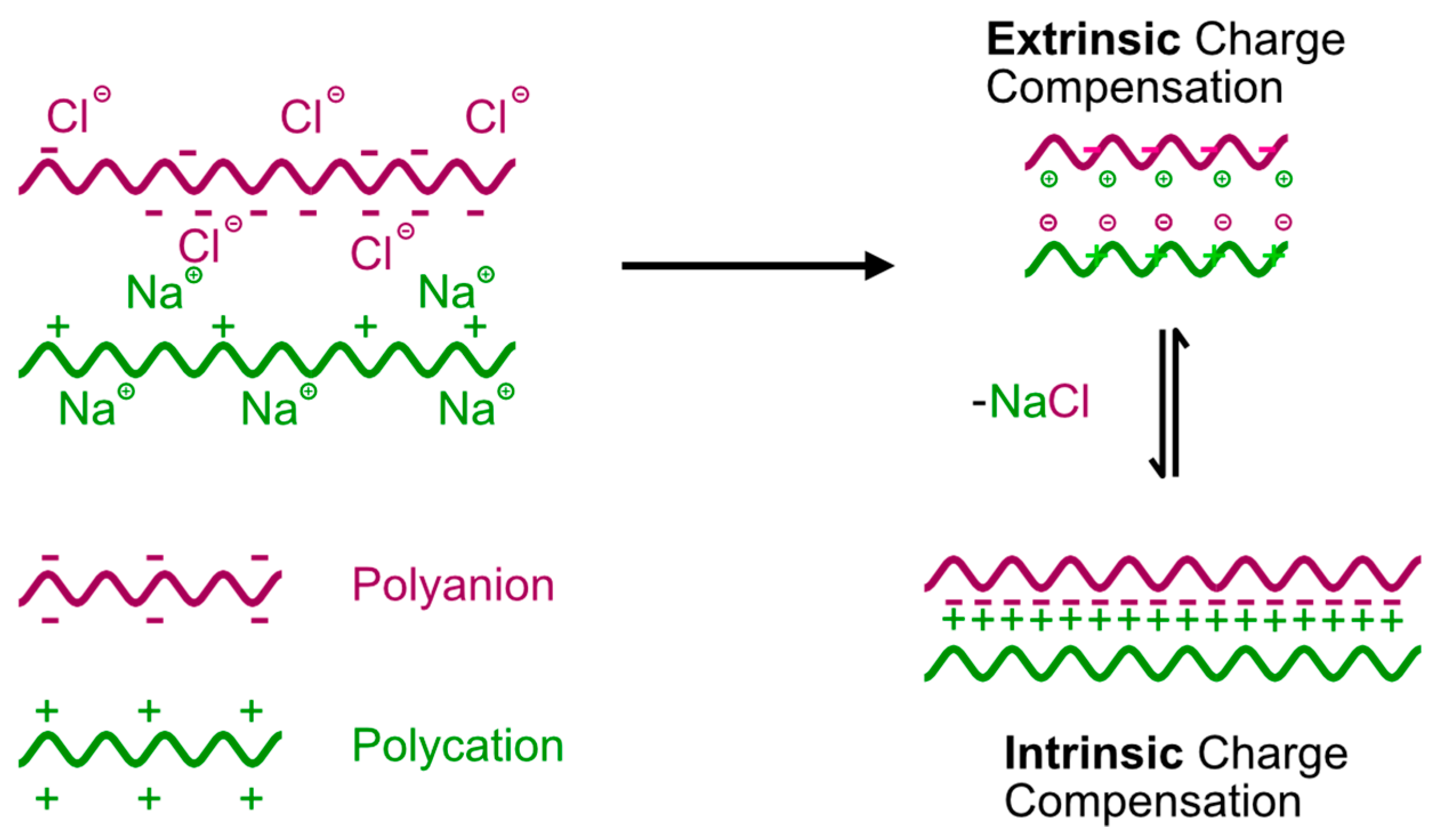
3.3.1. Dynamics of Polymer Chains and Charge Compensation in Multilayers
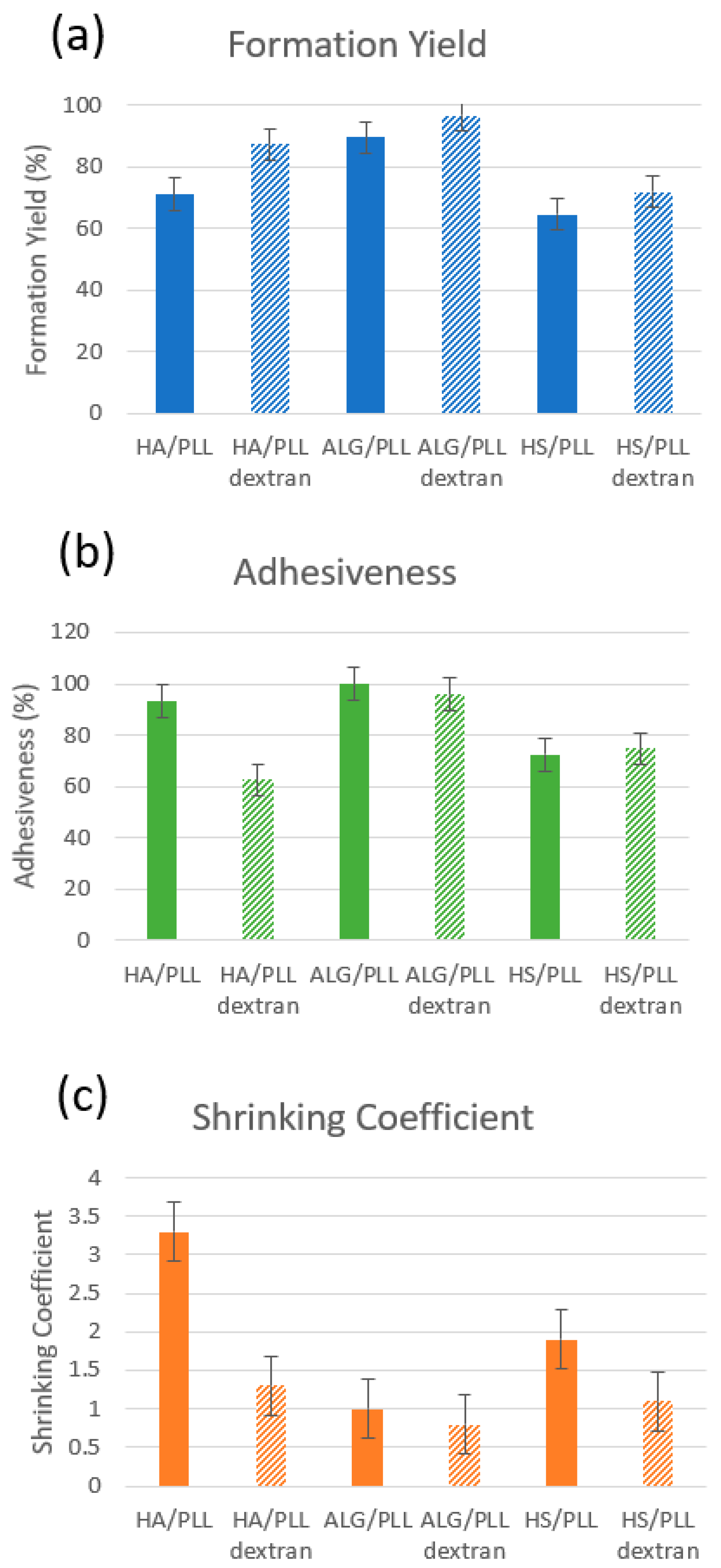
3.3.2. Main Properties of ss-MGs
3.4. Biodegradation of ss-MGs with Trypsin
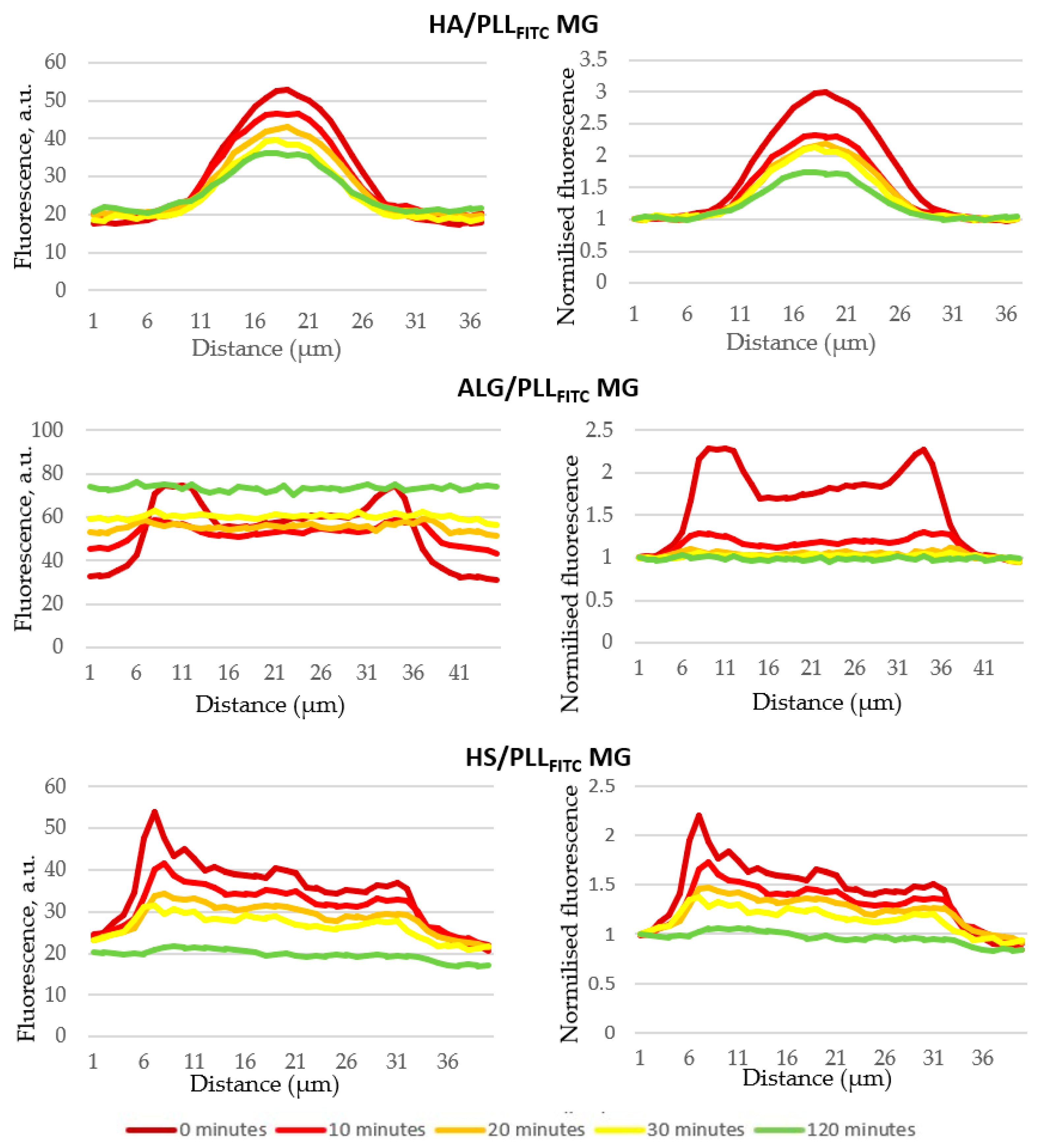
3.4.1. Fluorescence Imaging
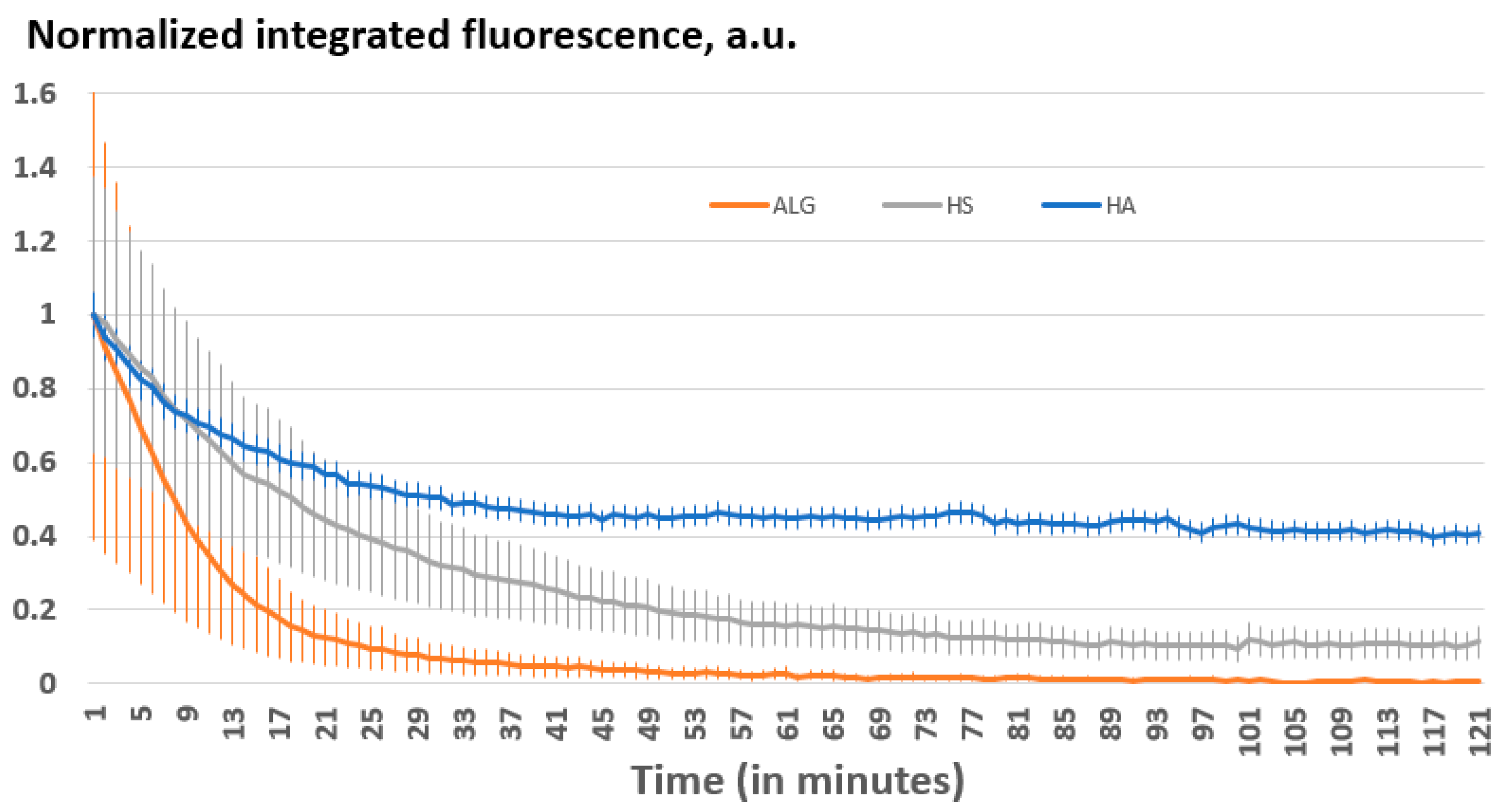
3.4.2. Biodegradation Kinetics and Mechanism
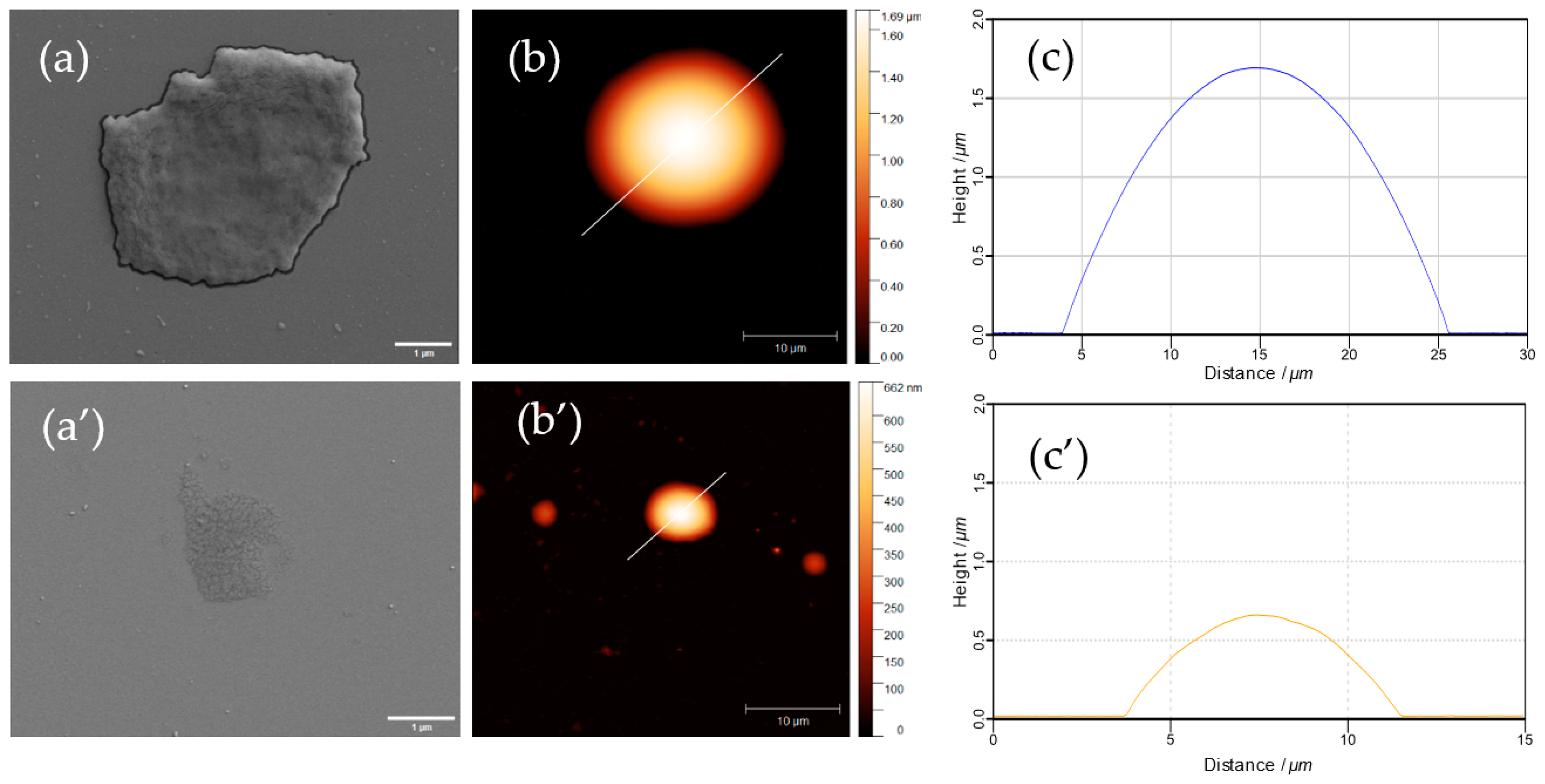
3.4.3. Formulation of Giant ss-MGs Using a One-Step ss-CaCO3 Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, J.; Abnett, J.; Kastania, G.; Volodkin, D.; Vikulina, A.S. Which Biopolymers Are Better for the Fabrication of Multilayer Capsules? A Comparative Study Using Vaterite CaCO3 as Templates. ACS Appl. Mater. Interfaces 2021, 13, 3259–3269. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, K.; Raichur, A.M. Biologically triggered exploding protein based microcapsules for drug delivery. Chem. Commun. 2012, 48, 2307–2309. [Google Scholar] [CrossRef] [PubMed]
- Ghiman, R.; Pop, R.; Rugina, D.; Focsan, M. Recent progress in preparation of microcapsules with tailored structures for bio-medical applications. J. Mol. Struct. 2022, 1248, 131366. [Google Scholar] [CrossRef]
- Matsusaki, M.; Ajiro, H.; Kida, T.; Serizawa, T.; Akashi, M. Layer-by-Layer Assembly Through Weak Interactions and Their Biomedical Applications. Adv. Mater. 2012, 24, 454–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lv, S.; Li, T.; He, Y.; Song, P. Fabricating Polymer Microspheres through CaCO3 Templates. Prog. Chem. 2016, 28, 75–82. [Google Scholar] [CrossRef]
- De Koker, S.; Hoogenboom, R.; De Geest, B.G. Polymeric multilayer capsules for drug delivery. Chem. Soc. Rev. 2012, 41, 2867–2884. [Google Scholar] [CrossRef] [PubMed]
- Nifontova, G.; Tsoi, T.; Karaulov, A.; Nabiev, I.; Sukhanova, A. Structure–function relationships in polymeric multilayer capsules designed for cancer drug delivery. Biomater. Sci. 2022, 10, 5092–5115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Parakhonskiy, B.V.; Skirtach, A.G. A decade of developing applications exploiting the properties of polyelectrolyte multilayer capsules. Chem. Commun. 2023, 59, 807–835. [Google Scholar] [CrossRef]
- Trushina, D.B.; Sulyanov, S.N.; Bukreeva, T.V.; Kovalchuk, M.V. Size control and structure features of spherical calcium carbonate particles. Crystallogr. Rep. 2015, 60, 570–577. [Google Scholar] [CrossRef]
- Vikulina, A.; Webster, J.; Voronin, D.; Ivanov, E.; Fakhrullin, R.; Vinokurov, V.; Volodkin, D. Mesoporous additive-free vaterite CaCO3 crystals of untypical sizes: From submicron to Giant. Mater. Des. 2021, 197, 109220. [Google Scholar] [CrossRef]
- Fadia, P.; Tyagi, S.; Bhagat, S.; Nair, A.; Panchal, P.; Dave, H.; Dang, S.; Singh, S. Calcium carbonate nano- and microparticles: Synthesis methods and biological applications. 3 Biotech 2021, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Vikulina, A.; Voronin, D.; Fakhrullin, R.; Vinokurov, V.; Volodkin, D. Naturally derived nano- and micro-drug delivery vehicles: Halloysite, vaterite and nanocellulose. New J. Chem. 2020, 44, 5638–5655. [Google Scholar] [CrossRef]
- Shenoy, D.B.; Antipov, A.A.; Sukhorukov, G.B.; Möhwald, H. Layer-by-Layer Engineering of Biocompatible, Decomposable Core−Shell Structures. Biomacromolecules 2003, 4, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Vikulina, A.S.; Campbell, J. Biopolymer-Based Multilayer Capsules and Beads Made via Templating: Advantages, Hurdles and Perspectives. Nanomaterials 2021, 11, 2502. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, L.; Bell, M.; Ashwell, R.; Volodkin, D.; Vikulina, A.S. Internal Structure of Matrix-Type Multilayer Capsules Templated on Porous Vaterite CaCO3 Crystals as Probed by Staining with a Fluorescence Dye. Micromachines 2018, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Nagaraja, A.T.; McShane, M.J. Fabrication of Nanocapsule Carriers from Multilayer-Coated Vaterite Calcium Carbonate Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 21193–21201. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, N.; Rose, J.; Prokopović, V.Z.; Vikulina, A.S.; Skirtach, A.; Volodkin, D. Controlling the Vaterite CaCO3 Crystal Pores. Design of Tailor-Made Polymer Based Microcapsules by Hard Templating. Langmuir 2016, 32, 4229–4238. [Google Scholar] [CrossRef] [PubMed]
- Donantan, S.; Yashchenok, A.; Khan, N.; Parakhonskiy, B.; Cocquyt, M.; Pinchasik, B.; Khalenkow, D.; Mohwald, H.; Konrad, M.; Skirtach, A. Loading Capacity versus Enzyme Activity in Anisotropic and Spherical Calcium Carbonate Microparticles. ACS Appl. Mater. Interfaces 2016, 8, 14284–14292. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Borodina, T.N.; Belova, D.D.; Belyakov, S.; Antipina, M.N. Heat-driven size reduction of biodegradable polyelectrolyte multilayer hollow capsules assembled on CaCO3 template. Colloids Surf. B 2018, 170, 312–321. [Google Scholar] [CrossRef]
- She, Z.; Antipina, M.N.; Li, J.; Surkhoruko, G.B. Mechanism of Protein Release from Polyelectrolyte Multilayer Microcapsules. Biomacromolecules 2010, 11, 1241–1247. [Google Scholar] [CrossRef]
- Marchenko, I.; Yashchenok, A.; Borodina, T.; Bukreeva, T.; Konrad, M.; Möhwald, H.; Skirtach, A. Controlled enzyme-catalyzed degradation of polymeric capsules templated on CaCO3: Influence of the number of LbL layers, conditions of degradation, and disassembly of multicompartments. J. Control. Release 2012, 162, 599–605. [Google Scholar] [CrossRef]
- Sun, L.; Xiong, X.; Zou, Q.; Ouyang, P.; Burkhardt, C.; Krastev, R. Design of intelligent chitosan/heparin hollow microcapsules for drug delivery. J. Appl. Polym. Sci. 2016, 134, 44425. [Google Scholar] [CrossRef]
- Yakovlev, N.L.; Kiryukhhin, M.V.; Antipina, M.N.; Susanto, T.T.; Ravi, S.; Adithyavairavan, M.; Sukhorukov, G.B. Secondary Ion Mass Spectrometry of Macromolecules Loading in Individual Polyelectrolyte Multilayer Microcapsules. Aust. J. Chem. 2011, 64, 1295–1298. [Google Scholar] [CrossRef]
- Berth, G.; Voigt, A.; Dautzenberg, H.; Donath, E.; Möhwald, H. Polyelectrolyte Complexes and Layer-by-Layer Capsules from Chitosan/Chitosan Sulfate. Biomacromolecules 2002, 3, 579–590. [Google Scholar] [CrossRef]
- Fan, L.; Körte, F.; Rudt, A.; Jung, O.; Burkhardt, C.; Barbeck, M.; Xiong, X. Encapsulated vaterite-calcite CaCO3 particles loaded with Mg2+ and Cu2+ ions with sustained release promoting osteogenesis and angiogenesis. Front. Bioeng. Biotechnol. 2022, 10, 983988. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Feoktistova, N.A.; Balabushevich, N.G.; Von Klitzing, R.; Volodkin, D. Cooling-Triggered Release from Mesoporous Poly(N-isopropylacrylamide) Microgels at Physiological Conditions. ACS Appl. Mater. Interfaces 2020, 12, 57401–57409. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Stetsyura, I.Y.; Onses, M.S.; Yilmaz, E.; Skirtach, A.G.; Volodkin, D. Mesoporous One-Component Gold Microshells as 3D SERS Substrates. Biosensors 2021, 11, 380. [Google Scholar] [CrossRef]
- Song, J.; Vikulina, A.S.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of hybrid materials. Part-II: The place of organics-on-inorganics in it, their composition and applications. Front. Chem. 2023, 11, 1078840. [Google Scholar] [CrossRef]
- Li, W.; Gao, C. Efficiently Stabilized Spherical Vaterite CaCO3 Crystals by Carbon Nanotubes in Biomimetic Mineralization. Langmuir 2007, 23, 4575–4582. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Vikulina, A.; Cave, G.W.V.; Loughlin, M.; Puddu, V.; Volodkin, D. Vaterite-nanosilver hybrids with antibacterial properties and pH-triggered release. Mater. Today Chem. 2023, 30, 101586. [Google Scholar] [CrossRef]
- Saveleva, M.; Prikhozhdenko, E.; Gorin, D.; Skirtach, A.G.; Yashchenok, A.; Parakhonskiy, B. Polycaprolactone-Based, Porous CaCO3 and Ag Nanoparticle Modified Scaffolds as a SERS Platform With Molecule-Specific Adsorption. Front. Chem. 2020, 7, 888. [Google Scholar] [CrossRef] [PubMed]
- Parakhonskiy, B.V.; Abalymov, A.; Ivanova, A.; Khalenkow, D.; Skirtach, A.G. Magnetic and silver nanoparticle functionalized calcium carbonate particles—Dual functionality of versatile, movable delivery carriers which can surface-enhance Raman signals. J. Appl. Phys. 2019, 126, 203102. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, J.; Groll, J.; Matsusaki, M. Layer-by-layer assembly methods and their biomedical applications. Biomater. Sci. 2022, 10, 4077–4094. [Google Scholar] [CrossRef] [PubMed]
- Szarpak, A.; Cui, D.; Dubreuil, F.; De Geest, B.G.; De Cock, L.J.; Picart, C.; Auze, R. Designing Hyaluronic Acid-Based Layer-by-Layer Capsules as a Carrier for Intracellular Drug Delivery. Biomacromolecules 2010, 11, 713–720. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Vandenbroucke, R.; Guenther, A.; Sukhorukov, G.; Hennink, W.; Sanders, N.; Demeester, J.; De Smedt, S. Intracellularly Degradable Polyelectrolyte Microcapsules. Adv. Mater. 2006, 18, 1005–1009. [Google Scholar] [CrossRef]
- An, Q.; Zhou, Y.; Zhang, Y.; Zhang, Y.; Shi, F. A facile method for the fabrication of covalently linked PAH/PSS layer-by-layer films. RSC Adv. 2014, 4, 5683–5688. [Google Scholar] [CrossRef]
- Schlenoff, J.; Decher, G. Multilayer Thin Film: Sequential Assembly of Nanocomposite Material; Wiley-VCH: Weinheim, Germany, 2006; p. 543. [Google Scholar]
- Becker, A.L.; Johnston, A.P.R.; Caruso, F. Layer-By-Layer-Assembled Capsules and Films for Therapeutic Delivery. Small 2010, 6, 1836–1852. [Google Scholar] [CrossRef]
- Knipe, J.M.; Chen, F.; Peppas, N.A. Enzymatic Biodegradation of Hydrogels for Protein Delivery Targeted to the Small Intestine. Biomacromolecules 2015, 16, 962–972. [Google Scholar] [CrossRef]
- Kurapati, R.; Groth, T.W.; Raichur, A.M. Recent Developments in Layer-by-Layer Technique for Drug Delivery Applications. ACS Appl. Bio Mater. 2019, 2, 5512–5527. [Google Scholar] [CrossRef]
- Sergeeva, A.; Vikulina, A.S.; Volodkin, D. Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals. Micromachines 2019, 10, 357. [Google Scholar] [CrossRef]
- Wulf, A.; Mendgaziev, R.I.; Fakhrullin, R.; Vinokurov, V.; Volodkin, D.; Vikulina, A.S. Porous Alginate Scaffolds Designed by Calcium Carbonate Leaching Technique. Adv. Func. Mater. 2022, 32, 2109824. [Google Scholar] [CrossRef]
- Prokopovic, V.Z.; Vikulina, A.S.; Sustr, D.; Shchukina, E.M.; Shchukin, D.G.; Volodkin, D.V. Binding Mechanism of the Model Charged Dye Carboxyfluorescein to Hyaluronan/Polylysine Multilayers. ACS Appl. Mater. Interfaces 2017, 9, 38908–38918. [Google Scholar] [CrossRef]
- Schnäckel, A.; Hiller, S.; Reibetanz, U.; Donath, E. Fluorescent bead arrays by means of layer-by-layer polyelectrolyte adsorption. Soft Matter 2007, 3, 200–206. [Google Scholar] [CrossRef]
- Pabbathi, A.; Patra, S.; Samanta, A. Structural Transformation of Bovine Serum Albumin Induced by Dimethyl Sulfoxide and Probed by Fluorescence Correlation Spectroscopy and Additional Methods. Chemphyschem 2013, 14, 2441–2449. [Google Scholar] [CrossRef]
- Moody, H.R.; Brown, C.P.; Bowden, J.C.; Crawford, R.W.; McElwain, D.L.S.; Oloyede, A.O. In vitro degradation of articular cartilage: Does trypsin treatment produce consistent results? J. Anat. 2006, 209, 259–267. [Google Scholar] [CrossRef]




| HA/PLL MGs | ALG/PLL MGs | HS/PLL MGs | |
|---|---|---|---|
| ss-CaCO3 | 18.1 ± 1.3 | 16.9 ± 0.8 | 14.6 ± 0.7 |
| ss-MGs | 5.5 ± 1.0 | 15.4 ± 0.8 | 7.8 ± 1.6 |
| DEX-FITC loaded ss-CaCO3 | 9.3 ± 0.7 | 8.3 ± 0.7 | 9.9 ± 0.6 |
| DEX-FITC loaded ss-MGs | 7.3 ± 0.6 | 9.9 ± 0.6 | 9.2 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mammen, M.; Hogg, C.; Craske, D.; Volodkin, D. Formulation and Biodegradation of Surface-Supported Biopolymer-Based Microgels Formed via Hard Templating onto Vaterite CaCO3 Crystals. Materials 2024, 17, 103. https://doi.org/10.3390/ma17010103
Mammen M, Hogg C, Craske D, Volodkin D. Formulation and Biodegradation of Surface-Supported Biopolymer-Based Microgels Formed via Hard Templating onto Vaterite CaCO3 Crystals. Materials. 2024; 17(1):103. https://doi.org/10.3390/ma17010103
Chicago/Turabian StyleMammen, Mariam, Cain Hogg, Dominic Craske, and Dmitry Volodkin. 2024. "Formulation and Biodegradation of Surface-Supported Biopolymer-Based Microgels Formed via Hard Templating onto Vaterite CaCO3 Crystals" Materials 17, no. 1: 103. https://doi.org/10.3390/ma17010103





